Abstract
Purpose
Minor salivary gland cancers are rare and account for roughly 2% to 3% of all head and neck tumors. This is a retrospective review in a modern cohort of patients treated for this rare cancer with surgery and adjuvant radiation therapy.
Materials and Methods
Between February 1990 and December 2010, 98 patients with cancer of the minor salivary glands were identified and treated at a single institution. The median radiation dose was 63 Gy. Outcomes assessed included local control (LC), locoregional control (LRC), and overall survival (OS). Toxicity was graded using the Common Terminology Criteria for Adverse Events, version 3.0. Competing-risk analysis using the Gray test was performed, with death as the competing risk. Overall survival was calculated by the Kaplan-Meier method.
Results
With a median follow-up of 7.3 years, the 5- and 10-year LC and LRC rates were 87.9% and 83%, and 80.5% and 73.7%, respectively. Higher T-stage and adenocarcinoma histology were the significant negative prognostic factors for both LC and LRC. Freedom from distant metastasis at 5 and 10 years were 83% and 63%, respectively. The median OS was 19.6 years. Overall no grade 4 or 5 toxicities occurred, and 20% of the cohort experienced an acute grade 3 toxicity, and 6% with a grade 3 late toxicity.
Conclusion
In a modern cohort treated with surgery and radiotherapy excellent outcomes can be achieved with lower toxicity rates compared with older published series.
Keywords: Minor Salivary Gland, Cancer, Radiation Therapy, Surgery
INTRODUCTION
Minor salivary gland tumors are rare and account for approximately 2% to 3% of all head and neck tumors.1 They are usually found in the oral cavity, and less often in the nasal cavity, paranasal sinuses, pharynx, and larynx. There are nearly 40 histological subtypes, many being exceptionally rare.2,3 In contrast to their major salivary gland counterparts, minor salivary gland tumors have a higher propensity to be malignant.4,5 The most common malignant minor salivary gland subtypes include mucoepidermoid carcinomas and adenoid cystic carcinomas.6 Despite the variety of histologic subtypes and anatomic locations of disease, there are similarities in regard to treatment and clinical outcomes.
Major salivary gland tumors, which benefit from a relative wealth of literature, have been the model to justify the utility of postoperative radiotherapy (RT). Postoperative RT for major salivary gland cancers has shown benefit in patients with locally advanced disease, recurrent disease, high-grade histological types, and lesions with positive margins.7,8 Extrapolating from this knowledge, minor salivary gland lesions have the potential to benefit from adjuvant RT as well.
Surgery has been the mainstay of treatment for patients with tumors of the minor salivary glands.9 Postoperative RT is not as well defined due to the scarcity of studies on the subject matter. Some of the largest series using RT for minor salivary gland cancers comes from data from patients treated during the 1960s, so the advances in RT, surgery, imaging, and chemotherapy are not reflected in their outcomes.
The aim of this study was to review our institution’s experience in patients with minor salivary gland cancers treated with postoperative RT, and limit our report to a modern cohort. Furthermore, we sought to identify factors predictive of local control (LC), locoregional control (LRC), and distant metastasis (DM) outcomes for this group of patients treated by both surgery and postoperative RT.
Methods
Patients
Between February 1990 and December 2010, 773 salivary gland cancer patients were treated by physicians at Memorial Sloan-Kettering Cancer Center (MSKCC), of which 127 patients had minor salivary gland cancer. The records of 98 consecutive minor salivary gland cancer patients who received adjuvant RT at MSKCC were identified. Table 1 shows baseline characteristics for our cohort, with a median age of 52.5 years at diagnosis (range of 18-92 years). Among these patients there were 43 men and 55 women.
Table 1.
Baseline Characteristics
| Gender | N | % | |
|---|---|---|---|
| Male | 43 | 53.06 | |
| Female | 55 | 56.12 | |
| Age | |||
| Median | 52.5 | ||
| Range | 18-92 | ||
| Years of treatment | |||
| 1990-1995 | 11 | 11.22 | |
| 1995-2000 | 22 | 22.45 | |
| 2000-2005 | 27 | 27.55 | |
| 2005+ | 38 | 38.78 | |
| Chemotherapy | |||
| Yes | 16 | 16.33 | |
| No | 82 | 83.67 | |
| Nodal disease | |||
| Yes | 27 | 27.6 | |
| No | 71 | 72.4 | |
| Recurrent disease* | |||
| Yes | 12 | 12.2 | |
| No | 86 | 87.8 | |
Patients had recurrent disease after surgery, not from upfront radiotherapy.
No patient received prior RT for the cancer of interest and none had DM at presentation. The median follow up was 87 months, ranging from 1 to 246 months. Prior to the initiation of therapy, all patients underwent a complete history and physical examination along with necessary imaging such as computed tomography (CT) and magnetic resonance imaging. More recently, positron-emission tomography was frequently used in the staging and evaluation of these patients.
