Abstract
Background
Exercise has been shown to reduce symptoms of anxiety, but few studies have studied exercise in individuals pre-selected because of their high anxiety.
Purpose
To review and critically evaluate studies of exercise training in adults with either high levels of anxiety or an anxiety disorder.
Methods
We conducted a systematic review of randomized clinical trials (RCTs) in which anxious adults were randomized to an exercise or non-exercise control condition. Data were extracted concerning anxiety outcomes and study design. Existing meta-analyses were also reviewed.
Results
Evidence from 12 RCTs suggested benefits of exercise, for select groups, similar to established treatments and greater than placebo. However, most studies had significant methodological limitations, including small sample sizes, concurrent therapies, and inadequate assessment of adherence and fitness levels.
Conclusions
Exercise may be a useful treatment for anxiety, but lack of data from rigorous, methodologically sound RCTs precludes any definitive conclusions about its effectiveness.
Keywords: Exercise, Physical activity, Anxiety, Anxiety disorders, Systematic review
INTRODUCTION
Anxiety, a psychological state characterized by apprehensive expectation or fear, is among the most commonly experienced psychiatric symptoms (1). Data from the National Comorbidity Study-Replication suggest that in the United States, the lifetime prevalence of any anxiety disorder is approximately one in three, more than any other diagnostic category (2). Some elevation in anxiety symptoms, whether affective (fear, apprehension) or physiological (racing heart, trembling, etc.), is a criterion common to all of these disorders. However, other diagnostic criteria for anxiety disorders can be quite heterogeneous, such as the frequency and severity of symptoms as well as whether triggers for these symptoms are specific or more generalized. Subsyndromal anxiety symptoms also can impair individuals’ psychosocial functioning and can necessitate use of health care resources (3). Anxiety represents a risk factor for lower health-related quality of life (4), increased risk of all-cause mortality (5), and a variety of physical health problems, particularly cardiovascular disease (CVD).
Findings from a number of prospective epidemiological studies report a strong association of anxiety with mortality in healthy individuals (5, 6, 7) and in CVD patients (8, 9, 10, 11, 12, 13, 14). It has been shown that elevated anxiety scores were associated with increased risk of mortality after accounting for established risk factors in 934 men and women with CVD (15). Moreover, elevated anxiety symptoms have been shown to be associated with a 2-fold increased risk of mortality in coronary bypass patients (11, 12, 13) and in outpatients with CVD (9, 16, 17). One study (10) reported that CVD patients with Generalized Anxiety Disorder (GAD) assessed two months following hospital discharge showed a 2.3-fold increased risk of adverse cardiac events, and another (17) reported a 2.8-fold increased risk of adverse events in acute post-MI patients in which anxiety was measured one month following hospital discharge. Similarly, a 2-fold increased risk of adverse events was observed in stable CVD patients with elevated anxiety during annual clinic visits (18). High anxiety has also been associated with increased risk for the development of hypertension (19), heart disease (20), and of increased cancer mortality in longitudinal studies of early adults (5, 21), even when controlling for other medical risk factors. Among healthy individuals, anxiety can be associated with unhealthy behaviors such as physical inactivity, smoking, and poor diet, leading to increased risk for developing health issues (22, 23).
Several empirically-supported treatments have shown efficacy for anxiety reduction, including cognitive-behavioral therapy (CBT) (24, 25, 26) and psychotropic medications, particularly selective serotonin reuptake inhibitors (SSRIs) (25, 27, 28, 29). However, these treatments may be associated with significant drawbacks and treatment barriers. For example, although SSRIs can be effective for some, they are not effective for everyone and may be associated with adverse side effects (30), which can lead to treatment dropout (31). Although CBT also has empirical support as a structured intervention for anxiety disorders, access can be limited by a myriad of factors, including availability of trained providers and economical considerations (32). Such issues represent barriers to effective treatment for individuals with elevated anxiety, creating the need for alternative therapies.
Exercise may represent a promising, affordable, and easily accessible treatment option for individuals with anxiety. Exercise is distinguished from other forms of physical activity in that it is a planned, structured, repetitive endeavor with the goal of improving physical fitness (33). A number of observational studies document an inverse association of exercise and symptoms of anxiety. For example, in one study of 8098 adults (34), individuals exercising “regularly” had a reduced risk of being diagnosed with an anxiety disorder compared to their sedentary counterparts (odds ratios [OR] from 0.64 to 0.78 for exercisers). In another study of 19,288 participants in the Netherlands Twin Registry (35), individuals reporting 240 minutes a week of moderate exercise reported less anxiety and neuroticism compared with non-exercisers. Although encouraging, data from observational studies cannot prove that exercise caused reduced risk for an anxiety disorder. Anxious persons may be less likely to be physically active and engage in exercise (35).
Interventional studies of healthy individuals without an anxiety disorder have demonstrated reductions in state anxiety immediately after performing single bouts of exercise (36, 37, 38, 39). However, such studies do not address the question of whether accumulated bouts of exercise reduce anxiety levels, nor do they address whether individuals with an anxiety disorder could benefit from repeated bouts of exercise. Interventional studies also have examined the effects of multiple bouts of exercise on anxiety, albeit primarily among individuals who were not selected on the basis of high anxiety levels. For example, one study of 357 older adults found that assignment to regular exercise participation was associated with significant anxiety reduction at 12 months compared to assignment to control (40). Similarly, state and trait anxiety decreased among participants with elevated blood pressure in a study of a 12-week Tai Chi program, compared with sedentary controls (41). However, participants in these trials were not experiencing clinically significant anxiety prior to intervention.
Depression may co-occur with anxiety (2, 9, 42, 43), and a number of comprehensive reviews of the literature on exercise for depression have been conducted (44, 45, 46). However, there have been relatively few reviews of the literature on exercise for anxiety. Those that exist have not distinguished studies that targeted anxious individuals a priori, nor have they provided a critical analysis of the quality of the research. Reviews that included results of exercise interventions on anxiety outcomes typically have found a robust and beneficial relationship (47, 48). However, these reviews have primarily included studies of non-anxious participants, and the clinical significance of reduced anxiety in non-anxious individuals is questionable. Other reviews have failed to differentiate single-bout and chronic exercise (48) or anxious and non-anxious individuals (49). In one recent review (50), only studies of individuals with diagnosed anxiety disorders were included, possibly omitting important information from those with significant anxiety but without a specific diagnosis. A critical review is needed in order to establish the quality and quantity of available evidence that this potentially valuable intervention is effective for treating anxious individuals.
The purpose of this review is to describe and critically evaluate published exercise interventions targeting individuals with elevated anxiety or with a diagnosis of an anxiety disorder. We also provide a review of meta-analyses of research concerning the relationship between exercise and anxiety, to complement our review of the randomized clinical trials (RCTs) that have been conducted targeting participants with elevated anxiety levels.
METHOD
We conducted a systematic search (July 2014) for randomized clinical trials (RCTs) in which participants were pre-selected on the basis of either a diagnosis of an anxiety disorder or elevated symptoms of anxiety and then randomized to treatment with exercise as one of the treatment arms of the trial. These criteria were chosen so that all experimental data concerning exercise as an intervention for anxious individuals would be included.
Inclusion Criteria
We included all RCTs that met the following criteria: (1) article published in English in a peer-reviewed journal; (2) participants were at least 18 years old; (3) participants had elevated anxiety symptoms using a validated assessment instrument, or were diagnosed with an anxiety disorder; (4) exercise intervention consisted of >1 exercise sessions; and (5) anxiety was an outcome measure. Several studies have examined the immediate effects of single bouts of exercise on anxiety (37, 38, 51), which were not included in this review. We did include RCTs of participants with post-traumatic stress disorder (PTSD), although DSM-5 now classifies PTSD separately from anxiety disorders (1).
Procedures
We first conducted a comprehensive search using CINAHL, EMBASE, MEDLINE, PsycINFO, and the Cochrane Library. Articles were identified with titles or abstracts that included terms, or variants thereof, from each of the following groups: (1) physical activity or exercise; (2) anxiety or phobia or panic; (3) randomly or randomized or clinical trial. These articles were compiled in an EndNote database. Next, every unique abstract in the database was examined to determine whether any inclusion criteria for the review were unequivocally unmet (e.g., study of children or non-humans, participants without elevated anxiety, etc.). Those articles were discarded, and the remaining studies were retained for data extraction. A random subset of articles (N = 25) was selected for double review, to confirm that the articles should be excluded and to establish inter-rater reliability. No discrepancies were observed during this double review (100% agreement). If there was any uncertainty about whether inclusion criteria were met, the article was retained for further examination.
Next, two randomly assigned raters independently reviewed the full text of each remaining article and removed those in which basic inclusion criteria were not met (e.g., absence of an exercise intervention). We also removed publications from consideration that either did not report anxiety outcomes or did not compare exercise to a control or comparison group. This level of review was conducted using a standardized form enumerating the inclusion criteria. Some of the studies reviewed during this step allowed individuals to participate if they had either elevated anxiety or another condition (e.g., depression, medical comorbidities). In these cases, we eliminated studies that did not provide data on anxiety-related outcomes specifically for the participants with elevated pre-treatment anxiety.
Each of the remaining publications was randomly assigned to two reviewers for data extraction. Information gathered in this step included study sample characteristics, experimental design, measures of anxiety, modality, intensity, and duration of exercise, methodological features such as intention to treat, blinding, and allocation concealment, primary (i.e., anxiety-related) and secondary outcomes, and results. Extracted data were compiled on a standardized form and reviewed for inter-rater discrepancies, which reviewing authors resolved via mutual discussion. In the rare instances in which discrepancies remained after this discussion, these were resolved by a third independent rater.
