Abstract
Background
Natriuretic peptides (NP) are hormones with natriuretic, diuretic, and vasodilatory effects. Experimental NP deficiency promotes salt-sensitive hypertension and cardiac hypertrophy, conditions that are more common among black individuals. We hypothesized that black individuals have lower N-terminal pro B-type natriuretic peptide (Nt-proBNP) levels than white and Hispanic individuals.
Objectives
To assess whether Nt-proBNP levels differ according to race/ethnicity.
Methods
We examined plasma Nt-proBNP levels according to race/ethnicity in 3,148 individuals (51% black, 31% white, 18% Hispanic) free of prevalent cardiovascular disease in the Dallas Heart Study. Nt-proBNP values in the bottom sex-specific quartile were defined as low. Multivariable linear and logistic regression analyses were performed adjusting for clinical covariates and MRI measurements of cardiac structure and function.
Results
Hypertension was present in 41%, 25%, and 16% of black, white, and Hispanic individuals, respectively. Unadjusted Nt-proBNP levels were lowest in blacks (median 24 pg/ml; IQR 10, 52) as compared with Hispanic (30 pg/ml; IQR 14, 59) and white individuals (32 pg/ml; IQR 16, 62), P < 0.0001. In multivariable-adjusted models, black individuals still had significantly lower Nt-proBNP levels (-39% [95%CI -46%, -31%]; P < 0.0001) and greater odds of having low Nt-proBNP (OR: 2.46, [95% CI 1.86, 3.26]), compared with whites. In contrast, Nt-proBNP levels did not significantly differ between Hispanic and white individuals (P = 0.28). The finding of lower Nt-proBNP levels in blacks was similar when analyses were restricted to healthy participants without cardiovascular risk factors.
Conclusions
In this multi-ethnic cohort, Nt-proBNP levels differ substantially according to race/ethnicity. Despite a higher prevalence of hypertension, blacks had significantly lower NP levels than white and Hispanic individuals. A relative NP “deficiency” among black individuals may lead to greater susceptibility to salt retention and hypertension.
Keywords: Natriuretic peptides, race, hypertension, deficiency
Introduction
Natriuretic peptides are hormones with natriuretic, diuretic, and vasodilatory effects (1). They are produced in response to increased cardiac wall stress, as seen in conditions such as hypertension and heart failure (2-10). Data from animal studies and human genetic studies suggest that impaired natriuretic peptide production can promote salt-sensitive hypertension and cardiac hypertrophy (11-15).
It has been proposed that conditions such as obesity may lead to a “relative deficiency” in natriuretic peptides, increasing susceptibility to salt retention and related disorders (1). Such a deficiency may be characterized by low or undetectable concentrations of natriuretic peptides in healthy individuals, or lower than expected concentrations in individuals with conditions (such as hypertension) in which higher levels are normally found. The impact of race and ethnicity on the natriuretic peptide axis is not well studied, but is important to understand because hypertension and cardiac hypertrophy disproportionately affect certain racial/ethnic groups. A few reports suggest that black individuals have lower natriuretic peptide levels than whites (16-20), but these studies were limited in that they were performed in clinically referred populations and/or were not designed to specifically focus on race/ethnic differences in natriuretic peptide levels and thus did not fully account for potential confounding factors.
Therefore, we examined the association on race/ethnicity and natriuretic peptides in the Dallas Heart Study, a large multi-ethnic cohort with extensive characterization of cardiovascular risk factors and cardiac structure and function. We hypothesized that black individuals have lower natriuretic peptide levels than white and Hispanic individuals.
Methods
Study population
The Dallas Heart Study is a multi-ethnic, probability-based, population cohort study of Dallas County adults, with a deliberate oversampling of black individuals for which detailed methods have been previously reported (21). In brief, between 2000 and 2002, a total of 6,101 individuals were enrolled. N-terminal pro B-type natriuretic peptide (Nt-proBNP) was measured in 3,527 individuals, as described below. Of these, 269 had self-reported prevalent cardiovascular disease, defined as myocardial infarction, heart failure, or stroke, and were excluded from this analysis. Individuals self-reporting race as “other” were also excluded (n=l 10), yielding 3,148 individuals in the final analysis. The protocol was approved by the institutional review board of the University of Texas Southwestern Medical Center and participants provided written informed consent.