Table 2 shows the histopathologic baseline characteristics of the cohort. The oral cavity was the most common involved site while adenoid cystic carcinoma was the most common histologic subtype. According to the American Joint Committee on Cancer (AJCC) 7th edition, 23 patients had T1 disease, 27 had T2 disease, 12 had T3 disease, and the remaining 36 had T4 disease. Importantly, salivary gland tumors are staged according to the site in which they involved and do not have their own independent staging system. Regional nodal disease was present in 27 patients, of whom 12 were N1, 12 were N2b, and 3 had N2c disease. Twelve patients had recurrent disease as the indication for postoperative RT.
Table 2.
Histopathologic Baseline Characteristics
| Subsite | N | % | |
|---|---|---|---|
| Oral cavity | 38 | 38.7 | |
| Sinuses/nasal cavity | 26 | 26.5 | |
| Oropharynx | 17 | 17.3 | |
| Nasopharynx | 1 | 1.0 | |
| Larynx/hypopharynx | 0 | 0 | |
| Other | 11 | 11.2 | |
| Unknown | 5 | 5.1 | |
| Histology | |||
| Adenoid Cystic | 46 | 46.9 | |
| Mucoepidermoid | 28 | 28.5 | |
| Myoepithelial | 1 | 1.0 | |
| Adenocarcinoma | 20 | 20.4 | |
| Other | 3 | 3.0 | |
| T stage | |||
| T1 | 23 | 23.4 | |
| T2 | 28 | 28.5 | |
| T3 | 12 | 12.2 | |
| T4 | 34 | 34.6 | |
| LN involvement | |||
| Yes | 27 | 27.5 | |
| No | 71 | 72.4 | |
| Margin status | |||
| Negative | 31 | 31.6 | |
| Close | 14 | 14.2 | |
| Positive | 44 | 44. | |
| Missing | 9 | 9.1 | |
| Grade | |||
| Low | 23 | 23.5 | |
| Intermediate | 10 | 10.2 | |
| High | 18 | 18.4 | |
| Missing | 47 | 48.0 |
Among the 38 oral cavity lesions, 18 were of the hard palate, 8 in the buccal mucosa, 8 floor of mouth, 2 gingival, 2 oral tongue. The 17 oropharynx lesions consisted of 14 base of tongue, 2 soft palate, and 1 tonsil. Of the 26 nasal cavity/sinus lesions, 14 were of the maxillary sinus, 3 of the ethmoid sinus, and 9 of the nasal cavity.
The surgical procedures performed on these patients were site dependent. Postoperative margin status was as follows: 44 positive, 14 close, 31 negative, and 9 with missing margin information. For patients at presumed high risk for local or distant recurrence, chemotherapy (n = 16 patients) was given at the choice of the treating medical oncologist.
All patients received RT at MSKCC. The doses prescribed typically were between 60 and 70 Gy, with a median of 63 Gy. Eighty-four patients were treated with 6 MV photon beams alone, 11 with a mixture of photon and electron beam, and the remaining with cobalt ± electron beam. Intensity-modulated radiation therapy was generally used in patients treated after year 2003 and was used for the majority of patients treated with photon-beam–only RT. The treated volume included the postoperative bed and, if regional lymph nodes were involved, or high-risk features were present, elective nodal radiation was performed. Thirty-seven patients had RT to their nodal basins, of which in 20 cases this was elective nodal treatment. Fourteen patients had a low anterior neck field to treat their bilateral lower neck.
Actuarial likelihood estimates for LC, LRC, and DM-free survival were determined using the competing-risk method with death as a competing risk. Univariate hazard ratios and 95% confidence intervals (95% CI) for LC, LRC, and DM were calculated using a competing-risks regression model. Multivariate analysis was not performed due to the limited number of events. Grading of toxicities was performed using the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. Two-sided P values ≤0.05 were considered statistically significant. Statistical analysis was performed using R version 2.13.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
For the entire cohort, the 5-year LC rate using competing-risk analysis was 87.9% and the 10-year LC rate was 80.5% (Figure 1a). On univariate analysis, T stage predicted for worse LC (P = 0.03, hazard ratio (HR) 1.75 [95% confidence interval (CI) 1.06 - 2.89]). Adenocarcinoma histology also predicted for worse LC (P = 0.02, HR 3.48 [1.15 - 5.12]). Margin status and N stage did not predict for local failure. Figure 2a shows the Kaplan Meier curve by histology, with myoepithelial, “other”, and adenoid cystic histologies having the greatest local control. As seen in Figure 2b, missed treatments resulting in an increased time to complete radiotherapy correlated with a trend towards worse local control (≥ 50 days vs <50 days, p=0.06).
Fig 1.
