Abstract
Renal resistive index (RRI) measured by Doppler ultrasonography is associated with cardiovascular events and mortality in hypertensive, diabetic, and elderly patients. We studied the factors associated with high RRI (≥0.70) and its associations with mortality in CKD patients without renal artery stenosis. We included 1,962 patients with an eGFR 15-59 ml/min/1.73 m2 who also had RRI measured (January 1, 2005 - October 2011) from an existing CKD registry. Participants with renal artery stenosis (60-99% or renal artery occlusion) were excluded. Multivariable logistic regression model was used to study factors associated with high RRI (≥0.70) and its association with mortality was studied using Kaplan-Meier plots and Cox proportional hazards model. Hypertension was prevalent in >90% of the patients. In the multivariable logistic regression, older age, female gender, diabetes mellitus, coronary artery disease, peripheral vascular disease, higher systolic blood pressure and use of beta blockers were associated with higher odds of having RRI ≥0.70. During a median follow-up of 2.2 years, 428 patients died. After adjusting for covariates, RRI ≥0.70 was associated with increased mortality (adjusted HR 1.29, 95% CI, 1.02- 1.65, P< 0.05). This association was more pronounced among younger patients and those with stage 3 CKD. Non-cardiovascular/non-malignancy related deaths were higher in those with RRI ≥0.70. RRI ≥0.70 is associated with higher mortality in hypertensive CKD patients without clinically significant renal artery stenosis after accounting for other significant risk factors. Its evaluation may allow early identification of those who are at risk thereby potentially preventing or delaying adverse outcomes.
Keywords: chronic kidney disease, renal resistive index, mortality, renal artery stenosis, cardiovascular disease
Background
Chronic kidney disease is a public health problem and is associated with higher risk of cardiovascular disease and mortality1-3. While kidney function measures, such as estimated glomerular filtration rate (eGFR) and albuminuria serve as markers to assess cardiovascular and mortality risk, other novel measures to predict these outcomes in kidney disease are warranted4. Clinically, data from renal Doppler ultrasonography is usually utilized for assessing the patency of renal arteries during the work-up of secondary hypertension or decline in kidney function. Renal resistive index (RRI), calculated as [peak systolic velocity–end-diastolic velocity]/peak systolic velocity, is a non-invasive measure obtained from a renal Doppler study to investigate renal hemodynamics. In addition, RRI provides prognostic information relating to systemic vasculature and an elevated RRI is associated with adverse outcomes in hypertensive, diabetic, and elderly patients5-8.
Few studies have examined the utility of the RRI parameter in those with kidney disease. Previous reports suggest that RRI might serve as a surrogate measure for assessing renal arteriolosclerosis and interstitial damage9. Further, RRI is reported to predict the outcome of therapy in those with renal artery stenosis and transplant dysfunction10-12. A recent study reported that a high RRI is associated with kidney disease progression in those without renal artery stenosis5. However, whether it could serve as a prognostic marker to predict mortality risk in CKD is less studied. Hence, we hypothesized that an elevated RRI will be associated with higher mortality in CKD patients without renal artery stenosis. To test this hypothesis, we examined the associations between RRI and all-cause mortality among CKD patients who underwent renal Doppler ultrasound in our health care system. In addition, we studied if these associations are modified by age, gender, and stage of kidney disease. We also report the cause-specific death details for patients who resided in the State of Ohio for whom we had the cause of death details.
Methods
This analysis was conducted using a pre-existing Electronic Health Record (EHR)-based CKD registry at our institution. The development and validation of our EHR-based CKD registry at Cleveland Clinic has been described in detail elsewhere13.
Study population
Patients who met the following criteria from January 1, 2005 through October 31, 2011 were included: 1) had at least one face-to-face outpatient encounter with a Cleveland Clinic health care provider, 2) had two eGFR 15-59.9 ml/min/1.73 m2 that were at least 90 days apart (using the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] equation)14, 3) had RRI measured using Renal Doppler ultrasonography within 1 year, prior to second eGFR<60 ml/min/1.73 m2 ,or during study follow up. Patients with renal artery stenosis (60-99% stenosis or occlusion), solitary kidney, end stage renal disease needing dialysis, and renal transplant prior to CKD diagnosis were excluded.
Definitions and outcome measures
Variables
Demographic details were extracted from the EHR. Diabetes mellitus, hypertension, coronary artery disease, and other comorbidities were defined using pre-specified criteria and validated13. Relevant outpatient laboratory values were obtained from the EHR. Medication details were obtained from the EHR as “history of any prior use” at inception, except for the use of ACE/ARB and aldosterone antagonists, (which are current use at the time of renal Doppler study) and were validated by one of the investigators (CT).
