Abstract
The epidermal growth factor receptor deletion variant EGFRvIII is known to be expressed in a subset of patients with glioblastoma (GBM) tumors that enhances tumorigenicity and also accounts for radiation and chemotherapy resistance. Targeting the EGFRvIII deletion mutant may lead to improved GBM therapy and better patient prognosis. Multifunctional magnetic nanoparticles serve as a potential clinical tool that can provide cancer cell targeted drug delivery, imaging, and therapy. Our previous studies have shown that an EGFRvIII-specific antibody and cetuximab (an EGFR- and EGFRvIII-specific antibody), when bioconjugated to IONPs (EGFRvIII-IONPs or cetuximab-IONPs respectively), can simultaneously provide sensitive cancer cell detection by magnetic resonance imaging (MRI) and targeted therapy of experimental GBM. In this study, we investigated whether cetuximab-IONPs can additionally allow for the radiosensitivity enhancement of GBM. Cetuximab-IONPs were used in combination with single (10Gy x 1) or multiple fractions (10Gy x 2) of ionizing radiation (IR) for radiosensitization of EGFRvIII-overexpressing human GBM cells in vitro and in vivo after convection-enhanced delivery (CED). A significant GBM antitumor effect was observed in vitro after treatment with cetuximab-IONPs and subsequent single or fractionated IR. A significant increase in overall survival of nude mice implanted with human GBM xenografts was found after treatment by cetuximab-IONP CED and subsequent fractionated IR. Increased DNA double strands breaks (DSBs), as well as increased reactive oxygen species (ROS) formation, were felt to represent the mediators of the observed radiosensitization effect with the combination therapy of IR and cetuximab-IONPs treatment.
Keywords: Glioblastoma, epidermal growth factor receptor, iron oxide nanoparticles, convection-enhanced delivery, magnetic resonance imaging, ionizing radiation
Introduction
Glioblastoma (GBM) is the most common and aggressive malignant glioma in adults [1]. The standard treatment of care for GBM patients consists of surgical resection followed by postoperative radiotherapy with concurrent and adjuvant chemotherapy [2]. Resistance to conventional therapies (e.g., radiotherapy and chemotherapy) contributes to the poor prognosis of GBM patients [3]. Further optimization of standard therapies, as well as novel therapeutic approaches are urgently needed.
The epidermal growth factor receptor variant III (EGFRvIII) is a specific mutation commonly expressed in GBM [4]. Several studies have demonstrated that EGFRvIII expression promotes enhanced tumorigenicity and accounts for radiation and chemotherapy resistance contributing to the poor prognosis in GBM patients [5,6]. The GBM-specific EGFRvIII deletion mutant found exclusively on GBM cells and not in the normal brain represents an ideal target for enhancing the effects of radio- and chemotherapy.
Cetuximab (Erbitux, IMC-C225) is a well-known recombinant monoclonal antibody, specific to EGFR (including EGFRvIII), that is FDA approved for treatment of patients with EGFR-expressing metastatic colorectal and head/neck cancers [7–9]. Cetuximab has a 10-fold higher affinity to the extracellular domain of EGFR than endogenous ligands and inhibits downstream signal transduction pathways [10]. Preclinical and clinical studies have shown a modest inhibitory effect on EGFR-expressing GBM tumors after treatment with cetuximab [11–13].
Recently, we have reported that magnetic iron-oxide nanoparticles (IONPs) bioconjugated to cetuximab (cetuximab-IONPs), have a significantly enhanced therapeutic effect against glioblastoma (GBM) when compared to cetuximab alone [14]. We have determined that cetuximab-IONPs bind to both the wtEGFR and the EGFRvIII deletion mutant on patient-derived GBM cells (including glioma stem-like cells (GSCs)), inhibit EGFR cell signaling, are internalized by the tumor cells, and promote internalization of the EGFR resulting in enhanced apoptosis. In multiple rodent glioma models, we have found that convection-enhanced delivery (CED) of cetuximab-IONPs is safe and significantly increases the survival of rodents intracranially implanted with human EGFR-expressing GBM xenografts [14]. Moreover, we have shown that intra- and peritumoral distribution of cetuximab-IONPs after CED can be tracked by magnetic resonance imaging (MRI) [15].
Magnetic IONPs are currently being used clinically for the treatment of recurrent GBM in combination with radiotherapy [16]. High concentrations of non-targeted IONPs can generate local hyperthermia when exposed to alternating magnetic fields (AMFs) [17,18]. IONPs in combination with radiotherapy has been shown to increase overall and progression-free survival in patients with GBM prospectively. Furthermore, human clinical studies have demonstrated minimal toxicity of IONPs [16] as they are biodegradable, making them attractive for widespread future clinical applications [19].
The main goal of this study was to investigate whether EGFR-targeted magnetic nanoparticles (cetuximab-IONPs), in combination with fractionated radiotherapy, can lead to the radiosensitivity enhancement of human GBM in a rodent glioma model. Highly tumorigenic and invasive EGFRvIII-expressing human GBM cells were used in this study. Mechanistic studies, including DNA double strand break (DSB) repair and reactive oxygen species (ROS) formation, were performed to determine the radiosensitivity enhancement effect. In addition, toxicity studies were performed with normal human astrocytes.