During this process, if multiple publications were found to be referring to the same RCT, a final round of data extraction was conducted. A third reviewer, treating the articles as a single study, extracted data that was submitted for discrepancy resolution with the preceding reviewers. Thus, if critical information had only been reported in one of the publications, the quality of the RCT was evaluated on the basis of all available study data.
Study quality and relative risk of bias from design of each RCTs were also assessed using the PEDro Scale (52), a widely used instrument to rate the overall quality of RCTs. PEDro scores are summarized across domains and those with higher scores (range: 0-10) are considered to have better quality. Briefly, after the first PEDro item, specification of eligibility criteria, the remaining 10 items are worth 1 point apiece and comprise the following criteria: random allocation of participants to groups; allocation concealment; similarity of groups at baseline; blinding of participants; blinding of interventionists; blinding of assessors of at least one key outcome; obtainment of a key outcome variable from 85% or more participants; intention-to-treat data collection and analysis; results reported for between-group differences on at least one key outcome; point measurements and measurements of variability reported for at least one key outcome. Because of our selection criteria, all studies automatically received a point each for randomizing participants to conditions and for reporting between-group differences on a key outcome. However, all studies also lost 2 points total for items 5 (blinding of all subjects) and 6 (blinding of all therapists), which were not relevant, as participants were randomized to receive exercise and the interventionists delivering the exercise treatment obviously could not be blinded to treatment condition.
Review of Meta-Analyses
In addition to our review of individual RCTs, we also surveyed the existing meta-analyses on exercise and anxiety. Our article search initially returned 203 unique records that were either review articles or meta-analyses. We included meta-analyses identified through our search that met similar criteria to the RCTs: anxiety as a primary focus of the meta-analysis, exercise as an intervention for anxiety, adult participants, and random assignment to treatment groups, in which at least one treatment was exercise. Our literature search did not reveal any meta-analyses that concerned both elevated anxiety symptoms (not diagnoses alone) and multiple sessions of exercise. Because of the few meta-analyses and critical reviews in the area, we elected not to exclude meta-analyses that permitted single-bout exercise interventions.
RESULTS
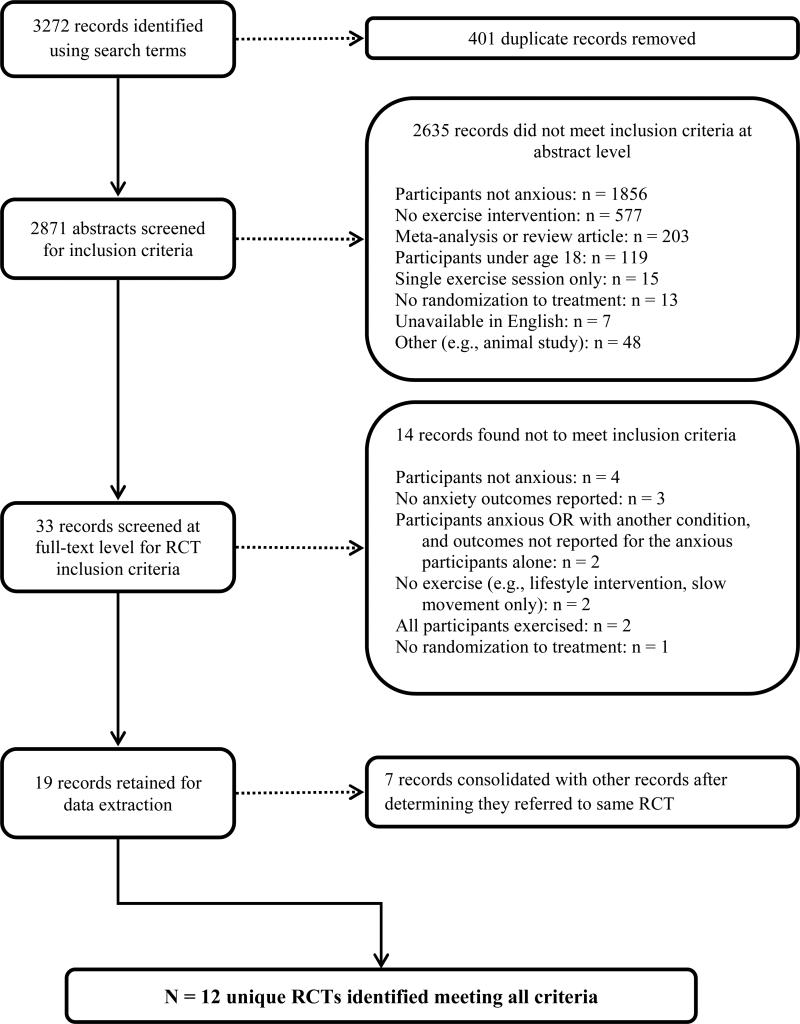
Figure 1 displays the process by which articles were selected for inclusion in this review. A total of 3272 records were collected using our search methods. A total of 401 duplicate records were identified and removed, yielding 2871 unique articles reviewed at the abstract level. The majority of studies (n = 1856, 56.7%) were excluded at the abstract level because elevated anxiety or a diagnosis of anxiety was not identified as one of the participant selection criteria. After abstract-level review, 33 of the 2871 publications (1.1%) remained and were examined at the full-text level. Full-text review resulted in exclusion of an additional 14 publications. Reasons for exclusion were the following: patients did not have elevated anxiety (53, 54, 55); anxiety was not measured or reported as an outcome (56, 57, 58); participants were not randomized or there was no explicit indication that participants were randomized to treatment (59); exercise was not clearly required as part of the intervention (60, 61). Notably, in two studies we identified for full-text review, all study groups engaged in some form of exercise, so that there was no valid non-exercise comparison group. In one trial, 22 individuals participating in cardiac rehabilitation (CR) with high anxiety related to outdoor walking were assigned to either walking or cycling at equal work output, with no non-exercising control (62). In the other trial, 70 psychiatric inpatients were randomized to either aerobic exercise (walking, jogging) or resistance exercise (strength training) (63). Finally, three studies were removed because outcomes were not reported specifically on anxious participants. One trial (64) included elevated anxiety among many other possible inclusion criteria, such as physical health problems or depression, and anxiety outcomes were not reported separately. Similarly, one study (65) selected participants on the basis of any non-psychotic DSM diagnosis, and another study (66) enrolled patients with elevated symptoms of anxiety or depression, and these groups were not distinguished from one another.
Fig. 1.
Flowchart for the selection of RCTs to include in the review, with reasons for exclusion
As a result, 19 articles concerning exercise RCTs among anxious individuals were identified. In the process of data extraction, we determined that in seven cases, multiple publications referred to the same trial. These cases comprised the following: a study among 46 individuals with diagnoses of Panic Disorder with Agoraphobia comparing jogging, clomipramine, and waitlist control (67, 68, 69); a study comparing unsupervised gym exercise to mindfulness-based stress reduction among 56 individuals with Social Anxiety Disorder (SAD) (70, 71, 72); a study of 30 individuals with GAD assigned to resistance exercise, aerobic exercise, or waitlist control (73, 74); a study of 85 individuals with diagnoses of GAD, Panic Disorder, or SAD who participated in both group CBT and either unsupervised exercise or healthy eating education (75, 76); a study of 201 patients with elevated anxiety or depression and recent acute myocardial infarction assigned to CR or usual care (77, 78). As a result, we ultimately identified 12 RCTs that met our inclusion criteria (Table 1).
Table 1.