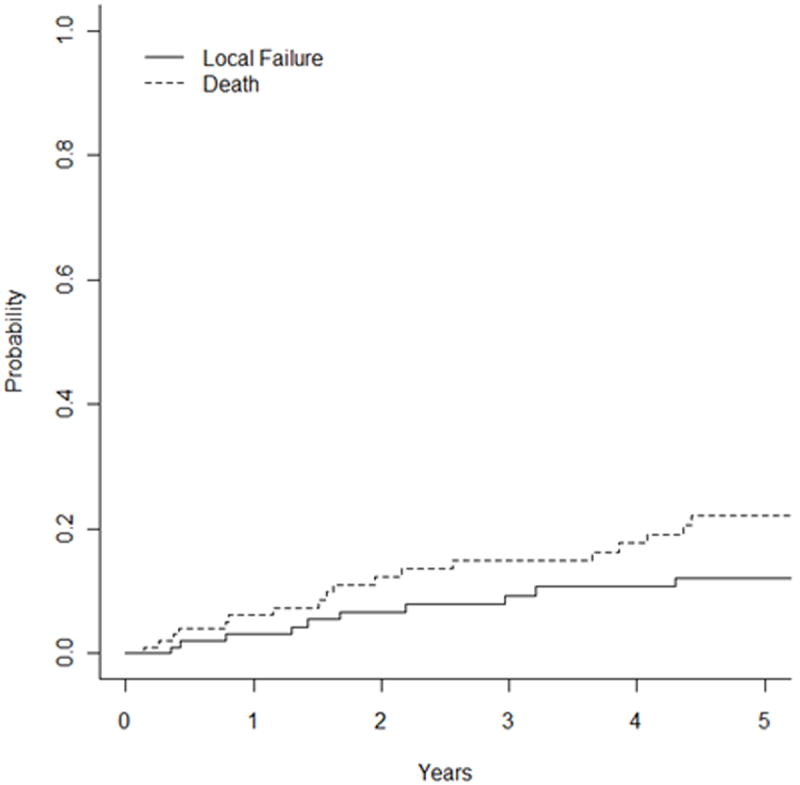
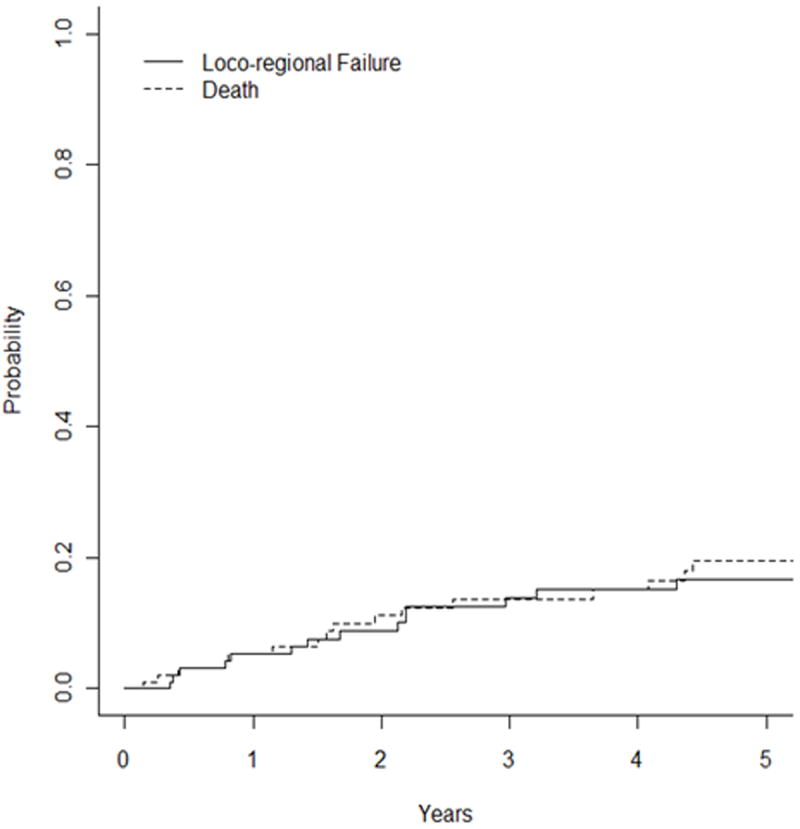
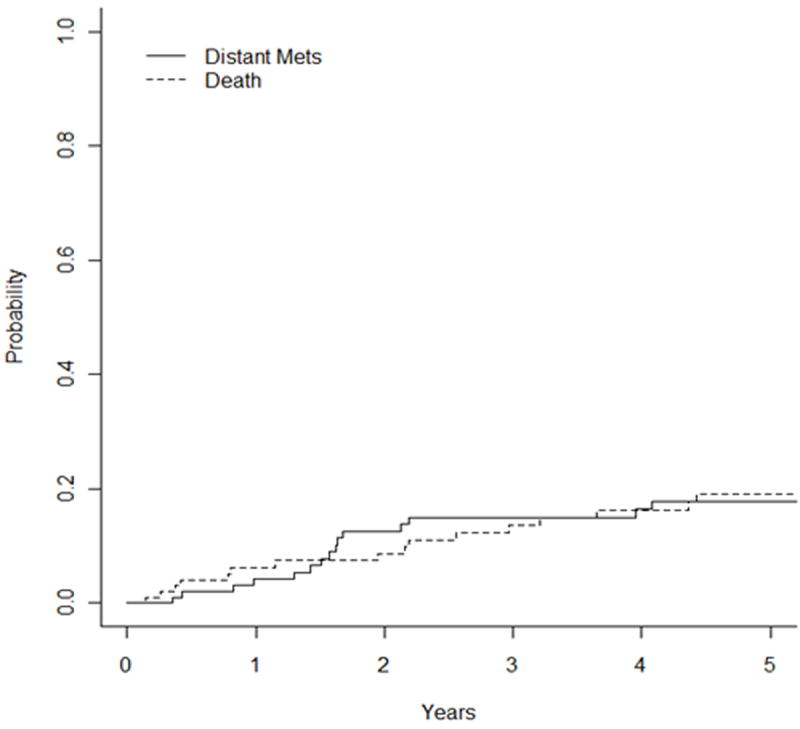
Unadjusted Kaplan-Meier curves with competing-risk analysis for (a) local failure, (b) locoregional failure, and (c) distant metastases.
