Abstract
Galacto-N-biose (GNB) derivatives were efficiently synthesized from galactose derivatives via a one-pot two-enzyme system containing two promiscuous enzymes from Bifidobacterium infantis: a galactokinase (BiGalK) and a D-galactosyl-β1–3-N-acetyl-D-hexosamine phosphorylase (BiGalHexNAcP). Mono-sialyl and di-sialyl galacto-N-biose derivatives were then prepared using a one-pot two-enzyme system containing a CMP-sialic acid synthetase and an α2–3-sialyltransferase or an α2–6-sialyltransferase.
Graphical abstract
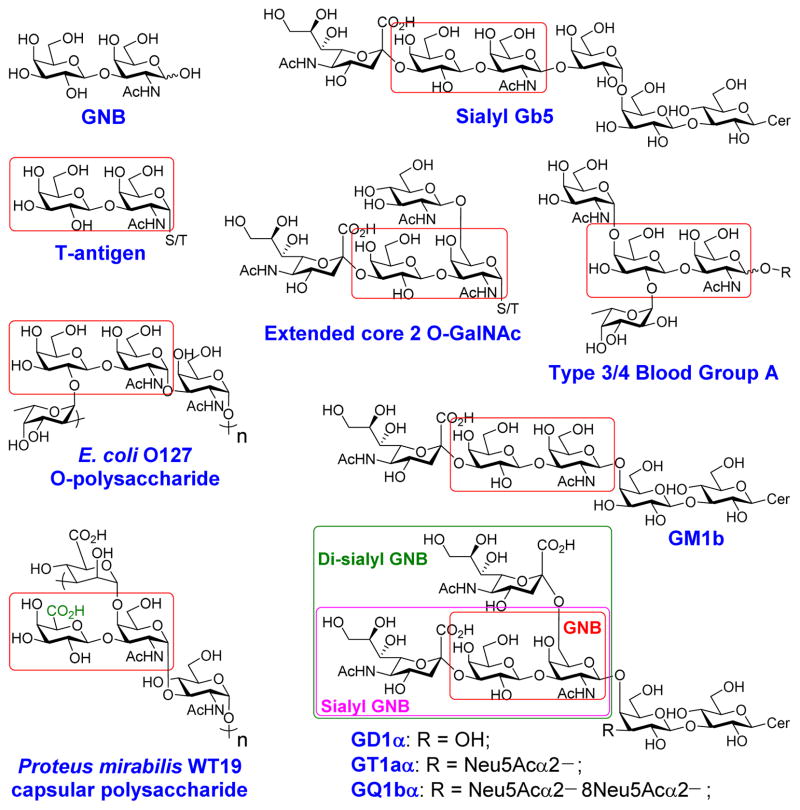
Disaccharide galacto-N-biose (GNB, Galβ1–3GalNAc) is a common glycan structure in nature. GNB with an α-configuration at the reducing end linked to the serine or threonine residue in glycoproteins (Galβ1–3GalNAcαSer/Thr) is named T-antigen and is one of the most common tumor-associated carbohydrate antigens (TATCs) found overexpressed on human carcinoma cells, including those of lung cancer, prostate cancer, breast cancer, etc.1 T-antigen is also a key component of core 1, core 2, and their extended forms of mucin-type O-GalNAc glycoproteins.2 In addition, GNB with an α-configuration at the reducing end is part of type 3 human blood group antigens3 (Fig. 1). On the other hand, GNB with a β-configuration at the reducing end (Galβ1–3GalNAcβ) is an essential part of globo-series (e.g. Globo-H, Gb5, sialyl Gb5) and ganglio-series (e.g. GA1, GM1, GD1, GT1, GP1, GQ1, etc) glycosphingolipids,4 and type 4 human blood group antigens3. Furthermore, GNB and its derivatives [e. g. GalAβ1–3GalNAc with the galactose (Gal) residue at the non-reducing end in GNB being replaced by galacturonic acid (GalA)] have been found in cell surface oligosaccharides or polysaccharides of various bacteria5 (Fig. 1). Sialyl GNB (Neu5Acα2–3Galβ1–3GalNAc) and di-sialyl GNB [Neu5Acα2–3Galβ1–3(Neu5Acα2–6)GalNAc], which are common terminal glycan structures found in gangliosides (Fig. 1), play significant roles in intermolecular interactions. For example, the sialyl GNB-terminated ganglioside GM1b serves as a receptor for H3N2 influenza virus during infection.6 It is also closely related to Guillain-Barré syndrome.7 On the other hand, the terminal di-sialyl GNB component of gangliosides GD1α, GT1aα and GQ1bα is the minimal binding epitope of myelin-associated glycoprotein (MAG) ligands,8 and is considered as a leading target for developing MAG inhibitors to enhance regeneration of central nervous system axons after injury in adult mammals.9
Figure 1.