Summary of Randomized Clinical Trials
| Author | N | Country | Anxiety inclusion criteria | % Female | Age (M) | Exercise condition(s) | Control condition(s) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Modality | Co-intervention | Frequency | Duration / Intensity | |||||||
| Broman-Fulks et al., 2008 |
35 | USA | Symptoms of high anxiety sensitivity |
79 | 19 | Aerobic: brisk walking or jogging |
None | 6 sessions over 2 weeks |
20 min, 60-90% HRR |
Waitlist |
|
Broocks et al., 1998; Bandelow et al., 2000; Broocks et al., 2003 |
46 | Germany | Diagnosis of PD with Agoraphobia |
50 | 33 | Aerobic: running | None | 4+ weekly sessions over 10 weeks |
4-mile route, gradual pace increase encouraged |
1.Clomipramine 2.Placebo |
| Carmeli et al., 2009 | 24 | Israel | “Diagnosed with anxiety” |
62 | 51 | Aerobic: bicycle or treadmill |
None | 3 sessions weekly for 26 weeks |
35 min, 50-70% HRR |
1.Leisure program focused on stability, flexibility, balance 2.Vocational activities |
|
Goldin et al., 2012; Jazaieri et al., 2012; Goldin et al., 2013 |
56 | USA | Diagnosis of SAD, moderate or more; fear in 5+ social situations |
52 | 33 | Aerobic: gym exercise |
None | 3 weekly sessions for 8 weeks |
Not specified, unsupervised |
Mindfulness-based stress reduction - 1-day retreat and 8 weekly sessions, 150 mina |
| Gutierrez et al., 2012 | 60 | Spain | Symptoms of anxiety, moderate or more; comorbid depression |
100 | 64 | Aerobic: group exercise |
None | 6 months of increasing sessions, 1 to 3 weekly |
50-60 min, 60-85% HRR at end of study |
Waitlist |
|
Herring et al., 2011; Herring et al., 2012 |
30 | USA | Diagnosis of GAD | 100 | 23 | Resistance (RET) group: leg presses, curls, extensions; Aerobic (AET) group: cycling |
None | 12 sessions over 6 weeks |
RET: 16 min, gradual increase from 50% of 1RM AET: equal work output to RET |
Waitlist |
| Hovland et al., 2013 | 36 | Norway | Diagnosis of PD | 81 | 38 | Combined aerobic and resistance: group exercise |
None | 3 weekly sessions for 12 weeks |
90 min, 60-80% HRR |
Group CBT for PD |
| Kim et al., 2013 | 28 | USA | Symptoms of PTSD |
97 | 46 | Mindfulness-based stretching and deep breathing exercise (MBX) |
None | 2 weekly sessions for 8 weeks |
60 min, gradually increasing intensity |
No intervention or waitlist identified |
|
Merom et al., 2008
Phongsavan et al., 2008 |
85 | Australia | Diagnosis of GAD, PD, or SAD |
78 | 39 | Participant's choice | Group CBT | 5 days a week for 8 weeks encouraged |
30 min, “moderate” intensity encouraged, unsupervised |
Group CBT plus healthy eating education (GCBT+ED) |
|
Oldridge et al., 1991; Oldridge et al., 1995 |
201 | USA | Symptoms of anxiety, moderate, with depressive symptoms |
11 | 54 | Aerobic: cardiac rehabilitation |
Group CBT, relaxation training |
2 weekly sessions for 8 weeks |
50 min | Usual care by local physician |
| Smits et al., 2008 | 60 | USA | Symptoms of high anxiety sensitivity |
75 | 21 | Aerobic: treadmill | Cognitive restructuring (sub-sample) |
6 sessions over 2 weeks |
26 min at 70% HRR | Waitlist |
| Wedekind et al, 2010 | 75 | Germany | Diagnosis of PD | 69 | 32 | Aerobic: running | Randomized to paroxetine or placebo |
3 weekly sessions for 10 weeks |
45 min each, gradual intensity increase encouraged |
Relaxation training, daily plus once weekly with a trainer |
| Author | ITT | % Attrition | Blinded outcome assessment | EX supervised | Fitness improved | PEDro scale score | Anxiety outcome | Findings |
|---|---|---|---|---|---|---|---|---|
| Broman-Fulks et al., 2008 | N | 31 | N/A | Y | ?c | 4 | ASI-R | ASI-R: Exercise better than waitlist. exercise 27.92 (15.36), waitlist 41.0 (25.68) |
|
Broocks et al., 1998; Bandelow et al., 2000; Broocks et al., 2003 |
Yb | 20 | Y | Partial | N | 7 | BAI CGI PAS HAM-A |
HAM-A, BAI: EX and clomipramine better than placebo; no difference between EX and CL. PAS (observer) and CGI: Clomipramine better than EX, both better than placebo. |
| Carmeli et al., 2009 | N | 33 | Y | Y | ? | 4 | HAM-A (modified) |
HAM-A: Decreased at post-test in exercise group (50%) and leisure program group (37.5%), but not control group (0%). |
|
Goldin et al., 2012; Jazaieri et al., 2012; Goldin et al., 2013 |
N | 25 | N/A | N | ? | 4 | LSAS-SR SIAS-S |
LSAS-SR, SIAS-S: both groups improved, no significant difference between groups. About 25% of participants had clinically significant improvement in SAD. |
| Gutierrez et al., 2012 | N | 5 | N/A | Y | N | 5 | HAM-A (Spanish) |
HAM-A: Exercise group significantly improved at post-test (p < 0.01 for participants with minor anxiety, p < 0.05 for major anxiety). No change in control group. |
|
Herring et al., 2011; Herring et al., 2012 |
Y | 0 | Y | Y | RET group only |
7 | POMS PSWQ |
POMS-Tension scores: RET better than waitlist. NS for AET. PSWQ: Exercise conditions better than waitlist when grouped, but pairwise NS. |
| Hovland et al., 2013 | Y | 3 | Y | Y | Y | 7 | ACQ BAI BSQ MI STAI |
STAI, BAI: No Group x Time effect. ACQ; BSQ; MI: CBT showed more anxiety reduction than exercise. |
| Kim et al., 2013 | Y | 5 | N/A | Y | ? | 5 | PCL-C | PCL-C: MBX better than aerobic exercise group (reduced by mean of - 13.6) at post-test; effect maintained 8 weeks after intervention |
|
Merom et al., 2008
Phongsavan et al., 2008 |
N | 52 | N | N | N | 5 | DASS-21 | DASS-21: All groups improved. No significant group differences in anxiety; change scores were -6.1 (exercise) vs -4.6 (control) at post-test. |
|
Oldridge et al., 1991; Oldridge et al., 1995 |
N | 7 | N/A | Y | Y | 5 | POMS STAI |
STAI-State: Exercise better than UC at 8 weeks but NS at 4, 8, and 12 months. POMS tension-anxiety: exercise better than UC at 8 weeks but NS at 12 months. |
| Smits et al., 2008 | Y | 20 | N/A | Y | ? | 5 | ASI BAI |
BAI, ASI: Both exercise groups better than waitlist. No effect for restructuring. |
| Wedekind et al., 2010 | Y | 20 | Y | Partial | Y | 6 | CGI HAM-A PAS |
HAM-A: Exercise better than relaxation at week 4 only (p < 0.01). PAS: No group differences. CGI: Exercise + paroxetine better than relaxation + placebo (p < 0.05), but all groups improved. |
ASI Anxiety Sensitivity Inventory, ASI-R Anxiety Sensitivity Inventory-Revised, BAI Beck Anxiety Inventory, BSQ Body Sensations Questionnaire, CBT cognitive-behavioral therapy, CGI Clinical Global Impression, DASS-21 Depression Anxiety Stress Scale, GAD generalized anxiety disorder, HAM-A Hamilton Anxiety Rating Scale, ITT intent-to-treat, LSAS-SR Liebowitz Social Anxiety Scale-Self-Report, MI Mobility Inventory, PAS Panic and Agoraphobia Scale, PCL-C PTSD Checklist-Civilian version, PD panic disorder, POMS Profile of Mood States, PSWQ Penn State Worry Questionnaire, PTSD posttraumatic stress disorder, SAD social anxiety disorder, SIAS-S Social Interaction Anxiety Scale-Straightforward Scale
Aerobic exercise was considered the control group for the purposes of this RCT.
The authors reported an ITT analysis; however, not all patients randomized were included in the primary analysis, and most of the results focused on a completers analysis.
Improvements in fitness were either not measured or not reported.
Sample Characteristics
The samples included in these RCTs were conducted in North America, Europe, the Middle East, and Australia. Of the 12 RCTs identified, 6 were conducted in the United States, 2 in Germany, and 1 each in France, Israel, Norway, and Spain. Samples varied greatly in gender composition (range: 11-100% female) and age (range: 19-64 years). Most studies sampled from a general population of adults; two studies recruited patients from primary care settings; one study was conducted among individuals with intellectual disabilities at a residential center (79), and one recruited hospital nurses exclusively (80).
Diagnoses and anxiety symptoms used for study inclusion also varied greatly. Panic Disorder, the most common criterion for inclusion, was studied in four RCTs. High general anxiety symptoms (e.g., elevated scores on a self-report measure) or a Generalized Anxiety Disorder (GAD) diagnosis were only used as inclusion criteria for four studies (73, 74, 75, 76); (77, 78); (81). Two studies (82, 83) examined anxiety sensitivity rather than anxiety symptoms, and two other studies required that participants have concurrent anxiety and depression symptoms (77, 78, 81). We identified one RCT of participants with symptoms of PTSD (80). One RCT (77, 78) included individuals with elevated anxiety symptoms, depressive symptoms, or both; we included this in our review because not only were 92% of participants identified as anxious, but also primary outcomes were reported for those participants who were anxious specifically.
Six studies assessed pre-treatment anxiety using structured diagnostic interviews and five studies relied on self-report measures. One study reported that participants had been “diagnosed with anxiety” without providing further information (79).
Exercise Interventions
Aerobic exercise was the most common exercise modality, employed in 8 RCTs (66%) and in tandem or compared to resistance exercise in two others. Six of the RCTs reported exercise intensity, ranging from 50% to 90% of maximum heart rate or work output. The length of the exercise interventions was also highly variable, and ranged from two weeks to six months. In one RCT (70, 71, 72), participants were directed to exercise on their own for 24 sessions over 8 weeks, but participants were not observed and adherence to the exercise prescription apparently was not measured. In their study design, aerobic exercise was used a control group and compared to mindfulness-based stress reduction.
Exercise was supervised in eight of the RCTs. In two others (67, 68, 69, 84), participants had only one of three weekly running sessions supervised by an exercise trainer. Exercise was not supervised in the remaining two studies (70, 71, 72, 75, 76).
None of the RCTs compared different levels of exercise intensity or examined a dose-response relationship between exercise intensity and anxiety reduction.
Two studies (62, 63) of anxious participants involved multiple sessions of exercise, but were excluded in the final review because all participants exercised, leaving no appropriate comparison group. One study that was included (73, 74) compared two different modalities of exercise (aerobic vs. resistance), at equal work output, to a waitlist control. One other study (80) compared participants completing a mindfulness-based stretching exercise routine to individuals receiving no intervention. Although the intensity of exercise was not stipulated as part of the intervention, the routine was characterized as increasing in intensity over several weeks. Thus, we elected to include this study for further review.
