Abstract
Chitosan is a widely used biocompatible biomaterial in the tissue regeneration, but its utility and application in the tissue morphogenesis of salivary gland remains unclear. The study aimed to explore the effects of chitosan on the epithelial morphogenesis of submandibular gland (SMG). With chitosan, the branching morphogenesis of the whole SMG explant was facilitated, and the morphogenetic-promoting effects of mesenchymal tissue on SMG were further enhanced. Furthermore, chitosan was competent to induce recombined SMG epithelium to form branches in the serum-free condition independently. In the presence of chitosan, the morphogenetic efficacy of mesenchyme-derived growth factors responsible for epithelial morphogenesis including fibroblast growth factors 7, fibroblast growth factor 10 and hepatocyte growth factor increased. The specific epithelial phenotype induced by individual growth factor, which was required for the accomplishment of salivary epithelial morphogenesis, was promoted by chitosan. Moreover, the proliferative and the chemotactic properties of these growth factors towards the SMG epithelia were also reinforced by chitosan. Therefore, in orchestrating and intensifying the essential mesenchyme-derived growth factors, chitosan is versatile in mediating SMG epithelium to form a predetermined phenotype more efficiently and comprehensively. This study suggested that chitosan is a morphogenetic-regulating biomaterial for salivary tissue, which might be useful for the future salivary gland investigation and regeneration.
Keywords: chitosan, salivary gland, epithelium, morphogenesis, branching
Introduction
Salivary gland is an important glandular organ in regulating saliva production and secretion, which is essential for keeping oral and dental health, aiding digestion and nutrition and assisting mucosal regeneration [1]. The destruction of salivary gland, which frequently results from the disease or therapeutic treatments, might lead to xerostomia, a common clinical problem which largely deteriorates the quality of life. To date, the mainstream of treating dysfunctional salivary gland is based on the saliva substitutes [2]. Nonetheless, for the purpose of treating dysfunctional salivary gland, the replacement with new functional tissues might be more ideal.
The salivary gland is formed by branching morphogenesis, a ubiquitous developmental process resulted from the reciprocal interaction between epithelium and mesenchyme. For example, the murine submandibular gland (SMG), a major gland responsible for saliva secretion, is formed by branching morphogenesis [3]. It is initiated from a condensation of oral epithelium and forms a single epithelial bud with the appearance of clefts under the instruction of mesenchyme. Subsequently, the successive deepening of the clefts leads to the formation of new buds. After repeating the process for numerous times, the epithelial buds finally develop the ramified structures [3, 4].
Although the mesenchyme-free SMG epithelium is able to undergo branching when embedded in the components of basement membranes [5–7], the morphogenetic information originating from the mesenchyme is still critical to the developmental process. During SMG development, mesenchyme might serve a major role in the morphogenetic direction and growth support [8–10]. For example, in the heterotypic tissue recombination of salivary gland mesenchyme, the recombined epithelia from other tissues take on the branching patterns mimicking the salivary glands [11–14]. Therefore, the interaction between highly plastic epithelium and surrounding mesenchyme is requisite for the establishment of complete branching [3].
Therefore, for the purpose of regenerating functional salivary glands, the recapitulation of branching process, which creates adequate numbers of functional units in the organized glandular structures, is required. Without the aid of branching process, the numbers of cells and the space for regenerating a functional salivary gland largely increase. Although the intrinsic regulating mechanisms of salivary gland branching have been well explored [3, 15], the biomaterial approaches, which aim to reproduce the branching process for tissue regeneration, have seldom been investigated. Therefore, for salivary gland regeneration, to find out the potential biomaterial which is capable of enhancing the mesenchymal functions in regulating the epithelial branching might be a promising approach. Previously, we have shown that the chitosan membranes were competent to promote salivary gland branching by providing an interactive environment for cultured salivary tissues [16]. It was found that chitosan membrane was a tissue-favoured substratum, in which the salivary mesenchymal cells were prone to deposit essential extracellular matrices [16]. In the current work, in agreement with the dominant role of mesenchyme in instructing and supporting the morphogenesis of salivary gland, we tried to investigate the effects of chitosan on the SMG mesenchyme, the pivotal component in modulating SMG epithelial morphogenesis. The results demonstrated that, in the presence of chitosan, SMG epithelial cells responded to the mesenchymal morphogenetic information more efficiently, and formed the specific tissue phenotypes which were essential for the branch formation. This study provides a novel insight into the role of chitosan in facilitating SMG epithelial morphogenesis by regulating the corresponding mesenchyme. The results might be promising by demonstrating the potential of chitosan to be used as a morphogenetic regulator in the salivary gland organogenesis and regeneration.
Materials and methods
Medium and reagents
The SMG medium was composed of Dulbecco’s modified eagle medium: nutrient mixture F-12 (DMEM/F12) medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 150 μg/ml vitamin C and 50 μg/ml transferrin as previously described [15]. In the following assays, the SMG medium was used and presented as the negative control SMG medium. Chitosan (448869, Mr: 612 kD) was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). A 2 wt.% (W/V) chitosan solution was prepared by dissolving chitosan in 1 M acetic acid. To prepare the chitosan-containing SMG medium (Chi-M), chitosan solution was mixed with SMG culture medium (0.3 mg/ml) and neutralized with NaOH [17, 18]. The Chi-M without additives was prepared in the same way, but by mixing with the SMG medium without the supplement of transferrin and vitamin C. On the other hand, the mock control SMG medium (M) was prepared as that of the Chi-M having same amount of acetic acid and NaOH added but without chitosan. The mock control medium with serum was prepared by adding 10% fetal calf serum (Gibco, Gaithersburg, MD, USA) in the mock control medium.