Renal function
We applied the CKD-EPI equation to patients who had two outpatient serum creatinine levels between January 1, 2005 and October 31, 2011 in our health system to calculate eGFR. All creatinine measurements were performed by the modified kinetic Jaffe reaction, using a Hitachi D 2400 Modular Chemistry Analyzer thereafter (Roche Diagnostics, Indianapolis, IN) in our laboratory. CKD was defined according to current guidelines as follows: stage 3 CKD (eGFR 30-59 ml/min/1.73 m2) and stage 4 CKD (eGFR 15-29 ml/min/1.73 m2). We further categorized stage 3 into CKD stage 3a (eGFR 45-59 ml/min/1.73 m2) and stage 3b (eGFR 30-44 ml/min/1.73 m2).
Doppler Ultrasonography and Renal Resistive Index
The renal Doppler studies were performed within the accredited non-invasive vascular laboratory or the radiology department of Cleveland Clinic by certified ultrasound personnel using a low-frequency ultrasound transducer and a standardized diagnostic scanning protocol for image acquisition. Intraparenchymal renal vessels were assessed by color and spectral Doppler analysis. Using pulsed wave Doppler, blood flow velocities were measured from segmental arteries located in the upper, middle, and lower third of the kidney for RRI analysis. The peak systolic velocity (in centimeters per second) and the end-diastolic velocity (in centimeters per second) of each vessel were measured at the time of the study to calculate the RRI using the formula: RRI = [peak systolic velocity–end-diastolic velocity]/peak systolic velocity. The three values were averaged to obtain the mean RRI for each side of the kidney. The average value between the right and left kidney was the RRI used for analysis. Ultrasound parameters used in our laboratory for diagnosis of renal artery stenosis of 60-99% have been previously described and validated15.
Mortality
The primary outcome of interest, all-cause mortality, was ascertained from our EHR and linkage of our CKD registry with the Social Security Death Index. We censored patients on November 2011. For cause-specific death analysis, we linked data for the State of Ohio residents with the Ohio death index mortality data. The underlying cause of death was coded according to the International Classification of Diseases, Tenth Revision (ICD-10). We grouped the underlying causes of death as per the National Center for Health Statistics for each coding system, except for some changes as outlined below16. We classified deaths into the following 3 major categories: a) cardiovascular deaths, b) malignancy, and c) non-cardiovascular/ non-malignancy related deaths. We defined cardiovascular deaths as deaths due to diseases of the heart, essential hypertension, cerebrovascular disease, atherosclerosis, or other diseases of the circulatory system (ICD-10 codes I00–I78).
Statistical analysis
We compared baseline characteristics between patients with RRI <0.70 and those ≥0.70 using Chi-square and t-tests for categorical and continuous variables, respectively. We used a logistic regression model to evaluate the factors associated with having a RRI ≥0.70. Variables that were significantly different between patients with high and normal RRI were included in the logistic model. We also ran an additional logistic regression model to evaluate the factors associated with having a RRI ≥0.70 by including proteinuria in the multivariable model. We used a Kaplan-Meier curve and the log-rank test to evaluate the relationship between RRI ≥0.70 and mortality. We also used unadjusted and adjusted Cox proportional hazards models to evaluate this relationship while adjusting for age, gender, race, eGFR, diabetes, smoking, coronary artery disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, BMI group, systolic blood pressure, diastolic blood pressure, ACE/ARB use, aldosterone antagonist use, and statin use. We tested two way interactions between RRI ≥0.70 and age ≥65, gender, race, eGFR ≥30, BMI ≥30 kg/m2 and diabetes. Inception point for survival analysis was the date of the second eGFR<60 ml/min/1.73 m2 or date of the renal ultrasound; whichever was last.
Seven percent of patients were missing smoking status, 2% were missing BMI and 7% were missing blood pressure data. We used multiple imputations (SAS proc MI) with the Markov Chain Monte Carlo method and a single chain to impute 5 datasets. All logistic and Cox models were performed on each of the 5 imputed datasets, and parameter estimates were combined using SAS MIanalyze17. To evaluate the effect of using multiple imputations, we fit the logistic and cox models on complete cases only. We also fit a similar adjusted cox model while considering RRI as a continuous variable. We performed a sensitivity analysis among the subset of patients for whom we had proteinuria data. For patients who were residents of the State of Ohio, we used a poisson model to estimate and compare the age/gender adjusted cause specific mortality rates by high and low RRI. We also tabulated the leading causes of death by high and low RRI.
All analyses were conducted using Unix SAS version 9.2 (SAS Institute, Cary, NC), and graphs were created using R 3.0.1 (The R Foundation for Statistical Computing, Vienna, Austria). The CKD registry and this study were approved by the Cleveland Clinic Institutional Review Board.
