Key Clinical Message
TAFRO syndrome have been proposed as a rare variant of Castleman's disease. This article reports a case of a 56-year-old man with TAFRO syndrome who was successfully treated with thalidomide in spite of the refractoriness to prednisolone and tocilizumab. Thalidomide may be one of the treatment options for TAFRO syndrome.
Keywords: Castleman's disease, Interleukin-6, TAFRO syndrome, thalidomide, tocilizumab, vascular endothelial growth factor
Introduction
Castleman's disease (CD) is a relatively rare lymphoproliferative disorder with excessive inflammatory features. Castleman's disease has been histopathologically subclassified into three types, that is, hyaline-vascular (HV), plasma-cell (PC), and mixed types, while the disease has been also subclassified into two entities from the perspective of their clinical presentations, that is, unicentric CD and multicentric CD (MCD). In general, while the former is a localized disease, is often asymptomatic, and is curable by surgical excision of the involved lymph node(s), the latter with systemic lymphadenopathy is frequently accompanied by various systemic manifestations, such as pyrexia, fatigue, organomegaly, and systemic fluid retention. In addition, CD has been etiologically subcategorized into two subtypes based upon the positive- and negative- associations with human immunodeficiency virus (HIV) infection. While most HIV-associated CD patients are positive for human herpes virus (HHV)-8, HHV-8 has been shown to be associated with 40–50% of HIV-negative CD patients. Recently, the newly proposed classification distinguished disease subtypes of MCD based on HHV-8 infection status, which is more closely associated with pathogenesis and response to treatments. Fajgenbaum DC et al. 1 defined HIV-negative and HHV-8-negative MCD as idiopathic MCD (iMCD), and they suggested that one or more underlying pathologic conditions, that is, systemic inflammatory disease, paraneoplastic syndrome and/or a non-HHV-8 viral infection, might drive iMCD with hypercytokinemia. Nevertheless, the precise pathophysiology of MCD remains not to be fully defined, however, the deregulated interplay of various inflammatory soluble factors has been considered to contribute to the development and progression of MCD. In particular, interleukin-6 (IL-6) has been shown to act as a key player in the pathogenesis of MCD 2. Indeed, while anti-HHV therapy and rituximab with or without chemotherapy are required for the treatment of HIV-positive MCD 3, treatments using corticosteroids and/or the anti-IL-6 targeting therapies, that is, tocilizumab (an anti-IL-6 receptor antibody), or siltuximab (an anti-IL-6 antibody), have been highly efficacious against HIV-negative MCD 4,5.
Recent studies have proposed a new disease entity of TAFRO syndrome as a rare variant of CD which is characterized by concomitant thrombocytopenia, anasarca, myelofibrosis, renal dysfunction and organomegaly, in addition to the typical clinical/histological features of MCD 6. While HIV or HHV-8 infection has no relevant association with TAFRO syndrome 7,8, elevation of serum IL-6 has been associated with TAFRO syndrome 9. It has been also reported that the complete remission rates following therapies with corticosteroids and/or IL-6-targeting strategies are lower in TAFRO syndrome than in classical MCD 8, suggesting the possible involvement of various proinflammatory cytokines other than IL-6 in TAFRO syndrome.
Thalidomide is an immunomodulatory therapeutic arsenal against plasma cell malignancies, especially for multiple myeloma, wherein IL-6 plays a crucial role in disease activity. Thalidomide has been shown to be potent in inhibiting tumor necrosis factor (TNF)-α, IL-1, IL-12, and VEGF in addition to IL-6, and can stimulate T cells via its interaction with cereblon 10,11. It has been also demonstrated that thalidomide is capable of decreasing IL-6 levels, lowering C-reactive protein, and, thereby, inducing remission in MCD. In this article, we report a case of a patient with TAFRO syndrome who was successfully treated with thalidomide, in addition to corticosteroid and tocilizumab. Although the initial therapy with prednisolone (PSL) and tocilizumab only achieved partial remission and failed to resolve the ascites, the addition of thalidomide efficiently resolved the intractable ascites.