In half of the studies, RCTs compared exercise interventions to a waitlist condition or no intervention or usual care. In the other studies, the efficacy of exercise was compared to that of psychological interventions such as CBT (75, 76, 85), relaxation training (84), mindfulness-based stress reduction (70, 71, 72), psychiatric medication or placebo (67, 68, 69), or non-exercise physical activity and vocational activities (79).
Study Quality
We compiled information concerning study design to determine relative strength and quality of the RCTs. Blinded outcomes assessments were conducted in 5 of the 12 studies; 5 of the remaining 7 used self-report outcome measures exclusively. Similarly, only 5 studies controlled for outside protocol interventions for anxiety, directing participants to discontinue or avoid starting another treatment prior to entering the study. One of the RCTs (73, 74) found that 4 of 30 participants had been exposed to an outside intervention during the study, although post hoc analyses showed no significant difference in primary outcomes between these and other participants. A third of the studies included a co-intervention for some or all participants, either medication or talk therapy.
With respect to sample size, only one of the 12 RCTs had a sample size over 100 participants (77, 78); in half of the studies, fewer than 50 participants were included in the trial, with no more than 19 participants assigned to exercise. The participant attrition rate tended to be high, ranging from 0 to 52% (mean attrition = 18.4%). Half the studies reported 20% or greater attrition, with three studies having more than a 30% dropout rate. Intent-to-treat (ITT) analysis was employed in only 50% of studies. Of note, at least one study indicated that an ITT analysis was performed, but reported primarily an analysis of study completers (67, 68, 69). Only one of the RCTs (85) made reference to allocation concealment during randomization.
Regarding quality ratings, PEDro scores were calculated using all applicable methodological characteristics, such as randomization, blinding, attrition rate, analysis, and risk of reporting bias. All PEDro scores for the RCTs fell within the low to medium range, between 4 and 7. However, it is important to note that each study started with a minimum of 2 points based on the eligibility criteria for inclusion in our review (random assignment to groups, outcomes reported on key variable), and studies were penalized 2 points because participants and interventionists could not be blinded to treatment condition (exercise versus non-exercise control). In addition, it should be noted that summary scores provide a limited assessment of study bias, particularly regarding threats to internal validity (86, 87, 88). For example, contamination of exercise with concurrent therapies was observed in some of the RCTs, such as mindfulness training (80) and CBT (77, 78); although this is not counted as a methodological weakness within the PEDro scoring system, it poses a major threat to the internal validity of the study.
Anxiety Measures
Measures used to assess anxiety outcomes were highly variable. The most common instruments were the Hamilton Anxiety Rating Scale (HAM-A) (89), with an original, modified, or translated version used in 4 of the 12 studies, and the Beck Anxiety Inventory (BAI) (90), used in 3 of the 12 studies. Other instruments included the Agoraphobia Cognitions Questionnaire (ACQ) (91), Anxiety Sensitivity Inventory (ASI) (92) and ASI-Revised (ASI-R) (93); Body Sensations Questionnaire (BSQ) (91); Clinical Global Impression (CGI); (94); Depression Anxiety Stress Scale-21 (DASS-21) (95); Liebowitz Social Anxiety Scale-Self-Report (LSAS-SR) (96); Mobility Inventory (MI) (97); Panic and Agoraphobia Scale (PAS) (98); PTSD Checklist-Civilian version (PCL-C) (99, 100); Profile of Mood States (POMS) (101); Penn State Worry Questionnaire (PSWQ); (102); Social Interaction Anxiety Scale-Straightforward Scale (SIAS-S) (103); State-Trait Anxiety Inventory (STAI) (104). Of note, five studies employed measures that were not used in any of the other RCTs.
Results of the Exercise Interventions
Regarding the effectiveness of exercise interventions, 4 of the 12 studies reported that the group receiving an exercise intervention showed superior anxiety outcomes compared to those of the control group, such as reduction in anxiety symptoms (80, 81) or reduction in anxiety sensitivity (82, 83). Three RCTs showed no significant post-test difference between exercise and no-exercise groups (71, 72, 75, 76, 85). In one of these cases (85), participants who performed aerobic and resistance showed comparable improvements on general anxiety symptom measures to those who received group CBT. However, on several measures of panic and agoraphobia, the CBT group showed greater improvement compared to the exercise group.
The remaining five studies also had mixed results. One study of patients with intellectual disability in a residential setting (79) compared aerobic exercise with a leisure program consisting of large body movements focused on stability, flexibility, and balance, as well as vocational activities as a control condition. The aerobic or leisure programs, but not vocational activities, were associated with significant reductions in anxiety symptoms. Another RCT found that resistance exercise, but not aerobic exercise, yielded significant reductions in symptoms of GAD (73, 74). In a study of patients recovering from acute myocardial infarction, participants in CR and usual care both exhibited similar reductions in anxiety symptoms, though those who engaged in the rehabilitation achieved improvement sooner (77, 78). Patients who enrolled in CR also participated in group-based CBT, however, so one cannot attribute these benefits specifically to exercise. In a study of individuals with Panic Disorder (67, 68, 69), clomipramine showed equivalent or superior outcomes to aerobic exercise, although both treatments performed better than placebo. Finally, in a comparison of exercise and relaxation training for treating Panic Disorder (84), both treatment groups improved. Exercise showed greater improvement than relaxation training at 4 weeks, but this effect did not persist after 6, 8, and 10 weeks.
Surprisingly, only four of the 12 RCTs reported significant improvements in fitness after exercise treatment. One study (67, 68, 69) observed statistically significant improvements in HAM-A and BAI scores for participants who either ran or took clomipramine for 10 weeks, compared to controls, although a measure of fitness—timed running distance—was comparable at post-test for all study groups, including the non-exercisers. In another study, Herring and colleagues (73, 74) observed improvements in leg strength for resistance exercisers but not aerobic exercisers. In three other studies, exercise and control groups showed no significant differences in fitness levels after training. In five studies, fitness levels or post-exercise improvements were not reported or measured.
Only two of the 12 RCTs included post-treatment follow-up to assess the sustained effects of the intervention. In one study (80), mindfulness-based exercise, but not aerobic exercise, showed a benefit at 8 weeks after training was completed. In the other study (77, 78) that found CR patients to show improvements in anxiety compared to patients in usual care, this difference did not persist after the completion of the 8-week program. The extent to which participants continued to exercise after the intervention period was not assessed in either study.
Review of Meta-Analyses
From the 203 records identified as reviews or meta-analyses, we identified 5 published meta-analyses of studies of exercise interventions on anxiety (47, 48, 49, 105, 106) (Table 2). The most inclusive of these meta-analyses included any type of exercise intervention (106), whereas the most restrictive analysis required a 3-week minimum of exercise participation (47). Of the five meta-analyses, four concluded that exercise is an effective treatment for anxiety, with effect sizes ranging from 0.22 (small) to 0.56 (moderate). However, participants without elevated anxiety or with no anxiety diagnosis were included in these analyses. Because only one meta-analysis restricted its scope to include only participants without elevated anxiety (48), the utility of exercise to ameliorate anxiety in clinical samples could not be determined.
Table 2.
Meta-Analyses Concerning Exercise-Anxiety Relationship
| Study | Inclusion Criteria | Sample | Results | ||||
|---|---|---|---|---|---|---|---|
| RCTs Only? | Exercise | Control | Other | Studies | N | ||
| Bartley et al., 2013 | Yes | More than 1 session | Any alternative intervention | Anxiety d/o other than PTSD | 7 | 407 | Exercise is similar, ES = 0.02. |
| Conn, 2010 | No | Any non-acute intervention to increase physical activity | N/A | No elevated anxiety or anxiety d/o | 19 | 3,789 | Exercise is superior, ES = 0.22. |
| Herring et al., 2010 | Yes | At least 3 weeks of any exercise | Nonexercise comparison conditions only | Sedentary adults with chronic illness | 40 | 2,914 | Exercise is superior, ES = 0.29. |
| Petruzzello et al., 1991 | No | Any exercise, single or multiple bouts | N/A | N/A | 104 | 3,048 | Exercise is effective for state anxiety (ES = 0.24), trait anxiety (ES = 0.34), and psychophysiological correlates of anxiety (ES = 0.56). |
| Wipfli et al, 2008 | Yes | Any exercise, single or multiple bouts | Any alternative intervention | No co-intervention for exercise condition | 49 | 3,566 | Exercise is superior, ES = −0.48. |
The remaining meta-analysis by Bartley and colleagues (105) addressed exercise RCTs among individuals with any diagnosed anxiety disorder (aside from PTSD). Results found no significant difference between exercise and control conditions for anxiety outcomes (ES = 0.02). Moderator analyses suggest that this non-significant finding could be attributed to between-study heterogeneity. When analysis was restricted to studies comparing exercise with placebo or waitlist controls, exercise showed a significant effect (SMD = 1.42), but no significant difference was found for exercise compared to other anxiolytic treatments such as CBT (SMD = -0.28). Thus, results of the meta-analysis suggested that exercise provided similar benefit to other established treatments for anxiety and resulted in superior outcomes compared with treatment or placebo treatment. It should be noted that moderator analyses based on a subsample of available studies is problematic because only 7 studies met inclusion criteria for the full analysis, and the analysis combined a heterogeneous group of anxiety disorders into a single entity. RCTs among individuals with elevated anxiety symptoms but without an anxiety diagnosis were not included, and the diagnoses present were similarly heterogeneous to those found in our current review.