Submandibular glands ex vivo organ culture
SMG explants were dissected from E13 ICR mice. Animal protocols were approved by the National Taiwan University Animal Care and Use Committee. The explants were cultured on the membranes (Nuclepore®, Whatman, Clifton, NJ, USA) in a submerged fashion of culture medium with an air/medium interface [19, 20]. All explants were cultured at 37°C in a humidified 5% CO2/95% air atmosphere. Explants were photographed and the branching numbers were measured at the indicated time. SMG branching numbers were presented for each explant as the fold change of bud, which was obtained from dividing the buds measured at each time-point by the buds measured at 0 hour. In the coculture assay, the dissected SMG explants were cultured in mock control and Chi-M, respectively. The mesenchymal rudiments dissected from the peripheral tissue adjacent to SMG were evenly placed in both mock control and Chi-M [15]. Each experimental set was repeated at least three times. The statistic analysis of the SMG budding numbers were counted and evaluated by t-test, paired t-test and ANOVA, where appropriate.
Culture of SMG epithelium
SMG explants were isolated from E13 embryos of ICR mice. After isolation, the explants were placed in Hanks’ balanced salt solution (Roche Molecular Biochemicals, Indianapolis, IN, USA). After treatment with 1.6 U/ml dispase (BD Bioscience, San Jose, CA, USA) at 37°C for 20 min epithelia were mechanically separated from mesenchyme with fine forceps in Hanks’ solution containing 10% bovine serum albumin. The epithelial rudiments were placed upon the culture dish, covered with growth factor-reduced Matrigel (BD Bioscience, diluted in 1:1 with mock control medium) [5, 20]. Explants were cultured in the medium at 37°C in 5% CO2/air. In the experiments with exogenous growth factors, one lobule of mesenchyme-free SMG epithelium dissected from E13 SMG explants was prepared as mentioned above. The recombinant fibroblast growth factor 7 (FGF7), fibroblast growth factor 10 (FGF10) (R&D Systems, Minneapolis, MN, USA) and hepatocyte growth factor (HGF) (Sigma-Aldrich) were supplemented with indicated concentrations, respectively. A range of dose was examined preliminarily for individual exogenous growth factor, but only the lowest concentration sufficient to induce the formation of distinct morphogenesis was presented and analysed.
Mesenchyme recombination assay
In the tissue recombination assays, the procedure was performed as described previously with some modifications [21]. In brief, dissected E13 SMG rudiments were treated with dispase (1.6 U/ml, BD Bioscience) at 37°C for 20 min. After incubation, the tissue fragments were thoroughly washed and carefully separated mechanically to isolate the epithelial and mesenchymal parts. Next, an isolated SMG epithelial explant was recombined with SMG mesenchymal rudiments. Subsequently, the recombinants were cultured on the membranes with mock control, mock control with serum and chitosan-containing medium in the same manner as that of the whole explant culture. Photographs were taken by using microscopy in a time-lapse manner.
Cell proliferation assay
To label the proliferative cells after culture, the SMG epithelial explants cultured in the indicated experimental conditions for 48 hrs were incubated with 10 μM 5-bromo-2′-deoxy-uridine (BrdU) (BrdU Labeling and Detection Kit 1, Roche Molecular Biochemicals, Mannheim, Germany) for 90 min. at 37°C. After incubation, the epithelial rudiments were washed thoroughly in phosphate buffered saline (PBS) and were subsequently fixed in 50 mM glycine in 70% ethanol, pH 2.0, for 20 min. at –20°C. After fixation and washing, SMG epithelium was incubated with anti-BrdU antibody (1:10) for 2 hrs at 37°C. Finally, the fluorescence of labelled cells was developed by incubation with secondary antibody. Immunofluorescent-labelled cells were detected and photographed by a confocal microscope (Leica SP-5), and the representative sections were shown. For quantification of the proliferative activities, the BrdU staining was analysed by MetaMorph software. The green fluorescent pixels were measured and expressed as a ratio relative to nuclei-stained pixels per unit area from all optical sections of the explants [20].
Chemotactic assay with HGF-soaked beads
To investigate the chemotactic properties of HGF, one lobule of mesenchyme-free SMG epithelium dissected from E13 SMG explants was prepared. The chemotactic assay was performed with some modifications [22]. In brief, the acrylic beads (Sigma-Aldrich) were washed thoroughly with PBS first. Then the beads with the similar diameters, around 150 μm, were selected for chemotactic assays. The M and Chi-M beads were prepared by immersing the beads in mock control and Chi-M for 24 hrs. Then the beads were soaked with 0.1 μg/ml purified recombinant HGF protein (Sigma-Aldrich) for another 24 hrs to prepare HGF M and HGF Chi-M beads. During chemotactic assays, the isolated epithelium was placed at a distance of 500 μm away from the beads, and the entire setting was embedded in Matrigel (BD Bioscience). After gelation, the whole system was cultured in SMG medium, and the migration of epithelium was photographed every 24 hrs.
Results
Chitosan promotes branching morphogenesis of SMG explants
SMG explants were first cultured in medium for 24 hrs. In the assays of Fig. 1, there were two control groups. The explants cultured in the SMG medium was marked as the negative control group while those cultured in the mock control SMG medium were presented as the mock control group. Between the negative control group and the mock control group, no significant difference of the SMG branching number was noted (P > 0.05). When chitosan was added in the culture, SMG branching was accelerated. The increase of SMG branch number was statistically significant, compared to that of the mock control group (P < 0.001). Furthermore, because transferrin and vitamin C were routinely used in the SMG explants culture medium [15], the branching morphogenesis of SMG explants were also cultured in the Chi-M without any additive. In the setting, the branching-promoting effects of chitosan were still found, indicating that the result was not simply an enhancing effect of the culture additives caused by chitosan.
Figure 1.
