Abstract
Skin cancers are by far the most common human malignancies. Retinoids have shown promising preventive and therapeutic effects against a variety of human malignancies. The aim of this study was to investigate the apoptosis-inducing effect of acitretin on human skin squamous cell carcinoma (SCC) SCL-1 cells. We found that acitretin preferentially inhibited the growth of SCL-1 cells in a dose- and time-dependent manner, but not of non-malignant keratinocyte HaCaT cells. This inhibition appeared to be due to induction of apoptosis as revealed by enzyme-linked immunosorbent assay. AnnexinV/propidium iodide assay and morphological observation confirmed the pro-apoptotic effect of acitretin on SCL-1 cells. We further demonstrated that apoptosis was induced within 1–2 days and involved activation of caspases-8, -9, -3 and poly (ADP-ribose) polymerase (PARP). Caspase-8 inhibitor effectively suppressed acitretin-induced apoptosis whereas caspase-9 inhibitor did not. Acitretin increased the levels of CD95 (Fas), CD95-ligand and Fas-associated death domain. Neutralizing ZB4 anti-Fas antibody significantly inhibited the apoptosis in SCL-1 cells induced by acitretin. These results suggest that acitretin is able to induce apoptosis in skin cancer cells possibly via death receptor CD95 apoptosis pathway without affecting the viability of normal keratinocyte.
Keywords: retinoid, apoptosis, death receptor CD95 apoptosis pathway, SCC
Introduction
The incidence and prevalence of pre-malignant and malignant skin lesions have been alarmingly increasing worldwide, creating a major medical and economic problems. Squamous cell carcinomas (SCCs) in particular, have rapidly increased in incidence during the past several decades [1]. DNA damage, gene mutations and various chemicals have all been implicated as etiologic factors in the development of SCC. In addition, solid organ transplant recipients (OTR) are a susceptible population due to the long-term immunosuppressant therapy [2]. SCC is the most common skin neoplasia and may affect as much as 30–58% of transplanted recipients, usually exhibiting an aggressive course with metastasis [3]. Actinic keratosis, which can eventually develop into invasive SCC, also exhibits an increased incidence in the OTR patients [3]. Therefore, prevention and management of skin cancer in OTR patients become a priority in the field of dermatology and oncology.
Retinoids are the structural and functional analogues of vitamin A with a wide range of biologic activities. Retinoids are active in most tissues and have major impacts on both normal and diseased skin [4]. Systemic retinoids, specifically acitretin, are effective, therapeutic and chemopreventive agents for some non-melanoma skin cancers. Lebwohl et al.[5] described a psoriatic patient developed multiple SCCs after long-term treatment with psoralen plus ultraviolet A and oral acitretin was administered to prevent the recurrence of skin cancer after surgical removal. Kuan et al.[6] successfully treated a patient who had multiple, extensive verrucous carcinomas with a single regimen of acitretin. Acitretin have also shown to be effective in controlling and reducing actinic keratosis [3] and preventing the development of SCC in OTR [2, 7].
Some studies suggested that retinoid could be used as a chemopreventive and chemotherapeutic agent in various malignancies because of its anti-proliferating effect through induction of cell apoptosis [8–10]. Other hypotheses postulate that retinoids may affect growth factors, down-regulate proto-oncogenes and increase ceramides [11–13].
Apoptosis plays a critical role in carcinogenesis, its signalling pathways include death receptor (extrinsic) and mitochondrial (intrinsic) pathway [14]. The Fas (CD95)/Fas ligand (FasL; CD95L) system is an important extracellular pathway. The binding of FasL to the Fas receptor activates caspase-8 (an initiator caspase) via Fas-associated death domain protein (FADD), and the two types of cells have been defined. In type I cells, caspase-8 is sufficient to ensure execution of the final steps of apoptosis through direct activation of caspase-3 [15, 16]. In type II cells, activation of caspase-8 leads to activation of mitochondria, which in turn results in cleavage of caspases-9, -3 [17, 18]. On the other hand, in intrinsic pathway, caspase-9 (another initiator caspase) activates caspase-3, contributing to apoptotic cell death [19, 20].
Although two major apoptotic pathways have been well defined in mammalian cells, the involvement of these two apoptotic pathways in acitretin-induced apoptosis has not been previously reported. Therefore, this study was to investigate the apoptosis-inducing effect of acitretin on human SCC cell line SCL-1 and HaCaT cells.
Materials and methods
Antibodies and reagents
Antibodies to caspase-3 (sc-7272), -9 (sc-8355), Fas (sc-715), FasL (sc-834) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and antibodies to human FADD, PARP, from NeoMarkers (Fremont, CA, USA). Fluorescein isothiocyanate (FITC)-conjugated anti-human Fas antibody (DX2) and antibodies to caspase-8 (551242) were purchased from BD PharMingen (San Diego, CA, USA), antibodies to neutralizing Fas (ZB4), from Upstate Biotechnology (Waltham, MA, USA). Trizol was obtained from Invitrogen (San Diego, CA, USA), and Z-IETD-FMK (caspase-8 inhibitor) and Z-LEHD-FMK (caspase-9 inhibitor) from Santa Cruz Biotechnology.
Cell culture and acitretin treatment
SCL-1, a human SCC cell line [21, 22], as well as HaCaT, a spontaneously immortalized human keratinocyte cell line [22, 23], were originally provided by N.E. Fusenig (German Cancer Research Center, Heidelberg, Germany). Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) medium (Hyclone, South Logan, UT, USA) supplemented with 10% heat-inactivated foetal calf serum (Hyclone), 100 U/ml penicillin and 100 U/ml streptomycin in 75-cm2 tissue culture flasks (BD PharMingen). Acitretin was obtained from Chongqing Huapont Pharm. Co., LTD. (Chongqing, China), diluted in dimethylsulfoxide (DMSO) (Sigma, St Louis, MO, USA) and stored in light-protected vials at –80°C. Subsequent dilutions were made in normal culture medium with a final DMSO concentration of 0.05%. The control was supplied with DMSO of a final concentration of 0.05%.