Materials and Methods
Cell lines
The human GBM cell line U87MG, overexpressing the EGFRvIII deletion mutant (U87MGEGFRvIII), as well as human astrocytes were used (supplementary data). Over-expression of the U87MG human GBM cell line with the EGFRvIII deletion mutant has been previously described from our group [20]. The U87MGEGFRvIII cell line selection was made based on the fact that over-expression of the EGFRvIII deletion mutant confers enhanced tumorigenicity resulting in formation of aggressive and invasive xenografts in immunocompromised rodents [21,22]. Furthermore, U87EGFRvIII cells are radioresistant.
IONPs, EGFR antibody, bioconjugation
IONPs were covalently conjugated to the human-mouse chimeric monoclonal antibody cetuximab by using the appropriate conjugation kit provided by the manufacturer (supplementary data).
In vitro studies
In vitro studies for viability, apoptosis, DNA damage and oxidative stress were performed on human GBM cells treated with control (PBS), IONPs, cetuximab and cetuximab-IONPs in combination with single or fractionated IR (supplementary data). Cell toxicity studies were additionally performed on human astrocytes undergone the same treatments in combination with IR (supplementary data).
Animal Experiments
All animals underwent intracerebral stereotactic inoculation of human GBM cells, followed by CED of PBS, cetuximab, or cetuximab-IONPs, and subsequent whole brain IR (supplementary data). Imaging, histology, and survival studies were performed (supplementary data).
Results
Bioconjugation of cetuximab to IONPs
Cetuximab was covalently conjugated to IONPs (10nm in core size-5mg/ml concentration, Ocean NanoTech). Bioconjugation was performed between the amino-terminal group of the antibody and carboxyl groups on the copolymer IONPs coating. Confirmation of successful conjugation of cetuximab to IONPs was performed by mobility shift in agarose gel electrophoresis analysis as has already been shown in our recent publication [14].
Selective antitumor effect of cetuximab-IONPs combined with IR on human GBM cells in vitro
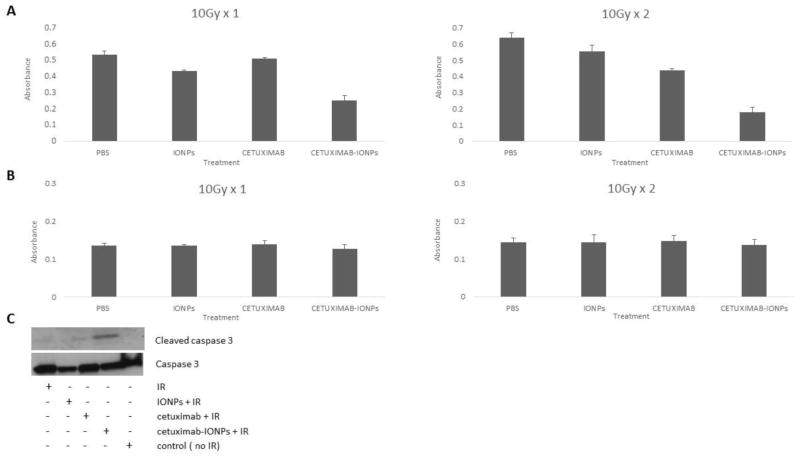
Human GBM cells (U87MGEGFRvIII) and human astrocytes were treated with PBS (control), IONPs, cetuximab, and cetuximab-IONPs at a concentration of 0.3mg/ml for 24 hours, followed by either a single IR dose of 10Gy or fractionated IR of 10Gy x 2 with a 24 h interval between IR doses. Cell viability assay was performed 1, 2 and 3 days after the last IR dose treatment. A statistically significant (p<0.05) antitumor effect was observed 72 hours after the last IR dose in GBM cells treated with cetuximab-IONPs prior to application of either single or fractionated IR, compared to other treatment groups (Fig. 1a). Human astrocytes revealed no significant toxicity 24 hours after treatment with cetuximab-IONPs and subsequent single and fractionated IR (Fig. 1b). This result was also found to be statistical significant (p<0.05).
Fig. 1.
a & b Cell proliferation analysis (MTT Assay) of U87MGEGFRvIII cells (5 × 103 cells/well) (a) and Normal Human Astrocytes (5 × 103 cells/well) (b) after treatment with control (PBS), IONPs (0.3mg/ml), cetuximab (0.3mg/ml) and cetuximab-IONPs (0.3mg/ml) in combination with IR (p<0.05). C. Western blot analysis for expression of cleaved caspase 3 and caspase 3 of U87MGEGFRvIII cells after treatment with control (PBS), IONPs (0.3mg/ml), cetuximab (0.3mg/ml) and cetuximab-IONPs (0.3mg/ml) in combination with IR.
Cetuximab-IONPs promote radiosensitivity enhancement of human GBM cells leading to apoptosis
Human GBM cells were incubated with PBS (control), IONPs, cetuximab and cetuximab-IONPs at a concentration of 0.3mg/ml for 24 hours. Cells were then treated with fractionated IR (10Gy x 2). Western blot analysis performed 24 hours after the second IR dose revealed elevated levels of cleaved caspase-3 and apoptosis in cells treated with the combination therapy of cetuximab-IONPs and IR (Fig. 1c). Levels of cleaved caspase-3 were not found after treatment of cells with PBS, IONPs, or cetuximab followed by IR (Fig. 1c).