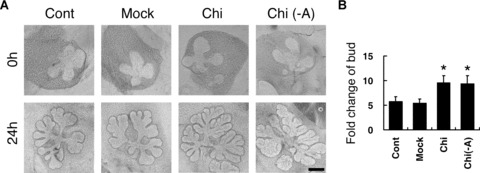
(A) Effects of chitosan on branching morphogenesis of SMG explants. SMG explants were cultured in negative control SMG medium (Cont), mock control SMG medium (Mock), chitosan-containing SMG medium (Chi-M), and chitosan-containing SMG medium without additives (Chi(-A)), respectively. Photographs were taken at 0 and 24 hours of culture. (B) Quantification was performed at 24 hours and was presented as the fold change of bud. Asterisks (*) denote significant differences (P < 0.001) compared with the mock control. Scale bars = 100 μm.
The branching-promoting effects of mesenchyme were enhanced by chitosan
To further explore the effects of chitosan on the developing SMG, the mesenchyme-free epithelia were cultured with chitosan. It has been reported that mesenchyme-free SMG epithelium might degrade without the supplement of mesenchyme-derived growth factors [3]. In accordance with the previous study, in the culture without exogenous growth factors, the mesenchyme-free SMG epithelial explants were unable to develop branching phenotypes, even in the Chi-M (Fig. 2A). On the other hand, in the coculture with the mesenchymal rudiments dissected from SMG peripheral tissue, the SMG budding ratio increased [22]. The effects were significantly greater in the Chi-M compared to those of the mock control group (P < 0.01; Fig. 2B). During SMG epithelial morphogenesis, the SMG mesenchyme played dominant roles in guiding the formation of branches [11]. It had also been found that the adjacent mesenchymal tissue of SMG exerted positive effects on the morphogenesis and the branch formation of the whole SMG explants [22, 23]. Thus, the results suggested the branching-promoting effects of chitosan on the SMG morphogenesis might stem from its cooperation with the surrounding mesenchyme. It raised the possibility that, although chitosan alone could not completely substitute the role of mesenchyme, the branching-promoting effects of mesenchyme for SMG morphogenesis could be enhanced in the presence of chitosan.
Figure 2.
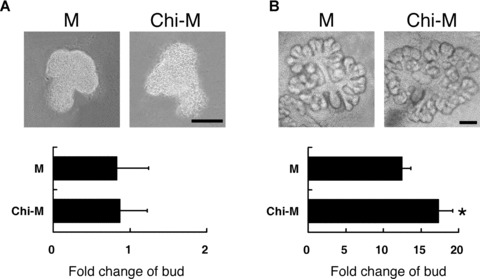
(A) Effects of chitosan on the morphogenesis of SMG epithelium. Representative pictures of SMG epithelium cultured in mock control (M) and chitosan-containing SMG medium (Chi-M) without exogenous growth factors were shown after 24 hours of culture. Quantification was performed at 24 hours and was presented as the fold change of bud. Scale bar = 100 μm. (B) Effects of chitosan on the branching morphogenesis of SMG explants cocultured with the SMG adjacent mesenchymal tissue. The SMG explants were cultured in mock control (M) and chitosan-containing SMG medium (Chi-M) for 24 hours. Quantification was performed at 24 hours and was presented as the fold change of bud. Asterisk (*) denotes a significant difference (P < 0.01) compared with the mock control. Scale bars = 100 μm.
Chitosan effects on SMG epithelium morphogenesis with homotypic mesenchymal recombination in serum-free culture
Because the branching-promoting effects of chitosan presumably originated from the SMG mesenchyme, the dissected SMG mesenchyme was recombined with SMG epithelium in the presence of chitosan. Previously, the recombination assay of SMG had been routinely performed in the serum-containing medium [21, 24, 25]. In order to compare the effect of chitosan in the serum-free culture with that of the serum-containing culture, the serum-containing medium was also prepared and assayed (Fig. 3). In the combination with SMG mesenchymal rudiments, SMG epithelium demonstrated tissue plasticity in remodelling original phenotypes. After culturing for 24 hrs, the epithelial cells proceeded with shape remodelling and the whole epithelial cells reorganized to form an epithelial spheroid in all groups (Fig. 3). Meanwhile, the recombined mesenchymal rudiments were also rearranged and organized into a mesenchymal mass surrounding the epithelium. In accordance with the previous studies [21, 24, 25], in the serum-containing group, the size of the epithelial explants increased compared to the original size and the epithelial budding initiated (Fig. 3). After 48 hrs, in the mock control group, the epithelial explant remained the original morphology but with a shrinkage in the epithelial size. Nonetheless, somewhat surprisingly, the explants cultured in Chi-M not only increased epithelial size, but also started to exhibit some distinct branches and clefts around the periphery of the explants, inferring a possibility of the initiation of branches formation (Fig. 3). After 72 hrs, in the mock control group, the sizes of the recombinants were reduced without any morphogenetic alteration. However, in the chitosan-containing group, the explants demonstrated remarkable morphological changes with well-developed branches. In our culture systems, the medium used for cultivating SMG explants were serum-free. Thus, the enhancing morphogenetic effects of chitosan were proposed to originate from the combined mesenchyme. Thus, these results provided evidence that, in the serum-free culture system, the epithelial morphogenesis induced by mesenchyme could be maintained and promoted by chitosan. In the presence of chitosan, SMG epithelium was able to interact with recombined mesenchyme, and proceeded morphological reorganization and formed branches without serum.
Figure 3.