Assay for cell viability
Cell viability was determined by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) cell proliferation assay. Briefly, SCL-1 cells and HaCaT cells were seeded at a density 3000 cells per well in 96-well tissue culture plates (BD PharMingen). After 24 hrs, cells were treated with five different concentrations of acitretin; control cultures received DMSO (0.05%). At designated time points after treatment, the cells were treated in sequence with MTT (Sigma) and DMSO. The absorbance was measured at 490 nm. Cell viability was expressed as the percentage of exposed cells to controls.
Determination of DNA fragmentation by ELISA
The cytoplasmic histone-associated DNA fragments were measured in SCL-1 cells and HaCaT cells using the Cell Death Detection ELISA (Roche Diagnostics, GmbH, Germany). Briefly, acitretin-treated cells were lysed and centrifuged at 200 ×g for 10 min. at 4°C. Twenty microlitres of the resulting supernatant were added to a streptavidin-coated microplate, followed by incubation with immunoreagent (containing anti-histone and anti-DNA) under moderate shaking for 2 hrs. The wells were then washed three times and 100 μl substrate solutions added and incubated for 15 min. at room temperature. The absorption was measured at 405 nm using an ELISA reader. All experiments were performed in duplicate and were repeated at least three times.
AnnexinV/propidium iodide (PI) assay by flow cytometry
Apoptotic cells were quantified by annexinV-FITC and PI double staining using a staining kit from Roche Diagnostics. Briefly, SCL-1 cells and HaCaT cells were treated with either DMSO or retinoids dissolved in DMSO for the designated times, and then 1.0 × 106 cells were washed with ice-cold phosphate buffer saline (PBS) and resuspended with 100 μl binding buffer. Two microlitres FITC-conjugated annexinV and 2 μl PI were added to the resuspended cells and incubated for 15 min. in the dark. The cells were then resuspended in 0.5 ml of binding buffer and analysed by flow cytometry.
Apoptotic morphological changes
Ultrastructural changes in cell apoptosis were determined by transmission electron microscopy. Briefly, treated and control cells in 75-cm2 flasks were trypsinized, washed with PBS, fixed in 2.5% glutaraldehyde in 0.15 M sodium cacodylate buffer for 30 min., post-fixed in 1% aqueous osmium tetroxide for 30 min. and embedded in Epon-Araldite. Thin sections were stained with uranyl acetate and lead citrate and examined under a Philips 300 electron microscope (Philips, Eindhoven, the Netherlands) at 90 kV. Micrographs were taken randomly for both acitretin-treated and control cultures and were blindly examined by an independent pathologist.
Detection of proteins by Western blotting
Cells were washed with PBS and then suspended in an extraction buffer (20 mM Tris-Cl, pH 7.4, 100 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 5 mM MgCl2, 0.1 mM phenylmethylsulfonyl fluoride, 0.1 mM pepstatin A, 0.1 mM antipain, 0.1 mM chymostatin, 0.2 mM leupeptin, 10 μg/ml aprotinin, 0.5 mg/ml soybean trypsin inhibitor and 1 mM benzamidine) on ice for 15 min. Lysates were spun down at 14,000 rpm for 20 min. Protein concentration was determined using the BCA Protein Assay Reagent (Pierce Chemical Company, Rockford, IL, USA). Equal amounts of cell extracts were resolved on SDS-polyacrylamide denaturing gels, transferred onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany) overnight at 4°C. The membrane was blocked in Tris-buffered saline containing 2% non-fat dry milk (Bio-Rad, Hercules, CA, USA) and 0.05% Tween 20 (Merck-Schuchardt, Hohenbrunn, Germany) for 1 hr and probed with an appropriate primary and horseradish peroxidase-conjugated secondary antibody. Protein bands were visualized using an enhanced chemiluminescence system (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Real-time PCR analysis
Real-time PCR was performed to assess Fas and FasL expression in SCL-1 cells. Briefly, total RNA was extracted from treated or untreated SCL-1 cells using Trizol reagent. Two micrograms of total RNA were reversely transcribed with TIANscript M-MLV transcriptase (Tiangen Biotechnology, Beijing, China) using random primers. The quantification of apoptosis-related genes was carried out in triplicate for each gene. Primers for Fas receptor were sense, 5′-AAGTGACTGACATCAACTCC-3′; and antisense, 5′-CCACTTCTAAGCCATGTCC-3′, amplicon size was 270 bp. Primers for the FasL were sense, 5′-ACATGAGGAACTCTAAGTATCCC-3′; and antisense, 5′-CAAAATTGACCAGAGAGAGC-3′, amplicon size was 174 bp. Primers for β-actin (house keeper gene) were sense, 5′-TCATCACCATTGGCAATGAGC-3′; and antisense, 5′-CAGCACTGTGTTGGCGTACAGGT-3′, amplicon size was 158 bp. The optimal PCR reaction was established using the Real Master Mix (SYBR Green) PCR Kit (Tiangen Biotechnology) according to the manufacturer’s instructions. A relative quantification analysis (two standard curves method) was carried out with Rotor Gene 3000 software, version 6.0 (Corbett Research, Mortlake, Australia). The ratio of a target DNA sequence to a reference β-actin sequence in samples from untreated cells was served as a ‘calibrator’. The results are expressed as a normalized ratio.
