Abstract
Our aim was to quantitatively assess the angiogenetic effects of VEGF and bFGF immobilized in a fibrin-based drug delivery system in a suitable subcutaneous rat model. After evaluation of a suitable implantation technique (6 rats), four teflon isolation chambers containing fibrin gel matrices were implanted subcutaneously in an upside-down fashion on the back of 30 Lewis rats. The matrices consisted of 500 μl fibrin gel with two different fibrinogen concentrations (10 mg/ml or 40 mg/ml fibrinogen) and 2 I.U./ml thrombin and contained VEGF and bFGF in five different concentrations (0 to 250 ng/ml each). At 3, 7 and 14 days after implantation, matrices were explanted and subjected to histological and morphometrical analysis. At 1 week, the volume of the fibrin clots was significantly smaller in the 100 and 250 ng/ml VEGF and bFGF groups in comparison to lower concentrated growth factors. At 1 and 2 weeks, the use of growth factors in low concentrations (25 ng/ml VEGF and bFGF) significantly increased the amount of fibrovascular tissue, average fraction of blood vessels and number of blood vessels at the matrix–host interface in comparison to growth factor-free controls. Higher concentrations were neither associated with further increase of tissue formation nor with increased sprouting of blood vessels in this model. This study demonstrates that fibrin gel-immobilized angioinductive growth factors efficiently stimulate generation of fibrovascular tissue and sprouting of blood vessels in a newly developed subcutaneous upside-down isolation chamber model with an optimum between 25 and 100 ng/ml.
Keywords: growth factors, VEGF, bFGF, fibrin, tissue engineering, rat
Introduction
Tissue engineering has developed rapidly since 1987, when the term was first coined [1]. Even though significant advances have been made and products are already available for clinical use, problems remain [2]. One of the major challenges in tissue engineering is the generation of large vascularized three-dimensional structures [2, 3]. Therefore, modulation of angiogenetic processes in three-dimensional matrices is of great interest in the field of tissue engineering.
Cells situated in the centre of large constructs suffer from suboptimal initial vascularization [4, 5]. Cells can survive by diffusion only when they are within a distance of 150–200 μm of a capillary [4, 6]. Prevascularization of three-dimensional matrices may help to increase survival of cells after implantation. Using angioinductive growth factors, the period between implantation and vascularization of matrices may be shortened [7, 8]. Angioinductive growth factors like basic fibrobast growth factor (bFGF) and vascular endothelial growth factor (VEGF) are bioactive proteins involved in regulation of new blood vessel formation through angiogenesis [9]. bFGF stimulates angiogenesis in vivo and in vitro[10]. VEGF, originally named vascular permeability factor, exerts angioinductive effects by enhancing microvascular permeability and endothelial cell proliferation [11, 12]. Beside their ability to stimulate and support angiogenesis [13, 14], fibrin sealants can act as a slow release system for certain growth factors including VEGF [15, 16] and bFGF [16, 17]. The use of growth factor combinations (e.g. VEGF and bFGF) shows an improvement in terms of increased neovascularization with formation of more mature vessels compared to the application of single growth factors [9].
Although VEGF and bFGF immobilized in a fibrin matrix have already been used in more complex models, e.g. the AV loop model [18], no data are available about the most efficient growth factor concentrations in vivo. The aim of this study was to evaluate a suitable subcutaneous implantation technique in rats as an in vivo screening tool and to quantitatively evaluate the angiogenetic effects of VEGF and bFGF immobilized in a fibrin-based drug delivery system in the subcutaneous rat model.
Materials and methods
Experimental design
In phase 1, a suitable subcutaneous implantation technique was evaluated. For this purpose, six male Lewis rats were divided into two identical groups (A and B). In animals from group A, two teflon isolation chambers containing fibrin gel matrices were implanted subcutaneously in conventional implantation technique with the opening of the chamber facing the dorsal skin of the rat and without any fixation of the chambers [19, 20] (Fig. 1A and B). In group B, the chambers were implanted in an upside-down fashion on the back of the rats with the opening of the chamber facing the dorsal muscle and with fixation of the chambers (Fig. 1C and D). In both groups, the matrices consisted of 500 μl fibrin gel with identical fibrinogen and thrombin concentrations (10 mg/ml fibrinogen, 2 I.U./ml thrombin). The explantation interval was 10 days after the initial operation for all groups (including 6 matrices per group and time-point).
Figure 1.
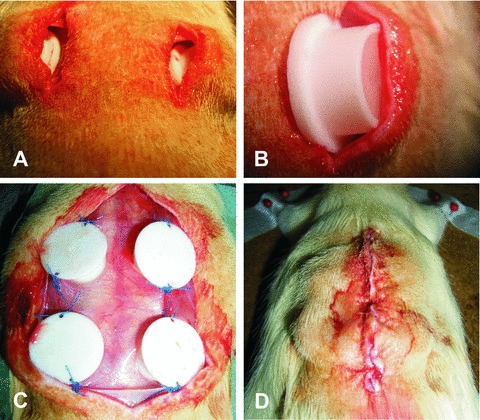
(A, B) The conventional implantation technique with the opening of the chamber (Teflon isolation chamber, inner diameter 10 mm, height 6 mm) facing the dorsal skin of the rat and without any fixation of the chambers. (C, D) The upside down fashion on the back of the rats with the opening of the chamber facing the dorsal muscle of the rat and with fixation of the chambers.