Enhanced DNA DSBs formation after cetuximab-IONP treatment of GBM
DNA DSBs after IR are well known to occur and can lead to cancer cell death [23]. The induction and repair of DNA DSBs in human GBM cells were analyzed in vitro by γH2AX foci visualization. γH2AX foci have been described to be a sensitive marker of DNA DSBs caused by IR [24]. γH2AX expression was evaluated in U87MGEGFRvIII cells treated with PBS (control), IONPs, cetuximab, and cetuximab-IONPs at a concentration of 0.3mg/ml for 24 hours, followed by a single IR dose of 2Gy. γH2AX foci visualization and analysis was performed 30 minutes post IR. Exposure of cells to the cetuximab-IONPs prior to IR was found to induce an increase in the number of γH2AX foci as detected 30 minutes post IR, compared to other combination treatments. Representative immunofluorescence images of γH2AX foci and DAPI for each treatment revealed enhanced γH2AX foci accumulation in the nuclei of cells treated with cetuximab-IONPs and IR (Fig. 2a). In an effort to quantify this observed effect, the γH2AX foci were measured in approximately 30 nuclei from each treatment group by semi-automated image analysis. The density of γH2AX foci (number of foci per square inch of nucleus) formed in the cells was found to be statistically significantly (p<0.05) higher with the cetuximab-IONP and IR group in comparison to other treatment groups (Fig. 2b).
Fig. 2.
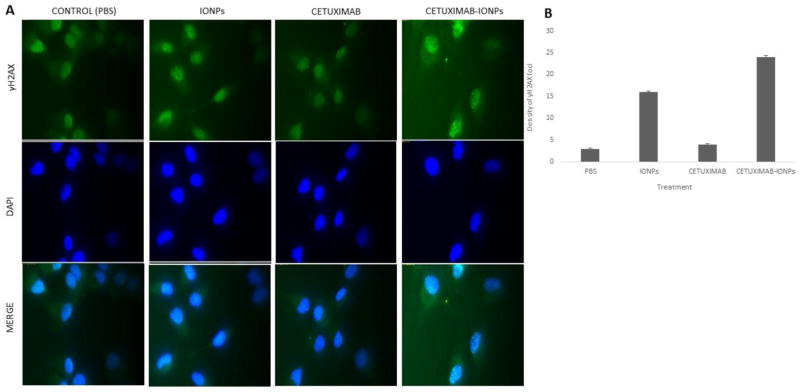
a Immunofluorescent staining of U87MGEGFRvIII cells (20 × 103/well) after treatment with control (PBS), IONPs (0.3mg/ml), cetuximab (0.3mg/ml), and cetuximab-IONPs (0.3mg/ml) and subsequent single IR dose of 2Gy 24 h post treatment. Fixation of cells 30 minutes post IR. Green: anti-γH2AX, Blue: DAPI. b Semi-automated density of γH2AX foci (number of foci/square inch of nucleus) for each treatment (p<0.05). Fixation of cells 30 minutes post IR
Enhanced ROS generation after cetuximab-IONP treatment of GBM
Enhanced ROS generation has been described as a possible mechanism of anticancer efficacy with IR [25]. ROS generation has also been found with IONP treatment of cells [26]. The ROS production in human GBM cells was measured in vitro using carboxy-H2DCFDA after IR treatment. This compound enters live cells and after subsequent intracellular deacetylation it reacts with ROS to form a highly fluorescent compound that emits green fluorescence. GBM cells were pre-treated with PBS (control), IONPs, cetuximab, and cetuximab-IONPs at a concentration of 0.3mg/ml for 24 hours, prior to a single IR dose of 10Gy. ROS generation was evaluated 3 hours post IR. Incubation of cells with cetuximab-IONPs prior to IR application was determined to result in enhanced intracellular ROS production compared to other treatment groups as showed in representative fluorescent microscopy images for each treatment (Fig. 3a). Cells were also stained with the ROS inducer compound TBHP which served as a positive control (Fig. 3b left) and for baseline intracellular ROS expression (negative control) (Fig. 3b right).
Fig. 3.