Assays for Fas
The expression of cell surface Fas was measured by immunofluorescence flow cytometry. After various treatments, a total of 1.0 × 106 cells was collected by centrifugation, and washed twice with ice-cold PBS containing 1% bovine serum albumin. Cells were then incubated with 100 μl of FITC-conjugated anti-Fas antibody or control mouse IgG-FITC antibody for 40 min. at 4°C. After incubation in the dark, cells were washed twice and resuspended in ice-cold PBS. Immunofluorescence staining of cell surface Fas was analysed by flow cytometry. For Fas inhibitor assay, neutralizing ZB4 anti-Fas antibody was added at 1 μg/ml 10 min. before treatment with acitretin for 24 hrs. Apoptosis was analysed by the ELISA assay as described above.
Inhibition of caspase activity
SCL-1 cells that were induced to undergo apoptosis were cultured in the presence or absence of caspase inhibitors that bind to the active site of their respective protease. The following caspase inhibitors were used: Z-IETD-FMK (caspase-8 inhibitor), Z-LEHD-FMK (caspase-9 inhibitor). Inhibitors were dissolved in DMSO at a concentration of 10 μM and diluted with normal culture medium to a final concentration of 10 nM. Cells were pre-incubated with caspase inhibitors for 10 min. at 37°C before adding acitretin (1 × 10−5 M) for 24 hrs at 37°C. Control cells were incubated in DMSO diluted 1:2000 in culture medium for 48 hrs at 37°C and 5% CO2. At the end of incubation, floating cells and attached cells were combined and analysed for apoptosis by the ELISA assay.
Statistical analyses
All statistical analyses were performed using Excel software (Microsoft, Redmond, WA, USA). Data are presented as median, analysed using the Mann-Whitney U test or Kruskal–Wallis H test. P-values less than 0.05 were considered statistically significant.
Results
Effects of acitretin on the cell proliferation of cell line SCL-1
We investigated the effects of acitretin on cell proliferation in SCL-1 and HaCaT at five different concentrations of acitretin for 3 days and 10−5 M of acitretin for 1, 3, 5 days using a MTT assay (Fig. 1A and B). The results showed that acitretin inhibited cell growth of SCL-1 in a dose- and time-dependent manner. In contrast, acitretin exhibited a few inhibitory effects on the proliferation of HaCaT cells, indicating that acitretin showed less toxicity to non-malignant keratinocytes.
Figure 1.
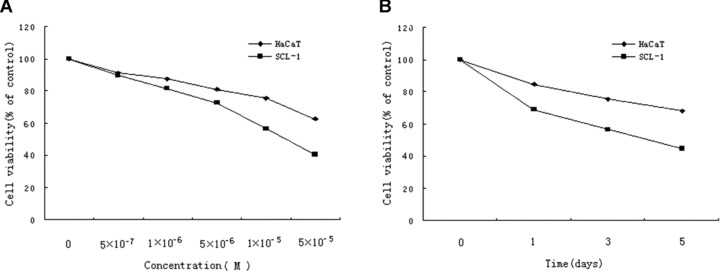
Effects of acitretin on cell growth of SCL-1 cells. (A) SCL-1 cells and HaCaT cells were treated with acitretin at five different concentrations for 3 days. (B) SCL-1 cells and HaCaT cells were treated with 10−5 M of acitretin for various times. Cell proliferation was assessed by MTT assay. Cell viability was estimated from the equation: % of cell viability = 100 × At/Ac, where At and Ac are the absorbencies in treated and control cultures, respectively. Data are shown as median of three different independent experiments (each with three cultures).
Effects of acitretin on apoptosis in SCL-1 cells
The induction of apoptosis was determined by the Cell Death Detection ELISA, and the results showed acitretin induced apoptosis of SCL-1 cells in a dose- and time-dependent manner (Fig. 2A and B). However, the pro-apoptotic effect of acitretin on HaCaT was much less prominent than that on SCL-1. AnnexinV and PI double staining were performed to confirm the above findings in cultured SCL-1 cells and HaCaT cells incubated with either vehicle or acitretin for 1, 2 and 3 days. AnnexinV-positive apoptotic cells were found to increase time dependently in acitretin-treated SCL-1 cells, consistent with the results of ELISA (Fig. 2C and D). Ultrastructural changes were examined to further confirm the apoptosis. Under transmission electron microscopy, the nucleus of SCL-1 cells treated with acitretin for 5 days showed features of apoptosis, including disappeared villi on cell membrane, nucleus shrinkage, dense aggregation and margination of chromatin. In control group, SCL-1 cells displayed normal morphology with rich villi on the cell membrane, normal shape of nucleus and nuclear chromatin which evenly distributed.
Figure 2.