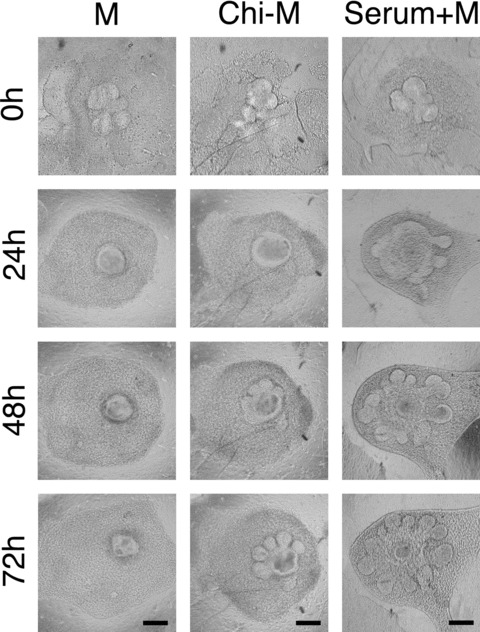
Recombination assay of SMG epithelium with homotypic mesenchyme. Isolated SMG epithelial explants were recombined with mesenchymal rudiments. The recombinants were cultured in mock control medium (M), chitosan-containing medium (Chi-M), or mock control medium added with serum (Serum+M). The photographs were taken in a time-lapse manner with a 24-hour interval. Scale bars = 100 μm.
Chitosan effects on SMG epithelial morphogenesis induced by FGF7, FGF10 and HGF
Because the morphogenetic effects of chitosan presumably stemmed from the cooperation with the mesenchymal tissues, the mesenchyme-derived morphogenetic factors, such as FGF7, FGF10 and HGF, were examined, respectively [20, 22, 26]. In the current assay, although chitosan was also effective in higher amounts of growth factors (data not shown), only the lowest concentration which was sufficient to induce the morphogenesis of SMG epithelium was presented and analysed. Thus, the morphogenetic effects of individual factors were not saturated and the differences of the phenotypes between each group could be observed more clearly. When 10 ng/ml FGF7 was added in the mock control medium, SMG epithelial explants were unable to develop well-shaped lobes and only formed tiny epithelial protrusions (Fig. 4). On the contrary, with the same concentration of FGF7, SMG epithelial explants developed well-organized lobes in the Chi-M (Fig. 4). For FGF10, the effects of ductal elongation and stalk formation were not remarkable in the mock control medium with a concentration of 200 ng/ml. Nonetheless, in the presence of chitosan, SMG epithelial explants showed duct elongation and formation (Fig. 5). Similarly, the Chi-M with 100 ng/ml HGF successfully promoted SMG epithelial explants to spread and to enlarge explant size, which was not obviously discernible in mock control medium with similar HGF concentrations (Fig. 6). Together, the results indicated that, in the presence of chitosan, the distinct morphogenetic processes induced by particular branching growth factors were largely augmented by chitosan. The results suggested a possible role of chitosan to serve as a progression factor for mesenchyme-derived factors in conducting morphogenesis of SMG epithelium.
Figure 4.
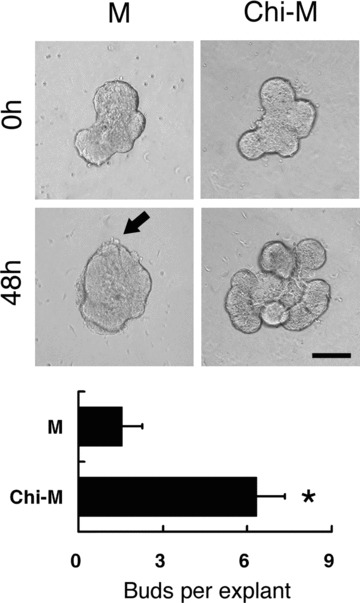
SMG epithelial explants cultured with 10 ng/ml FGF7. Paired epithelial explants were cultured in mock control medium (M) and chitosan-containing medium (Chi-M) respectively. With the addition of FGF7, remarkable lobular formation was found in the chitosan-containing group. In the mock control group, no new lobes were formed, but only with an epithelial protruding on the surface (arrow). Quantification was performed by counting the number of buds per explant at 48 hours of culture. Asterisk (*) denotes a significant difference compared with the mock control group (P < 0.01). Scale bar = 100 μm.
Figure 5.
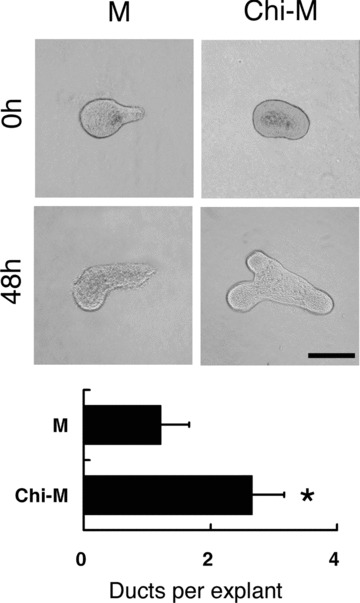
Effects of chitosan in FGF10-induced morphogenesis of SMG epithelium. One lobule of paired SMG epithelial explants was cultured for 48 hours in either mock control medium (M) or chitosan-containing medium (Chi-M) containing 200 ng/ml FGF10. With FGF10, the formation and elongation of duct-like structures were found in the chitosan-containing group. In the mock control group, the elongation of duct-like structure was found without new duct formation. The result of quantification was shown in bar graphs by counting the number of duct-like structures per explants at 48 hours of culture. Asterisk (*) denotes a significant difference compared with the mock control group (P < 0.01). Scale bar = 100 μm.
Figure 6.
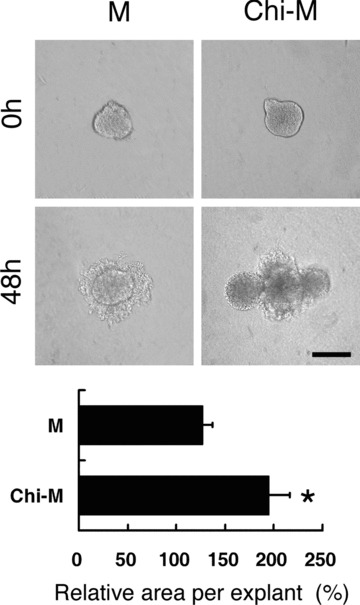
Effects of chitosan in HGF-induced SMG epithelial morphogenesis. One lobule of paired SMG epithelial explant was cultured for 48 hours in either mock control medium (M) or chitosan-containing medium (Chi-M) with 100 ng/ml HGF. With HGF, the increased epithelial area and protrusions were found in the chitosan-containing group. In the mock control group, the area of explants extended to a lesser extent. The relative area was presented as the area ratio by counting explants areas of 48 hours over 0 hour. Asterisk (*) denotes a significant difference compared with the mock control group (P < 0.01). Scale bar = 100 μm.
