Abstract
Objective
As sex differences in substance dependence may impinge upon the perception and regulation of emotion, we assess Emotional Intelligence (EI) as a function of gender, menstrual cycle (MC) phase and hormonal changes in early abstinent cocaine dependent individuals who abuse alcohol (CDA).
Methods
Study 1: The Mayer, Salovey, and Caruso Emotional Intelligence Test (MSCEIT) was administered to 98 CDA (55M/43F) and 56 healthy (28M/28F) individuals. Performance in women was also assessed by MC phase. Study 2: The MSCEIT was administered to 18 CDA (19M/9F) who received exogenous progesterone (400mg/day) versus placebo for 7 days. (Study 2).
Results
Study 1: Healthy females were better than healthy males at facilitating thought and managing emotions. This gender discrepancy was not observed in the CDA group. Additionally, all women in the high compared with the low progesterone phase of their MC were better at managing their emotions. Study 2: Exogenous progesterone improved ability to facilitate thought in both males and females.
Conclusions
CDA women may be vulnerable to difficulties managing and regulating emotions. Gonadal hormones may contribute to this gender effect, as increases in both endogenous and exogenous progesterone improved selective aspects of EI.
Keywords: Progesterone, Cocaine Dependence, MSCEIT, Emotional Intelligence, Gender
INTRODUCTION
Due to societal changes the prevalence of cocaine dependence is increasing in women to similar levels as men (SAMHSA, 2007). As women are more vulnerable than men to the problems associated with addiction (Anker and Carroll, 2010), the need to establish better tailored treatments for both men and women is critical. In view of this, gender, menstrual cycle (MC) phase and gonadal hormones are all associated with subjective and cognitive regulatory processes (Sinha et al., 2007b; Fox et al., 2008c, 2013; Sofuoglu et al., 1999, 2001, 2004, 2011; Quinones-Jenab, 2006) integral to addiction outcome (Fox et al., 2007, 2008b; Blume and Marlatt, 2009; Sofuoglu et al., 2013; Streeter et al., 2008). Establishing the manner in which these sex-specific variables affect ability to structure, manage and perceive emotions may therefore help elucidate important targets for treatment development in cocaine dependent men and women. As cocaine dependence is often co-morbid with alcohol misuse (SAMHSA, 2007), the current study examines pertinent aspects of emotional intelligence (EI) by gender and MC in an ecologically valid group of early-abstinent cocaine dependent women who also abuse alcohol and healthy controls (Study 1). We then examine the efficacy of exogenous progesterone as a viable agent for improving aspects of EI in both early-abstinent cocaine dependent men and women who also abuse alcohol (Study 2).
Conceptually, EI is known to involve the recognition, use, understanding, and management of one’s own and others’ emotional states (Mayer and Salovey, 1997; Salovey and Mayer, 1990), all of which are skills known to attenuate emotional sensitivity and assist in cognitive control. Not only are these processes integral to relapse during early abstinence, they are also robustly associated with MC fluctuations and levels of sex hormones in animals and humans (McCormick and Mathews, 2007 for review; Kirschbaum et al., 1999). Findings from our own laboratory have shown that cocaine dependent women demonstrate attenuated anxiety and sympathetic response to a cocaine cue during the mid-luteal phase of their MC characterized by high progesterone, compared with the early follicular phase, defined by low progesterone levels (Sinha et al., 2007b). In addition, exogenous progesterone compared with placebo, has been shown to reduce stress-related negative mood and enhance cognitive control processes in cocaine dependent women who also abuse alcohol (Fox et al., 2013) as well as in smokers (Sofuoglu et al., 2011). Corroborating these findings, imaging studies have also indicated that fluctuations in progesterone levels directly strengthen communication between the amygdala and prefrontal cortical circuitry (van Wingen, 2008) intrinsic to the cognitive regulation of emotion (Quirk and Beer, 2006).
A better understanding of sex-specific processes involved in EI during early abstinence from cocaine, may also be particularly pertinent in terms of developing treatments in women. For example, prior research indicates that symptoms of cocaine withdrawal severity, including elevated stress, craving for multiple drugs, anxiety and negative mood (Kampman et al., 2001, 2006; Fox et al., 2012) are all enhanced in women compared with men (Fox et al., 2008a, Fox and Sinha, 2009, Potenza et al., 2012). Furthermore, almost twice the prevalence of anxiety disorders have been reported in treatment seeking cocaine dependent women compared with men (Rounsaville et al., 1991), and meta-analyses of international longitudinal surveys have shown that emotionally driven, internalizing disorders are predictive of drinking in women but not in men (Fillmore et al., 1997). As difficulties managing and regulating sensitized anxiety and negative mood may lead to a reinforcing cycle of craving and relapse (Sinha, 2007a) these components of EI may be a particularly salient target for intervention in recovering cocaine dependent women who also abuse alcohol.
In the current studies, we measure EI using the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT; Mayer, Salovey, and Caruso, 2002), which employs a 4-branch model assessing ability to (a) perceive emotion, (b) use emotion to facilitate thought, (c) understand emotions, and (d) manage emotion (Mayer and Salovey, 1997). Such aspects of EI are key to the maintenance of cocaine and also alcohol dependence which is characterized by an enhanced and persistent inability to regulate negative mood following stress (Fox and Sinha, 2009) possibly due to a conflict in regulatory goals (Kuhl and Koole, 2004; Tice et al., 2001). In view of this, poor EI has previously been associated with cognitive processes and behaviors integral to the craving state, including high levels of perceived stress and low impulse control (Fox et al., 2011), selective cognitive processes (Jausovec et al. 2001), depression and anxiety (Brackett and Salovey, 2006) and interpersonal difficulties (Lopes et al., 2003).