In phase 2, the angiogenetic effects of VEGF and bFGF immobilized in a fibrin-based drug delivery system were quantitatively assessed in the subcutaneous rat model (Fig. 2). Four teflon isolation chambers containing fibrin gel matrices were implanted subcutaneously in an upside-down fashion on the back of 30 Lewis rats. The matrices consisted of 500 μl fibrin gel with two different fibrinogen concentrations (10 mg/ml or 40 mg/ml fibrinogen, 2 I.U./ml thrombin), loaded with VEGF165 and bFGF in five different concentrations (0 to 250 ng/ml each). The explantation intervals were 3, 7 and 14 days after the initial operation for all groups (including 4 matrices per group and time-point).
Figure 2.
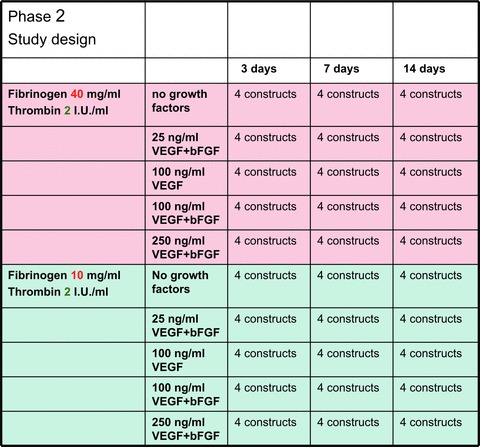
Study design of phase 2. Four teflon isolation chambers containing fibrin gel matrices were implanted subcutaneously in an upside- down fashion on the back of 30 Lewis rats. The matrices consisted of fibrin gel with two different fibrinogen concentrations, loaded with VEGF165 and bFGF in five different concentrations (0 to 250 ng/ml each). The explantation intervals were 3, 7 and 14 days after the initial operation for all groups.
Isolation chamber and composition of the fibrin matrix
The cylindrical chamber (inner diameter 10 mm, height 6 mm) was made of heat-resistant medical grade teflon (P. Greil, Department of Materials Science, Glass and Ceramics, University of Erlangen). In this study, the matrix was prepared from a fibrin sealant clinically approved in the United States and some European countries (Tisseel®, Baxter, Vienna, Austria). A final fibrinogen concentration of 10 mg/ml or 40 mg/ml, a thrombin concentration of 2 I.U./ml and an aprotinin concentration of 1500 K.I.E./ml to delay fibrinolysis were used. In phase 2 of this study, the matrix was loaded with VEGF165 and bFGF in five different concentrations (0, 25, 100 and 250 ng/ml bFGF and VEGF each and VEGF alone at 100 ng/ml) before implantation. Recombinant human VEGF165 (R&D Systems, Minneapolis, MN, USA) and recombinant human bFGF (Roche Diagnostics, Indianapolis, IN, USA) were used.
Animals
Syngenic male Lewis rats (Charles River Laboratories, Sulzfeld, Germany) were used in this study. German regulations for the care and use of laboratory animals were observed at all times. All experiments were approved by the animal care committee of the University of Erlangen and the Government of Mittelfranken, Germany. The animals were housed in the veterinary care facility of the University of Erlangen Medical Center and submitted to a12 hrs dark/light cycle with free access to standard chow (Altromin, Hamburg, Germany) and water.
Surgical procedures
All operations were performed by the same microsurgeon using an operative microscope (Karl Zeiss, Jena, Germany) under general anaesthesia with Isoflurane (Baxter, Unterschleiflheim, Germany).
For the conventional implantation technique, two small subcutaneous pockets were created on the back of the rats by two longitudinal skin incisions. Chambers were placed without any fixation in the pockets containing 500 μl fibrin gel with the opening of the chamber facing the dorsal skin. Haemostasis was assured and the wound was closed using Vicryl 5-0 (Ethicon, Norderstedt, Germany).
For the upside-down implanted chambers, one large subcutaneous pocket was created by a mid-line skin incision on the back of the rats. In the first phase, two, and in the second phase, four chambers containing 500 μl fibrin gel were implanted in the subcutaneous pocket with the opening of the chamber facing the dorsal muscle of the rats. In phase two, the fibrinogen component was loaded with VEGF and bFGF as described in detail in Figure 2. The chambers were fixed using Prolene 3-0 (Ethicon, Norderstedt, Germany) sutures. Haemostasis was assured and the wound was closed using Vicryl 5-0 (Ethicon, Norderstedt, Germany). All animals received 0.2 ml Benzylpenicillin-Benzathin (Tardomycel Comp®, Bayer, Leverkusen, Germany) and buprenorphin (0.3 mg/kg rat weight, Temgesic®, Essex Chemie AG, Luzern, Switzerland) postoperatively.