Results
Baseline Patient Characteristics
Between January 1, 2005 and October 31, 2011, 3,025 patients included in our CKD registry had Renal Doppler ultrasound with RRI data. We excluded 1,063 patients because they did not meet the specified inclusion criteria (Supplemental Figure 1) and among those, 525 patients were excluded because of renal artery stenosis. The clinical characteristics of the 1,962 patients at baseline based on RRI ≥0.70 vs. <0.70 are presented in Table 1. Patients with RRI ≥0.70 were older, and had lower mean eGFR and diastolic blood pressure. Comorbid conditions such as diabetes, hyperlipidemia, coronary artery disease, and congestive heart failure were higher in those with RRI ≥0.70.
Table 1.
Patient characteristics based on renal resistive index (RRI ≥0.70 vs. <0.70)
| Factor | N | RRI<0.70 (N=635) |
RRI>0.70 (N=1327) |
p-value |
|---|---|---|---|---|
| Age, Mean ± SD | 1962 | 65.7±13.4 | 73.3±10.4 | <0.001 |
| Male sex , No. (%) | 1962 | 419(66.0) | 692(52.1) | <0.001 |
| African American , No. (%) | 1962 | 135(21.3) | 277(20.9) | 0.84 |
| BMI, Mean ± SD | 1913 | 29.1±6.1 | 29.1±6.1 | 0.88 |
| BMI group , No. (%) | 1962 | 0.82 | ||
| <18.5 kg/m2 | 8(1.3) | 11(0.83) | ||
| 18.5-24.9 kg/m2 | 146(23.0) | 306(23.1) | ||
| 25-29.9 kg/m2 | 230(36.2) | 506(38.1) | ||
| >30 kg/m2 | 234(36.9) | 472(35.6) | ||
| Missing | 17(2.7) | 32(2.4) | ||
| eGFR ml/min/1.73 m2, Mean ± SD | 1962 | 40.6±12.3 | 38.7±11.9 | <0.001 |
| CKD stages , No. (%) | 1962 | 0.007 | ||
| 45-59 | 261(41.1) | 449(33.8) | ||
| 30-44 | 228(35.9) | 527(39.7) | ||
| 15-29 | 146(23.0) | 351(26.5) | ||
| SBP (mm Hg), Mean ± SD | 1817 | 137.0±24.7 | 139.9±26.8 | 0.025 |
| DBP (mm Hg), Mean ± SD | 1816 | 79.2±14.5 | 71.3±12.7 | <0.001 |
| Right Kidney size (cm), Mean ± SD | 1952 | 10.9±1.3 | 11.0±2.9 | 0.38 |
| Left kidney size (cm), Mean ± SD | 1936 | 11.1±1.4 | 11.0±1.4 | 0.44 |
| RI mean, Mean ± SD | 1962 | 0.65±0.04 | 0.76±0.05 | <0.001 |
| Diabetes , No. (%) | 1962 | 148(23.3) | 486(36.6) | <0.001 |
| Malignancy , No. (%) | 1962 | 105(16.5) | 266(20.0) | 0.063 |
| Hypertension , No. (%) | 1962 | 589(92.8) | 1249(94.1) | 0.24 |
| Hyperlipidemia , No. (%) | 1962 | 538(84.7) | 1170(88.2) | 0.033 |
| Coronary artery disease , No. (%) | 1962 | 162(25.5) | 533(40.2) | <0.001 |
| Congestive heart failure , No. (%) | 1962 | 75(11.8) | 224(16.9) | 0.003 |
| Peripheral vascular disease, No. (%) | 1962 | 24(3.8) | 117(8.8) | <0.001 |
| Cerebrovascular disease, No. (%) | 1962 | 146(14.7) | 242(24.9) | <0.001 |
| ACE/ARB use, No. (%) | 1962 | 369(58.1) | 829(62.5) | 0.064 |
| Aldosterone Antagonists use, No. (%) | 1962 | 29(4.6) | 89(6.7) | 0.062 |
| Statins use , No. (%) | 1962 | 473(74.5) | 1044(78.7) | 0.038 |
| Beta Blocker use, No. (%) | 1962 | 509(80.2) | 1164(87.7) | <0.001 |
| Smoking , No. (%) | 1962 | <0.001 | ||
| No | 498(78.4) | 1149(86.6) | ||
| Yes | 77(12.1) | 102(7.7) | ||
| Missing | 60(9.4) | 76(5.7) | ||
| Proteinuria , No. (%) | 1193 | 163(41.3) | 330(41.3) | 0.98 |
t-test for continuousvariables, and Chi-square test for categorical variables
Factors Associated with RRI ≥0.70
In the multivariable logistic regression model, older age, female gender, higher systolic blood pressure, coronary artery disease, peripheral vascular disease, and use of beta blockers were associated with higher odds of having RRI ≥0.70 while a higher diastolic blood pressure was associated with lower odds of having RRI ≥0.70 (Table 2). The sensitivity analysis using only complete cases showed similar results. In the model that included those who had proteinuria data, proteinuria was not associated with RRI ≥0.70 (Supplemental Table 1).