To this end, we first assessed emotional intelligence (EI) by gender and menstrual cycle in a group of early-abstinent cocaine dependent individuals who also abuse alcohol (CDA) and healthy controls (Study 1). We then examined the effects of exogenous progesterone on EI in both early-abstinent CDA men and women (Study 2). We hypothesize that selective changes in EI will be observed as a function of both gender and MC. In addition, we hypothesize that both endogenous and exogenous progesterone may improve these gender-specific processes in cocaine dependent individuals who also abuse alcohol in early abstinence, a period characterized by high craving.
METHODS
Two studies are presented
Study 1 represents extended analysis from a prior research study assessing EI dysregulation in cocaine dependent individuals who also abuse alcohol compared with a group of statistically well matched controls (Fox et al., 2011). Here, we present follow-up data showing the moderating effects of gender and MC on these early abstinent emotional adaptations. In Study 2, we present EI data collected as part of a larger laboratory study examining the effects of exogenous progesterone on stress system changes in cocaine dependent men and women who also abuse alcohol (Fox et al., 2013). In the case of both studies participants reported that cocaine was their primary drug of choice and, as such, defined themselves as being cocaine dependent. In support of the fact that polydrug users are becoming progressively more represented among substance-dependent populations (Patkar, 2004; SAMHSA, 2007), the current samples of cocaine dependent individuals also met abuse criteria for alcohol.
STUDY 1 – Effects of gender and endogenous progesterone levels on emotional intelligence in cocaine dependent men and women who also abuse alcohol
Participants
Ninety eight cocaine dependent individuals (55M/43F) and 56 healthy controls (28M/28F) were recruited through local advertisements. All participants were recruited as part of a larger study (Fox et al., 2011).
As data were collected prior to the publication of DSM V, cocaine dependent individuals were assessed using DSM-IV criteria, and tested positive for cocaine in urine toxicology screens upon entry into inpatient treatment. Participants were excluded if they met criteria for any current psychiatric disorder or current and/or lifetime dependence on substances other than cocaine, alcohol or nicotine. Healthy controls were excluded if they met current criteria for any psychiatric disorder or current and past diagnoses of any substance dependence, other than nicotine. In addition, women were excluded from the study if they were using any birth control or were peri- or postmenopausal. All participants were excluded if they required any prescribed medications or were not in good health. The study was approved by the Human Investigation Committee of the Yale University School of Medicine.
Procedures
Participants were all treatment-seeking for cocaine dependence and in early abstinence. They had all spent approximately 4 weeks on The Clinical Neuroscience Research Unit (CNRU) of the Connecticut Mental Health Center (CMHC) at the time of testing. The CNRU is a locked in-patient treatment facility with no access to alcohol or drugs and very limited access to visitors. Alcohol and drug testing is conducted regularly, 3 times per week, to ensure drug abstinence. Furthermore, as all participants were treatment seeking for cocaine dependence, they participated in four weeks of group counseling treatment for cocaine dependence using the standard 12-Step based Group Drug Counseling Manual as a guide (Project Match 12-Step Manual; Nowinski et al., 1994). All healthy control participants spent 3 days and 4 nights on the hospital research unit of the Yale Center for Clinical Investigation (YCCI), in a similar controlled environment and located a block away.
Following 3 to 4 weeks of abstinence the CDA individuals were administered the MSCEIT. Control participants were administered the test on day 1 of admission to the YCCI.
Assessments
Mayer-Salovey-Caruso Emotional Intelligence Test
An on-line version of the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT) (Mayer et al. 2002) distributed by Multi Health System’s (MHS) online assessment site and Scoring Organizer was administered to all participants following admission to the study. EI is measured across two main dimensions: Experiential Emotional Intelligence (EEIQ) and Strategic Emotional Intelligence (SEIQ). EEIQ is defined as the ability to perceive emotions (Branch 1) and the ability to utilize emotions in order to facilitate thought (Branch 2). SEIQ is defined as the ability to understand emotions (Branch 3) and the ability to manage emotions (Branch 4). Each “branch” of EI consists of two relevant subtests
A more detailed description of each branch and interpretation utilized in this study is outlined in Fox et al. 2011. In brief, MSCEIT is scored using a general consensus procedure based on the agreement of a large normative sample of more than 5000 people drawn from 50 research institutions in the United States and comprising heterogeneous respondents varying in sex, ethnicity, and age. Good sample sizes have been included for individuals from four ethnic classifications: Caucasian, African American, Asian and Hispanic. Furthermore, all age ranges from 17 to 50+ are adequately sampled for with regard to both age and gender. A large percentage of the sample has received some college or university education (Mayer et al., 2002).
A score is awarded to each potential response option based on the proportion of a prior screening sample who selected that item. For example, if 20% of individuals choose a particular response option, a score of .2 is awarded to that option (Legree et al., 2005). The MSCEIT determines an individual’s EI from several scores generated from responses to all eight subtasks: A Total score, two Area scores (EEIQ and SEIQ), four branch scores (Perceiving, Facilitating, Understanding and Managing emotions), and eight individual task scores.
Menstrual Cycle (MC) History
All women were required to complete a detailed MC history questionnaire providing self- report information regarding the dates and regularity of their last three MCs, the length of menses each time, and the onset of menses in the current cycle.
This MC information was used to group all women into either a High Progesterone group (luteal phase of the menstrual cycle, DAYS 20–25), or a Low Progesterone group (follicular phase, DAYS 1–19, and pre-menstrual phase of the menstrual cycle, DAYS 26–32) at the time of MSCEIT administration.