Galacto-N-biose (GNB) and natural glycans contain GNB or derivatives, and sialyl or di-sialyl GNB.
Given their significances, both chemical10 and enzymatic strategies11 were developed for the synthesis of these glycans. Previously, we cloned a novel D-galactosyl-β1–3-N-acetyl-D-hexosamine phosphorylase from Bifidobacterium infantis (BiGalHexNAcP), and developed a highly efficient one-pot two-enzyme system for rapid preparation of GNB and lacto-N-biose (LNB, Galβ1–3GlcNAc) as well as their derivatives.12 This system includes a galactokinase from E. coli (EcGalK) which catalyzes the formation of galactose-1-phosphate (Gal-1-P) from Gal, and BiGalHexNAcP that catalyzes the transfer of Gal from Gal-1-P to various acceptors including N-acetylgalactosamine (GalNAc), N-acetylglucosamine (GlcNAc) and their derivatives. It is worth nothing that this system is almost equally efficient in preparing GNB/LNB derivatives with both α- and β-configurations at the reducing end.12 Comparing to glycosyltransferase (GT)-catalyzed reactions, such a system not only avoids in situ re-generation of valuable sugar nucleotides (e.g. UDP-Gal), but also simplifies product purification since the reaction mixture excludes UDP/UTP. Other than the observed acceptor substrate specificity observed,12 a later success in utilizing this system to prepare GNB derivatives containing 6-deoxy-6-fluoro-Gal (Gal6F)13 indicates the promiscuity of BiGalHexNAcP towards sugar-1-phosphate donors. Nevertheless, the donor specificity of BiGalHexNAcP has not been studied in detail. Various GNB, sialyl GNB, and di-sialyl GNB derivatives are still in great demand as glycan probes or precursors for biological studies. In this study, we described an efficient one-pot two-enzyme system that combines BiGalHexNAcP and a promiscuous galactokinase from B. infantis (BiGalK) for the production of GNB derivatives containing Gal derivatives, including those with 2-deoxy-Gal, 6-deoxy-Gal, 6-azido-Gal, 6-fluoro-6-deoxy-Gal, and GalA. The obtained compounds were then used for acceptor substrate specificity studies of two bacterial sialyltransferases and for the preparative synthesis of novel sialyl GNB and di-sialyl GNB derivatives.
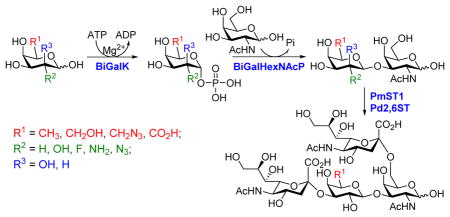
As shown in Scheme 1, the one-pot two-enzyme system contains: 1) BiGalK, which catalyzes the formation of Gal-1-P and derivatives from monosaccharides in the presence of ATP; 2) BiGalHexNAcP, which transfers monosaccharides onto GalNAc to form diverse GNB derivatives. EcGalK was originally used in the system to generate Gal-1-P. However, it was found that at the optimal reaction pH of BiGalHexNAcP (5.0–6.5), EcGalK showed dramatically reduced phosphorylation activity towards Gal.13 Thus, a two-step process was used previously in the one-pot two-enzyme synthesis of fluorinated T-antigens, where reaction pH was adjusted to meet the optimal pH of each enzyme in corresponding steps.13 BiGalK14 and another galactokinase from Meiothermus taiwanensis (MtGalK)15 are two recently discovered galactokinases with extremely high activity towards Gal. They also exhibit relaxed substrate specificity towards different Gal derivatives, for example, BiGalK can accept 2-deoxy-Gal (Gal2deoxy, 2), galactosamine (GalNH2, 3), 6-deoxy-Gal (7), and galacturonic acid (GalA, 8)14, and MtGalK