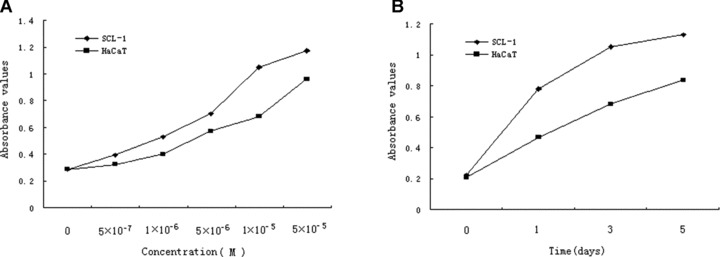
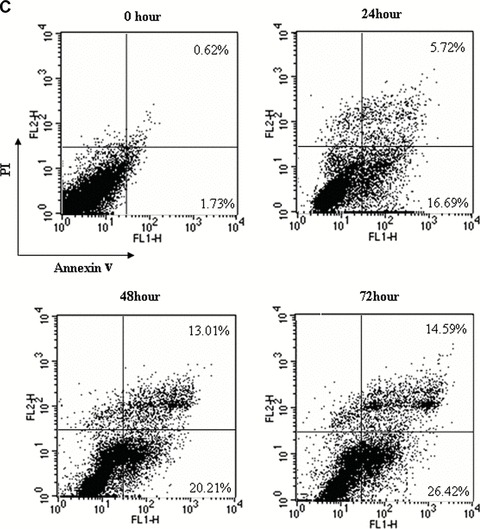
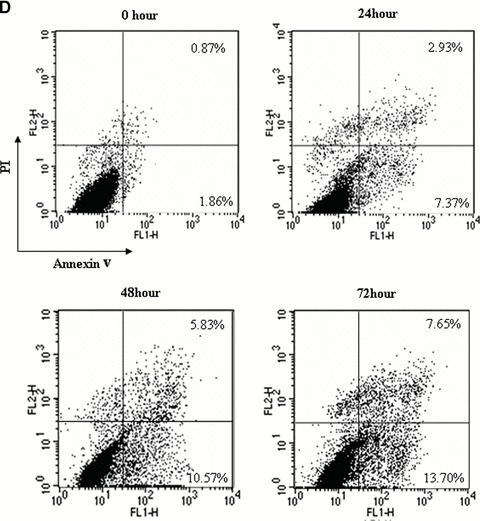
Effects of acitretin on apoptosis in SCL-1 cells. (A) SCL-1 cells and HaCaT cells were treated with acitretin at five different concentrations for 3 days. (B) SCL-1 cells and HaCaT cells were treated with 10−5 M of acitretin for various times. To determine and quantify the induction of apoptosis by acitretin in the SCL-1 cells and HaCaT cells, 20 μl of cell lysate was used for Cell Death Detection ELISA kit. DNA fragmentation was quantified at 405 nm. Data are shown as median of three different independent experiments. (C and D) SCL-1 cells (C) and HaCaT cells (D) were cultured with 10−5 M acitretin for 0, 24, 48 and 72 hrs. The cells were then stained with annexinV-FITC and PI labelling and were analysed by flow cytometry. The percentage of cells in each window is indicated. The percentage of annexinV-positive apoptotic cells in SCL-1 cells and in HaCaT cells both dramatically increased in a time-dependent manner. Three independent experiments were performed with similar results.
Effects of acitretin treatment on caspase activation
Since caspases play a pivotal role in the apoptosis signalling pathway [24, 25], we examined the activation of caspases-8, -9, -3 by both active and inactive forms using Western blot analysis. An active fragment of caspase-8 occurred 12 hrs after acitretin treatment, this fragment increased time dependently, whereas the corresponding caspase-9 and caspase-3 active fragments occurred 24 hrs after acitretin treatment (Fig. 3A). To confirm the presence of active caspase-3 in acitretin-treated cells, the cleavage of PARP was determined. After treatment with acitretin for 24 hrs, the full-length form of the PARP protein (116 kD), a substrate for caspase-3, was degraded into the cleaved form (85 kD; Fig. 3A). These results suggest that acitretin-induced apoptosis is associated with the activation of caspases-8, -9, -3 and caspase-8 may be the first caspase activated by acitretin in SCL-1 cells.
Figure 3.
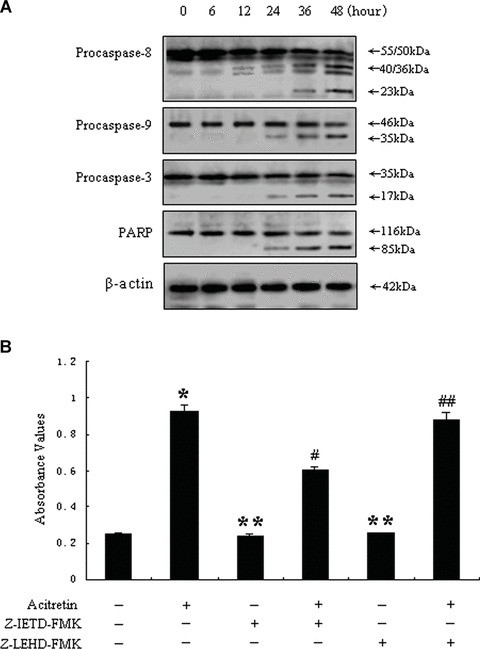
Effects of acitretin on caspase activation. (A) Time course of caspases-8, -9, -3 and PARP cleavage in acitretin-treated SCL-1 cells. Cells were cultured with 10−5 M acitretin for the indicated times, and then the cells were harvested. Twenty micrograms cell extracts were resolved by 7.5% SDS-polyacrylamide denaturing gels and analysed by Western blotting with specific antibodies for activation of caspases-9 and -3 and cleavage of the caspase-3 substrate PARP. Immunoblotting of the same membranes with antibodies to β-actin was used to control for loading. The results represent one of three independent experiments, which yielded similar results. (B) Cells were treated for 48 hrs with 10−5 M acitretin alone or the caspases-8 and -9 inhibitors (see ‘Materials and methods’) respectively, or the combinations of acitretin with each of the above caspase inhibitors. The SCL-1 cells were harvested and analysed for apoptosis by the Cell Death Detection ELISA. Cells treated with DMSO were used as control. Data are shown as median of three independent cultures in one representative experiment. The experiment shown is representative of three independent experiments showing similar results. *P < 0.01 versus untreated cells. **P > 0.01 versus untreated cells. #P < 0.01 versus acitretin-treated cells. ##P > 0.01 versus acitretin-treated cells.
In order to confirm the role of caspases in acitretin-induced apoptosis, Cell Death Detection ELISA was performed with inhibitors of caspases-8 and -9. These caspase inhibitors, when used alone, did not exert any effect on the apoptosis of SCL-1 cells. However, combined treatment with inhibitor of caspase-8 effectively blocked acitretin-induced apoptosis in SCL-1 cells, whereas with inhibitor of caspase-9 failed to do so (Fig. 3B). These results indicate that acitretin-induced apoptosis is dependent on the activation of caspase-8.
