Abstract
Objectives. We hypothesized that highly disordered neighborhoods would expose residents to environmental pressures, leading to reduced antiretroviral (ARV) medication adherence.
Methods. Using targeted sampling, we enrolled 503 socioeconomically disadvantaged HIV-positive substance users in urban South Florida between 2010 and 2012. Participants completed a 1-time standardized interview that took approximately 1 hour. We tested a multiple mediation model to examine the direct and indirect effects of neighborhood disorder on diversion-related nonadherence to ARVs; risky social networks and housing instability were examined as mediators of the disordered neighborhood environment.
Results. The total indirect effect in the model was statistically significant (P = .001), and the proportion of the total effect mediated was 53%. The model indicated substantial influence of neighborhood disorder on nonadherence to ARVs, operating through recent homelessness and diverter network size.
Conclusions. Long-term improvements in diversion-related ARV adherence will require initiatives to reduce demand for illicit ARV medications, as well as measures to reduce patient vulnerability to diversion, including increased resources for accessible housing, intensive treatment, and support services.
The past 15 years have witnessed increasing recognition by public health stakeholders that social and structural factors are key drivers of pervasive health inequalities, with poverty, social exclusion, stress, unemployment, and inadequate living conditions contributing to elevated disease burden among vulnerable populations.1–3 One aspect of the movement toward a social-ecological understanding of health has been a growing interest in neighborhood effects on illness and disease, with recognition that neighborhoods exert substantial influence on individuals’ psychological well-being and physical health.4 The examination of neighborhood-level factors in the disease process has gained particular momentum in the field of HIV, given that HIV infection tends to cluster geographically in areas of high poverty and high behavioral risk.5,6 Neighborhood factors have been associated with increased engagement in risky behaviors, as well as reduced access to HIV-related medical treatment and elevated AIDS-related mortality.7–9 In fact, a recent study demonstrated that neighborhoods with higher rates of poverty and unemployment, and those with higher racial segregation, were associated with poorer overall HIV disease management, manifested as lower CD4 counts.8
Neighborhoods have been viewed as operating on individual health through a variety of mechanisms, including exposure to risky social norms and networks, lower social capital, increased environmental stressors, and expanded opportunities for high-risk behavior.9 Neighborhood disorder theory emphasizes economic disadvantage as a driver of adverse health outcomes among residents; poverty and decay lead to the breakdown of physical and social order in the community, ultimately immersing the individual in stressful, hostile living conditions that weaken health.10,11 Communities with high levels of disorder are likely to be characterized by drug use, vandalism, and crime, and this disorder has been associated with poor sleep quality, psychological distress, depression, poor overall health, and increased risk for HIV.12–15
Crime, drug markets, and sex exchange venues thrive in disordered neighborhoods, which can intensify the environment of risk for HIV.16 Nevertheless, individuals’ experiences within neighborhoods have substantial heterogeneity and, as such, environmental conditions may be perceived in different ways and have differential impact on health behaviors and outcomes.17 We consider perceived neighborhood disorder as an indicator of an individual’s exposure to the local risk environment18 in which a variety of social, physical, and economic factors combine to influence drug- and disease-related harms.
Although neighborhood disorder has previously been implicated in increased HIV risk-taking behavior among injection drug users,19 to our knowledge previous research has not examined the impact of neighborhood disorder on behavioral disease management among HIV-positive individuals, which is critical for viral suppression.20 We hypothesized that location in a highly disordered neighborhood would expose HIV-positive residents to environmental pressures that have a negative impact on their adherence to antiretroviral (ARV) medications. In this regard, we recently documented the diversion (selling or trading) of ARV medications among high-needs HIV-positive substance abusers in South Florida,21 which was associated with reduced ARV adherence.