Histological and statistic analysis
Specimens were explanted in toto with the surrounding tissue and fixed in 3.5% formalin for histological processing. Afterwards, the constructs were dehydrated in graded ethanol and embedded in paraffin. Three μm cross-sections were obtained from two standardized planes (1 mm proximal and 1 mm distal of the central plane) using a Leica microtome (Leica Microsystems, Bensheim, Germany). Cross-sections were stained using haematoxylin and eosin according to standard protocols in phase 1 and 2. In phase 2, additional immuno-histochemical staining was performed using the lectin Bandeiraea Simplicifolia agglutinin (BS-1), alpha-smooth muscle actin and ED1. The images were evaluated by two independent and blinded observers.
All images of haematoxylin and eosin-stained cross-sections were generated with a Leica Microscope and Digital Camera under 25× magnification. In phase one, images were evaluated morphologically. In phase two, the matrix height and volume and the height and volume of the fibrovascular tissue adjacent to the matrix were calculated for each group and each time-point (Fig. 3).
Figure 3.
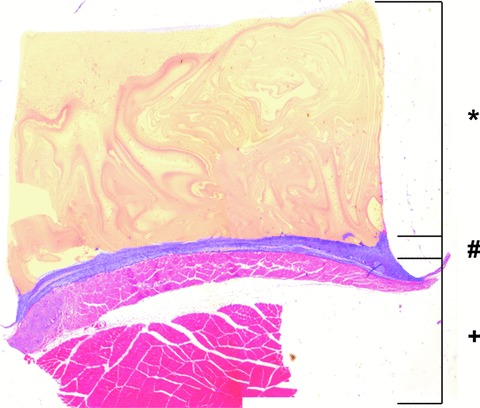
Histological evaluation of HE stained cross-sections of matrices explanted in phase two. The matrix height (*) and the height of the fibrovascular tissue adjacent to the matrix (#) were calculated for each group and each time. The dorsal muscles (+) are subjacent to the fibrovascular tissue.
The fibrovascular tissue adjacent to the matrix of all lectin-stained cross-sections was divided into 3 equal sectors, 2 at the periphery and 1 at the central part of the section (Fig. 4) and all images of each sector were acquired with a Leica Microscope and Digital Camera under 200× magnification. The images were rendered bimodal (standardized threshold) (WinQ, Leica Microsystems, Bensheim, Germany). The number of blood vessels, standard deviation of blood vessel size and average size of blood vessels was calculated for each group and each time. Results are given as mean ± standard deviation. Statistical analysis was performed using GraphPad Prism software (GraphPad Software, San Diego, CA, USA). Normal distribution was checked positive using the Kolmogorov-Smirnov test for all groups. Two-tailed unpaired Student’s t-test was applied for statistical analysis. The critical level of statistical significance chosen was P < 0.05.
Figure 4.
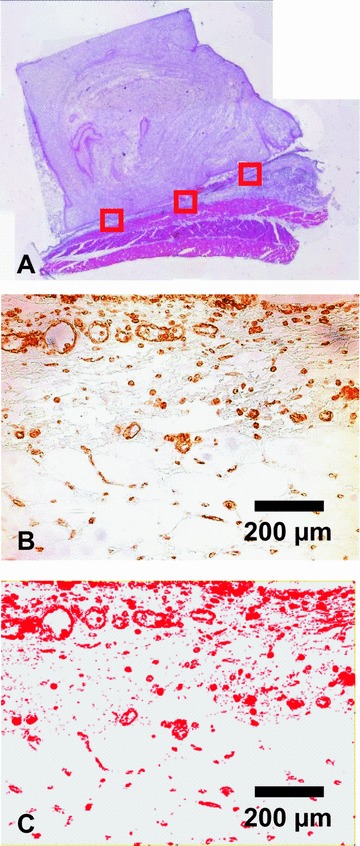
The fibrovascular tissue adjacent to the matrix of all lectin-stained cross-sections was divided into 3 equal sectors, 2 at the periphery and 1 at the central part of the section and all images were rendered bimodal (A) HE staining, magnification ×25; (B) Lectin staining, magnification ×200; (C) Bimodal rendered image, magnification ×200.
Immunohistology
Lectin
Rat endothelial cells were detected immuno-histochemically using the lectin Bandeiraea Simplicifolia agglutinin (BS-1) as described previously [21]. Paraffinated sections were treated in a xylol/ethanol sequence and after rinsing in PBS solution endogenous peroxidase activity was blocked in 3% H2O2 for 10 min. Blocking was completed by incubation with avidin and biotin 15 min. each (Vector Laboratories, Burlingame, CA, USA) as well as 5% normal goat serum for 1 hr Biotinylated lectin (BS-1, Sigma Chemical Co., St. Louis, MO, USA) was applied in a 1:100 dilution in PBS overnight at 4°C. Between incubation steps, slides were rinsed in PBS solution. For detection, Streptavidin AB Complex/HRP (Dako GmbH, Germany) was applied for 30 min., followed by development with DAB + Chromogen (Dako GmbH, Germany). Sections from heart muscle served as positive controls, and omission of the lectin as negative control.