Table 2.
Factors associated with RRI ≥0.70 in those with CKD
| Effect |
OR (95% CI)
*
N=1,962 |
Complete cases
OR (95% CI) N=1,714 |
|---|---|---|
| Age (per 10 year increase) | 1.48 (1.33, 1.64) | 1.50 (1.34, 1.68) |
| Female | 1.71 (1.37, 2.14) | 1.67 (1.31, 2.12) |
| eGFR (per 5 ml/min/1.73 m2) increase | 0.96 (0.92, 1.01) | 0.97 (0.92, 1.02) |
| Systolic blood pressure (per 1 mm Hg increase) |
1.03 (1.02, 1.03) | 1.03 (1.02, 1.03) |
| Diastolic blood pressure (per 1 mm Hg increase) |
0.94 (0.93, 0.95) | 0.94 (0.93, 0.95) |
| Diabetes | 1.85 (1.43, 2.39) | 1.93 (1.47, 2.54) |
| Coronary artery disease | 1.29 (1.004, 1.66) | 1.33 (1.02, 1.75) |
| Congestive heart failure | 1.29 (0.92, 1.81) | 1.36 (0.94, 1.95) |
| Hyperlipidemia | 0.90 (0.65, 1.25) | 0.86 (0.60, 1.22) |
| Peripheral vascular disease | 1.75 (1.06, 2.87) | 1.83 (1.09, 3.08) |
| Smoking | 0.71 (0.49, 1.01) | 0.71 (0.49, 1.02) |
| Beta blocker use | 1.40 (1.04, 1.88) | 1.33 (0.97, 1.82) |
Odds ratios shown were pooled using MIanalyze from 5 datasets created using multiple imputation
RRI and all-cause mortality
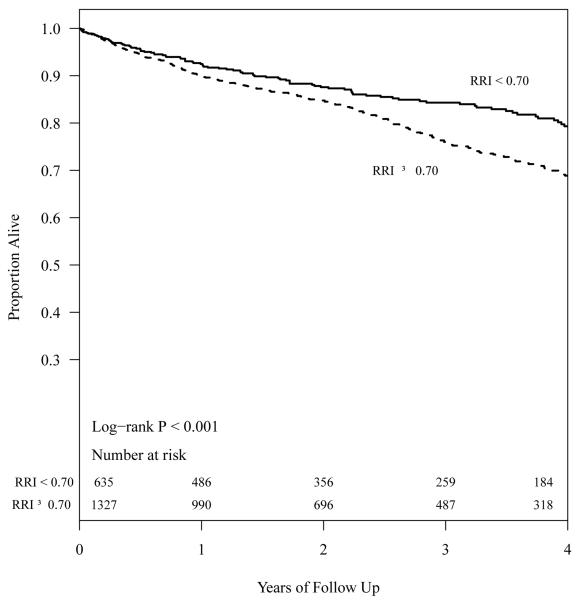
With a median follow up of 2.2 years, there were 428 deaths. The Kaplan-Meier analysis (Figure 1) showed significant differences in all-cause mortality for CKD patients with RRI ≥0.70 and RRI <0.70. After adjusting for age, gender, race, eGFR, systolic blood pressure, diastolic blood pressure, diabetes, coronary artery disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, smoking, BMI group, ACE/ARB, aldosterone antagonists, and statins, RRI ≥0.70 was associated with significantly higher hazards of mortality (HR 1.29, 95% CI, 1.02 - 1.65, P< 0.05). In a similarly adjusted model, each 0.05 unit increase in RRI was not significantly associated with mortality but the effect estimate pointed to a higher risk (HR 1.08, 95% CI, 0.99 - 1.17, P = 0.08). The sensitivity analysis with complete cases yielded very similar results except that each 0.05 unit increase in RRI was also significantly associated with higher hazard of mortality (Table 3).
Figure 1.
Survival of CKD patients with RRI ≥0.70 and <0.70
Table 3.