Chitosan effects on SMG cell proliferation induced by FGF7, FGF10 and HGF
Next, to further investigate the chitosan effects on SMG epithelium in the cellular level, cell proliferation labelled by BrdU incorporation was analysed. FGF7, FGF10 and HGF had been well explored for their capacities in inducing cell proliferation of SMG epithelium [20, 22]. In our results, SMG epithelium showed a proliferative feature with fluorescence-labelled cells located in the emerging lobules with FGF7. In the Chi-M, the number of fluorescent-labelled cells increased. These cells were more concentrated and showed a localized pattern over the emerging epithelium (Fig. 7). Similarly, in the SMG epithelium incubated with FGF10, the proliferative cells were distributed in the epithelial tips. Compared to those in the mock control group, more labelled cells were concentrated in the protruding parts of the epithelial explants in the chitosan-containing group (Fig. 7). In HGF-treated SMG epithelium, the proliferative cells were located in the periphery of explants in both groups. In the chitosan-containing group, more fluorescence-positive cells were found within the extending part of explants (Fig. 7). Quantitatively, with the supplement of exogenous growth factors, the proliferation increased significantly in the explants cultured in the Chi-M compared to those in the mock control medium in all groups, indicating that the mitogenic capacities of the growth factors were generally augmented (Fig. 7).
Figure 7.
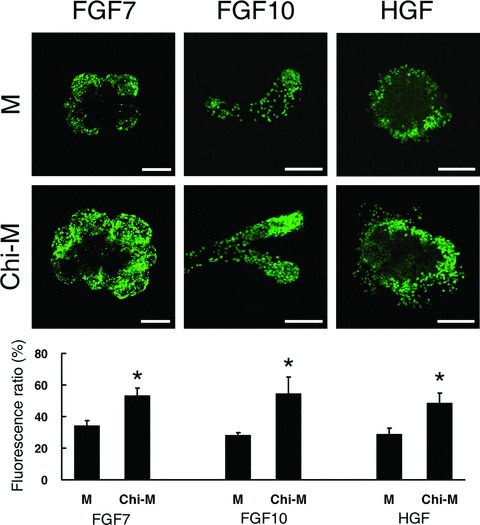
Chitosan effects on SMG epithelial proliferation induced by FGF7, FGF10, and HGF after 48 hours culture. FGF7 (10 ng/ml), FGF10 (200 ng/ml), and HGF (100 ng/ml) were added respectively in either mock control medium (M) or chitosan-containing medium (Chi-M). Each growth factor stimulated the epithelial proliferation, which is shown by BrdU labeling (green). The green fluorescent pixels were measured and expressed as a ratio relative to nuclei-stained pixels per unit area from all optical sections of the explants. Asterisks (*) denote significant differences (P < 0.01) compared to the explants cultured in the mock control medium of each group. Scale bars = 100 μm.
The chemotactic capacity of HGF beads with chitosan
Despite the promoting effects on cell proliferation, HGF was also shown to be chemotactic for SMG epithelium [22]. By using HGF-soaked beads, SMG epithelium demonstrated migratory abilities towards the source of HGF [22]. Therefore, in order to further elucidate whether chitosan also affected the chemotactic properties of HGF, the epithelium and bead were placed at a distance of 500 μm for an analysis of epithelial chemotaxis. Because the distance between the bead and the SMG epithelial rudiments in our study was farther than that previously described [22], the chemotactic effect of HGF with the same concentration might be diminished and the migration of SMG explants towards the bead might be limited. In the culture with HGF M bead, though with lesser effects, SMG epithelial rudiments started to proliferate and formed epithelial buds towards the bead, which was not found in the group with M bead (Fig. 8). On the contrary, in the HGF Chi-M beads, the size of the epithelial rudiment increased after 24 hrs of culture, further confirming that the proliferation properties of HGF were enhanced by chitosan. Moreover, apart from the increasing size, SMG epithelial explants exhibited a greater migratory ability towards the HGF Chi-M bead (Fig. 8). After 24 hrs incubation, the epithelial explant started to migrate towards the HGF-bead and had already moved away from the original site where it was initially located. Thereafter, the epithelial explant migrated more closely to the beads, and finally completely surrounded and engulfed the bead (Fig. 8). Thus, these results indicated that, in the presence of chitosan, the mitogenic and motogenic properties of HGF were further enhanced, which increased the size and promoted the chemotactic behaviours of SMG epithelial explants.
Figure 8.
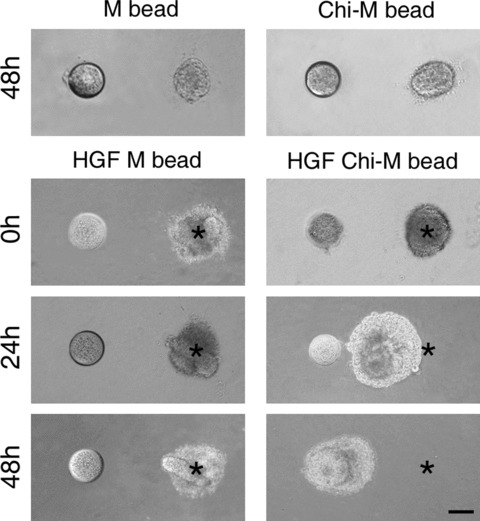
HGF induced chemotaxis and proliferation of SMG epithelium. One lobule of SMG epithelial explant and a bead were placed at a distance of 500 μm. The asterisks marked the original location of the epithelial explant. No proliferation or migration was found in the beads soaked with mock control medium (M bead) or chitosan-containing medium (Chi-M bead). The bead soaked with mock control medium and HGF (HGF M bead) demonstrated a weak chemotactic capacity toward epithelial explants. In the bead soaked with chitosan-containing medium and HGF (HGF Chi-M bead), the epithelial explant increased in size, migrated, and finally surrounded and engulfed the bead after 48-hour culture. Scale bar = 100 μm.