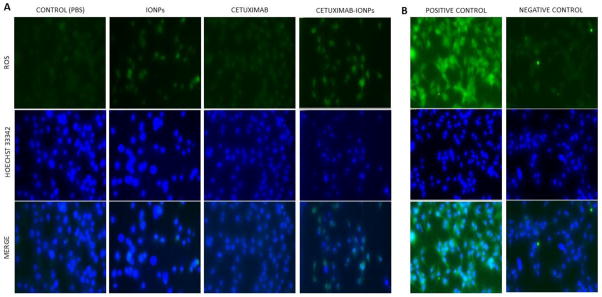
a ROS detection in live U87MGEGFRvIII cells (20 × 103/well) after treatment with control (PBS), IONPs (0.3mg/ml), cetuximab (0.3mg/ml) and cetuximab-IONPs (0.3mg/ml) and subsequent single IR dose of 10Gy 24 h post-treatment. Cells were stained 3 h post IR for ROS detection with 5-(and-6)-carboxy-2′,7′ dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA), a marker for ROS detection in live cells. Green: ROS, Blue: Hoechst 33342. b. Left: Induction of ROS in U87MGEGFRvIII cells by tert-butyl hydroperoxide (TBHP), an inducer of ROS production (positive control). Right: U87MGEGFRvIII stained for baseline ROS expression with 5-(and-6)-carboxy-2′,7′ dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA), a marker for ROS detection in live cells (negative control). Green: ROS, Blue: Hoechst 33342
Radiosensitivity enhancement of cetuximab-IONPs in an orthotopic EGFRvIII-expressing rodent GBM model
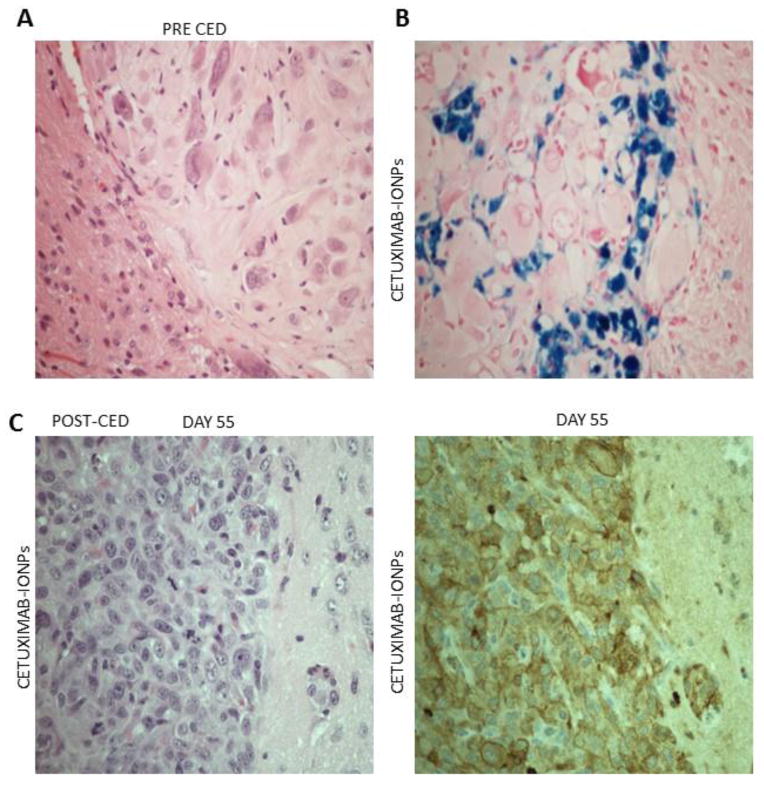
Athymic nude mice 6–8 weeks old underwent intracranial implantation of 2 × 105 human GBM cells per mouse on day 0. Five days after tumor inoculation, mice were randomly assigned into 3 treatment groups (n=7 for each group). Mice underwent CED of HBSS (untreated-control animals), cetuximab, and cetuximab-IONPs. The cetuximab-IONP concentration used in all treatment groups was 0.3mg/ml. Each CED treatment involved a total volume of 10μl infused at a rate of 0.5μl/min for a total of 20 minutes. Subsequent fractionated whole brain IR of 10Gy x 2 was performed 24 and 72 hours post CED. Histology (Hematoxylin & Eosin staining) was performed in brains harvested before CED to confirm intracranial xenograft formation (Fig. 4a). Prussian blue staining was able to confirm intratumoral and peritumoral distribution of cetuximab-IONPs after CED (Fig. 4b). Additional histology (H&E) was performed in mouse brains harvested several days post CED, confirming intracranial xenograft formation (Fig. 4c left). Immunohistochemistry for EGFRvIII was also performed confirming the presence of EGFRvIII expression in intracranial GBM xenografts (Fig. 4c right).
Fig. 4.
a Hematoxylin & Eosin (H&E) staining of intracranial human U87MGEGFRvIII GBM xenograft in athymic nude mouse confirms xenograft formation prior to CED (magnification 40x). b Prussian Blue staining of athymic nude mouse brain section showing intratumoral and peritumoral distribution of bioconjugated cetuximab-IONPs after CED (magnification 40x). c H&E staining (left) and immunostaining for EGFRvIII (right) of an athymic nude mouse which underwent intracranial human U87MGEGFRvIII GBM xenograft implantation and subsequent CED of bioconjugated cetuximab-IONPs (magnification 40x)
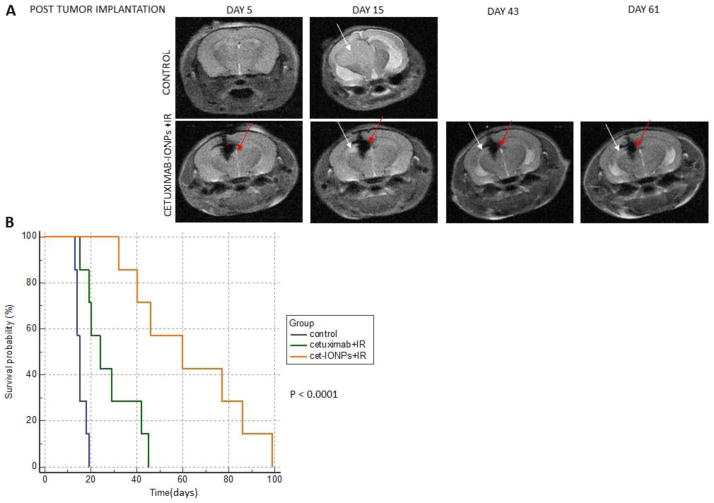
Brain MRI scans were initially performed immediately after CED of cetuximab-IONPs (day 5 after tumor implantation) to confirm the presence and determine the localization of the cetuximab-IONPs. Serial imaging was performed at frequent intervals up to 90 days post tumor implantation. Serial T2-weighted imaging of animals, which underwent CED of cetuximab-IONPs followed by IR, was able to show retention of the cetuximab-IONPs within the brain and delayed xenograft growth, compared to animals which underwent CED of HBSS (control animals) (Fig. 5a). Animal survival studies were performed and results were analyzed and plotted using Kaplan-Meier survival curve software. A statistically significant survival benefit was found in the animals which underwent CED of cetuximab-IONPs followed by IR compared to the animals treated by cetuximab combined with IR and to the control animals (Fig. 5b). The calculated median survival for the three animal groups was 60, 24, and 15 days respectively. Moreover, in two small pilot in vivo studies we performed, mice treated with CED of free IONPs in combination with IR showed a survival advantage similar to mice treated with cetuximab alone followed by IR.