Race/ethnicity and other covariates
Race/ethnicity was self-reported as black, Hispanic, or white. Conventional clinical definitions for hypertension and diabetes mellitus were used (22). Education and income were ascertained by self-report. Heart rate and blood pressure were measured as previously described (22). Body mass index was calculated from weight (kilograms)/height (meters)2. Lean and fat mass were determined by dual-energy x-ray absorptiometry (23). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as fasting insulin (μIU/ml) × fasting glucose (mmol/l)/22.5 (24). Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation (25) and urine microalbumin was measured as previously reported (26).
Cardiac MRI
Left ventricular (LV) mass and ejection fraction (EF) were determined from cardiac magnetic resonance imaging in a subset of participants (n=2,549), as previously described (27). Briefly, short-axis, breath-hold, electrocardiographic-gated cine magnetic resonance images were obtained on 1.5-T MRI systems (Phillips Medical) and analyzed offline to calculate LV mass and ejection fraction. LV mass was indexed to body surface area.
Nt-proBNP
Venous blood was collected in standard EDTA tubes. Samples were maintained at 4°C for ≤ 4 hours during which they were centrifuged (1,430g for 15 minutes). Plasma was then removed and frozen at -70°C until Nt-proBNP assays were performed using the Elecsys proBNP platform (Roche Diagnostics, Indianapolis, IN) (28). The lower limit of detection was 5 pg/ml. The coefficient of variation was 3.3% at a concentration of 282 pg/ml and 3.0% at concentration of 6,012 pg/ml. “Low” Nt-proBNP values were defined a priori as values at or below the sex-specific 25th percentile (≤ 7.3 pg/ml for men, ≤ 19.4 pg/ml for women).
Statistical analyses
Dallas Heart Study participants were categorized according to self-reported race/ethnicity. Summary statistics for covariates were calculated as percentages and median (25th,75th percentiles) for categorical and continuous data, respectively. Nt-proBNP levels were compared between race/ethnic groups by Kruskal-Wallis or Chi-squared tests, as appropriate. Sequential multivariable adjusted linear regression models were used to assess the associations between race/ethnicity (independent) and natural log transformed Nt-proBNP levels (dependent). The multiplicative effect (percent difference) on Nt-proBNP levels was estimated by the formula (eβ-l)*100, where β is the coefficient from linear regression models. Multivariable logistic regression models were used to calculate the adjusted odds of low Nt-proBNP levels by race. Based on prior reports, we selected the following variables for inclusion in adjusted models: age, sex, heart rate, anti-hypertensive medication use, systolic blood pressure, diabetes mellitus, body mass index, eGFR, urine microalbumin, education, income, LV mass, and LVEF. Multivariable models were repeated in the following sensitivity analyses: a) restricting the study population to participants without diabetes to allow adjustment for HOMA-IR, b) replacing BMI with lean and fat mass and c) restricting to healthy participants, defined as individuals with BMI 18-25 kg/m2, without hypertension, diabetes mellitus, insulin resistance, chronic kidney disease, or left ventricular hypertrophy (n=388). All statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC). For all statistical tests, 2-sided P values < 0.05 were considered significant without adjustment for multiple testing.
Results
Study sample
The study population was 51% black, 31% white, and 18% Hispanic (Table 1). Compared with white individuals, blacks and Hispanics were younger and more likely to be female. Blacks had the highest prevalence of hypertension (41%) compared with white (25%), and Hispanic (16%) individuals, P < 0.0001. LV mass index was also higher among black (median 83 g/m2; IQR 72, 96) compared with white (median 77 g/m2; IQR 68, 89) and Hispanic (median 78 g/m2; IQR 69,88) individuals, P < 0.0001.
Table 1.
Baseline characteristics of Dallas Heart Study participants without prevalent cardiovascular disease.