Associations of RRI with mortalityin those with CKD
| Factor | Unadjusted HR (95%CI) N=1,962 |
Mutliple imputation Adjusted HR (95%CI)* N=1,962 |
Complete cases Adjusted HR (95%CI)* N=1,682 |
|---|---|---|---|
| Model 1 | |||
| RRI≥0.70 vs. <0.70 | 1.56 (1.25, 1.94) | 1.29 (1.02, 1.65) | 1.42 (1.08, 1.88) |
| Model 2 | |||
| Per 0.05 unit higher RRI |
1.19 (1.11, 1.28) | 1.08 (0.99, 1.17) | 1.12 (1.02, 1.23) |
Adjusted for age, gender, race, eGFR, diabetes, coronary artery disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, smoking, BMI group, systolic blood pressure, diastolic blood pressure, ACE/ARB, aldosterone antagonists, and statins
Interactions
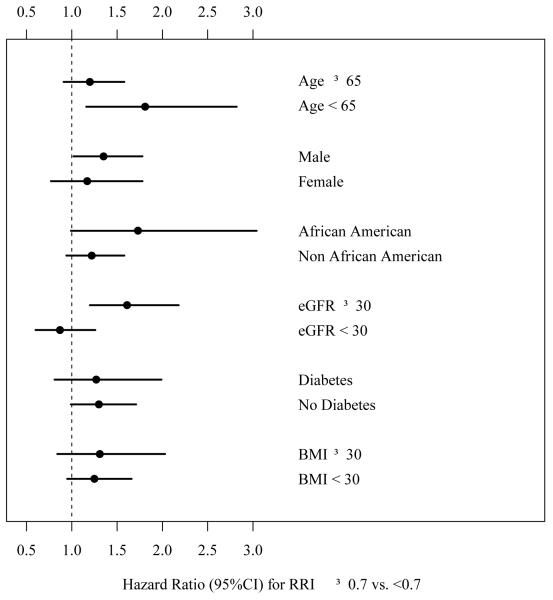
The interaction between RRI ≥0.70 and age > 65 vs. age < 65 was significant (P = 0.01) suggesting that the association between RRI ≥0.70 and mortality was stronger among younger patients, and less so among older ones (Figure 2). There was also an interaction between RRI ≥0.70 and eGFR ≥ 30 (P = 0.01) suggesting that the association between RRI ≥0.70 and mortality was significant only among those with eGFR ≥30 ml/min/1.73 m2 but not among those with eGFR <30 ml/min/1.73 m2. We did not find any significant interaction between RRI ≥0.70 and gender, race, diabetes or BMI ≥30 kg/m2.
Figure 2.
Associations between RRI and death based on age, gender, race, kidney function and BMI
Sensitivity analysis among patients with proteinuria data
In this cohort, 1,193 patients had proteinuria data and of those, 230 died during follow up. In this subset, each 0.05 unit increase in RRI was associated with a 13% higher risk of death (95% CI:1.01, 1.25; P = 0.03). Even though there was a trend toward significance, RRI ≥0.70 was not significantly associated with mortality in the adjusted model (HR 1.36, 95% CI, 0.97 - 1.90).
Causes of death
Overall, 1633/1692 (83%) were Ohio residents. 347 Ohio residents died during study follow up and 334/347 (96%) were found in the Ohio death index. We tabulated the age and gender adjusted rates of death per 1,000 years of follow up for the main causes of death for those with RRI ≥0.70 and <0.70. As noted in table 4, non-cardiovascular/non-malignancy related deaths were higher in those with RRI ≥0.70. Supplemental Table 2 shows the main causes of death for high and low RRI.
Table 4.
Age and gender adjusted mortality rates per 1,000 years of follow up in those with RRI ≥0.70 and <0.70(Ohio residents only)
| Cause of death | Age and gender adjusted mortality per 1,000 years RRI < 0.70 |
Age and gender adjusted mortality per 1,000 years RRI ≥0.70 |
P-value* |
|---|---|---|---|
| Cardiovascular | 25.8 (18.3,36.2) | 33.0 (26.6,40.8) | 0.22 |
| Malignancy | 15.3 (9.8,24.0) | 13.3 (9.6,18.5) | 0.62 |
| Non-cardiovascular and non- malignancy related deaths |
17.5 (11.6,26.6) | 33.3 (27.0,41.1) | 0.008 |
Age and gender adjusted Poisson model
Discussion
Renal resistive index data is readily available from renal Doppler studies performed for patients with and without kidney disease. In this clinical population, over 2/3rd of CKD patients (most of them with hypertension) without renal artery stenosis had RRI ≥0.70. Several factors such as older age, female gender and multiple comorbid conditions were associated with higher RRI. This analysis also showed that RRI ≥0.70 is significantly associated with increased all-cause mortality even after adjusting for various confounding variables. Furthermore, the association between RRI ≥0.70 and death was more pronounced among those who were younger and in those with stage 3 but not stage 4 CKD. Deaths due to non-cardiovascular/non-malignancy diseases were higher in those with RRI ≥0.70.