Case Report
A 56-year-old man was admitted to our hospital complaining of dyspnea, abdominal distension, pyrexia, and systemic lymphadenopathy. Computed tomography revealed the presence of mild hepatosplenomegaly, pleural effusion, pericardial effusion, massive ascites, and systemic lymphadenopathy. In addition, blood examination showed anemia (hemoglobin 8.1 g/dL), increased white blood cells (12.7 × 109/L containing 89% of neutrophils) and thrombocytopenia (76.0 × 109/L), while serological testing showed elevated C-reactive protein (CRP) (11.7 mg/dL (normal range; <0.3)) and alkaline phosphatase (ALP) (1007 IU/L (normal range; 80–260)), hypoalbuminemia of 2.0 g/dL (normal range; 4.1–5.1), renal dysfunction (serum creatinine 1.43 mg/dL (normal range; 0.6–1.1) and positivity for anti-nuclear antibody (Discrete-Speckled type (×1280) and Speckled type (×80) (normal range; < ×40)). The serum IL-6 was increased to 8.1 pg/mL (normal range; ≤4.0 pg/mL), and the plasma vascular endothelial cell growth factor (VEGF) level was elevated to 244 pg/mL (normal range; <115 pg/mL). He was negative for HIV infection. No monoclonal immunoglobulins were identified in his sera or urine. Albumin concentrations in the pleural effusion and ascites were increased to 2.4 g/dL and 3.0 g/dL, respectively, indicating increased vessel permeability. Bone marrow examination showed normal cellularity with slight megakaryocyte hyperplasia, but not plasmacytosis or myelofibrosis. Biopsy of the right inguinal lymph node revealed the histological diagnosis of PC-type MCD (Fig.1), while the in situ hybridization of HHV-8 was negative in the lymph node specimen. He was diagnosed as having TAFRO syndrome based on the pathological findings, laboratory tests and the clinical features. The initial treatment with 1 mg/kg of PSL failed to resolve the series of systemic symptoms. However, the additional therapy of four doses of 4 mg/kg tocilizumab given every other week ameliorated the systemic symptoms, including lymphadenopathy, or pleural effusion, and improved the laboratory data, including anemia, thrombocytopenia, hypoalbuminemia, and CRP elevation persisted. Prednisolone was tapered off during this period; however, massive ascites was refractory to the combination therapy with tocilizumab and PSL (Fig.2A). During this time period, the serum levels of IL-6 and VEGF were further elevated to 188 pg/mL and 996 pg/mL, respectively (Fig.3), which suggested the need for additional therapeutic intervention against the proinflammatory cytokines that might promote vessel hyperpermeability and cause intractable ascites even under treatment with tocilizumab plus PSL therapy. Supported by the previous reports demonstrating the inhibitory effect of thalidomide on proinflammatory cytokines, including VEGF, in patients with refractory ascites and pericardial effusion along with the angiogenic effects 12,13, thalidomide was initiated at 100 mg/day, every other week with the administration of tocilizumab because of the lack of any promising therapeutic strategy for the sublethal disease condition. Before starting thalidomide therapy, the use of thalidomide for this patient with TAFRO was approved by the institutional ethical committee, and the patient was fully given informed consent. His abdominal distention gradually subsided and complete resolution of the ascites was confirmed by CT after approximately 2 months of thalidomide therapy (Fig.2B). Although thalidomide was discontinued due to arthralgia and muscle cramps after 4 months of treatment, there has been no recurrence of ascites developed up to 2 years after the continuance of tocilizumab therapy for 2 years.
Figure 1.