Statistical Analyses
All statistical analyses were performed using SPSS software (SPSS Inc., Version 19, Chicago, IL, USA). Figures were created with Prism 5 (GraphPad Software Inc., San Diego, CA, USA). Group differences on all MSCEIT tests were assessed using linear mixed models. Gender (male vs. female) and group (CDA vs. healthy control) were used as fixed effects and subjects as random effects. Years of education has been identified as a salient indicator of IQ in many of our previous studies using substance abusers. As such, years of education was used as a covariate in order to account for its potential role as a confounder in MSCEIT performance. Independent samples t-tests and chi square analyses were used to analyze demographics and drug use.
RESULTS (Study 1)
Demographic Characteristics of Participants
Table 1: There were no significant differences in gender or age between the healthy control and CDA groups. However, the CDA group had a lower number of years of education (t (151)=8.38; p<0.001), significantly more smokers (t (151)=−13.29; p<0.001), comprised less Caucasians (p<0.001) and experienced a higher prevalence of lifetime anxiety with PTSD (t (145)=3.14; p=0.002) and lifetime anxiety without PTSD (t (145)=2.85; p=0.005). All factors were included as covariates. As expected, the CDA group had significantly more years of cocaine use (t (149)=1.25; p<0.001) and number of days of cocaine use in the past month (t (149)=12.45; p<0.001), as well as significantly higher number of years of alcohol use (t (146)=4.65; p<0.001) and number of drinking days in the past month (t (146)=4.88; p<0.001).
Table 1.
Study 1- Demographic Characteristics of Sample
| Healthy n=56 | Cocaine Dependent (CD) n=98 | |
|---|---|---|
| Gender – no. of males | 28 | 55 |
| Race | ||
| African American | 13 | 48 |
| Caucasian | 29 | 44 |
| Other | 14 | 6 |
| Age (s.d.) | 34.4 (9.9) | 36.4 (6.7) |
| Years spent in education (s.d.) | 14.8 (1.84) | 12.21 (1.88) |
| No. of regular smokers | 8 | 87 |
| Years of cocaine use (s.d.) | 0.05 (0.3) | 8.16 (5.9) |
| No. of days used in past month (s.d.) | 0 | 16.42 (9.9) |
| Years of alcohol use (s.d.) | 6.5 (8.1) | 13.1 (8.4) |
| No. of days used in past month (s.d.) | 4.45 (6.0) | 11.85 (10.1) |
| Lifetime depression | 6 | 10 |
| Lifetime anxiety (incl PTSD) | 4 | 28 |
| Lifetime anxiety (without PTSD) | 0 | 13 |
Gender variation in MSCEIT Scores (see Fig 1a–c)
Figure 1.
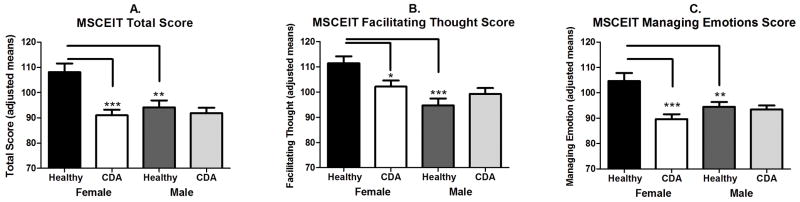
MSCEIT Performance as a Function of Gender (Female vs. Male) and Drug Use (Healthy vs. Cocaine Dependent with Alcohol Abuse (CDA)). * p<0.05; **p<0.01; ***p<0.001.
Total Score
A main effect of gender (F(1,150)=6.252; p=0.01) indicated that overall all women performed better on the MSCEIT than males. As expected, a main effect of group (F(1,150)=13.68; p<0.001) also indicated that the healthy control group performed significantly better than the CDA group. However, a gender x group interaction (F(1,150)=8.07; p=0.005) showed that healthy females demonstrated significantly higher overall scores than both healthy males (p=0.001) and CDA females (p<0.001). Notably, the significant variance observed between the female groups was not seen in the males.
Emotional Management
As shown in Fox et al. (2011), a main effect of group (F(1,150)=13.84; p<0.001) showed that CDA individuals performed significantly worse on this branch of the MSCEIT than the healthy group. In this extended analysis a gender x group interaction (F(1,150)=10.38; p=0.002) demonstrated that in the control group, females showed significantly higher scores than males (p=0.004), while in the CDA group, females performed significantly worse than males (p<0.001). CDA females also performed worse on this scale compared with control females (p<0.0001), a discrepancy not observed in the males
Facilitating Thought
A main effect of gender (F(1,150)=13.46; p<0.001) showed that again all females performed significantly better than all males on this subscale. However, again, a gender x group interaction (F(1,150)=6.65; p=0.01) indicated that the significant variation in gender was powered predominantly by the healthy females performing significantly better than both the healthy males (p<0.001) and the CDA females (p=0.02); Again no significant variation was observed between the male groups.
Effect of Menstrual Cycle on MSCEIT scores (see Fig 2a–c)
Figure 2.
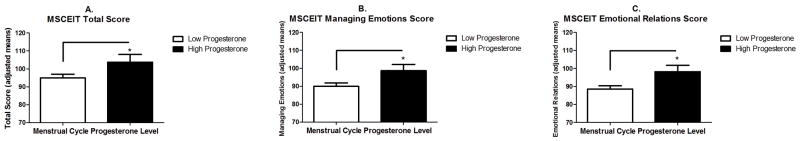
MSCEIT Performance as a Function of Menstrual Cycle Phase Progesterone Level. * p<0.05.