Prior studies have examined the association of RRI with kidney function decline in those with pre-existing kidney disease9,18. In other disease states such as diabetes and hypertension, RRI has been associated with cardiovascular events and death5,19-21. Studies examining the associations between RRI and death are limited, particularly in those with CKD. In newly diagnosed CKD patients with renal artery stenosis, Radermacher et al (n= 162) found that RRI > 0.80 was associated with an increased risk for renal function decline, progression to ESRD, and death22. Doi et al (n=426) demonstrated that a high RRI was independently associated with worse composite outcomes (mortality, cardiovascular events and renal events) in hypertensive patients5. Moreover, the association was stronger in those with an eGFR <60 mL/min/1.73 m2 and high RRI (vs. eGFR >60 mL/min/1.73 m2 and low RRI). Their study did not directly compare those with high and low RRI in the CKD population and our analysis provides prognostic significance of RRI in CKD by comparing these groups directly, along with providing cause-specific death details. It is also important to note that these associations were more pronounced in younger patients and those with earlier stages of CKD which have not been reported previously. Even though the clinical significance of such associations is not completely understood, interventions targeting this pathobiology through various measures such as appropriate blood pressure control and use of ACEi/ARBs in hypertensive kidney disease with high RRI can be further explored in prospective studies. Particularly, the observed associations are stronger in younger patients suggesting a possibility for earlier intervention.
Even though key questions such as what RRI represents and why a high value associates with worse outcomes have not been completely elucidated, several studies support the notion that vascular stiffness is the most likely factor23-25. RRI represents both renal arterial resistance and compliance. Bige et al demonstrated that a high RRI was associated with severe arteriosclerosis, which may explain its association with cardiovascular risk9. Furthermore, in patients with essential hypertension, RRI correlated with early signs of target organ damage such as carotid intimal-medial thickness and left ventricular hypertrophy7. Also, in kidney transplant patients, RRI of the allograft correlated more with the age of the recipient rather than that of the donor26, suggesting that extra-renal factors such as arterial stiffness contribute to RRI. These cardiovascular indices have long been established to correlate with worse cardiovascular outcomes, including mortality. In the cause-specific death analysis, we noted slightly higher cardiovascular deaths among those with RRI ≥0.70 but this was not statistically significant. On the other hand, we noted a higher proportion of non-cardiovascular/non-malignancy related deaths. Supplemental Table 2 shows the actual causes of death within this category and could also be a topic of future studies.
Similar to others, we found a positive correlation between RRI and older age. As shown in histological studies of the kidney, aging correlates with increased interstitial fibrosis and small vessel changes in the kidney9,27. Diastolic blood pressure also tends to decrease with age as vessels become less compliant. In this analysis, among those with RRI ≥0.70, diastolic blood pressure was noted to be lower and associate with RRI, suggesting the age-related effect on vascular compliance. Diabetics, at higher risk for atherosclerotic disease, also had higher odds of having a RRI ≥0.70. This corroborates the findings of Ohta et al, and the observed associations could possibly be explained by intrarenal atherosclerosis and arteriosclerosis25. We have adjusted for these parameters (age, diastolic blood pressure, and hyperlipidemia) in the models to examine the independent associations of RRI with death. We also found that women have higher odds of having an elevated RRI in our cohort as shown in previous studies5. Even though the exact reasons behind these associations are unclear, hormonal influences may play a role.
The strengths of our study include its large sample size by including stage 3 and 4 CKD patients belonging to various races thereby enhancing the generalizability of our results. However, as an observational study, it has some limitations. We included patients followed in our health system who underwent renal Doppler ultrasound for an indication (subjecting to selection bias) such as uncontrolled hypertension or worsening renal function, which places them at higher risk for death. We attempted to address this by adjusting for several confounding variables in the multivariable Cox regression analysis. Furthermore, the observational nature of this study limits causal inferences. It is a single-center study limiting its potential for external validity, but such associations in a large CKD cohort have not been reported previously. We had missing data for some variables, but performed multiple imputations and complete case analysis, methods which have been suggested to address the missing-ness in studies of kidney disease. The results remained consistent across these analyses. It is also important to point out that the comparison of high RRI among the published reports is difficult given the different cut-offs used by these studies. We chose the RRI value of 0.70 as the cut-off, but analysis by considering RRI as a continuous measure yielded similar results. We also lacked data about underlying atherosclerotic disease in the setting of <60% renal artery stenosis. Even though prior studies have shown associations between proteinuria and RRI, we didn’t find such associations which could be due to the lack of proteinuria data for about 40% of our study population (Table S1).