Fig. 5.
a Top: T2-weighted MRI of a control mouse implanted with EGFRvIII-expressing orthotopic human GBM xenograft (U87MGEGFRvIII) showing a hyperintense xenograft (white arrows) post tumor implantation. Bottom: Serial T2-weighted MRI of a mouse which underwent implantation of an EGFRvIII-expressing orthotopic human GBM xenograft (U87MGEGFRvIII) and subsequent CED of cetuximab-IONPs. Hypointense MRI signal drop after CED of cetuximab-IONPs (red arrows) and hyperintense EGFRvIII-expressing human GBM xenograft (white arrows). b Kaplan Meier survival curve comparison of athymic nude mice after intracranial implantation of U87MG EGFRvIII cells (2 × 105/mouse) receiving no treatment (control group) or combination treatment by CED of cetuximab (0.3mg/ml) or cetuximab-IONPs (0.3mg/ml) and subsequent IR (10 Gy x 2)
Discussion
Radiotherapy is considered the most effective conventional adjuvant treatment for GBM patients and is standard of care following surgery in combination with chemotherapy. However, GBM represents one of the most radio- and chemoresistant cancers. Despite these treatments, the majority of GBM patients develop recurrences at or near the site of their initial tumor that was treated. EGFRvIII expression in GBM, is known to promote significant radioresistance [27,28]. Moreover, it has been reported that IR-induced activation of the wtEGFR also contributes to enhanced radioresistance in tumor cells, through multiple mechanisms including accelerated cell proliferation, an antiapoptotic response, and improved DNA DSB repair after IR exposure [29,30]. New strategies are needed for radiosensitivity enhancement of GBM tumors.
The use of magnetic nanoparticles (MNPs) has already been applied to brain tumor imaging and therapy [31–33]. Conjugation of tumor specific ligands to MNPs represents an approach to increase tumor targeted therapeutic efficacy of MNPs and has been used in gliomas [34,35]. Our group initially reported that conjugation of an EGFRvIII specific antibody to IONPs (EGFRvIII-IONPs) can lead to simultaneous selective MRI contrast enhancement and targeted therapy of experimental infiltrative GBM cells both in vitro and in vivo after CED [20]. Recently, we have demonstrated that cetuximab conjugated IONPs can target both EGFR and the deletion mutant, EGFRvIII, in GBM and GBM stem-like cells, leading to greater antitumor efficacy when compared to cetuximab alone [14].
In this study, we chose to study the effects of IR in highly tumorigenic human GBM in combination with cetuximab-IONP treatment. Cetuximab alone can exert a radiosensitizing effect in GBM by promoting radiation-induced apoptosis, decreasing cell proliferation, and inhibiting radiation-induced damage repair [36,37]. Nontargeted free IONPs in high concentrations have also shown a radiosensitizing effect in recurrent GBM [16].
Highly radioresistant and tumorigenic EGFRvIII-expressing human GBM cells were used in this study. Our in vitro results revealed that cetuximab-IONPs in complication with IR induced significant radiosensitivity enhancement of GBM cells. Exposure of cells to cetuximab-IONPs for 24 h, followed by subsequent IR, resulted in a significant decrease in GBM cell survival compared to the control cells and to cells exposed to cetuximab alone or to free IONPs. Apoptosis was found to be the mode of GBM cell death after combination treatment of cetuximab-IONPs and IR. Given that a crucial cellular process in determining cell radiosensitivity is DNA DSBs formation and repair, we initially examined the formation of DNA DSBs in GBM cells after treatment with cetuximab-IONPs followed by IR. Our data showed enhanced γH2AX foci formation and accumulation in GBM cells treated with cetuximab-IONPs with IR compared to cells treated with cetuximab or free IONPs prior to IR application. This observation suggests a repair inhibition of IR-induced DNA DSBs caused by cetuximab-IONPs.