ED1
Macrophages were detected immuno-histochemically using ED1 stainings. Sections were deparaffinated and incubated for 30 min. with 1:300 anti-ED1 primary antibody (Serotec, Raleigh, NC, USA). A goat antimouse secondary antibody (Dako GmbH, Germany) was used at 1:20 for 30 min. After that, sections were incubated with 1:50 APAAP complex (alkaline phosphatase- anti-alkaline phosphatase) for 30 min. Enhancement was achieved by repeating incubation with secondary antibody and with the APAAP complex for 10 min. each. The enzyme was revealed with 0.2 mg/ml naphthol AS.MX (Sigma Chemical Co.), 0.002 mg/ml dimethyl formamide (Merck, Darmstadt, Germany) in 0.1 min. Tris pH 8.2, 1 mg/ml fast red TR salt (Sigma Chemical Co.) and 0.33 mg/ml levamisole (Sigma Chemical Co.). Sections were counterstained with haematoxylin (Merck, Darmstadt, Germany).
Alpha-smooth muscle actin (ASMA)
Sections were deparaffinated and incubated overnight at 4°C with 1:300 mouse anti-ASMA primary antibody (Dako GmbH, Germany). An antimouse anti-rabbit secondary antibody (Invitrogen, Carlsbad, CA, USA) was used for 30 min. at room temperature. The enzyme was revealed as described above.
Results
Surgery and animals
All 36 animals tolerated the surgical procedure well. There were no major postoperative complications such as infection, haematoma or wound dehiscence. No extrusion of the implants occurred over the observation period.
Phase 1
After opening the back of the rats, there were newly formed capsules of fibrous tissue around the chambers containing the matrices. After opening the capsules and removal of the chambers, all matrices implanted using the upside-down implantation technique were adherent to the subjacent tissue whereas matrices implanted using the conventional implantation technique were detached of the surrounding tissue.
Histologically, matrices of group A, which were implanted in a conventional manner, showed an inhomogeneous clot structure. Also, there was only a small area of contact between the clot and the adjacent fibrovascular tissue. In contrast, matrices implanted using the upside-down implantation technique displayed high adhesion of the fibrin clot to the subjacent tissue and a highly homogeneous structure (Fig. 5).
Figure 5.
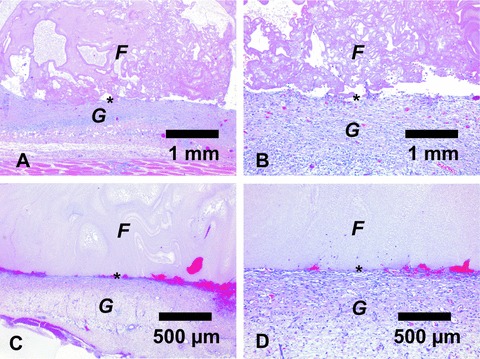
H&E stainings of matrices of phase one explanted after 10 days. Matrices of group A, which were implanted in a conventional manner, showed an inhomogeneous clot structure (A). Note the small area of contact between the clot and the adjacent fibrovascular tissue (B). In comparison, matrices implanted using the upside-down implantation technique displayed high adhesion of the fibrin clot to the subjacent tissue and a more homogeneous structure (C+D). A+C= magnification ×25; B+D= magnification ×100. F= Fibrin gel; G= Granulation tissue; *= Interface.
Phase 2
Different concentrations of fibrinogen did not influence tissue formation and vascularization patterns in this experimental setting. Therefore, only results using the diluted fibrinogen are presented in detail.
Macroscopically, all chambers in all groups were surrounded by a capsular layer of connective tissue. Upon day 3, all fibrin clots in all groups were still present without any fibrinolysis or degradation. At 1 week, volume of the fibrin clots was considerably smaller in the 100 and 250 ng/ml VEGF and bFGF groups compared to lower growth factor concentrations (Fig. 6). Almost complete resorption of the matrices had occurred upon day 14 after implantation in all groups.
Figure 6.
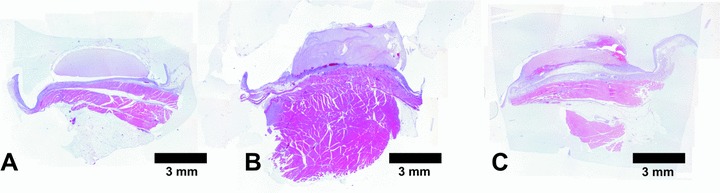
H&E stained cross sections of matrices explanted 1 week after the initial operation using no growth factors (A), 25 ng/ml VEGF and bFGF (B) and 250 ng/ml VEGF and bFGF (C). By the use of 250 ng/ml VEGF and bFGF there was a significant contraction of the fibrin gel matrix after 1 week. The use of growth factors increased height of fibrovascular tissue at the matrix-host interface. Magnification ×25.
Histologically, during the 14 days of implantation the fibrin gel was not invaded by blood vessels in any group. The fibrovascular tissue subjacent to the fibrin gel was composed of inflammatory cells, fibroblasts and blood vessels in all groups. Using ED1 immuno histochemical staining, some macrophages invading the fibrovascular tissue were visible independent of added growth factors. No significant foreign body reaction with presence of multinucleated giant cells was detected within the fibrin clots and the fibrovascular tissue. Vascularization of this tissue could be confirmed by immunohistochemical staining using the lectin Bandeiraea Simplicifolia agglutinin and alpha-smooth muscle actin (Fig. 7). Except for blood vessels there was no significant staining by ASMA in the fibrovascular tissue.