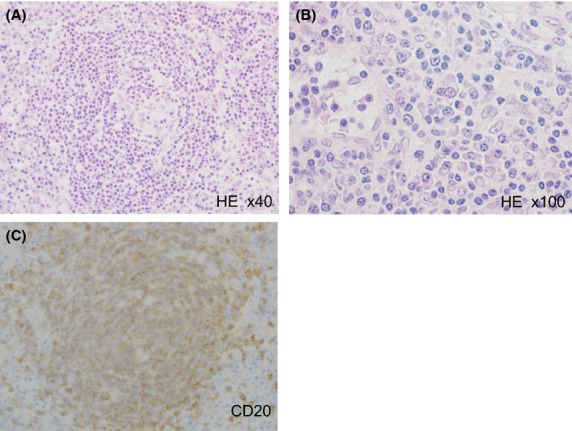
Histological and immunohistochemical findings of the biopsied specimen from the right inguinal lymph node. Hematoxylin and Eosin (HE) staining showed that the biopsied lymph node comprised slightly-indistinct large hyperplastic follicles with expanded mantle zones. Endothelial hyperplasia was also observed in the follicle (A). Prominent plasma cell infiltration was also noted in the interfollicular areas (B). CD20 immunostaining indicated that the follicle comprised B lymphocytes (C).
Figure 2.
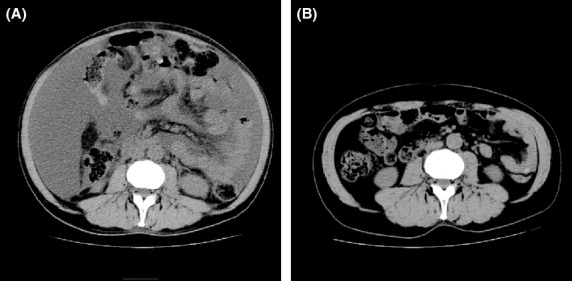
Computed tomography (CT) scan images. Abdominal CT scan views prior (A) and following (B) thalidomide treatment. The massive ascites at the earlier stage (A) was successfully resolved after 2 months of treatment with thalidomide (B).
Figure 3.
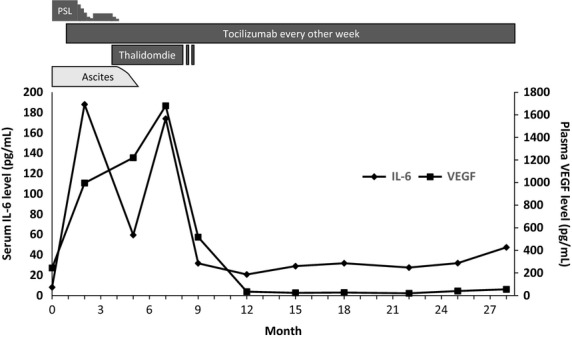
Treatment course and serum interleukin-6 (IL-6) and plasma vascular endothelial cell growth factor (VEGF) levels. The baseline serum IL-6 and plasma VEGF levels were 8.1 and 244 pg/mL, respectively, at diagnosis. These levels increased further to 188 and 1680 pg/mL after the initiation of tocilizumab therapy, and decreased gradually following the addition of thalidomide (Thal) therapy. PSL, prednisolone.