We additionally examined the effects of progesterone level as characterized by the menstrual cycle on MSCEIT performance in both CDA and healthy women, who were grouped into either a High Progesterone group (luteal phase (n=17; DAYS 20–25) or Low Progesterone group (follicular and pre-menstrual phase (n=53; DAYS 1–19 and 26–32, respectively). Findings indicated that women in the High Progesterone group demonstrated significantly higher MSCEIT scores on the overall Total scale compared with women in the Low Progesterone group (F(1,68)=4.1; p<0.05). This was due to the fact that High Progesterone women demonstrated significantly better ratings on the Managing Emotions scale (F(1,68)=4.85; p=0.03) and more specifically, in terms of interpersonal relations. Compared with Low Progesterone women, High Progesterone women rated more highly on the Managing Emotional Relations scale (F(1,68)=6.75; p=0.01).
In all of these measures, there was no significant effect of years in education.
STUDY 2 – Effects of exogenous progesterone on emotional intelligence in cocaine dependent men and women
Participants
Twenty eight treatment-seeking CDA individuals (19M/9F) were recruited through local advertisements, as outlined in Study 1. All participants were recruited as part of a larger study (Fox et al., 2013). In brief, CDA individuals were all treatment-seeking and spent 4 to 5 weeks on The Clinical Neuroscience Research Unit (CNRU) of the Connecticut Mental Health Center (CMHC). Following 2–3 weeks of inpatient stay, patients were administered either progesterone (9M/4F) or placebo (10M/5F) for 7 days, as outlined below. The MSCEIT was administered to all participants during early abstinence from cocaine, following 3 to 4 weeks on the CNRU.
Menstrual Cycle (MC) History
All women were required to complete a detailed MC history questionnaire as per Study 1. MC phase was also confirmed by obtaining serum levels of progesterone and estradiol upon admission to the inpatient unit and during the MSCEIT testing days. Serum levels and self- reported MC details were then compared to ensure accuracy. This information was used to ensure that women were in the early follicular phase of their menstrual cycle (DAYS 1- 9), which allowed for the initiation of progesterone administration in women when both progesterone and estradiol levels are endogenously low and similar to those typically observed in men (Zumoff et al., 1990).
Progesterone Administration
A placebo-controlled, double-blind experimental design was used in the current study. All micronized progesterone (Prometrium) pills (200 mg b.i.d.) were purchased from Solvay Pharmaceuticals, Marietta, Georgia, through the study pharmacy located at the CMHC. All active and placebo capsules used for medication administration appeared identical. Placebo pills contained lactose and abstinent dependent subjects were randomly assigned to placebo or progesterone conditions by the research pharmacist, experienced in Urn randomization procedures (Stout et al., 1994). All female participants were admitted to the inpatient unit approximately one to two weeks before the expected beginning of menstruation (MC day 1). The 7 day medication protocol was initiated between days 1–9 of their MC when both progesterone and estradiol levels are low and remain stable (approximately <85 pg/ml for estradiol and <1.1 ng/ml for progesterone; Chabbert-Buffet et al., 1998). In the case of male participants, treatment medication was administered after approximately 7–10 days of inpatient stay for consistency. Study medications were administered at 7:30 am and 9 pm daily for seven days (200 mgs b.i.d).
Safety and Side Effects Measures
All patients were evaluated on a) sitting and standing vital signs (HR/BP) taken every 15 minutes for one hour, every other day while on the medication, b) assessment of laboratory chemistry at study admission and discharge, including electrocardiography, renal, hepatic, and pancreatic tests, and c) assessment of any changes in physical and mental functioning every other day while on the medication, in order to evaluate any potential adverse events or side effects. The side effects were minimal and transient. For a detailed description, please refer to Fox et al. (2013).
Statistical Analyses
All statistical analyses were performed using SPSS software (SPSS Inc., Version 19, Chicago, IL, USA). Figures were created with Prism 5 (GraphPad Software Inc., San Diego, CA, USA). Group differences on all MSCEIT tests were assessed using linear mixed models. Gender (male vs. female) and group (progesterone vs. placebo) were used as fixed effects and subjects as random effects. Again, years of education was used as a covariate, in order to account for their possible role as confounder in MSCEIT performance. Progesterone levels at the time of MSCEIT testing and demographic characteristics of the subjects in each group were compared using the independent samples t-test, and group differences in race were assessed using chi square analysis.
RESULTS (Study 2)
Demographic Characteristics of Participants
Table 2: There were no significant between-group differences in any of the demographic characteristics described in Table 2, including gender distribution, race, age, years spent in education, smoking status, years of cocaine use and alcohol use, number of days in the past month on which cocaine or alcohol was used, lifetime depression or anxiety. As years of education was found to have no impact on the mixed effect model, it was subsequently removed.
Table 2.
Study 2- Demographic Characteristics of Sample
| Placebo n=15 | Progesterone n=13 | |
|---|---|---|
| Gender – no. of males | 10 | 9 |
| Race | ||
| African American | 10 | 10 |
| Caucasian | 4 | 2 |
| Other | 1 | 1 |
| Age (s.d.) | 40.53 (8.4) | 41.69 (6.6) |
| Years spent in education (s.d.) | 12.07 (1.75) | 12.17 (1.47) |
| No. of regular smokers | 14 | 13 |
| Years of cocaine use (s.d.) | 16.47 (9.2) | 16.17 (7.4) |
| No. of days used in past month (s.d.) | 14.2 (8.6) | 15.2 (8.7) |
| Years of alcohol use (s.d.) | 13.27 (9.9) | 16.96 (6.2) |
| No. of days used in past month (s.d.) | 11.73 (10.3) | 11.33 (10.4) |
| Lifetime depression | 0 | 1 |
| Lifetime anxiety (incl PTSD) | 3 | 5 |
| Lifetime anxiety (without PTSD) | 3 | 2 |
Progesterone Levels at Time of MSCEIT Testing
As expected, the progesterone group had significantly higher blood levels of progesterone than the placebo group (t (24)= 6.26; p<0.001) across gender with a mean level of progesterone at 31.35 (±5.7) ng/ml compared to 0.88 (±0.25) ng/ml in the placebo group.