Figure 7.
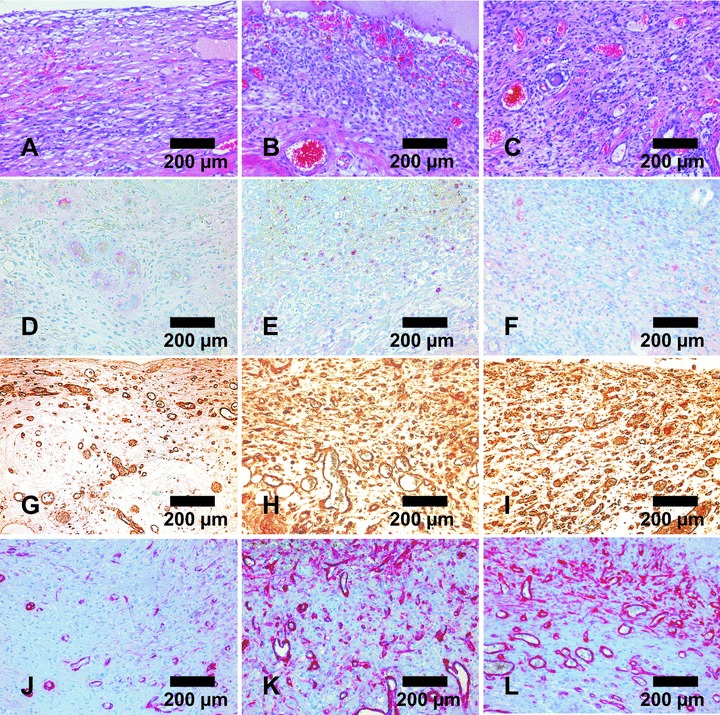
Representative micrographs of fibrovacular tissue adjacent to matrices explanted after one week without growth factors (A, D, G, J), with 25 ng/ml VEGF and bFGF (B, E, H, K) and with 100 ng/ml VEGF (C, F, I, L). (A, B, C) H&E stained cross sections; (D, E, F) ED1 staining of macrophages; (G, H, I) Lectin staining of endothelial cells; (J, K, L) Alpha-smooth muscle actin staining of blood vessels. Presence of macrophages was independent of added growth factors (D, E, F). Number of blood vessels increased when growth factors were used (G–L). Magnification ×200.
Morphometric analysis
In phase two, morphometric analysis of the constructs demonstrated a significant decrease of construct height following application of 100 and 250 ng/ml VEGF and bFGF with 1.75 ± 0.71 mm and 1.76 ± 0.9 mm in comparison to 25 ng/ml VEGF and bFGF with 3.65 ± 0.89 mm and 100 ng/ml VEGF with 5.65 ± 1.27 mm at 1 week (Fig. 8). In all groups, the matrix height did not signifi cantly differ 3 days after implantation. At day 14, all fibrin clots were almost completely degraded in all groups.
Figure 8.
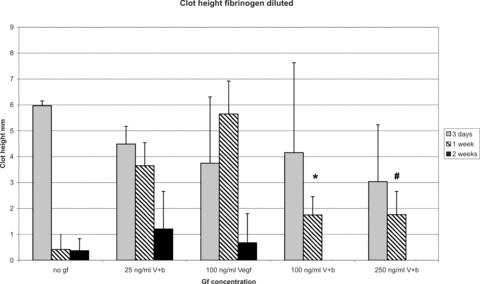
Morphometric analysis of construct height (fibrinogen diluted). There is a significant decrease of construct size and volume following application of 100 and 250 ng/ml VEGF and bFGF in comparison to 25 ng/ml VEGF and bFGF and 100 ng/ml VEGF alone at 1 week. In all groups, the matrix volume and size did not significantly differ 3 days after implantation. At day 14, all fibrin gels were almost completely degradated in all groups. *P < 0.05 versus 25 ng/ml VEGF and bFGF and 100 ng/ml VEGF; #P < 0.05 versus 25 ng/ml VEGF and bFGF and 100 ng/ml VEGF.
The use of growth factors with a concentration of 25 ng/ml VEGF and bFGF and 100 ng/ml VEGF significantly increased height of fibrovascular tissue at the matrix–host interface in comparison to growth factor-free controls at 1 week (0.87 ± 0.36 mm and 1.19 ± 0.58 mm versus 0.1 ± 0.12 mm) and at 2 weeks (1.11 ± 0.59 mm and 1.37 ± 0.66 mm versus 0.29 ± 0.12 mm). Higher concentrations did not further induce tissue formation (Fig. 9).
Figure 9.