Discussion
There are two major issues to be discussed in association with the clinical course of the present case. First, it is worthwhile to discuss the possible mechanism underlying the tocilizumab-refractory ascites despite the dramatic efficacy of tocilizumab for systemic symptoms other than ascites. When we looked back at the serum IL-6 level and the plasma VEGF level, the data showed prominent increases of both cytokines after the initiation of tocilizumab treatment (Fig.3). One possible explanation for the increase in IL-6 was that blockade of the IL-6 receptor by tocilizumab treatment stimulated the production of IL-6 via a feedback effect 14. Since it has also been suggested that the IL-6 signaling was pivotal in the promotion of VEGF production 15, the plasma VEGF level was expected to decrease following blockade of the IL-6 receptor by tocilizumab 16,17. However, in our case, the plasma level of VEGF continued to increase even after treatment with tocilizumab, and the ascites remained the only symptom that was refractory to tocilizumab, in contrast to other symptoms. It has been shown that several other proinflammatory cytokines, such as IL-1α, TNF-α, and thrombin, can induce VEGF synthesis in peritoneal mesothelial cells 18. Thus, it is possible that the local peritoneal inflammation associated with TAFRO syndrome and the increased VEGF synthesis might be induced not simply by IL-6, but also by other, as yet undetermined, proinflammatory cytokines that promote VEGF production in our case. The second issue to be debated is the mechanism of action of thalidomide on the ascites in our case. Thalidomide has been shown to exhibit a preventive effect on angiogenesis through inhibition of VEGF. Therefore, if blockade of VEGF was the central mechanism in resolving the tocilizumab-refractory ascites by thalidomide treatment, one could expect that the reduction in plasma VEGF would precede the improvement of ascites. However, in our case, the plasma VEGF level remained high with thalidomide therapy, despite the improvement of tocilizumab-resistant ascites, and eventually decreased after the improvement of ascites in our case. Considering the multifaceted immunomodulatory effects of thalidomide, that is, the inhibitory effect on basic fibroblast growth factor-2 and TNF-α, in addition to IL-6 and VEGF 19–21, we speculate that the overexpression/hyperactivation of various cytokines, other than IL-6 or VEGF, was involved in TAFRO syndrome-associated ascites, and that thalidomide resolved the tocilizumab-refractory ascites not by the inhibition of the IL-6/VEGF axis but by the inhibition of, as yet undetermined, proinflammatory soluble factors that function as the upstream mediators for VEGF production. Thus, it is possible that the coincident decline of both IL-6 and VEGF was not requisite for the improvement of ascites but represented the resultant phenomena after the improvement of peritoneal inflammation in our case.
While this report is the first to demonstrate the therapeutic effect of thalidomide for TAFRO syndrome, the effect of thalidomide on MCD has been reported in several cases (Table1) 13,22–27. In those series, the PC type was dominant histologically, and thalidomide was generally effective in both inducing and maintaining remission. Furthermore, thalidomide is effective for plasma cell dyscrasias, such as multiple myeloma, through its immunomodulatory effects. Thus, it is possible that thalidomide is also effective for TAFRO syndrome, which is a subtype of MCD. Indeed, while there has been no report of thalidomide treatment in TAFRO syndrome, other than our case, one MCD case with thrombocytopenia and anasarca reported by Lee et al. 13 seemed to at least partially meet the criteria of TAFRO syndrome, and was induced in partial remission by thalidomide therapy. On the other hand, TAFRO syndrome has been treated with various types of immunosuppressive therapies, including calcineurin inhibitors, corticosteroids, and tocilizumab (Table2) 7–9,28,29. While those immunosuppressive therapies have been largely effective for TAFRO syndrome, caution should be noted for the complication of severe infections. Since the standard therapy has not been established so far, thalidomide can be one of therapeutic options for TAFRO syndrome.
Table 1.