In an attempt to further investigate underlying mechanisms mediating the cetuximab-IONPs in vitro radiosensitization, intracellular ROS formation was examined. Cellular response to IR has been described to be mediated by the production of ROS which in turn results in damage to a variety of macromolecules such as DNA and proteins [38]. Our results demonstrated that intracellular ROS generation was significantly increased in GBM cells incubated with cetuximab-IONPs prior to IR treatment, compared to other pre-IR treatments such as cetuximab and free IONPs. We believe that enhanced intracellular ROS formation is an additional mechanism contributing to the cytotoxic effects of cetuximab-IONPs in GBM cells after IR, further accounting to the observed radiosensitivity enhancement. GBM cells treated with free IONPs combined with IR also demonstrated an enhanced antitumor effect and increased formation of intracellular ROS compared to the control group of cells and to cells treated with cetuximab alone prior to IR. The observed in vitro radiosensitization effect with free IONPs can be explained by the nonspecific uptake of the IONPs by the tumor cells, a phenomenon that has already been reported both in vitro and in vivo [39,40]. The enhanced ROS generated in GBM cells in vitro after exposure to free IONPs and subsequent IR has also been described as a method of IONP -mediated cytotoxicity [26,41].
Surface functionalization of MNPs plays an important role and contributes to enhanced targeting and internalization of IONPs in the tumor cells [42]. The targeted IONPs used in this study were bioconjugated to cetuximab and used in a 100-fold lower concentration than other studies utilizing free IONPs [16,18]. We have found that cetuximab-IONPs can more effectively target GBM cells, including GSCs, promote EGFR internalization, disrupt EGFR cell signaling, and ultimately increase apoptosis when compared to cetuximab alone [14].
One important aspect of this study was the ability to confirm the radiosensitivity enhancement of cetuximab-IONPs in vivo with radioresistant and invasive orthotopic GBM xenografts. The U87MGEGFRvIII-expressing GBM cells consistently formed very aggressive xenografts in rodents that were also radioresistant, sharing many similarities to naturally-occurring human GBM tumors. GBM xenografts were highly infiltrative and lethal, proven by the fact that all the animals which underwent CED of HBSS (control animals) were dead by day 18 after tumor implantation with a median survival of 15 days. Our animal survival studies demonstrated the ability of cetuximab-IONPs to promote radiosensitivity enhancement of xenograft tumors after CED by delaying tumor growth and significantly improving animal survival compared to cetuximab and control animal treatment groups. No toxicity was observed after cetuximab-IONPs treatment both in human astrocytes 24 hours after IR application in vitro and in animals after intracerebral administration of cetuximab-IONPs and subsequent whole brain IR. The significant selective killing of cetuximab-IONPs after IR application further supports our previously demonstrated basis of targeted therapy of GBM xenografts in the brain [14,20].
In this study, intratumoral administration of cetuximab-IONPs was performed by CED. This method of local delivery can bypass the BBB and overcome the obstacles associated with systemic delivery of IONPs to central nervous system (CNS). CED has already been used to deliver therapeutic agents for the treatment of malignant gliomas and in humans for the treatment of recurrent GBM [43,44]. CED permits homogenous delivery of high concentrations of therapeutic agents directly into targeted brain regions, avoiding toxicity to normal tissues and organs [43,45]. CED of cetuximab-IONPs in our mouse GBM model resulted in effective intratumoral and peritumoral distribution of cetuximab-IONPs confirmed by both MRI and prussian blue staining.
In conclusion, we have demonstrated that cetuximab-IONP treatment of radioresistant human GBM can promote radiosensitivity enhancement both in vitro and in vivo. The observed radiosensitization effect was found to be associated with both inhibition of DNA repair and enhancement of ROS formation. These results suggest that combination therapy of targeted cetuximab-IONPs and IR may offer a new strategy to augment the therapeutic effects of IR in GBM therapy.
Supplementary Material
Acknowledgments
We thank Dr. Robert C. Long for his significant contribution in acquiring and processing the MRI data and for helpful scientific image interpretations discussions. We also thank the Pathology Core Lab of Winship Cancer Institute of Emory University for helping with all the histology data. This work was supported by grants from the NIH (NS053454), Southeastern Brain Tumor Foundation (SBTF), Georgia Cancer Coalition Distinguished Cancer Clinicians and Scientific Program, Robbins Scholar Award from the Winship Cancer Institute of Emory University, AANS/CNS Section on Tumors/BrainLab International Research Fellowship and Dana Foundation.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Braun S, Oppermann H, Mueller A, Renner C, Hovhannisyan A, Baran-Schmidt R, Gebhardt R, Hipkiss A, Thiery J, Meixensberger J, Gaunitz F. Hedgehog signaling in glioblastoma multiforme. Cancer Biol Ther. 2012;13(7):487–495. doi: 10.4161/cbt.19591. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Omay SB, Vogelbaum MA. Current concepts and newer developments in the treatment of malignant gliomas. Indian J Cancer. 2009;46(2):88–95. doi: 10.4103/0019-509x.49146. [DOI] [PubMed] [Google Scholar]