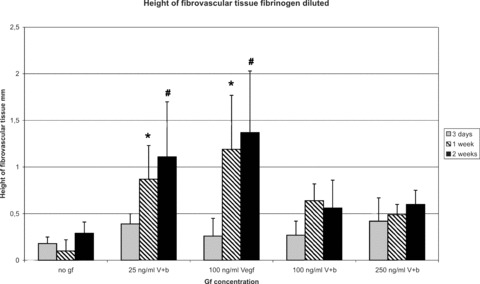
Morphometric analysis of height of fibrovascular tissue (fibrinogen diluted). The use of growth factors with a concentration of 25 ng/ml VEGF and bFGF and 100 ng/ml VEGF significantly increased height and volume of fibrovascular tissue at the matrix-host interface in comparison to growth factor-free controls at 1 and 2 weeks. Higher concentrations did not further increase tissue formation. *P < 0.05 versus no growth factors (1 week explantation time-point); #P < 0.05 versus no growth factors (2 weeks explantation time-point).
The total number of blood vessels counted in the fibrovascular tissue adjacent to the matrices of lectin stained cross-sections was significantly increased when using of 25 ng/ml VEGF and bFGF and 100 ng/ml VEGF in comparison to growth factor-free fibrin clots at 1 week (185 ± 45 vessels per field of view [FOV] and 168 ± 38 vessels per FOV versus 110 ± 21 vessels per FOV) and at 2 weeks (179 ± 64 vessels per FOV and 168 ± 42 vessels per FOV versus 99 ± 28 vessels per FOV). There was no further increase of blood vessel numbers when higher concentrations of growth factors were used (Fig. 10).
Figure 10.
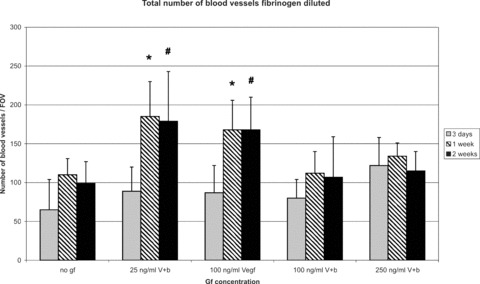
Morphometric analysis of total number of blood vessels (fibrinogen diluted). The number of blood vessels counted in the fibrovascular tissue adjacent to the matrices of lectin stained cross-sections was significantly increased by the use of 25 ng/ml VEGF and bFGF and 100 ng/ml VEGF in comparison to growth factor free fibrin clots at 1 and 2 weeks. There was no further increase of number of blood vessels by the use of higher concentrated growth factors. *P < 0.05 versus no growth factors (1 week explantation time-point); #P < 0.05 versus no growth factors (2 weeks explantation time-point).
Similar results could be obtained regarding average fraction of blood vessels and total area of blood vessels (data not shown).
There was no difference regarding average blood vessel size and standard deviation of blood vessel size between the different growth factor concentrations (data not shown).
Discussion
In phase one of this study, a technique for suitable subcutaneous implantation was evaluated. In the literature, mainly two implantation techniques are described for subcutaneous implantation into the dorsal space of small animals: first, the implantation without any chamber, using fibrin glue alone [22] or in combination with other matrices for example with decalcified spongiosa blocks to study tissue ingrowth into fibrin [23], and second, the subcutaneous implantation without any fixation of the chamber and with the opening of the chamber facing the dorsal skin of the rat, which is referred to as the conventional technique in our study [19, 20]. In comparison to these techniques, the upside-down implantation provides a fixation of the chamber to the subjacent tissue and the opening of the chamber faces the dorsal muscle of the rat.
Here we demonstrated that constructs implanted in the upside-down manner show a much better adhesion at the matrix–host interface and a much more homogeneous clot structure than constructs implanted using the conventional implantation technique. This could be explained by the reduced shear forces due to fixation of the chamber and by implantation of the construct in an upside-down fashion whereby a continuous contact of the fibrin gel to the host is provided. Another subcutaneous implantation model, which is described in the literature, uses chambers with a 10 hole-perforated bottom slip [24]. These chambers are not suitable for our study because our chamber should provide a high surface area for growth factor interactions with the subjacent tissue.
In phase two of this study, we demonstrate that VEGF and bFGF immobilized in a fibrin-based drug delivery system efficiently modulate angiogenesis in the subcutaneous model in the rat.
A known feature of the fibrin matrix is fibrinolysis and degradation over time [25], a phenomenon also observed in this study. To control the degradation of fibrin gel matrix, aprotinin was used in this study to slow down fibrinolysis via inhibition of plasmin. Because the degradation of the matrix is directly related to aprotinin concentration, we used the same aprotinin concentration of 1500 K.I.E./ml in all fibrin gel matrices in this study to delay fibrinolysis.
Fibrin gel is already established for delivery of growth factors such as VEGF and bFGF [9]. VEGF and bFGF bind specifically and saturably to fibrinogen [15, 17]. We used the 165 iso form of VEGF because of its constant and controlled release within the fibrin clot in contrast to VEGF 121, which binds very weakly with the clot [9]. bFGF interacts strongly with the fibrin clot and is released in a slow manner. It is well known that the greatest angiogenetic effect of VEGF is observed together with bFGF and the combination of VEGF and bFGF shows synergistic activity on angiogenesis [9].