| White | Black | Hispanic | |
|---|---|---|---|
| N=969 | N=1,606 | N=573 | |
| Age, years | 45 (37, 52) | 43 (36, 52) | 38 (33, 46) |
| Female, % | 53 | 58 | 58 |
| Hypertension, % | 25 | 41 | 16 |
| Anti-hypertensive med, % | 16 | 21 | 9 |
| Diabetes mellitus, % | 6 | 13 | 11 |
| Heart rate, bpm | 73 (66, 80) | 76 (69, 83) | 72 (66, 79) |
| Systolic BP, mmHg | 118 (110, 127) | 126 (115, 139) | 116 (107, 126) |
| Diastolic BP, mmHg | 75 (70, 81) | 80 (74, 86) | 74 (68, 80) |
| BMI, kg/m2 | 27.3 (23.9, 31.5) | 29.2 (24.8, 34.2) | 28.5 (25.4, 32.3) |
| Lean mass, kg | 54.3 (44.4,64.2) | 56.2 (48.2,65.7) | 49.8 (41.9,60.0) |
| Fat mass, kg | 24.8 (19.0,32.3) | 27.2 (18.3,36.5) | 24.5 (19.1,30.8) |
| Fasting glucose, mg/dL | 91 (84, 99) | 92 (84, 103) | 95 (88, 103) |
| HOMA-IR | 2.28 (1.33, 4.12) | 3.23 (1.79, 5.53) | 3.34 (1.82, 5.48) |
| eGFR, ml/min/1.73m2 | 90 (80, 100) | 102 (88, 117) | 103 (92, 120) |
| Urine microalbumin, mg | 0.30 (0.20, 0.50) | 0.40 (0.20, 1.00) | 0.40 (0.20, 0.70) |
| LV mass index, g/m2 | 77 (68, 89) | 83 (72, 96) | 78 (69, 88) |
| LV ejection fraction, % | 72 (68, 77) | 73 (68, 77) | 75 (70, 79) |
| Nt-proBNP, pg/ml | 32 (16, 61) | 24 (10, 52) | 30 (14, 59) |
| Low Nt-proBNP, % | 17 | 32 | 19 |
Results presented are median (25th, 75th percentiles) for continuous or percentage for categorical variables. Low Nt-proBNP defined as the lowest sex-specific quartile (≤ 7.3 pg/ml for men; ≤ 19.4 pg/ml for women). Total n for HOMA-IR = 2,710. Total n for LV mass index and LVEF = 2,549. Med = medication, BPM = beats per minute, BP = blood pressure, BMI = body mass index, HOMA-IR = homeostatic model assessment for insulin resistance, eGFR = estimated glomerular filtration rate, LV = left ventricular. P < 0.0001 for all between group comparisons, except female (P = 0.017).
Despite the higher prevalence of hypertension and higher LV mass among black individuals, unadjusted Nt-proBNP levels were significantly lower in black individuals (median 24 pg/ml; IQR 10, 52) as compared with white (32 pg/ml; IQR 16, 62), and Hispanic (30 pg/ml; IQR 14, 59) individuals, P < 0.0001. Low Nt-proBNP levels (using the pre-specified definition) were observed nearly twice as often among black individuals (32%) than white (17%) or Hispanic (19%) individuals, P < 0.0001 (Table 1).
Race/ethnic differences in plasma Nt-proBNP levels: Multivariable analyses
In multivariable linear regression analyses, adjusted log Nt-proBNP levels were 39% lower in blacks (95% confidence interval [CI], -46% to -31%; P < 0.0001) compared with whites (Table 2). The addition of HOMA-IR to multivariable models did not attenuate the association between black race and Nt-proBNP levels (Table 2). In contrast, Nt-proBNP levels did not significantly differ between Hispanic and white individuals (Table 2). Results were similar in models substituting lean and fat mass for body mass index (data not shown).
Table 2.
Association between race/ethnicity and plasma Nt-proBNP levels in multivariable linear regression models.
| Model | White | Black | P | Hispanic | P |
|---|---|---|---|---|---|
| Age, sex | reference | -0.283 (-0.373,-0.192) |
< 0.0001 | 0.025 (-0.095,0.144) |
0.69 |
|
| |||||
| Age, sex, HR, anti-HTN med, SBP, DM, BMI, eGFR, microalbumin | reference | -0.390 (-0.483,-0.296) |
< 0.0001 | 0.010 (-0.108,0.127) |
0.87 |
|
| |||||
| Age, sex, HR, anti-HTN med, SBP, DM, BMI, eGFR, microalbumin, education, income | reference | -0.464 (-0.570,-0.359) |
< 0.0001 | -0.102 (-0.239,0.035) |
0.14 |
|
| |||||
| Age, sex, HR, anti-HTN med, SBP, DM, BMI, eGFR, microalbumin, education, income, LV mass index, LVEF | reference | -0.492 (-0.608,-0.376) |
< 0.0001 | -0.081 (-0.230,0.067) |
0.28 |
|
| |||||
| Age, sex, HR, anti-HTN med, SBP, HOMA-IR, BMI, eGFR, microalbumin, education, income, LV mass index, LVEF | reference | -0.502 (-0.624,-0.380) |
< 0.0001 | -0.084 (-0.240,0.072) |
0.29 |
Multivariable linear regression models with natural log transformed Nt-proBNP as the dependent variable and race/ethnicity (reference white) as the primary independent variable. Results shown are β-coefficients (95%CI). Model including HOMA-IR restricted to participants without diabetes mellitus. HR = heart rate, HTN = hypertension, SBP = systolic blood pressure, DM = diabetes mellitus, BMI = body mass index, eGFR = estimated glomerular filtration rate, LV = left ventricular, LVEF = left ventricular ejection fraction.