Effect of Exogenous progesterone on MSCEIT scores (Fig 3)
Figure 3.
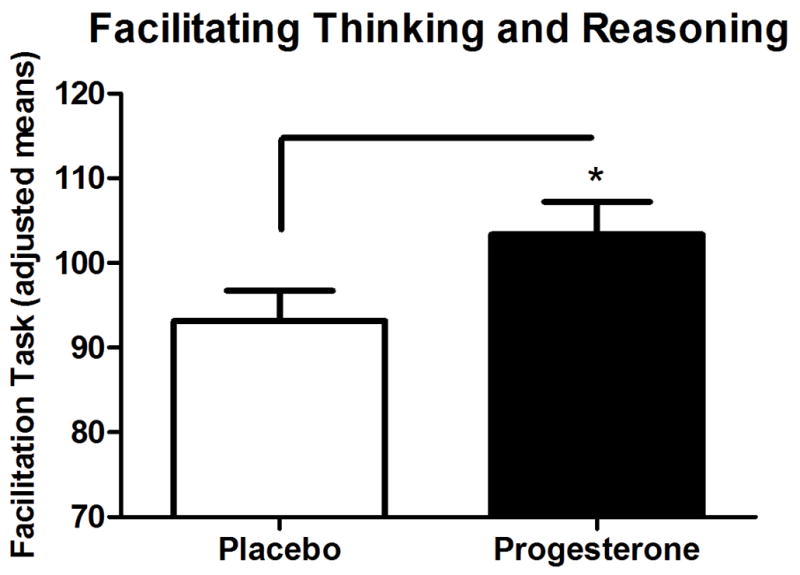
MSCEIT Performance as a Function of Exogenous Progesterone Treatment. * p<0.05.
When compared on all subscales of the MSCEIT, exogenous progesterone only improved scores on the Thought Facilitation Task scale compared with placebo in both males and females (F(1,24)=6.03; p=0.02). There was no gender x group interaction.
DISCUSSION
The present study examined the effect of a) gender and menstrual cycle on EI in a group of cocaine dependent individuals who also abused alcohol (CDA) and healthy controls and b) exogenous progesterone treatment on EI performance in CDA men and women. We found that healthy females performed better on most tests of EI compared to healthy males, however this discrepancy was not observed between the male and female CDA groups, due to the fact that the CDA women performed significantly more poorly than their female healthy controls. Additionally we found an improved ability to manage emotions in both healthy controls and CDA women with higher endogenous levels of progesterone compared to women with lower endogenous levels of progesterone, suggesting a potential role for endogenous progesterone in the strategizing of emotions. While data from a second study demonstrated some support for the effects of exogenous progesterone on specific components of emotional intelligence, effects were much less robust than those observed with endogenous progesterone and highly selective to the generation of emotions to facilitate thought processes. These findings imply that while EI and endogenous levels of progesterone may represent an important focus for therapy in substance abusing women, the effectiveness of exogenous progesterone in fully targeting these behavioral and biological systems remains unclear.
The greatest decrement in all factors of EI was observed in the CDA women compared with control women. This variation was not observed in the male groups who both performed worse than control females. This is consistent with other work that has demonstrated sex differences in emotional processing with females consistently performing better than males (Grossman and Wood, 1993; Wright et al, 2009). Social studies have also found gender differences in emotion regulation, such that women are more likely than men to be aware of their emotions and to use emotion regulation strategies to change their emotions (Nolen-Hoeksema, 2012). In light of current findings, however, increased emotional awareness in females (Boden and Berenbaum, 2012) may also represent a point of vulnerability during periods of challenge, or when the stress system is chronically perturbed, as in early abstinence from cocaine and alcohol (Fox and Sinha, 2009; Fox et al., 2008a; 2008b). Increased EI may also corroborate the idea that women are more prone to internalizing disorders compared with men (Kramer et al., 2008).
Current findings also indicated that both substance abusing and healthy women who were in the high progesterone phase of their MC were better able to manage, regulate and structure their emotions compared with women in the low MC phase. This was particularly in relation to interpersonal situations. These findings are consistent with studies which have found gonadal hormones to affect emotion regulation as well as the affective states that impinge upon these regulatory processes (for review see Van Wingen 2011). For example, fluctuations in endogenous progesterone levels during the MC have revealed direct effects on mood, where low progesterone levels are associated with basal negative mood in healthy women (Backstrom et al., 1983; Sveindottir and Backstrom, 2000) and increased anxiety in response to stress in cocaine dependent women (Sinha et al., 2007b). Furthermore, previous research has shown that fluctuations in endogenous progesterone levels during the menstrual cycle affect physiological, cognitive and subjective effects of both cocaine and alcohol (Sanz-Martin et al., 2012; Sofouglu et al., 1999; Evans et al., 2002; Evans and Foltin, 2006). Placebo-controlled progesterone administration studies have also shown that progesterone increases amygdala reactivity to threatening faces in women and strengthens the communication between the amygdala and the medial prefrontal cortex (mPFC) (van Wingen 2008), the key neural circuitry implicated in emotion regulation (Quirk and Beer 2006). High endogenous luteal progesterone levels are also associated with increased activity in the amygdala in response to negative emotion (Andreano and Cahill 2010).