Fig 2.
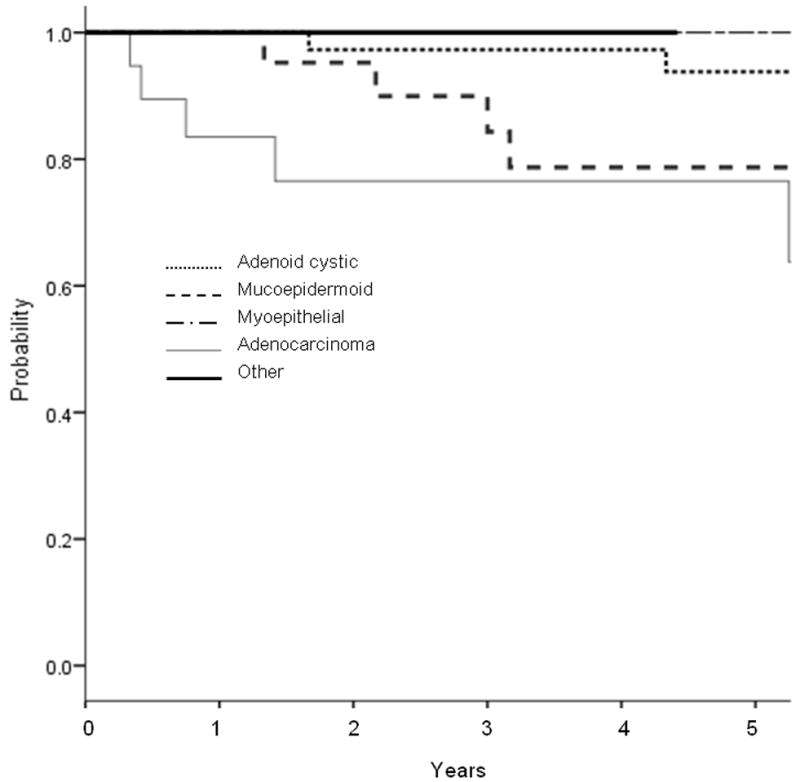
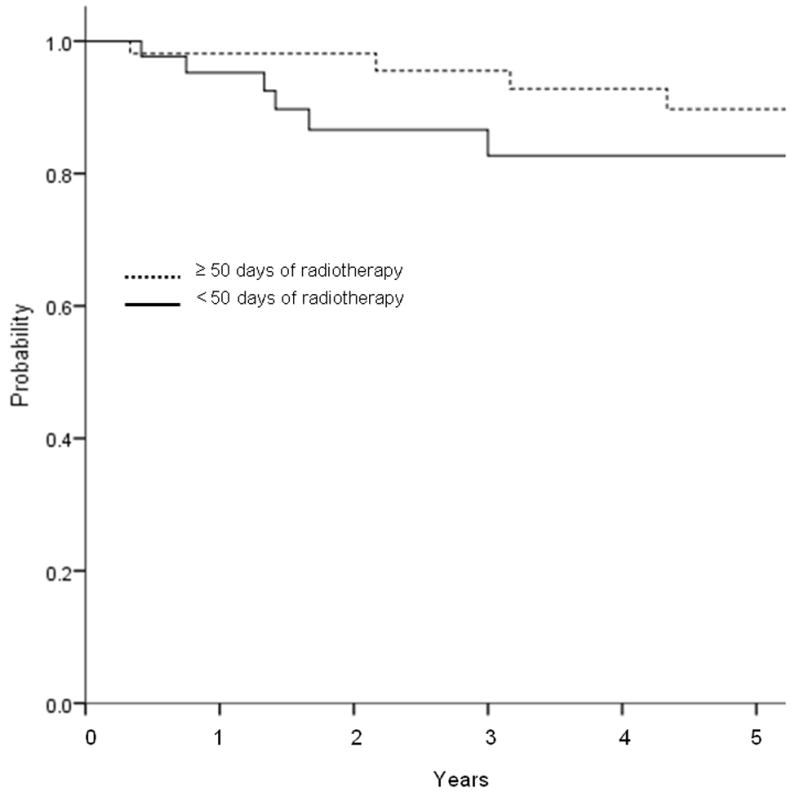
Unadjusted Kaplan-Meier curve for local control by (a) histology, and (b) duration of radiotherapy treatment. (a) Adenocarcinoma histology had significantly worse local control than the other histologies (p=0.01). (b) Treatment delay of ≥50 days had borderline significant detriment in local control (0.06).