A similar pattern was observed in analyses restricted to healthy individuals with normal cardiac structure and function. In this healthy subsample, Nt-proBNP levels were 41% lower in black individuals compared with white individuals (95% CI, -55% to -22%; P = 0.0002). There was no difference in Nt-proBNP levels between Hispanic and white individuals in this subsample, (-7%, 95% CI -34% to 30%; P = 0.50).
Multivariable logistic regression was performed to examine factors associated with “low” Nt-proBNP levels. Compared with whites, black individuals were more than twice as likely to have low Nt-proBNP levels (multivariable-adjusted OR: 2.46, 95% CI 1.86 to 3.26; P < 0.0001), which was consistent across multiple subgroups (Figure 1). The odds of low Nt-proBNP did not significantly differ between Hispanic and white individuals, (OR 1.00, 95% CI 0.69 to 1.45; P = 0.995).
Figure 1. Odds of low Nt-proBNP levels in black compared with white participants in the Dallas Heart Study, overall and in selected subgroups.
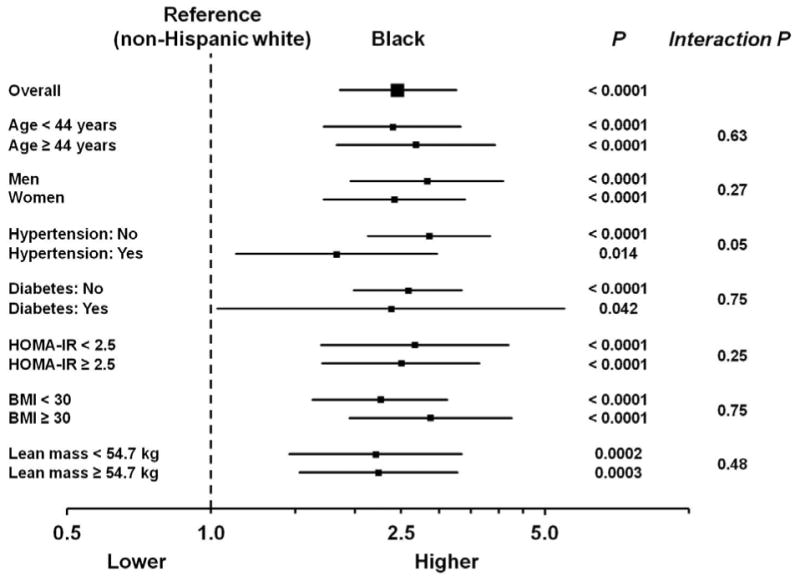
Odds ratios (95% CI) for low Nt-proBNP (defined as the lowest sex-specific quartile: ≤ 7.3 pg/ml for men; ≤ 19.4 pg/ml for women) for black compared with white individuals. Models are adjusted for race/ethnicity, age, sex, heart rate, anti-hypertensive medication use, systolic blood pressure, diabetes mellitus, body mass index, estimated glomerular filtration rate, urine microalbumin, education, and income. Analyses including HOMA-IR are restricted to individuals without diabetes mellitus. HOMA-IR = homeostatic assessment model for insulin resistance. For lean mass subgroup analyses, models included lean and fat mass in place of BMI. BMI = body mass index
Discussion
In this large, population-based, multi-ethnic study, we found that black individuals have considerably lower plasma natriuretic peptide levels than white individuals. The lower natriuretic peptide levels were specific to blacks, as differences were not consistently observed between other racial/ethnic groups. The lower levels in blacks were not explained by confounding from conditions that typically alter natriuretic peptide levels, such as hypertension, increased left ventricular mass, obesity, and older age. These findings support the hypothesis that black race is associated with a “relative deficiency” in natriuretic peptides. In the context of experimental evidence that natriuretic peptide deficiency is associated with salt-sensitive hypertension and left ventricular hypertrophy, our findings raise the possibility that a blunted natriuretic peptide system might contribute to the increased susceptibility to hypertension and related disorders in blacks (Figure 2).
Figure 2. Central illustration. Consequences of natriuretic peptide deficiency.