Review of thalidomide therapy for multicentric Castleman's disease and TAFRO syndrome
| Age | N | Histologic type | HIV | HHV-8 | Prior treatment | Thal (mg/day) | Concurrent Tx. with Thal | Response to Thal | Prognosis | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 37 | 1 | PC | − | − | CS | 200 | CS | PR | No relapse with Thal 300 (mg/day) | 13 |
| 33 | 1 | NA | + | + | CHOP, VP-16 | 200 | VP-16 | CR | No relapse with Thal 300 mg (mg/day) | 22 |
| 46 | 1 | PC variant | + | + | None | 200 | Rit, CS | CR | No relapse with Thal 100 mg (mg/day) | 23 |
| 30 | 1 | HV, Mixed | − | − | CS, IVIg | 200 | CS, CsA | CR | No relapse with Thal 200 mg (mg/day) | 24 |
| 38–60 | 3 | 3 PC | − | NA | 2 none, 1 CS+CY | 150 | CS | 3/3 CR | No relapse with Thal 50 (mg/day) | 25 |
| 32–60 | 11 | 6 PC | 7+ | 10+ | 10 none, 1 chrorambucil | 100 | Rit, CS | 10 CR, 1 PD | 3 relapsed with Thal 100 (mg/day), 1 died | 26 |
| 47 | 1 | NA | NA | NA | R-CHOP | 100–200 | CY, CS | PR | CR by Len | 27 |
| 56 | 1 | PC | − | − | Toc, CS | 100 | Toc, CS | CR | No relapse with Toc | Present case |
N, number of patients reported; PC, plasma-cell type; HV, hyaline-vascular type; HIV, human immunodeficiency virus infection; HHV-8, human herpes virus-8 involvement; Tx., therapy; CS; corticosteroid(s), CHOP, cyclophosphamide (CY), adriamycin, vincristine and prednisolone; VP-16, etoposide; Rit, rituximab; IVIg, intravenous immunoglobulin therapy; CsA, cyclosporine A; Toc, tocilizumab; PR, partial remission; CR, complete remission; PD, progressive disease; Thal, thalidomide; Len, lenalidomide; NA, not available.
Table 2.
Review of reported cases with TAFRO syndrome
| Age | N | Symptoms and Laboratory data | Treatment | Clinical course | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thrombo -cytopenia | Anasarca | Myelo -fibrosis | Renal dysfunction | Organo -megaly | IL-6 elevation | VEGF elevation | Histologic type | |||||
| 47–56 | 5 | 5+ | 5+ | 5+ | 2+ | 5+ | 4+ | 2+ | 1 HV | 3 CS, 3 CsA, 1 IVIg, 1 CY | 4 CR, 1 died of CMV infection | 9 |
| 43 | 1 | + | + | − | + | + | + | + | HV | CS, Rit, Toc | CR | 7 |
| 57, 73 | 2 | 2+ | 2+ | 1+ | 2+ | 2+ | 2+ | − | 1 MCD-like 1 mixed | 1 CS, 1 CHOP+VP-16 | 2 died of sepsis | 28 |
| 49 | 1 | + | + | + | − | + | + | + | MCD like | CS, CsA | CR | 29 |
| 47 | 1 | + | + | + | + | + | + | NA | PC | CS, Toc | CR | 8 |
| 56 | 1 | + | + | − | + | + | + | + | PC | CS, Toc, Thal | CR | Present case |
IL-6, interleukin-6; VEGF, vascular endothelial cell growth factor; MCD, multicentric Castleman's disease; CMV, cytomegalovirus.
In conclusion, we report a case of TAFRO syndrome that was successfully treated with thalidomide in addition to tocilizumab, and this case report suggests the possible therapeutic application of thalidomide for TAFRO syndrome for which a standard treatment strategy has not been established to date. As a novel disease concept, it is urgently needed to clarify the pathophysiology of TAFRO syndrome, so that we will be able to develop more rationalistic treatment strategy for TAFRO syndrome which possibly contains agents directed against both IL-6 signaling and VEGF.
Conflict of Interest
None declared.