The 5-year LRC rate using competing-risk analysis was 83% and the 10-year rate was 73.7% (Figure 1b). On univariate analysis, T stage (P = 0.01, HR 1.69 [1.11 – 2.59]) and adenocarcinoma histology (P = 0.007, HR 3.38 [2.68 – 3.61) predict for worse LRC. N stage and margin status did not predict for locoregional failure.
The 5-year DM rate with competing-risk analysis was 17% and the 10-year rate was 37% (Figure 1c). On univariate analysis, T stage trended for predicting worse DM (P = 0.08, HR 1.33 [0.96 – 1.86]). The median overall survival was 19.6 years, with an OS of 82% and 58% at 5 and 10 years, respectively (Figure 3).
Fig 3.
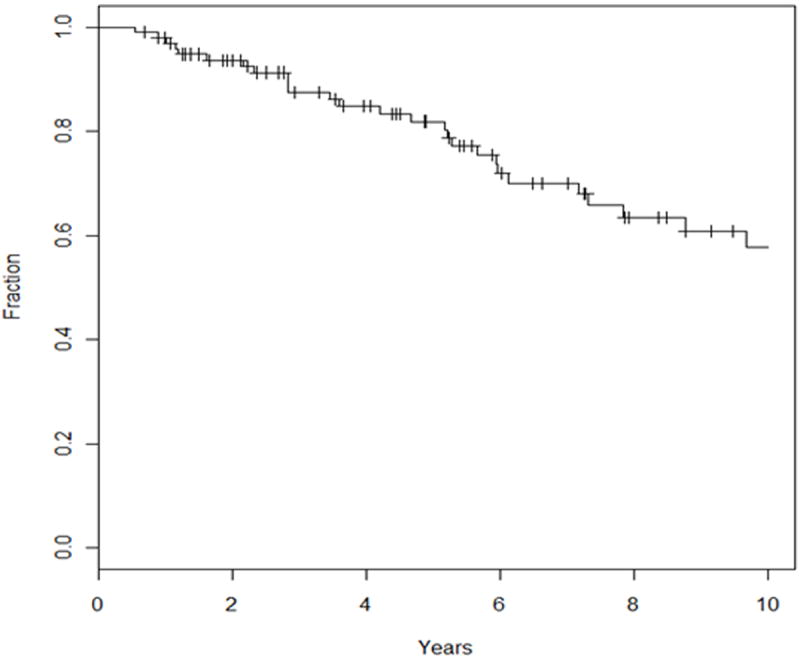
Unadjusted Kaplan-Meier curve for overall survival.
Toxicity grading for both acute and late toxicities can be found in Table 3. Acute toxicity experienced by the cohort include skin reaction (79 patients), mucositis (74), xerostomia (51), dysphagia/pharyngitis (39), fatigue (36), nausea (19), trismus (5), and laryngitis (4). Chronic toxicities included xerostomia (42), dysphagia (23), trismus (19), hearing loss (18), skin reaction (9), headache (8), blindness (3), mucositis (2), laryngitis (2), and osteoradionecrosis (2). Overall, no grade 4 or 5 toxicities occurred, and approximately 20% of the cohort experienced an acute and 6% a late grade 3 toxicity.
Table 3.
Acute and late toxicity grading
| Toxicity | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| Acute | |||||
| Dysphagia | 57 | 23 | 6 | 10 | - |
| Xerostomia | 45 | 37 | 13 | 1 | - |
| Larynx | 91 | 4 | - | - | - |
| Nausea | 75 | 19 | - | - | - |
| Fatigue | 61 | 22 | 11 | 3 | - |
| Skin | 17 | 49 | 27 | 3 | - |
| Mucositis | 22 | 38 | 28 | 8 | - |
| Trismus | 89 | 3 | 2 | - | - |
| Late | |||||
| Dysphagia | 68 | 12 | 8 | 3 | - |
| Xerostomia | 49 | 27 | 12 | 3 | - |
| Trismus | 71 | 13 | 5 | 1 | - |
| Radiotherapy necrosis | 88 | 2 | - | - | - |
| Hearing loss | 72 | 14 | 1 | 4 | - |
| Skin reaction | 88 | 9 | |||
| Vision change | 88 | 2 | 1 | - | - |
Discussion
Minor salivary gland tumors are rare and much of the reported literature includes patients treated prior to 1990.1 In the last two decades, significant advances have been made in surgical technique, chemotherapy, and RT. Computed tomography and magnetic resonance imaging were not routinely available prior to the 1980s, and the widespread implementation of these modalities has allowed better characterization of the extent of disease prior to treatment. Furthermore, positron-emission tomography imaging has played an important role in head and neck cancer in defining the extent of disease and for RT planning. Due to these advances, there has been stage migration towards more advanced stages being diagnosed prior to surgical resection. With these considerations we report excellent control rates with the modern day treatment of surgery followed by adjuvant RT, with reduced rates of toxicity compared with historical series.