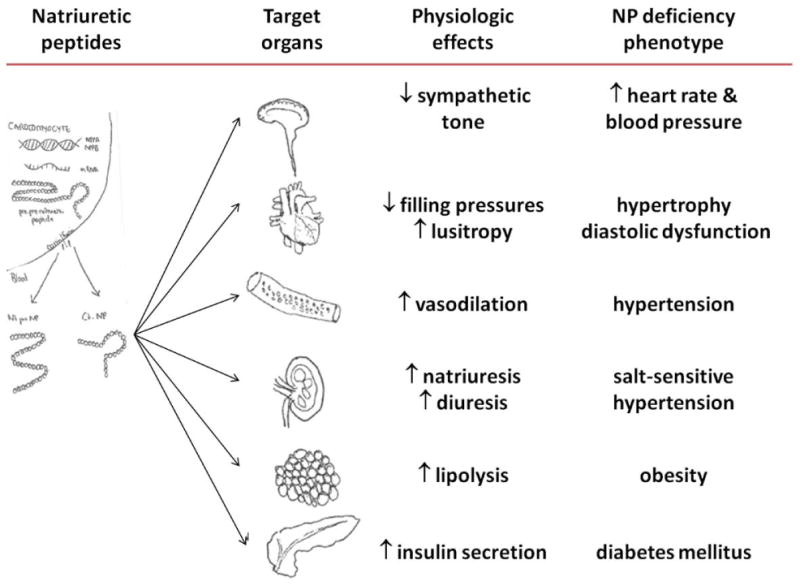
Natriuretic peptides are cardiac derived hormones that affect target organs resulting in cardiometabolic protective effects. Relative deficiencies of natriuretic peptides are associated with adverse phenotypes.
Reduced natriuretic peptide levels have previously been observed in a number of conditions including obesity, diabetes mellitus, and insulin resistance (28-33). These conditions are more common among black individuals and while some studies suggest that blacks may have lower natriuretic peptide levels than whites (16-20), these prior reports did not specifically focus on race/ethnic differences in natriuretic peptide levels while accounting for comorbidities. The measurement of Nt-proBNP in a multi-ethnic community dwelling cohort with extensive phenotype data available afforded the ability to examine whether natriuretic peptide levels differ by race/ethnicity and are lower in blacks independent of body mass index, diabetes mellitus, insulin resistance, and other factors previously demonstrated to influence natriuretic peptide levels. We found that Nt-proBNP levels were 40% lower in black compared with white individuals, even after accounting for other factors. This difference is substantial and likely to be clinically significant. For instance, obesity and insulin resistance, conditions in which lower natriuretic peptide levels have been widely corroborated, are associated with 6-20% and 10-30% lower Nt-proBNP levels, respectively (34). Genetic variants (rs5068 and rsl98358) in the natriuretic peptide precursor A and B gene loci provide insight into the consequences of variation in natriuretic peptide levels within the “normal” range. The alleles related to 20% lower circulating natriuretic peptides are associated with a 15% higher risk of hypertension (35). This association between genetic variants, lower natriuretic peptide levels, and increased risk of hypertension was present despite these lower natriuretic peptide levels being within a range that would be considered “normal” or not diagnostic of heart failure.
Genetic variation may contribute to lower natriuretic peptide levels in black individuals. Previous studies have demonstrated that natriuretic peptide levels are a heritable trait (36). However, there are limited data on racial differences in the genetics of the natriuretic peptide system (37,38). Corin is a cardiac serine protease involved in processing natriuretic peptide prohormones to yield active natriuretic peptides (39). Missense variants in the corin gene are more common in blacks than whites, and individuals with reduced function corin variants are at increased risk for hypertension and ventricular hypertrophy (37,40). Transgenic mice harboring the corin variant previously identified in black individuals demonstrate salt-sensitive hypertension and cardiac hypertrophy, thereby recapitulating the clinical phenotype (15). Furin is a serine endoprotease that is also involved in processing proBNP to Nt-proBNP and BNP (41). A single nucleotide polymorphism in furin (rs2521501) is associated with higher blood pressure and hypertension in both individuals of African and European ancestry (42). However, in the 1000 Genomes Project, this furin variant was present more commonly in persons of European compared with African ancestry (32% vs 21%), suggesting that it is unlikely to account for the lower NT-proBNP levels observed in black compared with white individuals in the Dallas Heart Study (43). Nonetheless, further studies are needed to clarify the relative contributions of synthesis, release, and clearance mechanisms to the lower natriuretic peptide levels observed in black individuals.