DISCUSSION
The present systematic review sought to describe what is known about the efficacy of exercise for treatment of anxiety. Our search identified 12 RCTs and 5 meta-analyses that satisfactorily addressed this issue. We systematically reviewed the extant studies and extracted data on sample characteristics, study design, key methodological features, and anxiety outcomes. The majority of studies concluded that, as a treatment for elevated anxiety or anxiety disorders, exercise offers benefits comparable to established treatments, including medication or CBT, and better than those of placebo or waitlist control. However, review of available RCTs and meta-analyses revealed that most studies suffer from significant methodological limitations that leave the issue of the use of exercise to treat anxiety unresolved.
Because of the heterogeneity in the definition of “anxiety” and “exercise” across the various RCTs, we believe that it is inappropriate to combine the data from these diverse studies in a quantitative way. We therefore elected to perform a comprehensive, qualitative review of the extant studies. This approach contrasts with prior reviews (49, 50), which have attempted to evaluate the exercise-anxiety relationship statistically, including through meta-analysis. Given the important methodological differences and shortcomings of the studies we identified, we instead elected to provide a more qualitative and critical analysis of the existing literature.
One recent review (50) limited its scope to exercise RCTs only among individuals with anxiety diagnoses. This group located a total of 8 RCTs meeting their inclusion criteria. Three of the trials evaluated by this group (67, 68, 69, 75, 76, 84) also met the inclusion criteria for the present review. The remaining five studies did not meet our inclusion criteria. These studies comprised trials (a) among individuals with any non-psychotic psychiatric diagnosis, not anxiety alone (65), (b) with a single-bout exercise intervention (37), (c) with no non-exercise control group (63), (d) with an intervention that did not require exercise for all participants receiving it (60), and (e) with an outcome measure encompassing general quality of life rather than anxiety specifically (57). Our perspective is that these studies by design cannot provide sufficient evidence either for or against the hypothesis that exercise reduces symptoms of anxiety in anxious individuals. In contrast, 9 additional RCTs were included in the present review. These studies comprised five exercise trials to reduce anxiety among individuals with anxiety diagnoses, to include Social Anxiety Disorder (70, 71, 72), GAD (73, 74), PTSD (80), Panic Disorder (85), and any anxiety diagnosis (79). In addition, our review included four trials among individuals with elevated symptoms of anxiety (77, 78, 81) or anxiety sensitivity (82, 83). We believe that including these trials more accurately represents the available evidence concerning exercise as a treatment for anxiety.
Unlike depression, for which many RCTs exist and have been the subject of many reviews and meta-analyses (44, 45, 46), research on exercise in patients with anxiety is significantly more limited. Furthermore, whereas depressive symptoms may be conceptualized along a single continuum (107, 108), anxiety disorders represent distinct clusters of symptoms within a broad constellation of diagnoses. One exception to this pattern would be GAD. However, GAD and broad anxiety symptoms were studied in only 3 RCTs. The most commonly represented disorder in our review, Panic Disorder (4 of 12 RCTs), has a lifetime prevalence of less than 5%, well below the lifetime 30% prevalence of anxiety disorders as a whole (2). Because of the great heterogeneity in inclusion criteria in exercise RCTs for anxiety, we cannot be confident that exercise will improve symptoms of anxiety in any given diagnostic group.
We also observed great variation in exercise interventions, with studies involving different modes of exercise for different durations and varied intensities. We were surprised to discover that aerobic fitness often was not measured, and when it was, it often did not improve after exercise interventions, despite the fact that anxiety was reduced. For example, participants in one study (67, 68, 69) showed lower HAM-A and BAI scores among participants assigned to running, but at 10-week post-test, their timed running distance was not different from controls. Interestingly, in the one RCT that compared two exercise modalities (73, 74), resistance exercise showed better fitness and lower POMS-T scores at post-test, but aerobic exercise did not. Most studies failed to document improvements in cardiorespiratory function associated with exercise training (e.g., changes in VO2 or submaximal heart rates at matching workloads), which also is problematic, as such changes in physical fitness would provide an important manipulation check to verify that the exercise intervention was sufficient to produce expected cardiopulmonary benefits and improved functional capacity. Similarly, few studies used the same instruments to measure anxiety outcomes, and in many cases, these measures appear to be specific to one population (e.g., those with panic symptoms or PTSD symptoms), limiting generalizability.
Existing RCTs also had other methodological problems that limit our ability to draw definitive conclusions. One third of the trials did not control for outside interventions for anxiety. For example, in the one RCT that had a sample of over 100 participants, participants assigned to exercise also received CBT and relaxation training concurrently (77, 78), which seriously detracted from the methodological quality of the study. Another RCT only delivered exercise in the context of a mindfulness intervention (80). Attrition of 20% or greater was noted in half of the trials. Intent-to-treat analysis was reported in only half of the studies, with one of these reporting primarily an analysis of completers (67, 68, 69). In many cases, self-report outcomes were the only posttest measures of anxiety employed. Taken together, examination of what are widely considered the most important methodological quality indicators revealed that existing studies have significant methodological weaknesses and a moderate risk of bias.
One way to determine the strength of exercise as an effective treatment for anxious individuals is to observe a dose-response effect. Such evidence would be critical to establishing a direct treatment effect of exercise on anxiety. Unfortunately, this important issue has been minimally explored in the literature. A prior meta-analysis (49), which found an overall benefit of exercise for anxiety reduction, attempted to quantify a dose-response relationship. One trial of individuals with Panic Disorder did compare directly the effects of a single bout of light or heavy physical activity on panic symptoms, finding that more intense activity did have a greater antipanic effect (37). However, this study enrolled a sample of only 18 participants and had no non-exercise control group. Because no rigorous RCTs have conducted a direct, experimental comparison of exercise volume (intensity and duration) on anxious participants, an optimal dose of exercise cannot be determined. Randomizing participants to differing intensities or frequencies of exercise is needed to determine the optimal dose of exercise to reduce anxiety. This approach has been used successfully in research on exercise for depression (109) but has not been investigated for anxiety.
Our review revealed that exercise interventions often were unsupervised and that intensity and frequency of exercise was typically either not reported or not manipulated as part of the study design. For example, some running interventions encouraged participants to increase their pace over several weeks, but only one session per week was supervised (67, 68, 69, 84), and in other RCTs, participants were directed to do exercise of their choosing (70, 71, 72). Of note, some trials of single-bout exercise have suggested that a dose-response effect may exist, though findings have been equivocal. For example, one study found that healthy individuals completing 20 minutes of low-intensity resistance exercise with weightlifting machines had immediate reductions in state anxiety, whereas anxiety increased among the high-intensity group (110). Another study found significant reductions in anxiety sensitivity for healthy individuals completing low- and high-intensity treadmill exercise, with greater reductions among the high-intensity group (111). Importantly, individuals can give differing self-reports of their affective response after a single bout of exercise, depending on how soon this self-report is solicited (112). Further research to address the optimal dose of exercise is needed.
Few studies have examined exercise as an adjunctive treatment. We identified one RCT (75, 76) that examined the effect of exercise compared to education control among anxious participants receiving group CBT; no treatment group differences were observed. Another study (84) used a 2x2 design to explore the utility of aerobic exercise and an SSRI (paroxetine), separately and combined, with no differences for combined versus unimodal treatment. Further research is needed to confirm whether adding exercise to other treatments confers a benefit to individuals with anxiety.
The issue of the mechanism(s) by which exercise affects anxiety seldom has been studied, although several potential pathways have been identified (113). Potential physiological explanations include regulation of the hypothalamic-pituitary-adrenal (HPA) axis, increases in serotonergic and noradrenergic levels in the brain, and endogenous opioid release. Psychological factors may also play a key role. For example, interventions such as CBT for anxiety often employ exposure to feared sensations or situations, combined with prevention of maladaptive responses that provide short-term relief (24). The mechanism by which CBT is effective remains poorly understood (114). However, in this case, the intervention is intended to promote habituation and a reduction in anxiety symptoms (24). Indeed, two studies we reviewed (82, 83) aimed to reduce anxiety sensitivity through aerobic exercise, which can create sensations similar to anxiety or panic (e.g., rapid heartbeat). Interestingly, one study that did not meet our inclusion criteria (62) involved individuals participating in CR who had elevated anxiety specific to walking. Anxiety improved among individuals who were assigned to walk for exercise but not in those assigned to cycling, suggesting that exposure to a specific feared stimulus may have helped. Alternatively, exercise may improve self-efficacy through progressive positive feedback, such as fitness gains (113). Existing evidence from RCTs does not adequately address whether exercise can reduce anxiety via improvements in fitness and related physiological changes, psychological changes, or a combination of factors; indeed, the relative paucity of evidence leaves open the question of whether a direct mechanism for exercise to reduce anxiety exists.
We included studies in our review in which participants’ anxiety symptoms did not necessarily meet criteria for anxiety disorders in DSM-5. For example, two of the identified RCTs address high anxiety sensitivity, a marker that may serve as a precursor to panic attacks or GAD. In addition, one RCT (80) was conducted among individuals with PTSD, a diagnosis which is now in a separate classification from anxiety disorders (1). We included these RCTs to address anxiety as broadly as possible, in light of the scarcity of the existing literature and the heterogeneity of populations sampled; we also did not identify any completed RCTs among individuals with DSM-5-diagnosed anxiety disorders.
Although a few RCTs included participants with one of several anxiety diagnoses, no single study addressed the issue of whether one anxiety disorder was more responsive to exercise than another, or whether situational anxiety symptoms responded as well to exercise as did persistent symptoms of anxiety. Testing for such moderator effects would be valuable but would require trials with larger samples than have been used to date.