Induction of cell surface Fas protein and mRNA expressions of Fas/FasL during acitretin-induced apoptosis
The fact that caspase-8 inhibitor was found to block acitretin-induced apoptosis dramatically in SCL-1 cells encourage us to further investigate whether the cell surface Fas receptor is affected by acitretin with flow cytometric analysis using an anti-Fas monoclonal antibody. The results revealed that the level of the cell surface Fas receptor was significantly increased in acitretin-treated cells compared with the untreated controls (Fig. 4). Accordingly, the results with relative quantification analysis also demonstrated that both Fas and FasL mRNAs increased at 12 hrs after the addition of acitretin and continued to increase substantially in a time-dependent manner (Fig. 5, Table 1).
Figure 4.
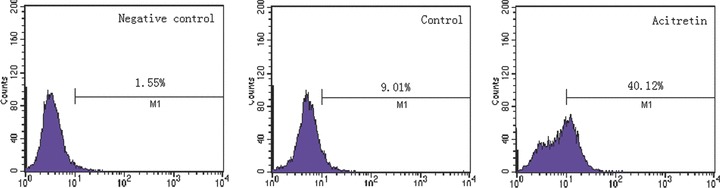
Evaluation of Fas receptor protein expression on the cell surface. After incubating for 48 hrs, SCL-1 cells with normal culture medium (control) or with acitretin (10−5 M) were stained with FITC-conjugated anti-Fas-specific antibody. Fas receptor protein expression on the cell surface was analysed by flow cytometry. For the sake of clarity, we used antimouse IgG-FITC antibody as the negative control. The percentages of FITC-positive cells are indicated. Three duplicate experiments were performed with similar results.
Figure 5.
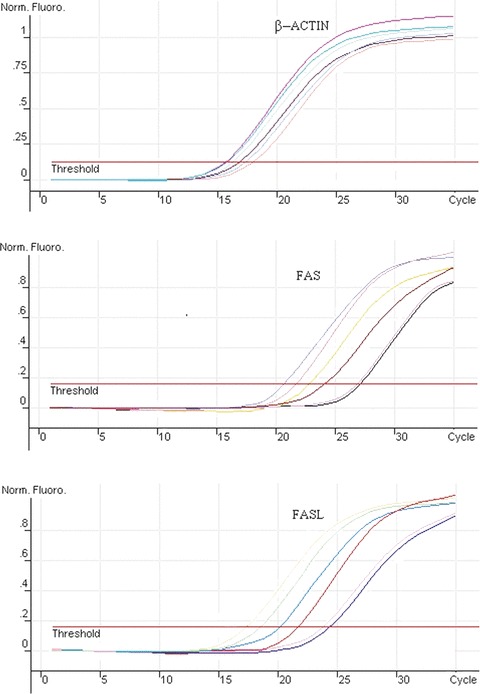
Results of mRNA amplified by real-time PCR. Total RNA was purified from SCL-1 cells treated with 10−5 M acitretin for 0, 6, 12, 24, 36 and 48 hrs. Two micrograms of total RNA were reversely transcribed and the levels of Fas, FasL and β-actin mRNA were determined by real-time PCR. A relative quantification analysis (two standard curves method) was carried out. The ratio of a target DNA sequence to a reference β-actin sequence in samples from untreated cells was served as a ‘calibrator’. Figures are representative of three independent experiments.
Table 1.
Relative quantification of Fas, FasL mRNA expression in SCL-1.
| Gene | Ratio of mRNA level | |||||
|---|---|---|---|---|---|---|
| 0 hr | 6 hrs | 12 hrs | 24 hrs | 36 hrs | 48 hrs | |
| Fas | 1* | 0.87* | 5.91* | 8.05* | 13.47* | 18.52* |
| (1–1) | (0.80–0.95) | (4.12–5.91) | (6.64–8.96) | (11.05–16.02) | (16.03–21.98) | |
| FasL | 1** | 0.91** | 6.96** | 10.47** | 16.93** | 47.09** |
| (1–1) | (0.83–1.04) | (4.87–8.69) | (8.54–11.97) | (13.06–20.57) | (40.22–53.37) | |
Values shown are median (interquartile range), analysed using Kruskal–Wallis H test.
P < 0.05, comparing Fas mRNA level in various times of 10−5 M acitretin-treated SCL-1 cells.
P < 0.05, comparing FasL mRNA level in various times of 10−5 M acitretin-treated SCL-1 cells.
Effects of acitretin on protein expressions in Fas apoptotic signalling pathway
To be sure whether acitretin affects the death receptor apoptotic pathway, we checked the status of Fas, FasL and FADD proteins. The results shown in Fig. 6 demonstrate the significantly increased levels of Fas, FasL, FADD in a time-dependent manner just after 12 hrs exposing to acitretin, and these changes were well matched to the appearance of the active caspase-8 fragment (Fig. 3A), presumably as a result of Fas/FasL binding. The results indicated that acitretin increased the levels of both Fas and FasL, which could initiate death signalling. To further test this possibility, we examined the effects of ZB4, an antagonistic Fas-antibody, on apoptosis in acitretin-treated cells by disturbing Fas–FasL interaction. As shown Fig. 7, ZB4 alone did not exert any effect on the apoptosis of SCL-1 cells; however, acitretin-induced apoptosis was effectively suppressed if the cells were pre-treated with ZB4 for 1 hr followed by concurrent treatment with acitretin and ZB4. These data suggested that the apoptotic pathway associated with Fas–FasL interaction is involved in acitretin-induced apoptosis.
Figure 6.