- 4.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gan HK, Kaye AH, Luwor RB. The EGFRvIII variant in glioblastoma multiforme. J Clin Neurosci. 2009;16(6):748–754. doi: 10.1016/j.jocn.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee B, McEllin B, Camacho CV, Tomimatsu N, Sirasanagandala S, Nannepaga S, Hatanpaa KJ, Mickey B, Madden C, Maher E, Boothman DA, Furnari F, Cavenee WK, Bachoo RM, Burma S. EGFRvIII and DNA double-strand break repair: a molecular mechanism for radioresistance in glioblastoma. Cancer Res. 2009;69(10):4252–4259. doi: 10.1158/0008-5472.can-08-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendelsohn J. Targeting the epidermal growth factor receptor for cancer therapy. J Clin Oncol. 2002;20(18 Suppl):1s–13s. [PubMed] [Google Scholar]
- 8.Garrett CR, Eng C. Cetuximab in the treatment of patients with colorectal cancer. Expert Opin Biol Ther. 2011;11(7):937–949. doi: 10.1517/14712598.2011.582464. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MH, Chen H, Shord S, Fuchs C, He K, Zhao H, Sickafuse S, Keegan P, Pazdur R. Approval summary: Cetuximab in combination with cisplatin or carboplatin and 5-fluorouracil for the first-line treatment of patients with recurrent locoregional or metastatic squamous cell head and neck cancer. Oncologist. 2013;18(4):460–466. doi: 10.1634/theoncologist.2012-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7(4):301–311. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Martens T, Laabs Y, Gunther HS, Kemming D, Zhu Z, Witte L, Hagel C, Westphal M, Lamszus K. Inhibition of glioblastoma growth in a highly invasive nude mouse model can be achieved by targeting epidermal growth factor receptor but not vascular endothelial growth factor receptor-2. Clin Cancer Res. 2008;14(17):5447–5458. doi: 10.1158/1078-0432.ccr-08-0147. [DOI] [PubMed] [Google Scholar]
- 12.Belda-Iniesta C, de Carpeno JC, Saenz EC, Gutierrez M, Perona R, Baron MG. Long term responses with cetuximab therapy in glioblastoma multiforme. Cancer Biol Ther. 2006;5(8):912–914. doi: 10.4161/cbt.5.8.3118. [DOI] [PubMed] [Google Scholar]
- 13.Eller JL, Longo SL, Hicklin DJ, Canute GW. Activity of anti-epidermal growth factor receptor monoclonal antibody C225 against glioblastoma multiforme. Neurosurgery. 2002;51(4):1005–1013. doi: 10.1097/00006123-200210000-00028. discussion 1013–1004. [DOI] [PubMed] [Google Scholar]
- 14.Kaluzova M, Bouras A, Machaidze R, Hadjipanayis CG. Targeted therapy of glioblastoma stem-like cells and tumor non-stem cells using cetuximab-conjugated iron-oxide nanoparticles. Oncotarget. 2015 doi: 10.18632/oncotarget.3554. advanced online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Platt S, Nduom E, Kent M, Freeman C, Machaidze R, Kaluzova M, Wang L, Mao H, Hadjipanayis CG. Canine model of convection-enhanced delivery of cetuximab-conjugated iron-oxide nanoparticles monitored with magnetic resonance imaging. Clin Neurosurg. 2012;59:107–113. doi: 10.1227/NEU.0b013e31826989ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Orawa H, Budach V, Jordan A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011;103(2):317–324. doi: 10.1007/s11060-010-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadjipanayis CG, Bonder MJ, Balakrishnan S, Wang X, Mao H, Hadjipanayis GC. Metallic iron nanoparticles for MRI contrast enhancement and local hyperthermia. Small. 2008;4(11):1925–1929. doi: 10.1002/smll.200800261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan A, Scholz R, Maier-Hauff K, van Landeghem FK, Waldoefner N, Teichgraeber U, Pinkernelle J, Bruhn H, Neumann F, Thiesen B, von Deimling A, Felix R. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J Neurooncol. 2006;78(1):7–14. doi: 10.1007/s11060-005-9059-z. [DOI] [PubMed] [Google Scholar]
- 19.Mahmoudi M, Hofmann H, Rothen-Rutishauser B, Petri-Fink A. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem Rev. 2012;112(4):2323–2338. doi: 10.1021/cr2002596. [DOI] [PubMed] [Google Scholar]
- 20.Hadjipanayis CG, Machaidze R, Kaluzova M, Wang L, Schuette AJ, Chen H, Wu X, Mao H. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010;70(15):6303–6312. doi: 10.1158/0008-5472.can-10-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagane M, Coufal F, Lin H, Bogler O, Cavenee WK, Huang HJ. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56(21):5079–5086. [PubMed] [Google Scholar]
- 22.Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, Huang HJ. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci U S A. 1994;91(16):7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461(7267):1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo LJ, Yang LX. Gamma-H2AX - a novel biomarker for DNA double-strand breaks. In Vivo. 2008;22(3):305–309. [PubMed] [Google Scholar]
- 25.Yamamori T, Yasui H, Yamazumi M, Wada Y, Nakamura Y, Nakamura H, Inanami O. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free Radic Biol Med. 2012;53(2):260–270. doi: 10.1016/j.freeradbiomed.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 26.Ahamed M, Alhadlaq HA, Khan MAM, Akhtar MJ. Selective killing of cancer cells by iron oxide nanoparticles mediated through reactive oxygen species via p53 pathway. Journal of Nanoparticle Research. 2013;15(1) doi: 10.1007/s11051-012-1225-6. [DOI] [Google Scholar]