The present review has several limitations. We did not personally contact researchers about unpublished RCTs, so the potential bias of the ‘file drawer effect’ (115, 116) could not be determined. However, because such unpublished studies were likely to suffer from significant methodological weaknesses or yield ‘null’ effects, it is unlikely that these additional data would alter our conclusions. Second, we limited our search to individuals of ages 18 years and over. Although we elected not include children in our review, we performed an additional search of our database for studies of exercise in anxious children. We failed to identify a single RCT of exercise training in persons under 18 years old that met our inclusion criteria. Therefore, we cannot comment on the potential benefits of exercise for children with elevated anxiety and suggest that this is an important, and understudied, area for future research.
In summary, findings from the present review suggest that exercise could be a useful, affordable, accessible treatment for anxiety. However, there appears to be a paucity of data from well-designed RCTs, and the methodological limitations in the existing trials of exercise preclude drawing definitive conclusions about its effectiveness. Indeed, the existing literature is marked by small trials with weak internal validity. At present, the existing body of evidence is not of sufficient scientific rigor to recommend it as a treatment among individuals with clinically elevated anxiety. Future research will require robust experimental designs and greater attention to critical methodological details including appropriate control groups, adequate sample sizes, use of intent-to-treat analysis, blinding of assessors, allocation concealment, monitoring of exercise adherence and intensity, documentation of aerobic “training effects,” and selection of well-validated instruments to assess anxiety before and following treatment.
Acknowledgements
The authors wish to acknowledge José Sandoval, Duke University, for his assistance with article review and data extraction. This manuscript was supported, in part, by Grant HL080664-01 from the National Institutes of Health, Bethesda, Maryland.
Footnotes
Authors’ Statement of Conflict of Interest:
Authors Stonerock, Hoffman, Smith, and Blumenthal declare that they have no conflicts of interest.
REFERENCES
- 1.Diagnostic and Statistical Manual of Mental Disorders: DSM-5. American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- 2.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International Journal of Methods in Psychiatric Research. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haller H, Cramer H, Lauche R, Gass F, Dobos G. The prevalence and burden of subthreshold generalized anxiety disorder: a systematic review. BMC Psychiatry. 2014;14:128. doi: 10.1186/1471-244X-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein MB, Roy-Byrne PP, Craske MG, et al. Functional impact and health utility of anxiety disorders in primary care outpatients. Medical Care. 2005;43:1164–1170. doi: 10.1097/01.mlr.0000185750.18119.fd. [DOI] [PubMed] [Google Scholar]
- 5.Tolmunen T, Lehto SM, Julkunen J, Hintikka J, Kauhanen J. Trait anxiety and somatic concerns associate with increased mortality risk: a 23-year follow-up in aging men. Annals of Epidemiology. 2014;24:463–468. doi: 10.1016/j.annepidem.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Roest AM, Martens EJ, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. Journal of the American College of Cardiology. 2010;56:38–46. doi: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Janszky I, Ahnve S, Lundberg I, Hemmingsson T. Early-onset depression, anxiety, and risk of subsequent coronary heart disease: 37-year follow-up of 49,321 young Swedish men. Journal of the American College of Cardiology. 2010;56:31–37. doi: 10.1016/j.jacc.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 8.Roest AM, Martens EJ, Denollet J, de Jonge P. Prognostic association of anxiety post myocardial infarction with mortality and new cardiac events: a meta-analysis. Psychosomatic Medicine. 2010;72:563–569. doi: 10.1097/PSY.0b013e3181dbff97. [DOI] [PubMed] [Google Scholar]
- 9.Frasure-Smith N, Lespérance F. Depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease. Archives of General Psychiatry. 2008;65:62–71. doi: 10.1001/archgenpsychiatry.2007.4. [DOI] [PubMed] [Google Scholar]
- 10.Frasure-Smith N. In-hospital symptoms of psychological stress as predictors of long-term outcome after acute myocardial infarction in men. The American Journal of Cardiology. 1991;67:121–127. doi: 10.1016/0002-9149(91)90432-k. [DOI] [PubMed] [Google Scholar]
- 11.Tully PJ, Baker RA, Knight JL. Anxiety and depression as risk factors for mortality after coronary artery bypass surgery. Journal of Psychosomatic Research. 2008;64:285–290. doi: 10.1016/j.jpsychores.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Tully P, Baker R, Turnbull D, Winefield H. The role of depression and anxiety symptoms in hospital readmissions after cardiac surgery. Journal of Behavioral Medicine. 2008;31:281–290. doi: 10.1007/s10865-008-9153-8. [DOI] [PubMed] [Google Scholar]
- 13.Szekely A, Balog P, Benko E, et al. Anxiety predicts mortality and morbidity after coronary artery and valve surgery—a 4-year follow-up study. Psychosomatic Medicine. 2007;69:625–631. doi: 10.1097/PSY.0b013e31814b8c0f. [DOI] [PubMed] [Google Scholar]
- 14.Moser DK, McKinley S, Riegel B, et al. Relationship of persistent symptoms of anxiety to morbidity and mortality outcomes in patients with coronary heart disease. Psychosomatic Medicine. 2011;73:803–809. doi: 10.1097/PSY.0b013e3182364992. [DOI] [PubMed] [Google Scholar]
- 15.Watkins LL, Koch GG, Sherwood A, et al. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. Journal of the American Heart Association. 2013;2 doi: 10.1161/JAHA.112.000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothenbacher D, Hahmann H, Wusten B, Koenig W, Brenner H. Symptoms of anxiety and depression in patients with stable coronary heart disease: prognostic value and consideration of pathogenetic links. European Journal of Cardiovascular Prevention and Rehabilitation. 2007;14:547–554. doi: 10.1097/HJR.0b013e3280142a02. [DOI] [PubMed] [Google Scholar]
- 17.Strik JJ, Denollet J, Lousberg R, Honig A. Comparing symptoms of depression and anxiety as predictors of cardiac events and increased health care consumption after myocardial infarction. Journal of the American College of Cardiology. 2003;42:1801–1807. doi: 10.1016/j.jacc.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Shibeshi WA, Young-Xu Y, Blatt CM. Anxiety worsens prognosis in patients with coronary artery disease. Journal of the American College of Cardiology. 2007;49:2021–2027. doi: 10.1016/j.jacc.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Markovitz JH, Matthews KA, Kannel WB, Cobb JL, D'Agostino RB. Psychological predictors of hypertension in the Framingham Study. Is there tension in hypertension? Journal of the American Medical Association. 1993;270:2439–2443. [PubMed] [Google Scholar]
- 20.Kawachi I, Sparrow D, Vokonas PS, Weiss ST. Symptoms of anxiety and risk of coronary heart disease. The Normative Aging Study. Circulation. 1994;90:2225–2229. doi: 10.1161/01.cir.90.5.2225. [DOI] [PubMed] [Google Scholar]
- 21.McCarron P, Gunnell D, Harrison GL, Okasha M, Davey Smith G. Temperament in young adulthood and later mortality: prospective observational study. Journal of Epidemiology and Community Health. 2003;57:888–892. doi: 10.1136/jech.57.11.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnet F, Irving K, Terra J-L, et al. Anxiety and depression are associated with unhealthy lifestyle in patients at risk of cardiovascular disease. Atherosclerosis. 2005;178:339–344. doi: 10.1016/j.atherosclerosis.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 23.Jonas BS, Franks P, Ingram DD. Are symptoms of anxiety and depression risk factors for hypertension? Longitudinal evidence from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Fam Med. 1997;6:43–49. doi: 10.1001/archfami.6.1.43. [DOI] [PubMed] [Google Scholar]
- 24.Barlow DH. Clinical Handbook of Psychological Disorders : A Step-By-Step Treatment Manual. 5th Ed. The Guilford Press; New York, NY: 2014. [Google Scholar]
- 25.Barlow DH, Gorman JM, Shear M, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. Journal of the American Medical Association. 2000;283:2529–2536. doi: 10.1001/jama.283.19.2529. [DOI] [PubMed] [Google Scholar]
- 26.Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive- behavioral therapy: a review of meta-analyses. Clinical Psychology Review. 2006;26:17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Kapczinski F, Lima MS, Souza JS, Schmitt R. Antidepressants for generalized anxiety disorder. Cochrane Database of Systematic Reviews. 2003 doi: 10.1002/14651858.CD003592. [DOI] [PubMed] [Google Scholar]
- 28.Stahl SM, Gergel I, Li D. Escitalopram in the treatment of panic disorder: a randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry. 2003;64:1322–1327. doi: 10.4088/jcp.v64n1107. [DOI] [PubMed] [Google Scholar]
- 29.Davidson JRT, Bose A, Korotzer A, Zheng H. Escitalopram in the treatment of generalized anxiety disorder: double-blind, placebo controlled, flexible-dose study. Depression and Anxiety. 2004;19:234–240. doi: 10.1002/da.10146. [DOI] [PubMed] [Google Scholar]