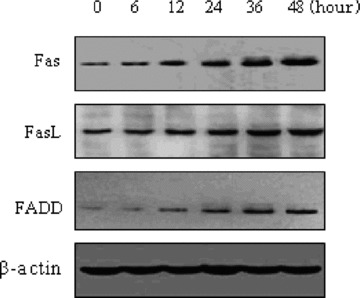
Effects of acitretin on the expression of Fas, FasL and FADD proteins in SCL-1 cells. Cells were treated with 10−5 M acitretin for the indicated times, and then the cells were harvested. 20 μg cell extracts were resolved by 7.5% SDS-polyacrylamide denaturing gels and analysed for Fas, FasL, FADD by Western blotting with anti-Fas, FasL and FADD antibodies. As control, antibodies to β-actin were used for loading equal amount of specimens. The experiment shown is representative of three independent experiments showing similar results.
Figure 7.
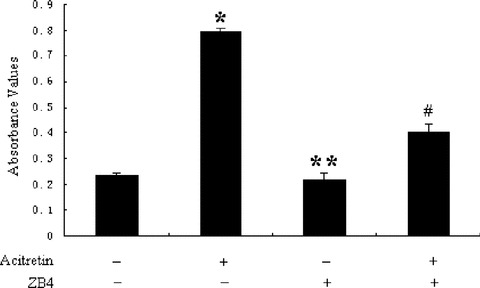
Fas–FasL interaction-dependent apoptosis by acitretin in SCL-1 cells. SCL-1 cells were treated with 1 μg/ml ZB4 for 10 min., then acitretin (10−5 M) was added, following by incubation for an additional 24 hrs. The cells were then analysed for apoptosis by the Cell Death Detection ELISA. Data are shown as median of three independent cultures in one representative experiment. The experiment shown is representative of three independent experiments showing similar results. *P < 0.01 versus untreated cells. **P > 0.01 versus untreated cells. #P < 0.01 versus acitretin- treated cells.
Discussion
Acitretin, the second generation of retinoids, was reported to exhibit anti-tumour activity in actinic keratosis, verrucous carcinomas, SCC without significant side effects or toxicity [2, 3, 5, 6]. However, the mechanism of its anti-carcinogenic effect is not fully understood. In this report, we demonstrate for the first time that acitretin-induced apoptosis in skin SCC cell line SCL-1 via CD95/CD95L apoptotic signalling pathway.
We found that SCL-1 cells were sensitive to the growth inhibitory effects of acitretin, which correlated with apoptosis induction. The apoptosis induction was further confirmed by annexinV/PI assay and apoptotic changes in morphology under electron microscope. Because caspases are central components in apoptotic signalling pathways [25, 26, 27], the involvement of caspases was examined. Among the caspases, caspases-3, -8 and -9 have been shown to play important roles in apoptosis [28], and caspase-3 is a key executioner involved in apoptosis. Caspase-3 activity is controlled by upstream regulators, such as caspase-9 or caspase-8, which modulates the mitochondria- and death receptor-dependent pathway, respectively [29]. Acitretin activated caspases-3, -8 and -9, it is plausible that both the mitochondrial and the death receptor pathways might be activated by acitretin. However, in our study, the inhibitor of caspase-9 had no significant effect on acitretin-induced apoptosis, indicating that intrinsic pathway did not play the major role in the apoptosis. Caspase-8 inhibitor effectively suppressed acitretin-induced apoptosis, implying that acitretin-induced apoptosis is dependent on the extrinsic pathway because caspase-8 is primarily activated during the death receptor apoptotic pathway. To determine whether the death receptor pathway is really involved in acitretin-induced apoptosis, we evaluated the status of the cell surface Fas receptor, which is a representative member of the death receptor family, is affected by acitretin-treated cells. Interestingly, the expression of Fas on the plasma membrane was significantly increased 48 hrs after acitretin treatment. Corresponding to the appearance of the active caspase-8 fragment (Fig. 3A), similar changes were also detected by quantitating both mRNA and protein for Fas receptor using RT-PCR and Western blot (Figs 5 and 6, Table 1); these results indicate that the apoptotic pathway related to Fas seems to be involved in acitretin-induced apoptosis.
Fas death receptor can be activated to undergo oligomerization by binding its ligand FasL, then the activated Fas recruits the adapter molecule FADD by the death domain, and procaspase-8 into a complex resulting in activated caspase-8. We demonstrated that FasL levels were increased in SCL-1 cells during the treatment with acitretin by RT-PCR and Western blotting (Figs 5 and 6, Table 1). In addition, up-regulation of Fas, FasL and FADD during acitretin treatment corresponded to activation of caspase-8 (Figs 3A and 6). These results suggest that signalling by the CD95/CD95L system may play an important role in the induction of apoptosis of SCL-1 cells by acitretin. The involvement of the Fas-mediated pathway in acitretin-induced apoptosis was corroborated by further investigating the effects of neutralizing ZB4 anti-Fas antibody on acitretin-induced apoptosis by disturbing the Fas–FasL interaction. ZB4 was found to inhibit the apoptosis induced by acitretin in SCL-1 cells, suggesting that Fas receptor–ligand interaction was involved in acitretin-induced apoptosis pathway.
In CD95/CD95L apoptotic signalling, the mitochondrial activation-mediated pathway is required in type II cells [30, 31]. Activation of caspase-8 induces the release of cytochrome c from the mitochondria [18]. Cytochrome c, with the help of apoptotic protease activating factor-1, activates caspase-9 and in turn activates caspase-3 [32], which is also transmitted in type I cells [33]. Because mitochondrial pathway is obligatory in mediating the Fas signalling in type II cells, any damage to mitochondrial pathway can only inhibit Fas-induced apoptosis in these cells, but not type I cells [34]. In this study acitretin substantially induced the activation of caspase-9; however, caspase-9 inhibitor failed to affect acitretin-induced apoptosis, whereas caspase-8 inhibitor or ZB4 effectively suppressed acitretin-induced apoptosis. From these findings, we conclude that activation of caspase-9 is triggered by Fas and acitretin enhances a type I CD95/Fas-mediated apoptotic signalling pathway in SCL-1 cells.