The use of growth factors in high concentrations (100 and 250 ng/ml VEGF and bFGF) led to a significant decrease of construct size after 1 week in comparison to lower growth factor concentrations maybe because of faster ingrowth of fibrovascular tissue with subsequent resorption of the matrix. Three days after implantation, no constructs displayed signs of fibrinolysis or degradation whereas after 2 weeks nearly all constructs were degraded.
The decreased fibrin gel clot size after 1 week following application of higher concentrated growth factors (100 and 250 ng/ml VEGF and bFGF) may have led to a shorter time of exposure of the fibrin clot and thereby to a reduced effect of the growth factors immobilized in the clot.
We demonstrated that fibrin gel-immobilized VEGF and bFGF efficiently stimulated generation of fibrovascular tissue and significantly enhanced the angiogenic response in the subcutaneous rat model. One and 2 weeks after implantation, number of blood vessels and average fraction of blood vessels detected in the fibrovascular tissue were significantly increased by the use of low concentrated growth factors (25 ng/ml VEGF and bFGF and 100 ng/ml VEGF) in comparison to growth factor-free groups. Higher concentrations did not further increase vascular sprouting in this model, which might be explained by the significantly decreased matrix size after 1 week and thus reduced time of exposure of VEGF and bFGF or by saturation kinetics of the growth factors.
Also volume and height of the fibrovascular tissue were significantly increased by the use of growth factors in low concentrations (25 ng/ml VEGF and bFGF and 100 ng/ml VEGF) in comparison to growth factor-free groups without further enhancement by higher concentrations of VEGF or bFGF.
Different concentrations of fibrinogen (40 mg/ml or 10 mg/ml) did neither influence blood vessel sprouting nor clot degradation time in this study. It is known that the release of bFGF and VEGF165 is nearly unaffected by different fibrinogen complex concentrations [9]. In addition, thrombin concentration at the time of clotting has the most profound influence on fibrin clot structure, a factor that remains constant for all groups in the study [26]. Because the diluted version of fibrinogen is better to handle, further experiments in different animal models will be performed using diluted fibrinogen.
Functionality of the newly grown vessels was confirmed by positive lectin staining. There was no difference regarding average blood vessel size and standard deviation of blood vessels’ size between the different growth factor concentrations. Histology though is unsuitable to accurately assess vascularization patterns and vessel morphology.
The subcutaneous implantation model represents an easy and efficient in vivo screening model for the effect of different growth factor concentrations immobilized in a fibrin matrix. In the future, the presented study will allow using VEGF and bFGF to reduce the time period between implantation and vascularization of matrices in more complex animal models, such as the AV loop model. The fibrin gel might also serve as a carrier of angiogenic growth factors such as VEGF and bFGF and other modulatory substances in combination with different types of matrices, i.e. hard matrices, which give the construct stability [27]. The fibrin gel can also serve as a cell carrier [21, 28] or as a local gene delivery system [29]. Eventually the implantation into ‘smart’ growth factor-enhanced matrices will facilitate upscaling of bioartificial devices for larger-volume applications.
Conclusion
Angioinductive growth factors immobilized in a commercially available fibrin gel efficiently stimulate generation of fibrovascular tissue and sprouting of blood vessels in the subcutaneous model. The angioinductive effects display a saturation kinetic with an optimum between 25 and 100 ng/ml. The increased tissue formation was accompanied by a faster resorption of the fibrin matrix. The application of angioinductive factors into given fibrin matrix volumes may eventually help generating vascularized tissue-engineered composites for application in reconstructive surgery.
Acknowledgments
This study was supported by research grants from Baxter Healthcare Corporation, Xue-Hong and Hans Georg Geis, as well as the University of Erlangen (ELAN Program). The authors thank Professor Peter Greil and Mr. Peter Reinhard for production of the Teflon chambers. This work contains parts of Andreas Saumweber’s and Jimmy Tjiawi’s doctoral thesis.
Note
Parts of this work have been presented at the annual meeting of the Tissue Engineering and Regenerative Medicine International Society (TERMIS), September 2007 in London, England.