Notably, improvements in the regulation and management component of EI in the high progesterone compared with low progesterone women were also specific to interpersonal, rather than non-social scenarios. Again, this holds some support for previous research that has observed endogenous levels of progesterone to be positively associated with social closeness (Brown et al., 2009) and affiliation motivation in both males and females (Wirth and Schultheiss, 2006; Schultheiss et al., 2004). Our findings also support the hypothesis that the up-regulation of progesterone during acute external stress in animals (Barbaccia et al., 1996) and prolonged internal stress during cocaine withdrawal in humans (Fox et al., 2008a) may play a protective role in encouraging affiliation for the purpose of stress reduction (Wirth and Schultheiss, 2006). As such, it may be that this social affiliation function is akin to the “tend and befriend” response to stress following oxytocin release (Taylor et al., 2000). Taken together with the current findings, endogenous progesterone may promote interpersonal affiliation via the ability to regulate and manage emotions within social settings.
Our study findings additionally imply that EI is important in women, and that endogenous progesterone improved some of the regulatory mechanisms in emotional processing. However, findings from our second progesterone treatment study indicate that exogenous progesterone seems to be working differently, in that it improves selective aspects of the perceptual, rather than strategic branches of the MSCEIT (See Figure 1). Specifically, present findings indicate that exogenous progesterone may improve the ability of early abstinent substance abusers to generate emotions and use them to facilitate the way they think. Although this may initially appear paradoxical, as the ability to generate and perceive emotions may be markedly dissociable from the ability to manage emotions, there may be some overlap in terms of reasoning and regulation processes and systems. For example, generating emotions to facilitate thought also relates to the manner in which individuals may reason with their emotions (Mayer, 2001) or rather use emotions to marshal attention allowing for a more rational, logical and creative thought process. Notably, while “managing emotions” has been described as reasoning about emotions, using “emotions to facilitate thought” has been described as pertaining more to the process of emotions enhancing reasoning (Brackett and Salovey, 2006).
Early abstinence from both cocaine and alcohol are associated with robust GABA-ergic and CRF adaptations within selective regions of the prefrontal cortex that are concomitant with cognitive and regulatory dysregulation (George et al., 2012; Peters et al., 2008). Furthermore, in view of the fact that progesterone is thought to strengthen emotional regulatory mechanisms via the actions of its metabolite allopregnanolone on GABA-activated inhibition of neural firing in dopaminergic regions of the prefrontal cortex (Fox et al., 2013; Becker, 1999; Becker and Hu, 2008; Russo et al., 2008), this may provide a possible broad underlying mechanism for the EI changes observed in the current paper.
It is important to note some limitations of the current study. First, biological markers of MC were not collected in Study 1. However, attempts were made to account for this by ascertaining the past 3 months of MC history. Second, while the CDA group in Study 1 had a higher number of participants with lifetime anxiety compared with the control group, symptoms could neither be current nor severe enough to require medication. Finally, the CDA group in Study 1 comprised more cigarette smokers and more years of alcohol use compared to the control group, and it is possible that MSCEIT performance may be a result of the combined effects of these drugs in addition to cocaine. While future research recruiting pure cocaine and/or alcohol dependent groups may be warranted to fully decipher the relative contribution of these different drugs on EI and menstrual cycle changes, it is important to note that participants enrolled in the current study reflected an ecologically valid sample of self-identifying cocaine dependent individuals.
Despite these limitations, this is one of the first studies to show that substance abusing women are particularly vulnerable to deficits in emotional intelligence during early abstinence compared with men. Furthermore, endogenous levels of progesterone during the luteal phase of the MC may contribute to improving these decrements. This potential role for endogenous progesterone is clinically relevant as deficits in managing emotions, particularly negative ones, and facilitating the necessary thinking and reasoning in response to these emotions may influence initiation, maintenance and relapse in cocaine and alcohol use. While both endogenous and exogenous progesterone enhanced selective components of EI, findings were markedly more robust in women with higher endogenous levels compared to those with lower levels. As baseline levels of EI may be disrupted in cocaine and alcohol dependent women, but not men, the effects of progesterone on improving EI may have a more therapeutic effect in women and future research is warranted to fully assess the moderating effects of gender.
Acknowledgments
This study was supported in part by Grants P50-DA16556 (Sinha); K01DA029040A (Fox); 1 R01 AA020095-01 (Fox); 1 R01 AA 20504-01 (Sinha) from the National Institutes of Health, Bethesda, MD, USA.
Footnotes
Conflicts of Interest: No conflicts of interest have been declared.