can accept GalNH2 (2), 2-deoxy-2-azido-Gal (GalN3, 4) and N-acetylglycosamine (GalNAc).15 Given that the yield of BiGalK (over 80 mg/L culture)14 is 4 times higher than that of MtGalK (16 mg/L culture),15 BiGalK was chosen in this study for the synthesis of GNB and derivatives. To further study the substrate specificity of BiGalK, and determine whether it can be used in the one-pot two-enzyme system, the phosphorylation activity of BiGalK towards a panel of C2 and C6 modified Gal were tested at pH 6.5. As shown in Table S1 and Figure S1, except for 2-deoxy-2-azido-Gal (GalN3, 4), 6-deoxy-Gal (Gal6deoxy, 7) and GalA (8), which had moderate yield (63%, 60% and 53% respectively), BiGalK could efficiently phosphorylate Gal (1) and all other derivatives (2, 3, 5, 6, 9) at pH 6.5 with yields of 76% to 96%. Even though specific activities of BiGalK towards C6 modified Gal are much lower than towards Gal (e.g. 0.0906 μmol min−1 mg−1 towards GalA), it is still efficient enough to prepare corresponding Gal-1-P derivatives on a preparative scales.16 These results indicate that BiGalK is promiscuous towards C2 or C6 modified Gal in mild acidic (pH 6.5) conditions, which is suitable for the proposed one-pot two-enzyme system (Scheme 1). Preparative scale synthesis and purification of corresponding Gal-1-P derivatives were carried out, and the structures were confirmed by MS and NMR analysis (Supporting Information).
Scheme 1.
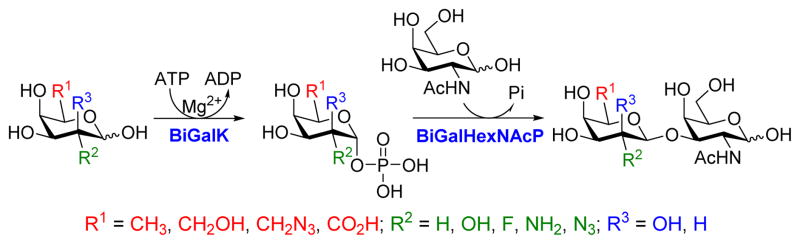
One-pot two-enzyme system for the synthesis of GNB and derivatives. BiGalK, galactokinase from Bifidobacterium infantis ATCC15697; BiGalHexNAcP, D-galactosyl-β1–3-N-acetyl-D-hexosamine phosphorylase from B. infantis ATCC15697.
The synthesis of GNB and derivatives was carried out using the one-pot two-enzyme system shown in Scheme 1. Excess amounts of BiGalK were added to achieve high phosphorylation efficiency towards each monosaccharide (see detailed methods and compounds characterization in Supporting Information). As listed in Table 1, the system was quite efficient in synthesizing GNB (12, 95%), 2-deoxy-Galβ1–3GalNAc (13, 80%), 6-deoxy-Galβ1–3GalNAc (18, 87%), GalAβ1–3GalNAc (19, 84%), and Gal6N3β1–3GalNAc (20, 76%) from corresponding Gal or derivatives (1, 2, 7, 8, 9). In contrast, the synthesis of GalNH2β1–3GalNAc (14), GalN3β1–3-GalNAc (15), Talβ1–3GalNAc (17) was not successful using the one-pot two-enzyme system. Most interestingly, GalFβ1–3GalNAc (16) and GalFβ1–3GlcNAc (45, Table S2) were able to be synthesized using either GalNAc or GlcNAc as acceptors, even though with relatively low yields (28%, 18.3 mg; 26%, 17.0 mg respectively). Preparation of such compounds using GTs catalyzed reactions was proven to be impossible, since UDP-GalF, the required donor, is usually an inhibitor of GTs.17 These 2-deoxy-2-fluoro modified glycans may found great application as inhibitors or probes in biological studies. Taken together with the high-yield synthesis of Gal6Fβ1–3GalNAc (21, 80%) reported previously,13 these results suggest that in donor substrate recognition, BiGalHexNAcP is quite relaxed towards C6 modifications of Gal, but relatively strict towards C2 modifications. Similar results were obtained when using GlcNAc as an acceptor in the one-pot two-enzyme system for the synthesis of LNB and derivatives (Table S2).