References
- 1.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Horch RE. Future perspectives in tissue engineering. J Cell Mol Med. 2006;10:4–6. doi: 10.1111/j.1582-4934.2006.tb00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiegel HC, Kaufmann PM, Bruns H, et al. Hepatic tissue engineering: from transplantation to customized cell-based liver directed therapies from the laboratory. J Cell Mol Med. 2008;12:56–66. doi: 10.1111/j.1582-4934.2007.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folkman J, Hochberg M. Self-regulation of growth in three dimensions. J Exp Med. 1973;138:745–53. doi: 10.1084/jem.138.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kneser U, Kaufmann PM, Fiegel HC, et al. Long-term differentiated function of heterotopically transplanted hepatocytes on three-dimensional polymer matrices. J Biomed Mater Res. 1999;47:494–503. doi: 10.1002/(sici)1097-4636(19991215)47:4<494::aid-jbm5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Colton CK. Implantable biohybrid artificial organs. Cell Transplant. 1995;4:415–36. doi: 10.1177/096368979500400413. [DOI] [PubMed] [Google Scholar]
- 7.Tabata Y. Tissue regeneration based on growth factor release. Tissue Eng. 2003;9:S5–15. doi: 10.1089/10763270360696941. [DOI] [PubMed] [Google Scholar]
- 8.Elcin YM, Dixit V, Gitnick G. Extensive in vivo angiogenesis following controlled release of human vascular endothelial cell growth factor: implications for tissue engineering and wound healing. Artif Organs. 2001;25:558–65. doi: 10.1046/j.1525-1594.2001.025007558.x. [DOI] [PubMed] [Google Scholar]
- 9.Wong C, Inman E, Spaethe R, et al. Fibrin-based biomaterials to deliver human growth factors. Thromb Haemost. 2003;89:573–82. [PubMed] [Google Scholar]
- 10.Basilico C, Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992;59:115–65. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- 11.Dvorak HF, Brown LF, Detmar M, et al. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–39. [PMC free article] [PubMed] [Google Scholar]
- 12.Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;9:777–94. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson WD, Stirk CM, Melvin WT, et al. Plasmin, fibrin degradation and angiogenesis. Nat Med. 1996;2:493. doi: 10.1038/nm0596-493. [DOI] [PubMed] [Google Scholar]
- 14.Van Hinsbergh VW, Collen A, Koolwijk P. Role of fibrin matrix in angiogenesis. Ann N Y Acad Sci. 2001;936:426–37. doi: 10.1111/j.1749-6632.2001.tb03526.x. [DOI] [PubMed] [Google Scholar]
- 15.Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96:3772–8. [PubMed] [Google Scholar]
- 16.Wilcke I, Lohmeyer JA, Liu S, et al. VEGF(165) and bFGF protein-based therapy in a slow release system to improve angiogenesis in a bioartificial dermal substitute in vitro and in vivo. Langenbecks Arch Surg. 2007;392:305–14. doi: 10.1007/s00423-007-0194-1. [DOI] [PubMed] [Google Scholar]
- 17.Sahni A, Odrljin T, Francis CW. Binding of basic fibroblast growth factor to fibrinogen and fibrin. J Biol Chem. 1998;273:7554–9. doi: 10.1074/jbc.273.13.7554. [DOI] [PubMed] [Google Scholar]
- 18.Arkudas A, Tjiawi J, Bleiziffer O, et al. Fibrin gel-immobilized VEGF and bFGF efficiently stimulate angiogenesis in the AV loop model. Mol Med. 2007;13:480–7. doi: 10.2119/2007-00057.Arkudas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapira L, Houri-Haddad Y, Frolov I, et al. The effect of stress on the inflammatory response to Porphyromonas gingivalis in a mouse subcutaneous chamber model. J Periodontol. 1999;70:289–93. doi: 10.1902/jop.1999.70.3.289. [DOI] [PubMed] [Google Scholar]
- 20.Houri-Haddad Y, Soskolne WA, Halabi A, et al. Repeat bacterial challenge in a subcutaneous chamber model results in augmented tumour necrosis factor-alpha and interferon-gamma response, and suppression of interleukin-10. Immunology. 2000;99:215–20. doi: 10.1046/j.1365-2567.2000.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arkudas A, Beier JP, Heidner K, et al. Axial prevascularization of porous matrices using an arteriovenous loop promotes survival and differentiation of transplanted autologous osteoblasts. Tissue Eng. 2007;13:1549–60. doi: 10.1089/ten.2006.0387. [DOI] [PubMed] [Google Scholar]
- 22.Romanos GE, Strub JR. Effect of Tissucol on connective tissue matrix during wound healing: an immunohistochemical study in rat skin. J Biomed Mater Res. 1998;39:462–8. doi: 10.1002/(sici)1097-4636(19980305)39:3<462::aid-jbm17>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 23.Dinges HP, Redl H, Thurnher M, et al. Morphometric studies on wound healing after systemic administration of adriamycin and local application of fibrin sealant. Application of a new wound healing model using spongiosa implants. Pathol Res Pract. 1986;181:746–54. doi: 10.1016/s0344-0338(86)80051-6. [DOI] [PubMed] [Google Scholar]
- 24.Hudlett P, Neuville A, Miternique A, et al. Angiogenesis and arteriogenesis are increased in fibrin gel chambers implanted in prehypertensive spontaneously hypertensive rats. J Hypertens. 2005;23:1559–64. doi: 10.1097/01.hjh.0000174607.18780.62. [DOI] [PubMed] [Google Scholar]
- 25.Ye Q, Zund G, Benedikt P, et al. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2000;17:587–91. doi: 10.1016/s1010-7940(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 26.Wolberg AS. Thrombin generation and fibrin clot structure. Blood Rev. 2007;21:131–42. doi: 10.1016/j.blre.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Kneser U, Schaefer DJ, Polykandriotis E, et al. Tissue engineering of bone: the reconstructive surgeon’s point of view. J Cell Mol Med. 2006;10:7–19. doi: 10.1111/j.1582-4934.2006.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bleiziffer O, Eriksson E, Yao F, et al. Gene transfer strategies in tissue engineering. J Cell Mol Med. 2007;11:206–23. doi: 10.1111/j.1582-4934.2007.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonadio J. 2000;78:303–11. doi: 10.1007/s001090000118. Tissue engineering via local gene delivery. [DOI] [PubMed] [Google Scholar]