References
- Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. NeuroImage. 2010;53(4):1286–1293. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker J, Carroll ME. The role of progestins in the behavioral effects of cocaine and other drugs of abuse: Human and animal research. Neuroscience & biobehavioral reviews. 2010;35(2):315–333. doi: 10.1016/j.neubiorev.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckström T, Sanders D, Leask R, Davidson D, Warner P, Bancroft J. Mood, sexuality, hormones, and the menstrual cycle. II. Hormone levels and their relationship to the premenstrual syndrome. Psychosomatic medicine; 1983;45(6):503–507. doi: 10.1097/00006842-198312000-00004. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63(2):166–72. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Bar-On R, Tranel D, Denburg NL, Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain. 2003;126(Pt 8):1790–800. doi: 10.1093/brain/awg177. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. Review. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:6–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume AW, Marlatt GA. The role of executive cognitive functions in changing substance use: What we know and what we need to know. Annals of behavioral medicine. 2009;37(2):117–125. doi: 10.1007/s12160-009-9093-8. [DOI] [PubMed] [Google Scholar]
- Boden MT, Gala S, Berenbaum H. Emotional awareness, gender, and peculiar body-related beliefs. Cogn Emot. 2012;27(5):942–51. doi: 10.1080/02699931.2012.752720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackett MA, Salovey P. Measuring emotional intelligence with the mayer-salovery-caruso emotional intelligence test (MSCEIT) Psicothema Revista De Psicologia. 2006;18(Suppl):34–41. [PubMed] [Google Scholar]
- Brown SL, Fredrickson BL, Wirth MM, Poulin MJ, Meier EA, Heaphy ED, Cohen MD, Schultheiss OC. Social closeness increases salivary progesterone in humans. Horm Behav. 2009;56(1):108–11. doi: 10.1016/j.yhbeh.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabbert Buffet N, Djakoure C, Maitre SC, Bouchard P. Regulation of the human menstrual cycle. Front Neuroendocrinol. 1998;19:151–86. doi: 10.1006/frne.1998.0167. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159(4):397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Pharmacokinetics of repeated doses of intravenous cocaine across the menstrual cycle in rhesus monkeys. Pharmacol Biochem Behav. 2006;83(1):56–66. doi: 10.1016/j.pbb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Fillmore KM, Golding JM, Leino EV. Patterns and trends in women’s and men’s drinking. In: Wilsnack RW, Wilsnack SC, editors. Gender and Alcohol: Individual and Social Perspectives. New Brunswick, NJ: Rutgers Center of Alcohol Studies; 1997. pp. 21–48. [Google Scholar]
- Fox HC, Garcia M, Jr, Kemp K, Milivojevic V, Kreek MJ, Sinha R. Gender differences in cardiovascular and corticoadrenal response to stress and drug cues in cocaine dependent individuals. Psychopharmacology (Berl) 2006;185(3):348–57. doi: 10.1007/s00213-005-0303-1. [DOI] [PubMed] [Google Scholar]
- Fox HC, Axelrod SR, Paliwal P, Sleeper J, Sinha R. Difficulties in emotion regulation and impulse control during cocaine abstinence. Drug and alcohol dependence. 2007;89(2–3):298–301. doi: 10.1016/j.drugalcdep.2006.12.026. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008a;33(4):796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Hong KA, Sinha R. Difficulties in emotion regulation and impulse control in recently abstinent alcoholics compared with social drinkers. Addictive behaviors. 2008b;33(2):388–394. doi: 10.1016/j.addbeh.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KA, Paliwal P, Morgan PT, Sinha R. Altered levels of sex and stress steroid hormones assessed daily over a 28-day cycle in early abstinent cocaine-dependent females. Psychopharmacology. 2008c;195:527–536. doi: 10.1007/s00213-007-0936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sinha R. Sex differences in drug-related stress system changes: implications for treatment in substance-abusing women. Harv Rev Psychiatry. 2009;17:103–19. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Casey J, Hong KA, Sinha R. Selective cocaine-related difficulties in emotional intelligence: relationship to stress and impulse control. Am J Addict. 2011;20(2):151–60. doi: 10.1111/j.1521-0391.2010.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sofuoglu M, Morgan PT, Tuit KL, Sinha R. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type. Psychoneuroendocrinology. 2013;38(9):1532–44. doi: 10.1016/j.psyneuen.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Wood W. Sex differences in intensity of emotional experience: a social role interpretation. Journal of personality and social psychology; 1993;65(5):1010–1022. doi: 10.1037//0022-3514.65.5.1010. [DOI] [PubMed] [Google Scholar]
- Jausovec N, Jausovec K, Gerlic I. Differences in event-related and induced eeg patterns in the theta and alpha frequency bands related to human emotional intelligence. Neuroscience Letters. 2001;311(2):93–96. doi: 10.1016/s0304-3940(01)02141-3. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, Mulvaney F, Alterman A, Cornish J, Gariti P, Cnaan A, Poole S, Muller E, Acosta T, Luce D, O’Brien C. Effectiveness of propanolol for cocaine deoendence treatment may depend on cocaine withdrawal symptom severity. Drug Alcohol Depend. 2001;63:69–78. doi: 10.1016/s0376-8716(00)00193-9. [DOI] [PubMed] [Google Scholar]
- Kampman KM1, Dackis C, Lynch KG, Pettinati H, Tirado C, Gariti P, Sparkman T, Atzram M, O’Brien CP. A double-blind, placebo-controlled trial of amantadine, propranolol, and their combination for the treatment of cocaine dependence in patients with severe cocaine withdrawal symptoms. Drug Alcohol Depend. 2006;85(2):129–37. doi: 10.1016/j.drugalcdep.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–62. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kramer MD, Krueger RF, Hicks BM. The role of internalizing and externalizing liability factors in accounting for gender differences in the prevalence of common psychopathological syndromes. Psychological Medicine. 2008;38(1):51–61. doi: 10.1017/S0033291707001572. [DOI] [PubMed] [Google Scholar]
- Kuhl J, Koole SL. Workings of the will. In: Greenberg J, Koole SL, Pyszczynski T, editors. Handbook of Experimental Existential Psychology. New York: Guildford Press; 2004. pp. 411–430. [Google Scholar]
- Legree PJ, Psotka J, Tremble T, Bourne DR. Using consensus based measurement to assess emotional intelligence. In: Schulze R, Roberts RD, editors. Emotional intelligence. An international handbook. Gottingen, Germany: Hogrefe & Huber; 2005. pp. 99–123. [Google Scholar]