Discussion
In the current work, the effect of chitosan on the morphogenesis of salivary gland was investigated. It was shown that chitosan was capable of stimulating salivary gland branching when it was supplemented in the culture system. Chitosan accelerated epithelial morphogenesis in the presence of mesenchymal tissues, even without serum in the culture. By incorporating with mesenchyme-derived branching factors, chitosan stimulated SMG epithelium to form distinct phenotypes more efficiently, suggesting that the desired biological functions of the factors were reinforced for the epithelial morphogenesis.
SMG morphogenesis results form the interaction between epithelium and mesenchyme [10]. It has been shown that, in the absence of mesenchyme, SMG epithelium alone is unable to grow and form branches [19]. On the other hand, with mesenchyme, no matter prepared from a recombination or separated from a filter, the epithelium grows and demonstrates prolific branching [19, 21]. Even in the completely dissociated SMG epithelial cells, they develop a more organized structure and elicit more robust branching in the presence of mesenchyme [27]. It is evident that during the ectodermal organogenesis, such as salivary glands, the morphogenesis is directed by mesenchyme [28]. In a heterotypic recombination of mesenchyme and epithelium, the heterotypic epithelium develops phenotypes which are seemingly reminiscent of the native epithelium belonging to the original mesenchymal tissue [21]. Therefore, although SMG epithelium plays a pivotal role in the morphogenesis, the growth support and the morphogenetic instructions from the mesenchyme are also critical to the establishment of salivary gland structures.
During branching morphogenesis, the mesenchyme-derived factors might act through autocrine or paracrine signalling, which appear as diffusible growth factors or extracellular matrix components. In the salivary glands, numerous growth factors have been implicated in the developmental processes, such as FGF7, FGF10 and HGF [6, 20, 22]. When they are added in the mesenchyme-free SMG explants, they are competent to facilitate the formation of specific epithelial phenotypes, which are altogether advantageous to the accomplishment of salivary epithelial morphogenesis. In addition to the diffusible growth factors, It is evident that glycosaminoglycan (GAG), a major component of SMG extracellular matrices, are essential in the salivary morphogenesis [29, 30]. During development, GAG accumulates at the epithelium with active branching [31], and serves as a reservoir of required growth factors [3, 24]. With GAG, growth factors are presented in an appropriate temporal and spatial manner, which is beneficial to the precisely controlled morphogenesis of SMG.
Because GAG plays a major role in the endogenous morphogenetic process of salivary tissues, to use the biomaterial which mimics GAG in structure and function might be a logical approach for salivary tissue regeneration. Accordingly, chitosan, a widely used biocompatible substrate sharing the similar structures with GAG, might be an appropriate candidate [32, 33]. Chitosan is a polymer composed of N-acetyl-glucosamine and glucosamine groups, and thereby largely resembles GAG in structure and serves as an analogue for related functions [34]. Therefore, it is likely that the branching-promoting effects of chitosan stem from its interaction with GAG, or like GAG, to recruit numerous essential branching growth factors for desired biological responses. Actually, chitosan is able to interact with GAG directly. They can easily form a complex ionically or covalently [35, 36]. On the other hand, it is evident that chitosan is competent to recruit and bind growth factors from surrounding environments by forming polyelectrolyte complexes [37, 38]. When bound to chitosan, these growth factors could be protected from degradation [39]. Therefore, by retaining and concentrating the essential factors, the desired biological functions are augmented by chitosan [40]. In the current work, the specific functions of the morphogenetic factors towards the SMG epithelium, such as FGF7, FGF10 and HGF, were specifically reinforced by chitosan (Figs 4–6), providing further evidence that chitosan serves as a progression factor in promoting SMG epithelial morphogenesis.
Based on the potential of chitosan in promoting the functions of mesenchymal-related growth factors, the current result indicates a promising application of chitosan in the culture system composed of the epithelial and mesenchymal components, which has been widely used in the tissue regeneration in which the desired biological functions are believed to be induced in a more native manner [41, 42]. During the endogenous development, the organogenesis of the exocrine tissue is regulated precisely by the surrounding mesenchyme [28]. Likewise, in the regeneration of salivary glands, the presence of mesenchyme is advantageous to the formation of epithelial structure [27]. With chitosan, the functions of mesenchyme and its derived factors could be augmented, which might accelerate the morphogenetic process more efficaciously and innately. Accordingly, chitosan not only serves as a constituting ingredient of the salivary-tissue favourable scaffold [16], but also regulates the functions of growth factors required for salivary epithelial morphogenesis, which are another important components of tissue regeneration [43]. Therefore, with the intensified morphogenetic instruction from mesenchyme promoted by chitosan, it is likely that the regenerative salivary tissue might be developed resembling the native structures.
In the previous reports of salivary tissue morphogenesis, most of the recombined salivary epithelium and mesenchyme were cultured in the medium with serum [21, 24, 25]. It is evident that the serum added in the culture system is beneficial to the morphogenesis of salivary epithelium by providing some essential components for the functions of diffusible growth factors [44]. Nonetheless, for the purpose of salivary tissue regeneration, the supplemented serum might be costly and raises the possibility of bioincompatibility. In the current study, in the presence of chitosan, we showed that the recombined SMG epithelium was able to sprout branches in a serum-free culture system. Although chitosan is not parallel with serum in the effectiveness, it is competent to facilitate SMG epithelial morphogenesis independently. Therefore, by using chitosan, a natural biodegradable and biocompatible substrate [45], the need of serum in the culture system of salivary tissues could be partly substituted, which possibly infers an important progress for the purpose of salivary tissue regeneration.