Table 1.
Synthesis of GNB and derivatives via the one-pot two-enzyme system shown in Scheme 1. ND, not detected.
| Donors | Acceptors | Products | Yields (%) |
|---|---|---|---|
 1 |
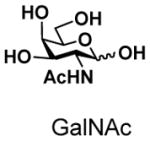 11 |
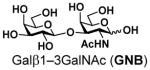 12 |
95 |
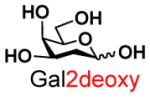 2 |
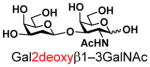 13 |
80 | |
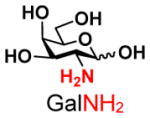 3 |
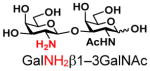 14 |
ND | |
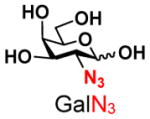 4 |
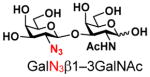 15 |
ND | |
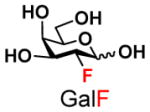 5 |
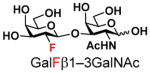 16 |
28 | |
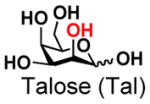 6 |
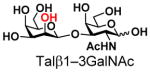 17 |
ND | |
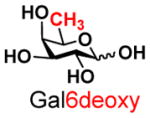 7 |
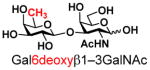 18 |
87 | |
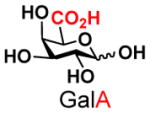 8 |
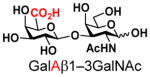 19 |
84 | |
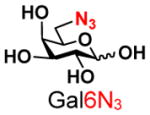 9 |
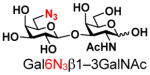 20 |
76 | |
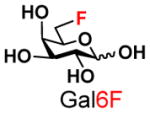 10 |
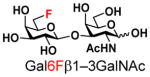 21 |
8013 |
Pasteurella multocida α2–3-sialyltranferase 1 (PmST1) and its mutants were found to be extremely useful in preparing bioactive α2–3-sialosides and derivatives.18 The donor substrate specificity of the enzyme has been extensively investigated, whereas the acceptor substrate specificity studies have been limited. Only those with a non-modified Gal (either free or linked to other glycans) or Gal6F (Gal6Fβ1–3GalNAc)17b at the non-reducing end have been tested. The access to various GNB derivatives with modifications on the Gal residue allowed us to further study the acceptor specificity of PmST1, and to synthesize novel α2–3-sialosides. As shown in Scheme 2, a one-pot two-enzyme system was used for this purpose. The sugar donor, CMP-Neu5Ac was first generated from N-acetylneuraminic acid (Neu5Ac) and cytidine 5′-triphosphate (CTP) by Neisseria meningitidis CMP-Sia synthetase (NmCSS)19 in presence of Mg2+. Secondly, the Neu5Ac on CMP-Neu5Ac was transferred onto the Gal via an α2–3-linkage to form sialyl GNB.
Scheme 2.
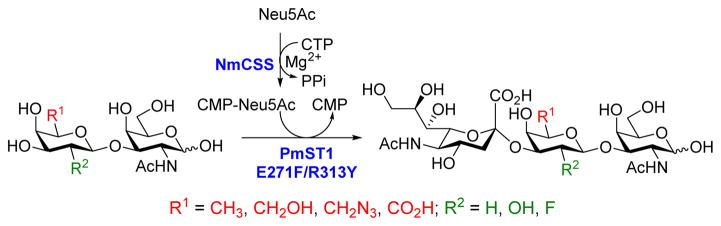
One-pot two-enzyme system for the synthesis of sialyl GNB and derivatives. NmCSS, CMP-Sia synthetase from Neisseria meningitidis; PmST1 E271F/R313Y, Pasteurella multocida α2–3-sialyltransferase 1 mutant with decreased sialidase activity.