- 30.Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. Journal of Psychopharmacology. 2005;19:567–596. doi: 10.1177/0269881105059253. [DOI] [PubMed] [Google Scholar]
- 31.Pampallona S, Bollini P, Tibaldi G, Kupelnick B, Munizza C. Patient adherence in the treatment of depression. The British Journal of Psychiatry. 2002;180:104–109. doi: 10.1192/bjp.180.2.104. [DOI] [PubMed] [Google Scholar]
- 32.Gunter RW, Whittal ML. Dissemination of cognitive-behavioral treatments for anxiety disorders: overcoming barriers and improving patient access. Clinical Psychology Review. 2010;30:194–202. doi: 10.1016/j.cpr.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Reports. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 34.Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Preventive Medicine. 2003;36:698–703. doi: 10.1016/s0091-7435(03)00042-2. [DOI] [PubMed] [Google Scholar]
- 35.De Moor MHM, Beem AL, Stubbe JH, Boomsma DI, De Geus EJC. Regular exercise, anxiety, depression and personality: a population-based study. Preventive Medicine. 2006;42:273–279. doi: 10.1016/j.ypmed.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Strickland JC, Smith MA. The anxiolytic effects of resistance exercise. Front Psychol. 2014;5:753. doi: 10.3389/fpsyg.2014.00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esquivel G, Diaz-Galvis J, Schruers K, et al. Acute exercise reduces the effects of a 35% CO2 challenge in patients with panic disorder. Journal of Affective Disorders. 2008;107:217–220. doi: 10.1016/j.jad.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 38.Yeung RR. The acute effects of exercise on mood state. Journal of Psychosomatic Research. 1996;40:123–141. doi: 10.1016/0022-3999(95)00554-4. [DOI] [PubMed] [Google Scholar]
- 39.Berger BG, Owen DR. Relation of low and moderate intensity exercise with acute mood change in college joggers. Perceptual and Motor Skills. 1998;87:611–621. doi: 10.2466/pms.1998.87.2.611. [DOI] [PubMed] [Google Scholar]
- 40.King AC, Taylor CB, Haskell WL. Effects of differing intensities and formats of 12 months of exercise training on psychological outcomes in older adults. Health Psychology. 1993;12:292–300. doi: 10.1037//0278-6133.12.4.292. [DOI] [PubMed] [Google Scholar]
- 41.Tsai J-C, Wang W-H, Chan P, et al. The beneficial effects of Tai Chi Chuan on blood pressure and lipid profile and anxiety status in a randomized controlled trial. The Journal of Alternative and Complementary Medicine. 2003;9:747–754. doi: 10.1089/107555303322524599. [DOI] [PubMed] [Google Scholar]
- 42.Stavrakaki C, Vargo B. The relationship of anxiety and depression: a review of the literature. The British Journal of Psychiatry. 1986;149:7–16. doi: 10.1192/bjp.149.1.7. [DOI] [PubMed] [Google Scholar]
- 43.Zung WW, Magruder-Habib K, Velez R, Alling W. The comorbidity of anxiety and depression in general medical patients: a longitudinal study. Journal of Clinical Psychiatry. 1990;51:77–80. [PubMed] [Google Scholar]
- 44.Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database of Systematic Reviews. 2013;9:Cd004366. doi: 10.1002/14651858.CD004366.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mead GE, Morley W, Campbell P, et al. Exercise for depression. Cochrane Database of Systematic Reviews. 2009:Cd004366. doi: 10.1002/14651858.CD004366.pub4. [DOI] [PubMed] [Google Scholar]
- 46.Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322:763. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herring MP, O'Connor PJ, Dishman RK. The effect of exercise training on anxiety symptoms among patients: a systematic review. Archives of Internal Medicine. 2010;170:321–331. doi: 10.1001/archinternmed.2009.530. [DOI] [PubMed] [Google Scholar]
- 48.Conn VS. Anxiety outcomes after physical activity interventions: meta-analysis findings. Nursing Research. 2010;59 doi: 10.1097/NNR.0b013e3181dbb2f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wipfli BM, Rethorst CD, Landers DM. The anxiolytic effects of exercise: a meta- analysis of randomized trials and dose-response analysis. Journal of Sport & Exercise Psychology. 2008;30:392–410. doi: 10.1123/jsep.30.4.392. [DOI] [PubMed] [Google Scholar]
- 50.Jayakody K, Gunadasa S, Hosker C. Exercise for anxiety disorders: systematic review. British Journal of Sports Medicine. 2014;48:187–196. doi: 10.1136/bjsports-2012-091287. [DOI] [PubMed] [Google Scholar]
- 51.Focht BC, Koltyn KF. Influence of resistance exercise of different intensities on state anxiety and blood pressure. Medicine and Science in Sports and Exercise. 1999;31:456–463. doi: 10.1097/00005768-199903000-00016. [DOI] [PubMed] [Google Scholar]
- 52.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Physical Therapy. 2003;83:713–721. [PubMed] [Google Scholar]
- 53.Annesi JJ, Gorjala S. Association of reduction in waist circumference with normalization of mood in obese women initiating exercise supported by the Coach Approach protocol. Southern Medical Journal. 2010;103:517–521. doi: 10.1097/SMJ.0b013e3181de0eb5. [DOI] [PubMed] [Google Scholar]
- 54.Hale BS, Raglin JS. State anxiety responses to acute resistance training and step aerobic exercise across eight weeks of training. Journal of Sports Medicine and Physical Fitness. 2002;42:108–112. [PubMed] [Google Scholar]
- 55.Smith JA, Greer T, Sheets T, Watson S. Is there more to yoga than exercise? Alternative therapies in health and medicine. 2011;17:22–29. [PubMed] [Google Scholar]
- 56.Mussgay L, Schmidt F, Morad E, Ruddel H. Does aerobic exercise modulate baroreflex sensitivity in patients with anxiety and somatization disorders? Homeostasis in Health and Disease. 2003;42:277–287. [Google Scholar]
- 57.Oeland A-M, Laessoe U, Olesen AV, Munk-Jorgensen P. Impact of exercise on patients with depression and anxiety. Nordic Journal of Psychiatry. 2010;64:210–217. doi: 10.3109/08039480903511373. [DOI] [PubMed] [Google Scholar]
- 58.Wedekind D, Sprute A, Broocks A, et al. Nocturnal urinary cortisol excretion over a randomized controlled trial with paroxetine vs. placebo combined with relaxation training or aerobic exercise in panic disorder. Current Pharmaceutical Design. 2008;14:3518–3524. doi: 10.2174/138161208786848757. [DOI] [PubMed] [Google Scholar]
- 59.Gaul-Alacova P, Boucek J, Stejskal P, et al. Assessment of the influence of exercise on heart rate variability in anxiety patients. Neuroendocrinology Letters. 2005;26:713–718. [PubMed] [Google Scholar]
- 60.Lambert RA, Harvey I, Poland F. A pragmatic, unblinded randomised controlled trial comparing an occupational therapy-led lifestyle approach and routine GP care for panic disorder treatment in primary care. Journal of Affective Disorders. 2007;99:63–71. doi: 10.1016/j.jad.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 61.Kim J-H, Yang H, Schroeppel S. A pilot study examining the effects of Kouk Sun Do on university students with anxiety symptoms. Stress and Health. 2013;29:99–107. doi: 10.1002/smi.2431. [DOI] [PubMed] [Google Scholar]
- 62.Faulkner J, Westrupp N, Rousseau J, Lark S. A randomized controlled trial to assess the effect of self-paced walking on task-specific anxiety in cardiac rehabilitation patients. Journal of Cardiopulmonary Rehabilitation and Prevention. 2013;33:292–296. doi: 10.1097/HCR.0b013e3182a0295c. [DOI] [PubMed] [Google Scholar]
- 63.Martinsen EW, Hoffart A, Solberg OY. Aerobic and non-aerobic forms of exercise in the treatment of anxiety disorders. Stress Medicine. 1989;5:115–120. [Google Scholar]
- 64.Murphy SM, Edwards RT, Williams N, et al. An evaluation of the effectiveness and cost effectiveness of the National Exercise Referral Scheme in Wales, UK: a randomised controlled trial of a public health policy initiative. Journal of Epidemiology and Community Health. 2012;66:745–753. doi: 10.1136/jech-2011-200689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sexton H, Maere A, Dahl NH. Exercise intensity and reduction in neurotic symptoms: a controlled follow-up study. Acta Psychiatrica Scandinavica. 1989;80:231–235. doi: 10.1111/j.1600-0447.1989.tb01332.x. [DOI] [PubMed] [Google Scholar]
- 66.Goldstein-Shirley J, Brown M. Communicating Nursing Research. Palm Springs; CA: 2002. Intervention of light, exercise and vitamins for mixed anxiety-depression. [Google Scholar]
- 67.Bandelow B, Broocks A, Pekrun G, et al. The use of the Panic and Agoraphobia Scale (P & A) in a controlled clinical trial. Pharmacopsychiatry. 2000;33:174–181. doi: 10.1055/s-2000-12982. [DOI] [PubMed] [Google Scholar]
- 68.Broocks A, Bandelow B, Pekrun G, et al. Comparison of aerobic exercise, clomipramine, and placebo in the treatment of panic disorder. American Journal of Psychiatry. 1998;155:603–609. doi: 10.1176/ajp.155.5.603. [DOI] [PubMed] [Google Scholar]
- 69.Broocks A, Meyer T, Opitz M, et al. 5-HT1A responsivity in patients with panic disorder before and after treatment with aerobic exercise, clomipramine or placebo. European Neuropsychopharmacology. 2003;13:153–164. doi: 10.1016/s0924-977x(02)00177-3. [DOI] [PubMed] [Google Scholar]