References
- Fajgenbaum DC, van Rhee F. Nabel CS. HHV-8-negative, idiopathic multicentric castleman disease: novel insights into biology, pathogenesis, and therapy. Blood. 2014;123:2924–2933. doi: 10.1182/blood-2013-12-545087. [DOI] [PubMed] [Google Scholar]
- El-Osta HE. Kurzrock R. Castleman's disease: from basic mechanisms to molecular therapeutics. Oncologist. 2011;16:497–511. doi: 10.1634/theoncologist.2010-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower M. How i treat HIV-associated multicentric castleman disease. Blood. 2010;116:4415–4421. doi: 10.1182/blood-2010-07-290213. [DOI] [PubMed] [Google Scholar]
- Nishimoto N, Kanakura Y, Aozasa K, Johkoh T, Nakamura M, Nakano S, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric castleman disease. Blood. 2005;106:2627–2632. doi: 10.1182/blood-2004-12-4602. [DOI] [PubMed] [Google Scholar]
- van Rhee F, Wong RS, Munshi N, Rossi JF, Ke XY, Fossa A, et al. Siltuximab for multicentric castleman's disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2014;15:966–974. doi: 10.1016/S1470-2045(14)70319-5. [DOI] [PubMed] [Google Scholar]
- Kawabata H, Takai K, Kojima M, Nakamura N, Aoki S, Nakamura S, et al. Castleman-kojima disease (TAFRO syndrome): a novel systemic inflammatory disease characterized by a constellation of symptoms, namely, thrombocytopenia, ascites (anasarca), microcytic anemia, myelofibrosis, renal dysfunction, and organomegaly: a status report and summary of Fukushima (6 June, 2012) and Nagoya meetings (22 September, 2012) J. Clin. Exp. Hematop. 2013;53:57–61. doi: 10.3960/jslrt.53.57. [DOI] [PubMed] [Google Scholar]
- Iwaki N, Sato Y, Takata K, Kondo E, Ohno K, Takeuchi M, et al. Atypical hyaline vascular-type castleman's disease with thrombocytopenia, anasarca, fever, and systemic lymphadenopathy. J. Clin. Exp. Hematop. 2013;53:87–93. doi: 10.3960/jslrt.53.87. [DOI] [PubMed] [Google Scholar]
- Kawabata H, Kotani S, Matsumura Y, Kondo T, Katsurada T, Haga H, et al. Successful treatment of a patient with multicentric castleman's disease who presented with thrombocytopenia, ascites, renal failure and myelofibrosis using tocilizumab, an anti-interleukin-6 receptor antibody. Intern. Med. 2013;52:1503–1507. doi: 10.2169/internalmedicine.52.9482. [DOI] [PubMed] [Google Scholar]
- Takai K, Nikkuni K, Momoi A, Nagai K, Igarashi N. Saeki T. Thrombocytopenia with reticulin fibrosis accompanied by fever, anasarca and hepatosplenomegaly: a clinical report of five cases. J. Clin. Exp. Hematop. 2013;53:63–68. doi: 10.3960/jslrt.53.63. [DOI] [PubMed] [Google Scholar]
- Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26:2326–2335. doi: 10.1038/leu.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichholz A, Merchant S. Gaya AM. Anti-angiogenesis therapies: their potential in cancer management. Onco. Targets Ther. 2010;3:69–82. doi: 10.2147/ott.s5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FC. Merchant SH. Alleviation of systemic manifestations of multicentric castleman's disease by thalidomide. Am. J. Hematol. 2003;73:48–53. doi: 10.1002/ajh.10310. [DOI] [PubMed] [Google Scholar]
- Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N. Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-il-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and castleman disease. Blood. 2008;112:3959–3964. doi: 10.1182/blood-2008-05-155846. [DOI] [PubMed] [Google Scholar]
- Cohen T, Nahari D, Cerem LW, Neufeld G. Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J. Biol. Chem. 1996;271:736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Song J, Sugimoto M, Hagihara K, Kishimoto T, Yoshizaki K, et al. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003;48:1521–1529. doi: 10.1002/art.11143. [DOI] [PubMed] [Google Scholar]
- Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (satori): significant reduction in disease activity and serum vascular endothelial growth factor by il-6 receptor inhibition therapy. Mod. Rheumatol. 2009;19:12–19. doi: 10.1007/s10165-008-0125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl-Weber S, Cohen CD, Haslinger B, Kretzler M. Sitter T. Vascular endothelial growth factor production and regulation in human peritoneal mesothelial cells. Kidney Int. 2002;61:570–578. doi: 10.1046/j.1523-1755.2002.00143.x. [DOI] [PubMed] [Google Scholar]
- Li X, Liu X, Wang J, Wang Z, Jiang W, Reed E, et al. Thalidomide down-regulates the expression of VEGF and BFGF in cisplatin-resistant human lung carcinoma cells. Anticancer Res. 2003;23:2481–2487. [PubMed] [Google Scholar]
- Shannon E, Noveck R, Sandoval F, Kamath B. Kearney M. Thalidomide suppressed interleukin-6 but not tumor necrosis factor-alpha in volunteers with experimental endotoxemia. Transl. Res. 2007;150:275–280. doi: 10.1016/j.trsl.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Lv P, Li HY, Ji SS, Li W. Fan LJ. Thalidomide alleviates acute pancreatitis-associated lung injury via down-regulation of NFκB induced TNF-α. Pathol. Res. Pract. 2014;210:558–564. doi: 10.1016/j.prp.2014.04.022. [DOI] [PubMed] [Google Scholar]
- Jung CP, Emmerich B, Goebel FD. Bogner JR. Successful treatment of a patient with HIV-associated multicentric castleman disease (MCD) with thalidomide. Am. J. Hematol. 2004;75:176–177. doi: 10.1002/ajh.10467. [DOI] [PubMed] [Google Scholar]
- Stary G, Kohrgruber N, Herneth AM, Gaiger A, Stingl G. Rieger A. Complete regression of HIV-associated multicentric castleman disease treated with rituximab and thalidomide. Aids. 2008;22:1232–1234. doi: 10.1097/QAD.0b013e3282fa75ce. [DOI] [PubMed] [Google Scholar]
- Miltenyi Z, Toth J, Gonda A, Tar I, Remenyik E. Illes A. Successful immunomodulatory therapy in castleman disease with paraneoplastic pemphigus vulgaris. Pathol. Oncol. Res. 2009;15:375–381. doi: 10.1007/s12253-008-9133-x. [DOI] [PubMed] [Google Scholar]
- Zhao X, Shi R, Jin X. Zheng J. Diffuse hyperpigmented plaques as cutaneous manifestation of multicentric castleman disease and treatment with thalidomide: report of three cases. J. Am. Acad. Dermatol. 2011;65:430–432. doi: 10.1016/j.jaad.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Ramasamy K, Gandhi S, Tenant-Flowers M, Ceesay M, Corderoy S, Marcus R, et al. Rituximab and thalidomide combination therapy for castleman disease. Br. J. Haematol. 2012;158:421–423. doi: 10.1111/j.1365-2141.2012.09157.x. [DOI] [PubMed] [Google Scholar]
- Szturz P, Adam Z, Rehak Z, Koukalova R, Kren L, Moulis M, et al. Salvage lenalidomide in four rare oncological diseases. Tumori. 2013;99:e251–e256. doi: 10.1177/030089161309900524. [DOI] [PubMed] [Google Scholar]
- Masaki Y, Nakajima A, Iwao H, Kurose N, Sato T, Nakamura T, et al. Japanese variant of multicentric castleman's disease associated with serositis and thrombocytopenia–a report of two cases: is TAFRO syndrome (Castleman-Kojima disease) a distinct clinicopathological entity? J. Clin. Exp. Hematop. 2013;53:79–85. doi: 10.3960/jslrt.53.79. [DOI] [PubMed] [Google Scholar]
- Inoue M, Ankou M, Hua J, Iwaki Y. Hagihara M. Complete resolution of TAFRO syndrome (thrombocytopenia, anasarca, fever, reticulin fibrosis and organomegaly) after immunosuppressive therapies using corticosteroids and Cyclosporin A: a case report. J. Clin. Exp. Hematop. 2013;53:95–99. doi: 10.3960/jslrt.53.95. [DOI] [PubMed] [Google Scholar]