As listed in Table 2, the system was highly efficient in sialylating all GNB derivatives expect for GalAβ1–3GalNAc (19) (see detailed methods and compounds characterization in Supporting Information). These results imply that PmST1 could tolerate modifications at the C2 of Gal (Compounds 13, 16), and most importantly, it has good tolerance towards modifications at the C6 of Gal including a deoxy substituent (Compound 18) or an azido substituent (Compound 20). However, PmST1 could hardly accept 19 (16% yield), which may be caused by the negative charge of the C6 carboxyl group in GalA.
Table 2.
Synthesis of sialyl GNB and derivatives via the one-pot two-enzyme α2–3-sialylation system shown in Scheme 2.
| Acceptors | Products | Yields (%) |
|---|---|---|
| 12 |
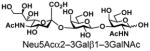 22 |
94 |
| 13 |
23 |
82 |
| 16 |
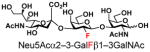 24 |
87 |
| 18 |
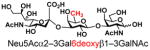 25 |
85 |
| 19 |
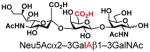 26 |
16 |
| 20 |
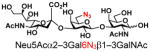 27 |
79 |
Given the significant roles that the terminal di-sialyl GNB play in ganglioside-MAG interaction, it is of great synthetic interest. Previously, Photobacterium damselae α2–6-sialyltransferase (Pd2,6ST)20 was used to generate di-sialyl GNB from sialyl GNB, however, a glycan mixture was obtained due to non-specific sialylation of either the Gal or the GlcNAc residue of GNB by Pd2,6ST.21 A “substrate engineering” strategy was then developed to overcome the problem,21 including: 1) chemical formation of 1,4-lactone between the C1″ carboxyl group of Neu5Ac and the C4′ hydroxyl group of Gal on sialyl GNB to prevent α2–6-sialylation of Gal, followed by 2) Pd2,6ST- catalyzed α2–6-sialylation of GalNAc and 3) saponification to yield di-sialyl GNB.
We propose that removing or replacing the C6 hydroxyl group of the Gal residue would prevent non-specific sialylation at the Gal, thus provide a direct approach for the formation of useful di-sialyl GNB derivatives. The access of three sialyl GNB derivative (25, 26, 27) with modifications at the C6 of the Gal allows us to test this approach. As shown in Scheme 3, the di-sialyl GNB derivatives were synthesized using a one-pot two-enzyme system, which contains 1) NmCSS for catalyzing the in situ generation of CMP-Neu5Ac, and 2) Pd2,6ST for catalyzing the α2–6-sialylation of the internal GalNAc residue of sialyl GNB derivatives. As expected, three corresponding di-sialyl GNB derivatives were successfully synthesized with moderate (29, 57%) to high yields (28, 81%; 30, 75%) (Table 3), and no by-products were found by either mass spectrometry (MS) or thin-layer chromatography (TLC) analysis. These results indicate that Pd2,6ST can tolerate sialyl-GNB with modifications at the C6 of Gal, and it is a practical and reliable approach to synthesize di-sialyl GNB with appropriated C6 modifications of the Gal residue. Such an approach not only provided well-controlled synthesis of di-sialyl GNB mimicking natural gangliosides, but also introduced functional groups (e.g. -N3) potentially useful for molecular probing. Furthermore, compare with the previously reported “substrate engineering” strategy,21 this approach excluded chemical protection/deprotection steps.
Scheme 3.
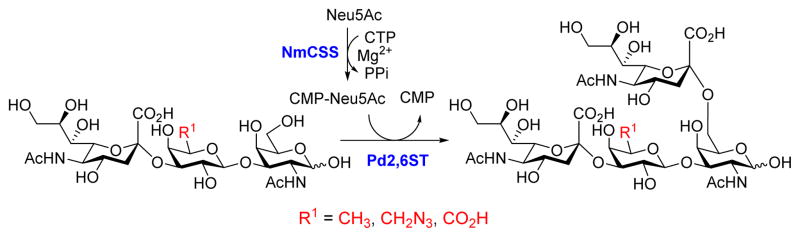
One-pot two-enzyme system for the synthesis of di-sialyl GNB derivatives. NmCSS, CMP-Sia synthetase from Neisseria meningitidis; Pd2,6ST, α2–6-sialyltransferase from Photobacterium damselae.
Table 3.