A relative natriuretic peptide deficiency may predispose to increased susceptibility to cardiovascular risk factors and disease in black individuals. Recently, the PARADIGM-HF trial demonstrated that combined neprilysin and angiotensin inhibition was superior to angiotensin inhibition alone in reducing heart failure progression and cardiovascular death in patients with heart failure and reduced ejection fraction, supporting chronic enhancement of the natriuretic peptide system as a therapeutic strategy (44,45). Augmenting the natriuretic peptide system may be an attractive and novel preventive and therapeutic approach to reduce the burden of cardiovascular risk factors and disease, particularly among high risk individuals with relative natriuretic peptide deficiencies, e.g. blacks. However, this hypothesis remains to be formally tested, especially because the small number of black individuals included in PARADIGM-HF trial precludes robust conclusions regarding the efficacy of neprilysin inhibition in this racial group.
Strengths and limitations
We evaluated racial/ethnic differences in Nt-proBNP levels in a well-phenotyped, multi-ethnic, community-dwelling adult population. The large sample size allowed the use of multivariable adjusted analyses to account for factors that influence natriuretic peptide levels, including LV mass and ejection fraction measured by cardiac magnetic resonance imaging. We were also able to demonstrate the consistency of the finding of lower Nt-proBNP in blacks across multiple clinically-relevant subgroups. Several limitations also merit comment.
Race/ethnicity was determined from participant self-report, which may result in misclassification bias. The proportion of Hispanic individuals in the Dallas Heart Study was relatively small (18%) compared to the other race/ethnic groups, which may have limited statistical power to detect a difference in Nt-proBNP levels. In a proportion of healthy individuals, plasma Nt-proBNP levels are below the detection limit. However, censoring of low levels would be expected to bias against the finding of lower natriuretic peptide levels in blacks versus whites. The mechanisms contributing to the racial differences in Nt-proBNP levels could not be determined in this observational cohort. Whether specific genetic variants contribute to the racial differences in natriuretic peptide levels is a question for further study. Racial differences in Nt-proBNP levels may also be related to natriuretic peptide processing. However, precise measurement of different natriuretic peptide isoforms would require mass spectrometry, which is beyond the scope of this study, but may be a future direction.
Conclusion
Despite a higher prevalence of hypertension and greater left ventricular mass, blacks have significantly lower natriuretic peptide levels than white individuals. Black individuals may have a relative natriuretic peptide deficiency that predisposes to cardiovascular risk. The natriuretic peptide system may represent a target for the prevention and treatment of cardiovascular disease, particularly in black individuals.
Perspectives
Competency in medical knowledge
Natriuretic peptides are cardiac derived hormones with protective cardio-metabolic effects. Deficiencies of the natriuretic peptide system are associated with the development of cardiovascular risk factors, such as hypertension, left ventricular hypertrophy, and hyperglycemia; conditions more common among black individuals. We found that Nt-proBNP levels are 40% lower in black compared with white individuals.
Translational outlook
Augmenting the natriuretic peptide system in individuals with relative natriuretic peptide deficiencies, e.g. blacks, may be an attractive and novel strategy for the prevention of cardiovascular risk factors and disease.
Acknowledgments
The authors thank the staff and participants of the Dallas Heart Study for their important contributions.
Funding sources: Nt-proBNP measurements were supported by investigator-initiated grants from Roche Diagnostics (Indianapolis, IN). Research reported in this publication was supported by the National Heart, Lung, and Blood Institute grants K12 HL109019 and R01-HL-102780; the National Center for Advancing Translational Sciences of the National Institutes of Health award numbers UL1TR000445 (Vanderbilt University) and UL1TR001105 (University of Texas-Southwestern); the American Heart Association Strategically Focused Research Network grants to Vanderbilt University (14SFRN20420046) and the University of Texas-Southwestern Medical Center (14SFRN20740000); and the American Heart Association grant (13GRNT14560079).