Skin cancers are by far the most common human malignancies [35], although the surgical removal is the major therapeutic modality, but it is practically limited in treating multiple lesions. Particularly in OTR patients with the high risk of transition from pre-malignant to malignant cutaneous lesions, the therapeutic options are limited to the local excision, cryotherapy, topical chemotherapeutic agents and radiotherapy [36]. The presence of multiple cancerous lesions makes it difficult to effectively treat cutaneous malignancies at the same time by dermatosurgery. Therefore, the preventative or suppressive modalities have been recommended in the population with high risk of SCC, and also with actinic keratosis, verrucous carcinomas. Oral retinoids, specifically acitretin, have been shown to have chemosuppressive effects against skin cancers in OTR [2]. Several uncontrolled trials, case reports and a few small controlled trials have supported the use of oral acitretin for chemoprevention of skin cancer in this population [7, 37, 38]. For instance, Bavinck et al.[39] reported that 2 of the 19 patients (11%) developing two SCCs in the treatment group as opposed to 9 of the 19 patients (47%) in the placebo group developed 18 new skin cancers (15 SCCs, 1 Bowen’s disease, 2 basal cell carcinomas-BCCs). The difference was significant both in terms of number of patients developing skin cancers and in terms of number of lesions developing. To date, retinol and the retinoids are the only compounds proved to be effective for chemoprevention of skin carcinogenesis [13]. In this study, acitretin remarkably inhibited cell proliferation of skin SCC cells by the induction of apoptosis, while maintaining the minimal toxicities to the cell growth and a few apoptotic effects of non-malignant keratinocytes, indicating that the effects of acitretin are specific to neoplastic cells derived from keratinocytes.
In summary, our findings demonstrate that acitretin is a potent inducer of apoptosis in human cutaneous SCC cell line SCL-1, and enhance a type I CD95/Fas-mediated apoptotic signalling pathway, provide biologic insight into the clinical results of acitretin in skin SCC. Our results suggest that further studies on the potential of this retinoid for prevention and therapy of skin SCC and the parallel clinical application are warranted.
Acknowledgments
We thank Professor Christoph C. Geilen, Professor Constantin E Orfanos and Dr. Jürgen Eberle (the former Free University of Berlin, Germany) for their kind helps in this experiment. We thank Chongqing Huapont Pharm. Co., LTD. China for acitretin. This work was supported in part by grants from the National Natural Science Foundation of China (30271198), the Project-sponsored by SRF for ROCS, SEM (2002247) and Program for Changjiang Scholars and Innovative Research Team in University (State Education Ministry, IRT0769 and Education Department of Liaoning Province).
References
- 1.Jagdeo J, Weinstock MA, Piepkorn M, et al. Reliability of the histopathologic diagnosis of keratinocyte carcinomas. J Am Acad Dermatol. 2007;57:279–84. doi: 10.1016/j.jaad.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Kovach BT, Murphy G, Otley CC, et al. Oral retinoids for chemoprevention of skin cancers in organ transplant recipients: results of a survey. Transplant Proc. 2006;38:1366–8. doi: 10.1016/j.transproceed.2006.02.119. [DOI] [PubMed] [Google Scholar]
- 3.Carneiro RV, Sotto MN, Azevedo LS, et al. Acitretin and skin cancer in kidney transplanted patients. Clinical and histological evaluation and immunohistochemical analysis of lymphocytes, natural killer cells and Langerhans cells in sun exposed and sun protected skin. Clin Transplant. 2005;19:115–21. doi: 10.1111/j.1399-0012.2004.00311.x. [DOI] [PubMed] [Google Scholar]
- 4.Cheepala SB, Syed Z, Trutschl M, et al. Retinoids and skin: microarrays shed new light on chemopreventive action of all-trans retinoic. Acid Mol Carcino. 2007;46:634–9. doi: 10.1002/mc.20346. [DOI] [PubMed] [Google Scholar]
- 5.Lebwohl M, Tannis C, Carrasco D. Acitretin suppression of squamous cell carcinoma case report and literature review. J Dermatol Treat. 2003;14:3–6. doi: 10.1080/jdt.14.s2.3.6. [DOI] [PubMed] [Google Scholar]
- 6.Kuan YZ, Hsu HC, Kuo TT, et al. Multiple verrucous carcinomas treated with acitretin. J Am Acad Dermatol. 2007;56:29–32. doi: 10.1016/j.jaad.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 7.De Graaf YG, Euvrard S, Bouwes Bavinck JN. Systemic and topical retinoids in the management of skin cancer in organ transplant recipients. Dermatol Surg. 2004;30:656–61. doi: 10.1111/j.1524-4725.2004.30152.x. [DOI] [PubMed] [Google Scholar]
- 8.Reilly P, DiGiovanna JJ. Retinoid chemoprevention in high-risk skin cancer patients. Dermatol Nurs. 2004;16:117–20. [PubMed] [Google Scholar]
- 9.Orlandi A, Bianchi L, Costanzo A, et al. Evidence of increased apoptosis and reduced proliferation in basal cell carcinoma treated with tazarotene. J Invest Dermatol. 2004;122:1037–41. doi: 10.1111/j.0022-202X.2004.22414.x. [DOI] [PubMed] [Google Scholar]
- 10.Harwood CA, Leedham-Green M, Leigh IM, et al. Low dose retinoids in the prevention of cutaneous squamous cell carcinomas in organ transplant recipients: a 16-year retrospective study. Arch Dermatol. 2005;141:456–64. doi: 10.1001/archderm.141.4.456. [DOI] [PubMed] [Google Scholar]