Synthesis of di-sialyl GNB derivatives via the one-pot two-enzyme α2–6-sialylation system shown in Scheme 3.
| Acceptors | Products | Yields (%) |
|---|---|---|
| 25 |
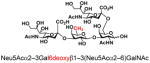 28 |
81 |
| 26 |
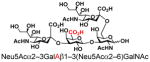 29 |
57 |
| 27 |
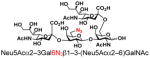 30 |
75 |
In summary, taking advantages of the acceptor substrate promiscuity of BiGalK and donor substrate promiscuity of BiGalHexNAcP, various novel GNB and LNB derivatives were synthesized using an efficient one-pot two-enzyme system. These compounds were used for acceptor substrate specificity study of PmST1 and efficient synthesis of sialyl GNB derivatives. Finally, well-controlled synthesis of di-sialyl GNB was achieved by selectively blocking one of the C6-glycosylation sites on Gal/GalNAc in the substrates. The compounds synthesized in this study are valuable probes for elucidating the biological roles of GNB, sialyl and di-sialyl GNB-containing glycans.
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China grant 31100587 (to L. Li), as well as National Institute of Health grants R01 GM085267 (to P.G. Wang) and R01 HD065122 (to X. Chen).
Footnotes
Electronic Supplementary Information (ESI) available: Experimental details for substrate specificity study and enzymatic synthesis, NMR and HRMS data and spectra. See DOI: 10.1039/c000000x/
Contributor Information
Lei Li, Email: lli22@gsu.edu.
Peng G Wang, Email: pwang11@gsu.edu.
References
- 1.Dube DH, Bertozzi CR. Nat Rev Drug Discov. 2005;4:477. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 2.Brockhausen I, Schachter H, Stanley P. In: Essentials of Glycobiology. 2. Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. ch 9. Cold Spring Harbor; New York: 2009. [PubMed] [Google Scholar]
- 3.Storry JR, Olsson ML. Immunohematology. 2009;25:48. [PubMed] [Google Scholar]
- 4.Schnaar RL, Suzuki A, Stanley P. In: Essentials of Glycobiology. 2. Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. ch 10. New York: 2009. [PubMed] [Google Scholar]
- 5.(a) Knirel YA, Perepelov AV, Kondakova AN, Senchenkova SN, Sidorczyk Z, Rozalski A, Kaca W. Innate Immun. 2011;17:70. doi: 10.1177/1753425909360668. [DOI] [PubMed] [Google Scholar]; (b) Wang Q, Perepelov AV, Feng L, Knirel YA, Li Y, Wang L. FEMS Immunol Med Microbiol. 2009;55:47. doi: 10.1111/j.1574-695X.2008.00494.x. [DOI] [PubMed] [Google Scholar]; (c) Widmalm G, Leontein K. Carbohydr Res. 1993;247:255. doi: 10.1016/0008-6215(93)84258-8. [DOI] [PubMed] [Google Scholar]; (d) Liu B, Knirel YA, Feng L, Perepelov AV, Senchenkova SN, Wang Q, Reeves PR, Wang L. FEMS Microbiol Rev. 2008;32:627. doi: 10.1111/j.1574-6976.2008.00114.x. [DOI] [PubMed] [Google Scholar]; (e) Knirel’Iu A, Kochetkov NK. Biokhimiia. 1994;59:1784. [PubMed] [Google Scholar]
- 6.Suzuki Y, Matsunaga M, Nagao Y, Taki T, Hirabayashi Y, Matsumoto M. Vaccine. 1985;3:201. doi: 10.1016/0264-410x(85)90104-5. [DOI] [PubMed] [Google Scholar]
- 7.Kusunoki S, Iwamori M, Chiba A, Hitoshi S, Arita M, Kanazawa I. Neurology. 1996;47:237. doi: 10.1212/wnl.47.1.237. [DOI] [PubMed] [Google Scholar]
- 8.(a) Schnaar RL. FEBS Lett. 2010;584:1741. doi: 10.1016/j.febslet.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kolter T, Proia RL, Sandhoff K. J Biol Chem. 2002;277:25859. doi: 10.1074/jbc.R200001200. [DOI] [PubMed] [Google Scholar]