Conclusions
Chitosan is a biomaterial competent to intensify the morphogenetic capacity of mesenchyme and enhances the biological functions of mesenchyme-derived factors in regulating SMG epithelial morphogenesis. By promoting specific cellular functions induced by growth factors, it is likely that chitosan is capable of regulating the morphogenesis of SMG epithelium more efficaciously and comprehensively. Therefore, this study showed an encouraging result which revealed a novel role of chitosan in regulating salivary glands morphogenesis. The branching-promoting effects of chitosan on SMG epithelium might be applied with great potential in the future investigation and regeneration of salivary glands.
Acknowledgments
The authors thank National Science Council of the Republic of China and National Taiwan University Hospital for their financial support of this research.
References
- 1.Vissink A, Jansma J, Spijkervet FK, et al. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212. doi: 10.1177/154411130301400305. [DOI] [PubMed] [Google Scholar]
- 2.Warde P, O’Sullivan B, Aslanidis J, et al. A phase III placebo-controlled trial of oral pilocarpine in patients undergoing radiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;54:9–13. doi: 10.1016/s0360-3016(02)02890-0. [DOI] [PubMed] [Google Scholar]
- 3.Patel VN, Rebustini IT, Hoffman MP. Salivary gland branching morphogenesis. Differentiation. 2006;74:349–64. doi: 10.1111/j.1432-0436.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 4.Jaskoll T, Chen H, Min Zhou Y, et al. Developmental expression of survivin during embryonic submandibular salivary gland development. BMC Dev Biol. 2001;1:5. doi: 10.1186/1471-213X-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nogawa H, Takahashi Y. Substitution for mesenchyme by basement-membrane-like substratum and epidermal growth factor in inducing branching morphogenesis of mouse salivary epithelium. Development. 1991;112:855–61. doi: 10.1242/dev.112.3.855. [DOI] [PubMed] [Google Scholar]
- 6.Morita K, Nogawa H. EGF-dependent lobule formation and FGF7-dependent stalk elongation in branching morphogenesis of mouse salivary epithelium in vitro. Dev Dyn. 1999;215:148–54. doi: 10.1002/(SICI)1097-0177(199906)215:2<148::AID-DVDY7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 7.Hosokawa Y, Takahashi Y, Kadoya Y, et al. Significant role of laminin-1 in branching morphogenesis of mouse salivary epithelium cultured in basement membrane matrix. Dev Growth Differ. 1999;41:207–16. doi: 10.1046/j.1440-169x.1999.00419.x. [DOI] [PubMed] [Google Scholar]
- 8.Larsen M, Wei C, Yamada KM. Cell and fibronectin dynamics during branching morphogenesis. J Cell Sci. 2006;119:3376–84. doi: 10.1242/jcs.03079. [DOI] [PubMed] [Google Scholar]
- 9.Kadoya Y, Yamashina S. Salivary gland morphogenesis and basement membranes. Anat Sci Int. 2005;80:71–9. doi: 10.1111/j.1447-073x.2005.00102.x. [DOI] [PubMed] [Google Scholar]
- 10.Hieda Y, Nakanishi Y. Epithelial morphogenesis in mouse embryonic submandibular gland: its relationships to the tissue organization of epithelium and mesenchyme. Dev Growth Differ. 1997;39:1–8. doi: 10.1046/j.1440-169x.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 11.Tucker AS. Salivary gland development. Semin Cell Dev Biol. 2007;18:237–44. doi: 10.1016/j.semcdb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Kratochwil K. Organ specificity in mesenchymal induction demonstrated in the embryonic development of the mammary gland of the mouse. Dev Biol. 1969;20:46–71. doi: 10.1016/0012-1606(69)90004-9. [DOI] [PubMed] [Google Scholar]
- 13.Sakakura T, Nishizuka Y, Dawe CJ. Mesenchyme-dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science. 1976;194:1439–41. doi: 10.1126/science.827022. [DOI] [PubMed] [Google Scholar]
- 14.Tyler MS, Koch WE. In vitro development of palatal tissues from embryonic mice. III. Interactions between palatal epithelium and heterotypic oral mesenchyme. J Embryol Exp Morphol. 1977;38:37–48. [PubMed] [Google Scholar]
- 15.Hoffman MP, Kidder BL, Steinberg ZL, et al. Gene expression profiles of mouse submandibular gland development: FGFR1 regulates branching morphogenesis in vitro through BMP- and FGF-dependent mechanisms. Development. 2002;129:5767–78. doi: 10.1242/dev.00172. [DOI] [PubMed] [Google Scholar]
- 16.Yang TL, Young TH. The enhancement of submandibular gland branch formation on chitosan membranes. Biomaterials. 2008;29:2501–8. doi: 10.1016/j.biomaterials.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Lu S, Song X, Cao D, et al. Preparation of water-soluble chitosan. J. Appl. Polym. Sci. 2004;91:1357–61. [Google Scholar]
- 18.Xie W, Xu P, Liu Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg Med Chem Lett. 2001;11:1699–701. doi: 10.1016/s0960-894x(01)00285-2. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi Y, Nogawa H. Branching morphogenesis of mouse salivary epithelium in basement membrane-like substratum separated from mesenchyme by the membrane filter. Development. 1991;111:327–35. doi: 10.1242/dev.111.2.327. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg Z, Myers C, Heim VM, et al. FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development. 2005;132:1223–34. doi: 10.1242/dev.01690. [DOI] [PubMed] [Google Scholar]