Prognostic Factors
Although the literature is scarce regarding the effects of postoperative RT on outcomes, several authors have looked at prognostic factors of minor salivary gland cancers. Historically, tumor stage, surgical margins, and lymph node involvement were utilized as predictive factors of disease-free survival. Two groups published the prognostic importance of nodal stage on outcomes. Anderson et al. reported on 95 patients (64 with oral cavity lesions) treated over a 35-year period,10 and Feinstein et al2 looked at 74 patients in a 16-year period, with 39% of them having N2 disease and 58% having N0-1 disease. Both authors concluded that nodal disease was strongly associated with worse outcomes, such as OS and disease-free survival. However, we report that, on univariate analysis, for the use of adjuvant RT in our cohort, N stage did not predict for either local or locoregional recurrence. It should be noted that, due to the limited number of events, multivariate analyses were not performed. Interestingly, of the node positive patients, 10 were oropharyngeal subsite, 10 from the oral cavity, only 1 from the paranasal sinus region, and none were node positive from the nasopharynx, larynx, hypopharynx, or nasal cavity.
Others have found grade to be a strong prognostic factor in regards to outcomes. In 2008, Loh at al11 published a study on 171 minor salivary gland cancer patients and found that the grade of the tumor was the only factor associated with disease-specific survival (DSS). They found that adenoid cystic carcinomas were associated with higher local and regional recurrence rates, at 42.9% and 6.5%, compared with adenocarcinomas (15.6% and 6.3%, respectively), and mucoepidermoid (10.5% and 5.3% respectively). Our study also found adenocarcinoma to have higher recurrence rates than the mucoepidermoid type; however, adenoid cystic histology had a lower recurrence rate than adenocarcinoma (Table 4). This may be due to differences in patient populations, as all our patients received surgery and postoperative radiotherapy, while Loh’s series contained patients treated with surgery alone, radiotherapy alone, or surgery and postoperative radiotherapy.
Table 4.
Univariate analysis for local failure
| Variable | Hazard Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| T Stage | 1.75 | 1.06 – 2.89 | 0.03 |
| N Stage | 1.59 | 0.50 – 5.12 | 0.43 |
| Histology (adenocarcinoma vs others) | 3.48 | 1.15 – 5.12 | 0.02 |
| Margin status | 1.08 | 0.58 – 2.00 | 0.80 |
In 2010 Iyer et al4 discussed outcomes and predictive factors of minor salivary gland cancers in the oropharynx. In this paper there was no statistically significant difference in OS or DSS among the different histologic types; however, there were some notable trends. Comparing mucoepidermoid and adenoid cystic carcinoma, OS and DSS were similar at 5 years, but at 10 years survival was worse for patients with adenoid cystic carcinoma. These patients presented with a 10-year OS of 35% for adenoid cystic carcinomas versus 65% for mucoepidermoid carcinoma. The study further indicated 51% DSS for adenoid cystic carcinomas versus 85% for the mucoepidermoid type. The authors also reported that clinical T stage and anatomic subsite were independent predictors for OS, and T stage also predicted for recurrence-free survival.4
Margin status has been studied, and for most cancers the conclusion from most reports is that there is a higher recurrence rate for positive margins. Triantafillidou et al. concluded that the best method of treatment for adenoid cystic carcinomas is radical resection combined with radiotherapy. In contrast to our own study, Triantafillidou et al. found that treatment failure was found to be associated with positive margins of the excised surgical specimen and named nerve involvement.12,13 With our cohort being significantly larger, we did not find margin status to predict for LC, LRC, or DM, and this may be secondary to differences in RT technique and target delineation.
Anatomic location also has been shown to be predictive for outcomes.4 Weber et al. identified prognostic factors concerning minor salivary gland cancers of the lip and buccal mucosa. These authors reported that lesions arising in the lip have higher recurrence rates than those arising in the buccal mucosa. Their indications for postoperative RT for malignant salivary gland tumors of the lip and buccal mucosa include high grade, close or positive margins, perineural invasion, deep invasion into muscle and bone, and lymph node metastasis.14
Based on our results we believe that, in the setting of postoperative RT, patients with adenocarcinoma histology, high T stage, and potentially other reported high-risk features including anatomic location, perineural invasion, high N stage, and close/positive margins, should be considered at increased risk of locoregional recurrence. In this high-risk population, the use of chemotherapy should be considered. Currently, there is an active multi-institutional Radiation Therapy Oncology Group (RTOG) study open that will attempt to define further the benefits of chemotherapy.
Role of Radiation Therapy
Despite the rarity of minor salivary gland cancers, studies have demonstrated RT to be of great importance in improving outcomes. Yorozu et al15 conducted a retrospective analysis of 31 patients who were treated with 4-6 MV photons at an average dose of 50 Gy in 16 fractions over 3 weeks. The study reported that definitive RT conferred a 5-year LC rate of 53%, that when allowing for salvage surgery rose to 69%. These results highlight that definitive RT is a treatment option in patients that are poor surgical candidates or for whom the operation would result in unacceptable morbidity and poor cosmetic result. However, as with most head and neck cancers that are readily surgically accessible, the combination of appropriate surgical resection with postoperative RT often produces superior results.