Perspectives
RRI ≥0.70 is associated with higher mortality in hypertensive CKD patients without clinically significant renal artery stenosis after accounting for other significant risk factors. Deaths due to non-cardiovascular/non-malignancy reasons account for the higher mortality burden in those with higher RRI. Its evaluation may allow for early identification of those who are at risk, thereby potentially preventing or delaying adverse outcomes in CKD. Our results highlight the need for further prospective studies to provide conclusive evidence to guide clinical practice.
Supplementary Material
Novelty and significance.
What is new?
Elevated renal resistive index (RRI) is associated with higher mortality in those with CKD (and patent renal arteries) independent of comorbidities including blood pressure and kidney function.
These associations are pronounced in those with stage 3 CKD and in those who are younger than 65 years old
What is relevant?
Often, the renal Doppler ultrasound data is reviewed by clinicians to know if patients had renal artery stenosis or not. This study results suggest that the RRI data (reflecting vascular stiffness) reported in the renal Doppler results would help clinicians identify high risk patients.
Summary
Renal resistive index data could help us to assess the underlying risk of death in those with kidney disease.
Acknowledgements
The authors wish to thank Welf Saupe, Vicky Konig and John Sharp of Cleveland Clinic who helped in data extraction during the development of the registry.
Grant support:
No research funding was obtained for the purposes of this manuscript. SDN is supported by R01DK101500; JVN: DK094112 and SEJ: 1K23DK091363. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The creation of the CCF CKD registry was funded by an unrestricted grant from Amgen, Inc to the Department of Nephrology and Hypertension Research and Education Fund.
Footnotes
The authors have no relevant financial interest in the study.
Parts of this manuscript was presented as a poster at the annual American Society of Nephrology meeting held on November 2014 at Philadelphia, PA.
Disclosures
NONE
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Chronic Kidney Disease Prognosis Consortium. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doi Y, Iwashima Y, Yoshihara F, Kamide K, Hayashi S, Kubota Y, Nakamura S, Horio T, Kawano Y. Renal resistive index and cardiovascular and renal outcomes in essential hypertension. Hypertension. 2012;60:770–777. doi: 10.1161/HYPERTENSIONAHA.112.196717. [DOI] [PubMed] [Google Scholar]
- 6.Tedesco MA, Natale F, Mocerino R, Tassinario G, Calabro R. Renal resistive index and cardiovascular organ damage in a large population of hypertensive patients. J Hum Hypertens. 2007;21:291–296. doi: 10.1038/sj.jhh.1002145. [DOI] [PubMed] [Google Scholar]
- 7.Pontremoli R, Viazzi F, Martinoli C, Ravera M, Nicolella C, Berruti V, Leoncini G, Ruello N, Zagami P, Bezante GP, Derchi LE, Deferrari G. Increased renal resistive index in patients with essential hypertension: a marker of target organ damage. Nephrol Dial Transplant. 1999;14:360–365. doi: 10.1093/ndt/14.2.360. [DOI] [PubMed] [Google Scholar]
- 8.Pearce JD, Craven TE, Edwards MS, Corriere MA, Crutchley TA, Fleming SH, Hansen KJ. Associations between renal duplex parameters and adverse cardiovascular events in the elderly: a prospective cohort study. Am J Kidney Dis. 2010;55:281–290. doi: 10.1053/j.ajkd.2009.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bige N, Levy PP, Callard P, Faintuch JM, Chigot V, Jousselin V, Ronco P, Boffa JJ. Renal arterial resistive index is associated with severe histological changes and poor renal outcome during chronic kidney disease. BMC Nephrol. 2012;13:139-2369–13-139. doi: 10.1186/1471-2369-13-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radermacher J, Chavan A, Bleck J, Vitzthum A, Stoess B, Gebel MJ, Galanski M, Koch KM, Haller H. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med. 2001;344:410–417. doi: 10.1056/NEJM200102083440603. [DOI] [PubMed] [Google Scholar]
- 11.Radermacher J, Haller H. The role of the intrarenal resistive index in kidney transplantation. N Engl J Med. 2013;369:1853–1855. doi: 10.1056/NEJMe1312281. [DOI] [PubMed] [Google Scholar]
- 12.Radermacher J, Mengel M, Ellis S, Stuht S, Hiss M, Schwarz A, Eisenberger U, Burg M, Luft FC, Gwinner W, Haller H. The renal arterial resistance index and renal allograft survival. N Engl J Med. 2003;349:115–124. doi: 10.1056/NEJMoa022602. [DOI] [PubMed] [Google Scholar]
- 13.Navaneethan SD, Jolly SE, Schold JD, Arrigain S, Saupe W, Sharp J, Lyons J, Simon JF, Schreiber MJ, Jr, Jain A, Nally JV., Jr Development and validation of an electronic health record-based chronic kidney disease registry. Clin J Am Soc Nephrol. 2011;6:40–49. doi: 10.2215/CJN.04230510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olin JW, Piedmonte MR, Young JR, DeAnna S, Grubb M, Childs MB. The utility of duplex ultrasound scanning of the renal arteries for diagnosing significant renal artery stenosis. Ann Intern Med. 1995;122:833–838. doi: 10.7326/0003-4819-122-11-199506010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Center for Disease Control and Prevention [Accessed September 15, 2014];Deaths: Preliminary data for 2011. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_06.pdf.