Abbreviations
- NP
natriuretic peptides
- Nt-proBNP
N-terminal pro B-type natriuretic peptide
- MRI
magnetic resonance imaging
- IQR
interquartile range
- OR
odds ratio
- HOMA-IR
homeostasis model assessment of insulin resistance
- eGFR
estimated glomerular filtration rate
- LV
left ventricular
- EF
ejection fraction
- BMI
body mass index
- CI
confidence interval
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang TJ. The natriuretic peptides and fat metabolism. N Engl J Med. 2012;367:377–8. doi: 10.1056/NEJMcibr1204796. [DOI] [PubMed] [Google Scholar]
- 2.Mukoyama M, Nakao K, Saito Y, et al. Increased human brain natriuretic peptide in congestive heart failure. N Engl J Med. 1990;323:757–8. doi: 10.1056/NEJM199009133231114. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb SS, Kukin ML, Ahern D, Packer M. Prognostic importance of atrial natriuretic peptide in patients with chronic heart failure. J Am Coll Cardiol. 1989;13:1534–9. doi: 10.1016/0735-1097(89)90344-6. [DOI] [PubMed] [Google Scholar]
- 4.de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345:1014–21. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 5.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–7. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 6.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–63. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 7.Mega JL, Morrow DA, De Lemos JA, et al. B-type natriuretic peptide at presentation and prognosis in patients with ST-segment elevation myocardial infarction: an ENTTRE-TIMI-23 substudy. J Am Coll Cardiol. 2004;44:335–9. doi: 10.1016/j.jacc.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 8.Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers and the risk of incident hypertension. Hypertension. 2007;49:432–8. doi: 10.1161/01.HYP.0000256956.61872.aa. [DOI] [PubMed] [Google Scholar]
- 9.de Lemos JA, McGuire DK, Khera A, et al. Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natriuretic peptides: results from the Dallas Heart Study. Am Heart J. 2009;157:746–53. e2. doi: 10.1016/j.ahj.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Cushman M, Judd SE, Howard VJ, et al. N-terminal pro-B-type natriuretic peptide and stroke risk: the Reasons for Geographic And Racial Differences in Stroke cohort. Stroke; a journal of cerebral circulation. 2014;45:1646–50. doi: 10.1161/STROKEAHA.114.004712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamura N, Ogawa Y, Chusho H, et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4239–44. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wada A, Tsutamoto T, Matsuda Y, Kinoshita M. Cardiorenal and neurohumoral effects of endogenous atrial natriuretic peptide in dogs with severe congestive heart failure using a specific antagonist for guanylate cyclase-coupled receptors. Circulation. 1994;89:2232–40. doi: 10.1161/01.cir.89.5.2232. [DOI] [PubMed] [Google Scholar]
- 13.John SW, Krege JH, Oliver PM, et al. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–81. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 14.Melo LG, Veress AT, Chong CK, Ackermann U, Sonnenberg H. Salt-sensitive hypertension in ANP knockout mice is prevented by AT1 receptor antagonist losartan. The American journal of physiology. 1999;277:R624–30. doi: 10.1152/ajpregu.1999.277.3.R624. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Cui Y, Shen J, et al. Salt-sensitive hypertension and cardiac hypertrophy in transgenic mice expressing a corin variant identified in blacks. Hypertension. 2012;60:1352–8. doi: 10.1161/HYPERTENSIONAHA.112.201244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krauser DG, Chen AA, Tung R, Anwaruddin S, Baggish AL, Januzzi JL., Jr Neither race nor gender influences the usefulness of amino-terminal pro-brain natriuretic peptide testing in dyspneic subjects: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. J Card Fail. 2006;12:452–7. doi: 10.1016/j.cardfail.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Montagnana M, Lippi G, Salvagno GL, Guidi GC. Reference ranges and diagnostic thresholds of laboratory markers of cardiac damage and dysfunction in a population of apparently healthy black Africans. Clin Chem Lab Med. 2008;46:714–6. doi: 10.1515/cclm.2008.130. [DOI] [PubMed] [Google Scholar]
- 18.Daniels LB, Clopton P, Potocki M, et al. Influence of age, race, sex, and body mass index on interpretation of midregional pro atrial natriuretic peptide for the diagnosis of acute heart failure: results from the BACH multinational study. Eur J Heart Fail. 2012;14:22–31. doi: 10.1093/eurjhf/hfr157. [DOI] [PubMed] [Google Scholar]