- 21.Iwai K, Hieda Y, Nakanishi Y. Effects of mesenchyme on epithelial tissue architecture revealed by tissue recombination experiments between the submandibular gland and lung of embryonic mice. Dev Growth Differ. 1998;40:327–34. doi: 10.1046/j.1440-169x.1998.t01-1-00008.x. [DOI] [PubMed] [Google Scholar]
- 22.Ikari T, Hiraki A, Seki K, et al. Involvement of hepatocyte growth factor in branching morphogenesis of murine salivary gland. Dev Dyn. 2003;228:173–84. doi: 10.1002/dvdy.10377. [DOI] [PubMed] [Google Scholar]
- 23.Ball WD. Development of the rat salivary glands. 3. Mesenchymal specificity in the morphogenesis of the embryonic submaxillary and sublingual glands of the rat. J Exp Zool. 1974;188:277–88. doi: 10.1002/jez.1401880304. [DOI] [PubMed] [Google Scholar]
- 24.Umeda Y, Miyazaki Y, Shiinoki H, et al. Involvement of heparin-binding EGF-like growth factor and its processing by metalloproteinases in early epithelial morphogenesis of the submandibular gland. Dev Biol. 2001;237:202–11. doi: 10.1006/dbio.2001.0351. [DOI] [PubMed] [Google Scholar]
- 25.Grobstein C. Inductive epitheliomesenchymal interaction in cultured organ rudiments of the mouse. Science. 1953;118:52–5. doi: 10.1126/science.118.3054.52. [DOI] [PubMed] [Google Scholar]
- 26.Bellusci S, Grindley J, Emoto H, et al. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–78. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- 27.Wei C, Larsen M, Hoffman MP, et al. Self-organization and branching morphogenesis of primary salivary epithelial cells. Tissue Eng. 2007;13:721–35. doi: 10.1089/ten.2006.0123. [DOI] [PubMed] [Google Scholar]
- 28.Pispa J, Thesleff I. Mechanisms of ectodermal organogenesis. Dev Biol. 2003;262:195–205. doi: 10.1016/s0012-1606(03)00325-7. [DOI] [PubMed] [Google Scholar]
- 29.Thompson HA, Spooner BS. Inhibition of branching morphogenesis and alteration of glycosaminoglycan biosynthesis in salivary glands treated with beta-D-xyloside. Dev Biol. 1982;89:417–24. doi: 10.1016/0012-1606(82)90330-x. [DOI] [PubMed] [Google Scholar]
- 30.Nakanishi Y, Uematsu J, Takamatsu H, et al. Removal of heparan sulfate chains halted epithelial branching morphogenesis of the developing mouse submandibular gland in vitro. Dev Growth Differ. 1993;35:371–84. doi: 10.1111/j.1440-169X.1993.00371.x. [DOI] [PubMed] [Google Scholar]
- 31.Bernfield MR, Banerjee SD. Acid mucopolysaccharide (glycosaminoglycan) at the epithelial-mesenchymal interface of mouse embryo salivary glands. J Cell Biol. 1972;52:664–73. doi: 10.1083/jcb.52.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishihara M, Obara K, Nakamura S, et al. Chitosan hydrogel as a drug delivery carrier to control angiogenesis. J Artif Organs. 2006;9:8–16. doi: 10.1007/s10047-005-0313-0. [DOI] [PubMed] [Google Scholar]
- 33.Di Martino A, Sittinger M, Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomater-ials. 2005;26:5983–90. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21:2589–98. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 35.Chandy T, Sharma CP. Chitosan as a biomaterial. Biomater Artif Cells Artif Organs. 1990;18:1–24. doi: 10.3109/10731199009117286. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi T. Biodegradable polymers for biomedical uses. Prog Poly Sci. 1994;19:663–702. [Google Scholar]
- 37.Ishihara M, Fujita M, Obara K, et al. Controlled releases of FGF-2 and paclitaxel from chitosan hydrogels and their subsequent effects on wound repair, angiogenesis, and tumor growth. Curr Drug Deliv. 2006;3:351–8. doi: 10.2174/156720106778559047. [DOI] [PubMed] [Google Scholar]
- 38.Mori T, Okumura M, Matsuura M, et al. Effects of chitin and its derivatives on the proliferation and cytokine production of fibroblasts in vitro. Biomaterials. 1997;18:947–51. doi: 10.1016/s0142-9612(97)00017-3. [DOI] [PubMed] [Google Scholar]
- 39.Masuoka K, Ishihara M, Asazuma T, et al. The interaction of chitosan with fibroblast growth factor-2 and its protection from inactivation. Biomaterials. 2005;26:3277–84. doi: 10.1016/j.biomaterials.2004.07.061. [DOI] [PubMed] [Google Scholar]
- 40.Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20:1133–42. doi: 10.1016/s0142-9612(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 41.Le Visage C, Dunham B, Flint P, et al. Coculture of mesenchymal stem cells and respiratory epithelial cells to engineer a human composite respiratory mucosa. Tissue Eng. 2004;10:1426–35. doi: 10.1089/ten.2004.10.1426. [DOI] [PubMed] [Google Scholar]
- 42.Honda MJ, Tsuchiya S, Sumita Y, et al. The sequential seeding of epithelial and mesenchymal cells for tissue-engineered tooth regeneration. Biomaterials. 2007;28:680–9. doi: 10.1016/j.biomaterials.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 43.Langer RS, Vacanti JP. Tissue engineering: the challenges ahead. Sci Am. 1999;280:86–9. doi: 10.1038/scientificamerican0499-86. [DOI] [PubMed] [Google Scholar]
- 44.Noguchi Y, Okamoto A, Kasama T, et al. Lysophosphatidic acid cooperates with EGF in inducing branching morphogenesis of embryonic mouse salivary epithelium. Dev Dyn. 2006;235:403–10. doi: 10.1002/dvdy.20651. [DOI] [PubMed] [Google Scholar]
- 45.Kim IY, Seo SJ, Moon HS, et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv. 2008;26:1–21. doi: 10.1016/j.biotechadv.2007.07.009. [DOI] [PubMed] [Google Scholar]