- 70.Goldin P, Ziv M, Jazaieri H, Gross JJ. Randomized controlled trial of mindfulness-based stress reduction versus aerobic exercise: effects on the self-referential brain network in social anxiety disorder. Frontiers in Human Neuroscience. 2012 doi: 10.3389/fnhum.2012.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldin P, Ziv M, Jazaieri H, Hahn K, Gross JJ. MBSR vs aerobic exercise in social anxiety: fMRI of emotion regulation of negative self-beliefs. Social Cognitive and Affective Neuroscience. 2013;8:65–72. doi: 10.1093/scan/nss054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jazaieri H, Goldin PR, Werner K, Ziv M, Gross JJ. A randomized trial of MBSR versus aerobic exercise for social anxiety disorder. Journal of Clinical Psychology. 2012;68:715–731. doi: 10.1002/jclp.21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herring MP, Jacob ML, Suveg C, Dishman RK, O'Connor PJ. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychotherapy and Psychosomatics. 2012;81:21–28. doi: 10.1159/000327898. [DOI] [PubMed] [Google Scholar]
- 74.Herring MP, Jacob ML, Suveg C, O'Connor PJ. Effects of short-term exercise training on signs and symptoms of generalized anxiety disorder. Mental Health and Physical Activity. 2011;4:71–77. [Google Scholar]
- 75.Merom D, Phongsavan P, Wagner R, et al. Promoting walking as an adjunct intervention to group cognitive behavioral therapy for anxiety disorders—a pilot group randomized trial. Journal of Anxiety Disorders. 2008;22:959–968. doi: 10.1016/j.janxdis.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 76.Phongsavan P, Merom D, Wagner R, et al. Process evaluation in an intervention designed to promote physical activity among adults with anxiety disorders: evidence of acceptability and adherence. Health Promotion Journal of Australia. 2008;19:137–143. doi: 10.1071/he08137. [DOI] [PubMed] [Google Scholar]
- 77.Oldridge N, Guyatt G, Jones N, et al. Effects on quality of life with comprehensive rehabilitation after acute myocardial infarction. American Journal of Cardiology. 1991;67:1084–1089. doi: 10.1016/0002-9149(91)90870-q. [DOI] [PubMed] [Google Scholar]
- 78.Oldridge N, Streiner D, Hoffmann R, Guyatt G. Profile of mood states and cardiac rehabilitation after acute myocardial infarction. Medicine and Science in Sports and Exercise. 1995;27:900–905. [PubMed] [Google Scholar]
- 79.Carmeli E, Barak S, Morad M, Kodesh E. Physical exercises can reduce anxiety and improve quality of life among adults with intellectual disability. International SportMed Journal. 2009;10:77–85. [Google Scholar]
- 80.Kim SH, Schneider SM, Bevans M, et al. PTSD symptom reduction with mindfulness- based stretching and deep breathing exercise: randomized controlled clinical trial of efficacy. Journal of Clinical Endocrinology and Metabolism. 2013;98:2984–2992. doi: 10.1210/jc.2012-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gutiérrez CV, Luque GT, Medina GMÁ, et al. Influence of exercise on mood in postmenopausal women. Journal of Clinical Nursing. 2012;21:923–928. doi: 10.1111/j.1365-2702.2011.03972.x. [DOI] [PubMed] [Google Scholar]
- 82.Broman-Fulks JJ, Storey KM. Evaluation of a brief aerobic exercise intervention for high anxiety sensitivity. Anxiety, Stress, & Coping. 2008;21:117–128. doi: 10.1080/10615800701762675. [DOI] [PubMed] [Google Scholar]
- 83.Smits JAJ, Berry AC, Rosenfield D, et al. Reducing anxiety sensitivity with exercise. Depression and Anxiety. 2008;25:689–699. doi: 10.1002/da.20411. [DOI] [PubMed] [Google Scholar]
- 84.Wedekind D, Broocks A, Weiss N, et al. A randomized, controlled trial of aerobic exercise in combination with paroxetine in the treatment of panic disorder. World Journal of Biological Psychiatry. 2010;11:904–913. doi: 10.3109/15622975.2010.489620. [DOI] [PubMed] [Google Scholar]
- 85.Hovland A, Nordhus IH, Sjobo T, et al. Comparing physical exercise in groups to group cognitive behaviour therapy for the treatment of panic disorder in a randomized controlled trial. Behavioural and Cognitive Psychotherapy. 2013;41:408–432. doi: 10.1017/S1352465812000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, 5.1.0 [updated March 2011]: The Cochrane Collaboration. 2011 Available at www.cochrane-handbook.org.
- 87.Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. Journal of the American Medical Association. 1999;282:1054–1060. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- 88.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 89.Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 90.Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. Psychological Corporation; San Antonio, TX: 1990. [Google Scholar]
- 91.Chambless DL, Caputo GC, Bright P, Gallagher R. Assessment of fear of fear in agoraphobics: the Body Sensations Questionnaire and the Agoraphobic Cognitions Questionnaire. Journal of Consulting and Clinical Psychology. 1984;52:1090–1097. doi: 10.1037//0022-006x.52.6.1090. [DOI] [PubMed] [Google Scholar]
- 92.Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behaviour Research and Therapy. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- 93.Taylor S, Cox BJ. An expanded Anxiety Sensitivity Index: evidence for a hierarchic structure in a clinical sample. Journal of Anxiety Disorders. 1998;12:463–483. doi: 10.1016/s0887-6185(98)00028-0. [DOI] [PubMed] [Google Scholar]
- 94.Guy W, editor. ECDEU Assessment Manual for Psychopharmacology-Revised. US Department of Heath, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration; Rockville, MD: 1976. [Google Scholar]
- 95.Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. Psychology Foundation of Australia; Sydney, N.S.W.: 1995. [Google Scholar]
- 96.Fresco DM, Coles ME, Heimberg RG, et al. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychological Medicine. 2001;31:1025–1035. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- 97.Chambless DL, Caputo GC, Jasin SE, Gracely EJ, Williams C. The Mobility Inventory for Agoraphobia. Behaviour Research and Therapy. 1985;23:35–44. doi: 10.1016/0005-7967(85)90140-8. [DOI] [PubMed] [Google Scholar]
- 98.Bandelow B. Assessing the efficacy of treatments for panic disorder and agoraphobia. II. the Panic and Agoraphobia Scale. International Clinical Psychopharmacology. 1995;10:73–81. doi: 10.1097/00004850-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 99.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL). Behaviour Research and Therapy. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 100.Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. Annual Convention of the International Society for Traumatic Stress Studies. San Antonio: 1993. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. [Google Scholar]
- 101.McNair DM, Lorr M, Droppleman L. POMS: Profile of Mood States. Multi-Health Systems Inc; North Tonawanda, NY: 1992. [Google Scholar]
- 102.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 103.Rodebaugh TL, Woods CM, Heimberg RG. The reverse of social anxiety is not always the opposite: the reverse-scored items of the Social Interaction Anxiety Scale do not belong. Behavior Therapy. 2007;38:192–206. doi: 10.1016/j.beth.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 104.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State- Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto: 1983. [Google Scholar]
- 105.Bartley CA, Hay M, Bloch MH. Meta-analysis: aerobic exercise for the treatment of anxiety disorders. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2013;45:34–39. doi: 10.1016/j.pnpbp.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 106.Petruzzello SJ, Landers DM, Hatfield BD, Kubitz KA, Salazar W. A meta-analysis on the anxiety-reducing effects of acute and chronic exercise: outcomes and mechanisms. Sports Medicine. 1991;11:143–182. doi: 10.2165/00007256-199111030-00002. [DOI] [PubMed] [Google Scholar]
- 107.Santor DA, Zuroff DC, Ramsay JO, Cervantes P, Palacios J. Examining scale discriminability in the BDI and CES-D as a function of depressive severity. Psychological Assessment. 1995;7:131–139. [Google Scholar]
- 108.Slade T, Andrews G. Latent structure of depression in a community sample: a taxometric analysis. Psychological Medicine. 2005;35:489–497. doi: 10.1017/s0033291704003708. [DOI] [PubMed] [Google Scholar]
- 109.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. American Journal of Preventive Medicine. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 110.Bartholomew J, Linder D. State anxiety following resistance exercise: the role of gender and exercise intensity. Journal of Behavioral Medicine. 1998;21:205–219. doi: 10.1023/a:1018732025340. [DOI] [PubMed] [Google Scholar]
- 111.Broman-Fulks JJ, Berman ME, Rabian BA, Webster MJ. Effects of aerobic exercise on anxiety sensitivity. Behaviour Research and Therapy. 2004;42:125–136. doi: 10.1016/S0005-7967(03)00103-7. [DOI] [PubMed] [Google Scholar]
- 112.Masters KS, Spielmans GI, LaCaille RA, et al. Effects of Home Exercise on Immediate and Delayed Affect and Mood Among Rural Individuals at Risk for Type 2 Diabetes. Journal of Social, Behavioral, and Health Sciences. 2011;5:1–16. [Google Scholar]
- 113.Anderson E, Shivakumar G. Effects of exercise and physical activity on anxiety. Front Psychiatry. 2013;4:27. doi: 10.3389/fpsyt.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arch JJ, Craske MG. Acceptance and commitment therapy and cognitive behavioral therapy for anxiety disorders: different treatments, similar mechanisms? Clinical Psychology: Science and Practice. 2008;15:263–279. [Google Scholar]
- 115.Rosenthal R. The file drawer problem and tolerance for null results. Psychological Bulletin. 1979;86:638–641. [Google Scholar]
- 116.Easterbrook PJ, Gopalan R, Berlin JA, Matthews DR. Publication bias in clinical research. The Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]