Li et al16 advocates that, although surgery with adequate margins leads to a favorable outcome in minor salivary gland tumors of the hard palate, postoperative RT, with doses of 49 to 70 Gy, is a useful adjunctive treatment. The authors noted that the benefit of RT is primarily useful for patients with poorly differentiated disease, cervical lymph node metastasis, positive or close margins, and large primary lesions. Furthermore, cervical lymph involvement is associated with decreased survival and is a strong indication for postoperative RT.17
The optimal radiation dose for minor salivary gland cancers has been derived from multiple series. MD Anderson performed a retrospective study over 31-years including 160 patients who received postoperative RT after gross removal of their tumors was performed.18 Of the entire cohort, 70% had lesions confined to the oral cavity and oropharynx, whereas the rest had lesions located in the nasal cavity and paranasal sinuses. This study concluded that for most patients a dose of 60 Gy in 30 fractions to the operative bed was the optimal treatment approach.18 Others have found success with this dose fractionation, specifically in adenoid cystic carcinomas of the minor salivary gland.13 At our institution we routinely prescribe 60 Gy to the postoperative bed if there are negative margins, 66 Gy to close and positive margin, and 70 Gy if gross residual disease remains. Nodal disease is treated to 54-60 Gy based on the suspicion for involvement.
Our OS rates at 5- and 10-year were 82% and 58% respectively, whereas Garden et al18 presented rates of 81% and 65%, Li et al.16 presented rates of 77.9% and 65%, and finally Le at al.19 at Stanford University Hospital reported 75% and 63% at the 5- and 10-year marks, respectively. Our OS rates are consistent with the reported literature and are improved upon select series. The 5-year LC rate from our cohort was 87.9% and the 10-year LC rate was 80.5%, whereas Garden et al. presented 96% and 86%. Our excellent local control rates may be partly due to our policy of comprehensive nerve coverage to skull base for adenoid cystic lesions. Finally, Cianchetti et. al. conducted a study that included definitive RT and post-surgical RT in the cohort and presented a 10-year local regional control rate of 61%.1 It is difficult to directly compare cohorts secondary to stage migration, and tremendous variability in stage, histology, margin status, and other prognostic features.
Finally, in 2008, Gomez et al.20 at MSKCC published a study looking at 59 patients with adenoid cystic carcinomas of the head and neck who between 1990 and 2004 received RT at MSKCC. All but five received postoperative RT and 17 patients received IMRT, 15 three-dimensional conformal therapy, and the remaining received conventional radiation therapy. He was able to show 5- and 10-year local control rates of 91% and 81%, respectively, as well as 5- and 10-year disease-free survival rates of 76% and 40% and finally overall survival rates at 5 and 10 years of 87% and 65%, respectively.
Toxicity
Despite RT’s pivotal role in the treatment of minor salivary gland cancers, it can lead to a variety of problems, many attributed to decreased salivary output. These include dryness of mouth, changes on oral function, and even ulcerations and injuries due to dryness of the oral mucosa,21 all which are consistent with some of the toxicities observed in our study. However, there is an even greater lack of studies reporting on the toxicity of RT.
Gomez et al.20 in 2008 in his study of 59 adenoid cystic minor salivary gland patients, showed that, acutely at least, all patients experienced mild mucositis, whereas 15 patients experienced grade 3 or higher toxicity. Chronically, six patients presented with grade 3 toxicity and none with grade 4.
Garden et al.18 reported an unspecified grade of hearing loss in 26 patients; visual problems in 22 patients, including keratitis (3), corneal ulcer (1), blepharitis (2), perforated globe (1), optic atrophy (4), nasolacrimal duct obstruction (1), retinal detachment (1), undocumented decreased vision (2), retinopathy (1), and cataract (1), 3 of whom underwent orbital exenteration; osteonecrosis in 12 patients; and brain necrosis in 3 patients. Many of these toxicities are grade 3 and 4 by modern CTCAE grading. In our cohort we identified less than 20% of patients who experienced grade 3 toxicity, and no patient experienced grade 4 or 5 toxicity.
Conclusion
Tumors of the minor salivary gland have good 5-year control rates with surgical resection and postoperative RT with select use of chemotherapy for high-risk patients. The strongest prognostic features for local and locoregional recurrence include higher T stage and adenocarcinoma histology. These features should be considered for more aggressive treatment even in the setting of postoperative RT. Toxicity is acceptable with the addition of RT, and the most common serious toxicities include osteoradionecrosis, vision deterioration, and hearing loss. IMRT likely is primarily responsible for the improved toxicity profile seen over historic series.
Footnotes
No conflicts of interest, including financial conflicts of interest, to disclose.
No funding to disclose.
Contributor Information
Lucas Resende Salgado, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, New York, NY.
Daniel E. Spratt, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, New York, NY.
Nadeem Riaz, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, New York, NY.
Paul B. Romesser, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, New York, NY.
Suzanne Wolden, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, New York, NY.
Shyam Rao, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, New York, NY.
Christine Chin, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, New York, NY.
Julian C. Hong, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, New York, NY.
Richard Wong, Department of Head and Neck Surgery, Memorial Sloan-Kettering Cancer Center, New York, NY.
Nancy Y. Lee, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, New York, NY.