- 27.Lammering G, Valerie K, Lin PS, Hewit TH, Schmidt-Ullrich RK. Radiation-induced activation of a common variant of EGFR confers enhanced radioresistance. Radiother Oncol. 2004;72(3):267–273. doi: 10.1016/j.radonc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Li B, Yuan M, Kim IA, Chang CM, Bernhard EJ, Shu HK. Mutant epidermal growth factor receptor displays increased signaling through the phosphatidylinositol-3 kinase/AKT pathway and promotes radioresistance in cells of astrocytic origin. Oncogene. 2004;23(26):4594–4602. doi: 10.1038/sj.onc.1207602. [DOI] [PubMed] [Google Scholar]
- 29.Lammering G, Hewit TH, Valerie K, Contessa JN, Amorino GP, Dent P, Schmidt-Ullrich RK. EGFRvIII-mediated radioresistance through a strong cytoprotective response. Oncogene. 2003;22(36):5545–5553. doi: 10.1038/sj.onc.1206788. [DOI] [PubMed] [Google Scholar]
- 30.Fan QW, Cheng CK, Gustafson WC, Charron E, Zipper P, Wong RA, Chen J, Lau J, Knobbe-Thomsen C, Weller M, Jura N, Reifenberger G, Shokat KM, Weiss WA. EGFR phosphorylates tumor-derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer Cell. 2013;24(4):438–449. doi: 10.1016/j.ccr.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orringer DA, Koo YE, Chen T, Kopelman R, Sagher O, Philbert MA. Small solutions for big problems: the application of nanoparticles to brain tumor diagnosis and therapy. Clin Pharmacol Ther. 2009;85(5):531–534. doi: 10.1038/clpt.2008.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Provenzale JM, Silva GA. Uses of nanoparticles for central nervous system imaging and therapy. AJNR Am J Neuroradiol. 2009;30(7):1293–1301. doi: 10.3174/ajnr.A1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caruso G, Caffo M, Alafaci C, Raudino G, Cafarella D, Lucerna S, Salpietro FM, Tomasello F. Could nanoparticle systems have a role in the treatment of cerebral gliomas? Nanomedicine. 2011;7(6):744–752. doi: 10.1016/j.nano.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Sun C, Veiseh O, Gunn J, Fang C, Hansen S, Lee D, Sze R, Ellenbogen RG, Olson J, Zhang M. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small. 2008;4(3):372–379. doi: 10.1002/smll.200700784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy GR, Bhojani MS, McConville P, Moody J, Moffat BA, Hall DE, Kim G, Koo YE, Woolliscroft MJ, Sugai JV, Johnson TD, Philbert MA, Kopelman R, Rehemtulla A, Ross BD. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin Cancer Res. 2006;12(22):6677–6686. doi: 10.1158/1078-0432.ccr-06-0946. [DOI] [PubMed] [Google Scholar]
- 36.Diaz Miqueli A, Rolff J, Lemm M, Fichtner I, Perez R, Montero E. Radiosensitisation of U87MG brain tumours by anti-epidermal growth factor receptor monoclonal antibodies. Br J Cancer. 2009;100(6):950–958. doi: 10.1038/sj.bjc.6604943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eller JL, Longo SL, Kyle MM, Bassano D, Hicklin DJ, Canute GW. Anti-epidermal growth factor receptor monoclonal antibody cetuximab augments radiation effects in glioblastoma multiforme in vitro and in vivo. Neurosurgery. 2005;56(1):155–162. doi: 10.1227/01.neu.0000145865.25689.55. discussion 162. [DOI] [PubMed] [Google Scholar]
- 38.Mikkelsen RB, Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22(37):5734–5754. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- 39.Zimmer C, Weissleder R, Poss K, Bogdanova A, Wright SC, Jr, Enochs WS. MR imaging of phagocytosis in experimental gliomas. Radiology. 1995;197(2):533–538. doi: 10.1148/radiology.197.2.7480707. [DOI] [PubMed] [Google Scholar]
- 40.Moore A, Marecos E, Bogdanov A, Jr, Weissleder R. Tumoral distribution of long-circulating dextran-coated iron oxide nanoparticles in a rodent model. Radiology. 2000;214(2):568–574. doi: 10.1148/radiology.214.2.r00fe19568. [DOI] [PubMed] [Google Scholar]
- 41.Klein S, Sommer A, Distel LV, Neuhuber W, Kryschi C. Superparamagnetic iron oxide nanoparticles as radiosensitizer via enhanced reactive oxygen species formation. Biochem Biophys Res Commun. 2012;425(2):393–397. doi: 10.1016/j.bbrc.2012.07.108. [DOI] [PubMed] [Google Scholar]
- 42.Villanueva A, Canete M, Roca AG, Calero M, Veintemillas-Verdaguer S, Serna CJ, del Morales MP, Miranda R. The influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cells. Nanotechnology. 2009;20(11):115103. doi: 10.1088/0957-4484/20/11/115103. [DOI] [PubMed] [Google Scholar]
- 43.Hadjipanayis CG, Fellows-Mayle W, Deluca NA. Therapeutic efficacy of a herpes simplex virus with radiation or temozolomide for intracranial glioblastoma after convection-enhanced delivery. Mol Ther. 2008;16(11):1783–1788. doi: 10.1038/mt.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sampson JH, Akabani G, Archer GE, Berger MS, Coleman RE, Friedman AH, Friedman HS, Greer K, Herndon JE, 2nd, Kunwar S, McLendon RE, Paolino A, Petry NA, Provenzale JM, Reardon DA, Wong TZ, Zalutsky MR, Pastan I, Bigner DD. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol. 2008;10(3):320–329. doi: 10.1215/15228517-2008-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91(6):2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.