- 17.Montez-Rath ME, Winkelmayer WC, Desai M. Addressing missing data in clinical studies of kidney diseases. Clin J Am Soc Nephrol. 2014;9:1328–1335. doi: 10.2215/CJN.10141013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nosadini R, Velussi M, Brocco E, Abaterusso C, Carraro A, Piarulli F, Morgia G, Satta A, Faedda R, Abhyankar A, Luthman H, Tonolo G. Increased renal arterial resistance predicts the course of renal function in type 2 diabetes with microalbuminuria. Diabetes. 2006;55:234–239. [PubMed] [Google Scholar]
- 19.Heine GH, Rogacev KS, Fliser D, Krumme B. Renal resistive index and cardiovascular and renal outcomes in essential hypertension. Hypertension. 2013;61:e22. doi: 10.1161/HYPERTENSIONAHA.111.00655. [DOI] [PubMed] [Google Scholar]
- 20.Hamano K, Nitta A, Ohtake T, Kobayashi S. Associations of renal vascular resistance with albuminuria and other macroangiopathy in type 2 diabetic patients. Diabetes Care. 2008;31:1853–1857. doi: 10.2337/dc08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ennezat PV, Marechaux S, Six-Carpentier M, Pincon C, Sediri I, Delsart P, Gras M, Mounier-Vehier C, Gautier C, Montaigne D, Jude B, Asseman P, Le Jemtel TH. Renal resistance index and its prognostic significance in patients with heart failure with preserved ejection fraction. Nephrol Dial Transplant. 2011;26:3908–3913. doi: 10.1093/ndt/gfr116. [DOI] [PubMed] [Google Scholar]
- 22.Radermacher J, Ellis S, Haller H. Renal resistance index and progression of renal disease. Hypertension. 2002;39:699–703. doi: 10.1161/hy0202.103782. [DOI] [PubMed] [Google Scholar]
- 23.Geraci G, Mule G, Geraci C, Mogavero M, D’Ignoto F, Morreale M, Foraci AC, Cottone S. Association of renal resistive index with aortic pulse wave velocity in hypertensive patients. Eur J Prev Cardiol. 2015;22:415–422. doi: 10.1177/2047487314524683. [DOI] [PubMed] [Google Scholar]
- 24.Kawai T, Kamide K, Onishi M, Yamamoto-Hanasaki H, Baba Y, Hongyo K, Shimaoka I, Tatara Y, Takeya Y, Ohishi M, Rakugi H. Usefulness of the resistive index in renal Doppler ultrasonography as an indicator of vascular damage in patients with risks of atherosclerosis. Nephrol Dial Transplant. 2011;26:3256–3262. doi: 10.1093/ndt/gfr054. [DOI] [PubMed] [Google Scholar]
- 25.Ohta Y, Fujii K, Arima H, Matsumura K, Tsuchihashi T, Tokumoto M, Tsuruya K, Kanai H, Iwase M, Hirakata H, Iida M. Increased renal resistive index in atherosclerosis and diabetic nephropathy assessed by Doppler sonography. J Hypertens. 2005;23:1905–1911. doi: 10.1097/01.hjh.0000181323.44162.01. [DOI] [PubMed] [Google Scholar]
- 26.Krumme B, Grotz W, Kirste G, Schollmeyer P, Rump LC. Determinants of intrarenal Doppler indices in stable renal allografts. J Am Soc Nephrol. 1997;8:813–816. doi: 10.1681/ASN.V85813. [DOI] [PubMed] [Google Scholar]
- 27.Ikee R, Kobayashi S, Hemmi N, Imakiire T, Kikuchi Y, Moriya H, Suzuki S, Miura S. Correlation between the resistive index by Doppler ultrasound and kidney function and histology. Am J Kidney Dis. 2005;46:603–609. doi: 10.1053/j.ajkd.2005.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.