- 19.Choi EY, Bahrami H, Wu CO, et al. N-terminal pro-B-type natriuretic peptide, left ventricular mass, and incident heart failure: Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2012;5:727–34. doi: 10.1161/CIRCHEARTFAILURE.112.968701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazo M, Young JH, Brancati FL, et al. NH2-Terminal Pro-Brain Natriuretic Peptide and Risk of Diabetes. Diabetes. 2013;62:3189–93. doi: 10.2337/db13-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–80. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 22.Deo R, Khera A, McGuire DK, et al. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol. 2004;44:1812–8. doi: 10.1016/j.jacc.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 23.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91:4459–66. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Adler S, Caggiula AW, et al. Effects of dietary protein restriction on the progression of advanced renal disease in the Modification of Diet in Renal Disease Study. American Journal of Kidney Diseases : the official journal of the National Kidney Foundation. 1996;27:652–63. doi: 10.1016/s0272-6386(96)90099-2. [DOI] [PubMed] [Google Scholar]
- 26.Baber U, de Lemos JA, Khera A, et al. Non-traditional risk factors predict coronary calcification in chronic kidney disease in a population-based cohort. Kidney international. 2008;73:615–21. doi: 10.1038/sj.ki.5002716. [DOI] [PubMed] [Google Scholar]
- 27.Drazner MH, Dries DL, Peshock RM, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46:124–9. doi: 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- 28.Das SR, Drazner MH, Dries DL, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112:2163–8. doi: 10.1161/CIRCULATIONAHA.105.555573. [DOI] [PubMed] [Google Scholar]
- 29.Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 30.Melander O, Frandsen E, Magnusson M, Grubb A, Jovinge S, Groop L. Nt-proANP in plasma, a marker of salt sensitivity, is reduced in type 2 diabetes patients. Journal of internal medicine. 2005;257:281–8. doi: 10.1111/j.1365-2796.2005.01449.x. [DOI] [PubMed] [Google Scholar]
- 31.Wang TJ, Larson MG, Keyes MJ, Levy D, Benjamin EJ, Vasan RS. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation. 2007;115:1345–53. doi: 10.1161/CIRCULATIONAHA.106.655142. [DOI] [PubMed] [Google Scholar]
- 32.Fox ER, Musani SK, Bidulescu A, et al. Relation of obesity to circulating B-type natriuretic peptide concentrations in blacks: the Jackson Heart Study. Circulation. 2011;124:1021–7. doi: 10.1161/CIRCULATIONAHA.110.991943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng S, Fox CS, Larson MG, et al. Relation of visceral adiposity to circulating natriuretic peptides in ambulatory individuals. Am J Cardiol. 2011;108:979–84. doi: 10.1016/j.amjcard.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan AM, Cheng S, Magnusson M, et al. Cardiac natriuretic peptides, obesity, and insulin resistance: evidence from two community-based studies. J Clin Endocrinol Metab. 2011;96:3242–9. doi: 10.1210/jc.2011-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newton-Cheh C, Larson MG, Vasan RS, et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nature genetics. 2009;41:348–53. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang TJ, Larson MG, Levy D, et al. Heritability and genetic linkage of plasma natriuretic peptide levels. Circulation. 2003;108:13–6. doi: 10.1161/01.CIR.0000081657.83724.A7. [DOI] [PubMed] [Google Scholar]
- 37.Dries DL, Victor RG, Rame JE, et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–10. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 38.Lanfear DE, Stoiker JM, Marsh S, Rich MW, McLeod HL. Genetic variation in the B-type natiuretic peptide pathway affects BNP levels. Cardiovasc Drugs Ther. 2007;21:55–62. doi: 10.1007/s10557-007-6007-5. [DOI] [PubMed] [Google Scholar]
- 39.Wang W, Liao X, Fukuda K, et al. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res. 2008;103:502–8. doi: 10.1161/CIRCRESAHA.108.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rame JE, Drazner MH, Post W, et al. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension. 2007;49:857–64. doi: 10.1161/01.HYP.0000258566.95867.9e. [DOI] [PubMed] [Google Scholar]
- 41.Semenov AG, Tamm NN, Seferian KR, et al. Processing of pro-B-type natriuretic peptide: furin and corin as candidate convertases. Clin Chem. 2010;56:1166–76. doi: 10.1373/clinchem.2010.143883. [DOI] [PubMed] [Google Scholar]
- 42.International Consortium for Blood Pressure Genome-Wide Association S. Ehret GB, Munroe PB, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–9. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.rs2521501 SNP. [Accessed January 12, 2015]; Available at: http://useast.ensembl.org/Homo_sapiens/Variation/Explore?db=core;r=15:90893658-90894658;v=rs2521501;vdb=variation;vf=2159702.
- 44.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 45.Packer M, McMurray JJ, Desai AS, et al. Angiotensin Receptor Neprilysin Inhibition Compared With Enalapril on the Risk of Clinical Progression in Surviving Patients With Heart Failure. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.114.013748. [DOI] [PubMed] [Google Scholar]