- 11.Schule R, Rangarajan P, Yang N, et al. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci USA. 1991;88:6092–6. doi: 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen LA, Sigman CC, Andreola F, et al. Retinoids in chemoprevention and differentiation therapy. Carcinogenesis. 2000;21:1271–9. [PubMed] [Google Scholar]
- 13.Wright TI, Spencer JM, Flowers FP. Chemoprevention of nonmelanoma skin cancer. J Am Acad Dermatol. 2006;54:933–46. doi: 10.1016/j.jaad.2005.08.062. [DOI] [PubMed] [Google Scholar]
- 14.Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer. 2005;5:231–7. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]
- 15.Barnhart BC, Alappat EC, Peter ME. The CD95 type I/type II model. Semin Immunol. 2003;15:185–93. doi: 10.1016/s1044-5323(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 16.Peter ME, Krammer PH. The CD95 (APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 17.Scaffidi C, Fulda S, Srinivasan , et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–87. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chun KH, Pfahl M, Lotan R. Induction of apoptosis by the synthetic retinoid MX3350–1 through extrinsic and intrinsic pathways in head and neck squamous carcinoma cells. Oncogene. 2005;24:3669–77. doi: 10.1038/sj.onc.1208339. [DOI] [PubMed] [Google Scholar]
- 19.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 20.Xian M, Ito K, Nakazato T, et al. Zerumbone, a bioactive sesquiterpene, induces G2/M cell cycle arrest and apoptosis in leukemia cells via a Fas- and mitochondria-mediated pathway. Cancer Sci. 2007;98:118–26. doi: 10.1111/j.1349-7006.2006.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tilgen W, Boukamp P, Breitkreutz D, et al. Preservation of morphological, functional, and karyotypic traits during long-term culture and in vivo passage of two human skin squamous cell carcinomas. Cancer Res. 1983;43:5995–6011. [PubMed] [Google Scholar]
- 22.Schön MP, Schön M, Klein CE, et al. Carcinoma-associated 38-kD membrane glycoprotein MH 99/KS 1/4 is related to proliferation and age of transformed epithelial cell lines. J Invest Dermatol. 1994;102:987–91. doi: 10.1111/1523-1747.ep12384258. [DOI] [PubMed] [Google Scholar]
- 23.Boukamp P, Petrussevska RT, Breitkreutz D, et al. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1998;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–6. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 25.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–6. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 26.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 27.Kim SG, Jong HS, Kim TY, et al. Transforming growth factor-β1 induces apoptosis through Fas ligand-independent activation of the Fas death pathway in human gastric SNU-620 carcinoma cells. Mol Biol Cell. 2004;15:420–34. doi: 10.1091/mbc.E03-04-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung JY, Kim WJ. Involvement of mitochondrial- and Fas-mediated dual mechanism in CoCl2-induced apoptosis of rat PC12 cells. Neurosci Lett. 2004;371:85–90. doi: 10.1016/j.neulet.2004.06.069. [DOI] [PubMed] [Google Scholar]
- 29.Kuida K, Zheng TS, Na S, et al. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–72. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 30.Park MT, Kang JA, Choi JA, et al. Phytosphingosine induces apoptotic cell death via caspase 8 activation and Bax translocation in human cancer cells. Clin Cancer Res. 2003;9:878–85. [PubMed] [Google Scholar]
- 31.Ito K, Nakazato T, Murakami A, et al. Induction of apoptosis in human myeloid leukemic cells by 1’-acetoxychavicol acetate through a mitochondrial- and Fas-mediated dual mechanism. Clin Cancer Res. 2004;15:2120–30. doi: 10.1158/1078-0432.ccr-1142-03. [DOI] [PubMed] [Google Scholar]
- 32.Zou H, Li Y, Liu X, et al. An APAF-1. Cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–56. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 33.Poulaki V, Mitsiades N, Romero ME, et al. Fas-mediated apoptosis in neuroblastoma requires mitochondrial activation and is inhibited by FLICE inhibitor protein and bcl-2. Cancer Res. 2001;61:4864–72. [PubMed] [Google Scholar]
- 34.Henkler F, Behrle E, Dennehy KM, et al. The extracellular domains of FasL and Fas are sufficient for the formation of supramolecular FasL-Fas clusters of high stability. J Cell Biol. 2005;168:1087–98. doi: 10.1083/jcb.200501048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filipowicz E, Adegboyega P, Sanchez RL, et al. Expression of CD95 (FAS) in Sun-Exposed Human skin and cutaneous carcinomas. Am Cancer Soc. 2002;94:814–9. doi: 10.1002/cncr.10277. [DOI] [PubMed] [Google Scholar]
- 36.De Sévaux RG, Smit JV, De Jong EM, et al. Acitretin treatment of premalignant and malignant skin disorders in renal transplant recipients: clinical effects of a randomized trial comparing two doses of acitretin. J Am Acad Dermatol. 2003;49:407–12. doi: 10.1067/s0190-9622(03)01831-0. [DOI] [PubMed] [Google Scholar]
- 37.Chen K, Craig JC, Shumack S. Oral retinoids for the prevention of skin cancers in solid organ transplant recipients: a systematic review of randomized controlled trials. Br J Dermatol. 2005;152:518–23. doi: 10.1111/j.1365-2133.2005.06347.x. [DOI] [PubMed] [Google Scholar]
- 38.Kovach BT, Sams HH, Stasko T. Systemic strategies for chemoprevention of skin cancers in transplant recipients. Clin Transplant. 2005;19:726–34. doi: 10.1111/j.1399-0012.2005.00412.x. [DOI] [PubMed] [Google Scholar]
- 39.Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind placebo controlled study. J Clin Oncol. 1995;13:1933–8. doi: 10.1200/JCO.1995.13.8.1933. [DOI] [PubMed] [Google Scholar]