- Lopes PN, Salovey P, Straus R. Emotional intelligence, personality and the perceived quality of social relationships. Personality and Individual Differences. 2003;3:641–659. [Google Scholar]
- Mayer JD, Salovey P. What is emotional intelligence? In: Salovey P, Sluyter D, editors. Emotional development and emotional intelligence: Educational implications. New York: BasicBooks; 1997. [Google Scholar]
- Mayer JD. Primary division of personality and their scientific contributions: From the triology-of-mind to the systems set. Journal for the Theory of Social Behavior. 2001;31:449–477. [Google Scholar]
- Mayer JD, Salovey P, Caruso DR. Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT). USER’S Manual. Toronto, ON: Multi-Health Systems, Inc; 2002. [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86(2):220–33. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Emotion Regulation and Psychopathology: The Role of Gender. Annual Review of Clinical Psychology. 2012;8:161–187. doi: 10.1146/annurev-clinpsy-032511-143109. [DOI] [PubMed] [Google Scholar]
- Nowinski J, Baker S, Carroll K. Twelve Step Facilitation Therapy Manual. In: Mattson ME, editor. Project Match Monograph Series. Vol. 1. Rockville: US Department of Health and Human Services; 1994. [Google Scholar]
- Patkar A. Comparison of pretreatment characteristics and treatment outcomes for alcohol-, cocaine-, and multisubstance-dependent patients. Journal of addictive diseases. 2004;23(1):93–109. doi: 10.1300/J069v23n01_08. [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic Prefrontal Cortex Is Responsible for Inhibiting Cocaine Seeking in Extinguished Rats. The Journal of Neuroscience. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Hong KA, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: Influences of sex and cocaine dependence. The American journal of psychiatry. 2012;169(4):406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Jenab V. Why are women from Venus and men from Mars when they abuse cocaine? Brain Res. 2006;1126:200–3. doi: 10.1016/j.brainres.2006.08.109. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Anton SF, Carroll K, Budde D, Prusoff BA, Gawin F. Psychiatric diagnoses of treatment-seeking cocaine abusers. Archives of general psychiatry; 1991;48(1):43–51. doi: 10.1001/archpsyc.1991.01810250045005. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Sun WL, Minerly AC, Weierstall K, Nazarian A, Festa ED, Niyomchai T, Akhavan A, Luine V, Jenab S, Quiñones-Jenab V. Progesterone attenuates cocaine-induced conditioned place preference in female rats. Brain Res. 2008;1189:229–235. doi: 10.1016/j.brainres.2007.10.057. [DOI] [PubMed] [Google Scholar]
- Salovey P, Mayer JD. Emotional intelligence. Imagination, Cognition and Personality. 1990;9:185–211. [Google Scholar]
- Sanz-Martin A, Hernández-González M, Guevara MA, Santana G, Gumá-Díaz E, Amezcua C. Effects of alcohol on the performance of the Tower of London task in relation to the menstrual cycle: an electroencephalographic study. Behav Pharmacol. 2012;23(7):637–49. doi: 10.1097/FBP.0b013e3283584748. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Wirth MM, Stanton SJ. Effects of affiliation and power motivation arousal on salivary progesterone and testosterone. Horm Behav. 2004;46(5):592–9. doi: 10.1016/j.yhbeh.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Current psychiatry reports. 2007a;9(5):388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Paliwal P, Hong KA, Morgan PT, Bergquist KL. Sex steroid hormones, stress response and drug craving in cocaine dependent women: implications for relapse susceptibility. Exp Clin Psychopharmacol. 2007b;15:445–452. doi: 10.1037/1064-1297.15.5.445. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Experimental and clinical psychopharmacology; 1999;7(3):274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. Pharmacology, biochemistry and behavior. 2001;69(1–2):299–304. doi: 10.1016/s0091-3057(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacology, biochemistry and behavior; 2004;78(4):699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mouratidis M, Mooney M. Progesterone improves cognitive performance and attenuates smoking urges in abstinent smokers. Psychoneuroendocrinology. 2011;36(1):123–132. doi: 10.1016/j.psyneuen.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito E, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Streeter CC, Terhune DB, Whitfield TH, Gruber S, Sarid-Segal O, Silveri MM, Tzilos G, Afshar M, Rousev ED, Tian H, Renshaw PF, Ciraulo DA, Yurgelun-Todd DA. Performance on the Stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology. 2008;33:827–36. doi: 10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2007. (Office of Applied Studies, NSDUH Series H-32, DHHS Publication No. SMA 07–4293) [Google Scholar]
- Sveindottir H, Bäckström T. Prevalence of menstrual cycle symptom cyclicity and premenstrual dysphoric disorder in a random sample of women using and not using oral contraceptives. Acta obstetricia et gynecologica Scandinavica. 2000;79(5):405–413. doi: 10.1080/j.1600-0412.2000.079005405.x. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Tice DM, Bratslavsky E, Baumeister RF. Emotional distress regulation takes precedence over impulse control: If you feel bad, do it! Journal of personality and social psychology. 2001;80(1):53–67. [PubMed] [Google Scholar]
- van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Bäckström T, Buitelaar JK, Fernández G. Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry. 2008;13:325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, Ossewaarde L, Bäckström T, Hermans EJ, Fernández G. Gonadal hormone regulation of the emotion circuitry in humans. Neuroscience. 2011;191:38–45. doi: 10.1016/j.neuroscience.2011.04.042. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Schultheiss OC. Effects of affiliation arousal (hope of closeness) and affiliation stress (fear of rejection) on progesterone and cortisol. Horm Behav. 2006;50(5):786–95. doi: 10.1016/j.yhbeh.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Wright SL, Langenecker SA, Deldin PJ, Rapport LJ, Nielson KA, Kade AM, Own LS, Akil H, Young EA, Zubieta J. Gender-specific disruptions in emotion processing in younger adults with depression. Depression and anxiety; 2009;26(2):182–189. doi: 10.1002/da.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumoff B, Miller L, Levin J, Levit CD, Miller EH, Heinz U, Kalin M, Denman H, Jandorek R, Rosenfeld RS. Follicular-phase serum progesterone levels of nonsmoking women do not differ from the levels of nonsmoking men. Steroids. 1990;55:557–9. doi: 10.1016/0039-128x(90)90052-d. [DOI] [PubMed] [Google Scholar]