References
- 1.Cianchetti M, Sandow PS, Scarborough LD, et al. Radiation therapy for minor salivary gland carcinoma. Laryngoscope. 2009;119:1334–8. doi: 10.1002/lary.20501. [DOI] [PubMed] [Google Scholar]
- 2.Feinstein TM, Lai SY, Lenzner D, et al. Prognostic factors in patients with high-risk locally advanced salivary gland cancers treated with surgery and postoperative radiotherapy. Head Neck. 2011;33:318–23. doi: 10.1002/hed.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speight PM. Update on diagnostic difficulties in lesions of the minor salivary glands. Head Neck Pathol. 2007;1:55–60. doi: 10.1007/s12105-007-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iyer NG, Kim L, Nixon IJ, et al. Factors predicting outcome in malignant minor salivary gland tumors of the oropharynx. Arch Otolaryngol Head Neck Surg. 2010;136:1240–7. doi: 10.1001/archoto.2010.213. [DOI] [PubMed] [Google Scholar]
- 5.Ganly I, Patel SG, Coleman M, et al. Malignant minor salivary gland tumors of the larynx. Arch Otolaryngol Head Neck Surg. 2006;132:767–70. doi: 10.1001/archotol.132.7.767. [DOI] [PubMed] [Google Scholar]
- 6.Yih WY, Kratochvil FJ, Stewart JC. Intraoral minor salivary gland neoplasms: review of 213 cases. J Oral Maxillofac Surg. 2005;63:805–10. doi: 10.1016/j.joms.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Mendenhall WM, Morris CG, Amdur RJ, et al. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck. 2004;26:154–62. doi: 10.1002/hed.10380. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong JG, Harrison LB, Spiro RH, et al. Malignant tumors of major salivary gland origin. A matched-pair analysis of the role of combined surgery and postoperative radiotherapy. Arch Otolaryngol Head Neck Surg. 1990;116:290–3. doi: 10.1001/archotol.1990.01870030054008. [DOI] [PubMed] [Google Scholar]
- 9.Spiro RH, Thaler HT, Hicks WF, et al. The importance of clinical staging of minor salivary gland carcinoma. Am J Surg. 1991;162:330–6. doi: 10.1016/0002-9610(91)90142-z. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JN, Jr, Beenken SW, Crowe R, et al. Prognostic factors in minor salivary gland cancer. Head Neck. 1995;17:480–6. doi: 10.1002/hed.2880170605. [DOI] [PubMed] [Google Scholar]
- 11.Loh KS, Barker E, Bruch G, et al. Prognostic factors in malignancy of the minor salivary glands. Head Neck. 2009;31:58–63. doi: 10.1002/hed.20924. [DOI] [PubMed] [Google Scholar]
- 12.Pons Vicente O, Almendros Marques N, Berini Aytes L, et al. Minor salivary gland tumors: A clinicopathological study of 18 cases. Med Oral Patol Oral Cir Bucal. 2008;13:E582–8. [PubMed] [Google Scholar]
- 13.Triantafillidou K, Dimitrakopoulos J, Iordanidis F, et al. Management of adenoid cystic carcinoma of minor salivary glands. J Oral Maxillofac Surg. 2006;64:1114–20. doi: 10.1016/j.joms.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Weber RS, Palmer JM, el-Naggar A, et al. Minor salivary gland tumors of the lip and buccal mucosa. Laryngoscope. 1989;99:6–9. doi: 10.1288/00005537-198901000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Yorozu A, Sykes AJ, Slevin NJ. Carcinoma of the hard palate treated with radiotherapy: a retrospective review of 31 cases. Oral Oncol. 2001;37:493–7. doi: 10.1016/s1368-8375(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Zhang XR, Liu XK, et al. Long-term treatment outcome of minor salivary gland carcinoma of the hard palate. Oral Oncol. 2012 doi: 10.1016/j.oraloncology.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd S, Yu JB, Ross DA, et al. A prognostic index for predicting lymph node metastasis in minor salivary gland cancer. Int J Radiat Oncol Biol Phys. 2010;76:169–75. doi: 10.1016/j.ijrobp.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Garden AS, Weber RS, Ang KK, et al. Postoperative radiation therapy for malignant tumors of minor salivary glands. Outcome and patterns of failure Cancer. 1994;73:2563–9. doi: 10.1002/1097-0142(19940515)73:10<2563::aid-cncr2820731018>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Le QT, Birdwell S, Terris DJ, et al. Postoperative irradiation of minor salivary gland malignancies of the head and neck. Radiother Oncol. 1999;52:165–71. doi: 10.1016/s0167-8140(99)00084-5. [DOI] [PubMed] [Google Scholar]
- 20.Gomez DR, Hoppe BS, Wolden SL, et al. Outcomes and prognostic variables in adenoid cystic carcinoma of the head and neck: a recent experience. Int J Radiat Oncol Biol Phys. 2008;70:1365–72. doi: 10.1016/j.ijrobp.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Vissink A, Mitchell JB, Baum BJ, et al. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys. 2010;78:983–91. doi: 10.1016/j.ijrobp.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]