- 9.(a) Ernst B, Schwardt O, Mesch S, Wittwer M, Rossato G, Vedani A. Chimia (Aarau) 2010;64:17. doi: 10.2533/chimia.2010.17. [DOI] [PubMed] [Google Scholar]; (b) Ernst B, Magnani JL. Nat Rev Drug Discov. 2009;8:661. doi: 10.1038/nrd2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilstermann M, Magnusson G. Carbohydr Res. 1995;272:1. doi: 10.1016/0008-6215(95)00026-p. [DOI] [PubMed] [Google Scholar]
- 11.(a) Hedbys L, Johansson E, Mosbach K, Larsson PO. Carbohydr Res. 1989;186:217. doi: 10.1016/0008-6215(89)84036-4. [DOI] [PubMed] [Google Scholar]; (b) Vetere A, Miletich M, Bosco M, Paoletti S. Eur J Biochem. 2000;267:942. doi: 10.1046/j.1432-1327.2000.01068.x. [DOI] [PubMed] [Google Scholar]; (c) Baisch G, Ohrlein R, Streiff M, Kolbinger F. Bioorg Med Chem Lett. 1998;8:751. doi: 10.1016/s0960-894x(98)00093-6. [DOI] [PubMed] [Google Scholar]; (d) Su DM, Eguchi H, Yi W, Li L, Wang PG, Xia C. Org Lett. 2008;10:1009. doi: 10.1021/ol703121h. [DOI] [PubMed] [Google Scholar]; (e) Wang Z, Gilbert M, Eguchi H, Yu H, Cheng J, Muthana S, Zhou L, Wang PG, Chen X, Huang X. Adv Synth Catal. 2008;350:1717. doi: 10.1002/adsc.200800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu H, Thon V, Lau K, Cai L, Chen Y, Mu S, Li Y, Wang PG, Chen X. Chem Commun (Camb) 2010;46:7507. doi: 10.1039/c0cc02850a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan J, Chen X, Wang F, Cao H. Org Biomol Chem. 2013;11:842. doi: 10.1039/c2ob26989a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Liu Y, Wang W, Cheng J, Zhao W, Wang P. Carbohydr Res. 2012;355:35. doi: 10.1016/j.carres.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Li SP, Hsiao WC, Yu CC, Chien WT, Lin HJ, Huang LD, Lin CH, Wu WL, Wu SH, Lin CC. Adv Synth Catal. 2014;356:3199. [Google Scholar]
- 16.Muthana MM, Qu J, Xue M, Klyuchnik T, Siu A, Li Y, Zhang L, Yu H, Li L, Wang PG, Chen X. Chem Commun (Camb) 2015;51:4595. doi: 10.1039/c4cc10306h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Hayashi T, Murray BW, Wang R, Wong CH. Bioorg Med Chem. 1997;5:497. doi: 10.1016/s0968-0896(96)00263-5. [DOI] [PubMed] [Google Scholar]; (b) Burkart MD, Vincent SP, Duffels A, Murray BW, Ley SV, Wong CH. Bioorg Med Chem. 2000;8:1937. doi: 10.1016/s0968-0896(00)00139-5. [DOI] [PubMed] [Google Scholar]
- 18.(a) Yu H, Chokhawala H, Karpel R, Yu H, Wu B, Zhang J, Zhang Y, Jia Q, Chen X. J Am Chem Soc. 2005;127:17618. doi: 10.1021/ja0561690. [DOI] [PubMed] [Google Scholar]; (b) Sugiarto G, Lau K, Li Y, Khedri Z, Yu H, Le DT, Chen X. Mol Biosyst. 2011;7:3021. doi: 10.1039/c1mb05182b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sugiarto G, Lau K, Qu J, Li Y, Lim S, Mu S, Ames JB, Fisher AJ, Chen X. ACS Chem Biol. 2012;7:1232. doi: 10.1021/cb300125k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H, Yu H, Karpel R, Chen X. Bioorg Med Chem. 2004;12:6427. doi: 10.1016/j.bmc.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Huang S, Chokhawala H, Sun M, Zheng H, Chen X. Angew Chem Int Ed Engl. 2006;45:3938. doi: 10.1002/anie.200600572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng X, Yao W, Cheng J, Zhang X, Jin L, Yu H, Chen X, Wang F, Cao H. J Am Chem Soc. 2014;136:5205. doi: 10.1021/ja5000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.