The diversion of ARVs to the illicit market appears to be driven by a variety of demand-related factors, including medication seeking among acutely disadvantaged HIV-positive individuals; in some instances, street purchases of ARVs serve as an informal mechanism for coping with limited access to, or gaps in, formal HIV care or medication insurance coverage and serve a need for urgent medication acquisition to replace lost, stolen, or ruined prescriptions.22 Of particular relevance to this analysis, much of the demand for illicit ARVs appears to be concentrated among networks of nonpatient pill brokers who seek out vulnerable HIV-positive individuals to buy their legitimately obtained ARVs, with the goal of acquiring unmarked bottles of expensive, frontline ARV medications that can be recycled back into the formal medication supply chain.22 Because ARV diversion is driven in large part by an organized system of pill brokers and local pharmacies targeting economically vulnerable patients for exploitation,21,22 we argue that highly disordered neighborhood environments increase exposure to such diversion activities and, as a result, reduce ARV adherence.
We examined risky social networks and housing instability as key elements of the disordered neighborhood environment that may mediate individual ARV diversion and adherence behaviors. High-risk personal networks have been demonstrated to act as significant sources of vulnerability for their members,23 increasing both risky needle-sharing behaviors and sexual risk for HIV.24,25 We propose that location in a disordered neighborhood environment increases an individual’s exposure to such risky social connections, which may promote ARV diversion and thereby inhibit full ARV medication compliance. Homeless individuals also have greater exposure to conditions on the streets than those who are housed9 and suffer from a range of health, economic, and social vulnerabilities.26–29 Because highly disordered neighborhoods are likely to have concentrations of residents who are unstably housed and economically disadvantaged, these individuals are likely to be targeted for ARV diversion and ultimately suffer from reduced adherence. We tested a multiple mediation model to examine the direct and indirect effects of neighborhood disorder on diversion-related nonadherence to ARVs.
METHODS
We enrolled socioeconomically disadvantaged HIV-positive substance users in urban South Florida between 2010 and 2012. Eligibility criteria for all participants were (1) aged 18 years or older; (2) active substance use, defined as 12 or more occasions of cocaine or heroin use in the 3 months preceding enrollment; (3) documented HIV-positive status; and (4) current prescription for ARV medication. The study design called for the enrollment of equal numbers of participants who diverted (sold or traded) their personal ARVs and participants who did not. For inclusion in the diverter sample, participants had to indicate engaging in at least 1 ARV sale or trade in the 3 months preceding the interview.
Study Recruitment
We recruited participants using modified targeted sampling techniques,30 which are widely used for contacting hard-to-reach populations. Recruitment was guided by 2 primary elements of targeted sampling. First, using county-level indicator data, we identified 6 specific geographic target areas in urban Miami (defined by zip code boundaries) that report intersecting and persistent high HIV prevalence and high poverty rates. Second, we collected information on ARV diversion from key informants in these target areas (including treatment professionals, community outreach workers, HIV service providers, and illicit drug users) to identify specific locales in which diversion activities were known to occur. Initial recruitment efforts targeted 6 geographically clustered areas to the north of downtown Miami. A team of professional field staff and outreach workers conducted direct outreach on at least a weekly basis to distribute study information cards and flyers to all major HIV service organizations within the identified target areas. On the basis of diversion activity reports from key informants, outreach teams also regularly distributed study recruitment materials in specific street venues and other identified service locations in the target areas. Following similar procedures, we subsequently expanded recruitment efforts to areas with high HIV prevalence and poverty in urban Ft. Lauderdale.
Study Procedures
Study recruitment materials contained contact information for the project, and potential participants were asked to participate in telephone screening for eligibility. Those meeting project eligibility requirements were scheduled for appointments at the field site, where they were rescreened. In total, 2112 individuals were screened for the study, 599 met study eligibility criteria, and 503 were enrolled. As mentioned, we enrolled approximately equal numbers diverting (n = 251) and not diverting (n = 252) their personal ARVs. After eligibility was confirmed, informed consent documents were reviewed with participants, and consent was obtained. A 1-time standardized interview assessment was then administered, which took approximately 1 hour to complete. Participants were paid a $30 stipend on completion of the interview and were provided educational and risk reduction materials, along with appropriate community resource referrals.
Data Collection and Measures
Trained interviewers conducted computer-assisted personal interviews. Interviews were offered in either English or Spanish, according to the participant’s language preference. The Global Appraisal of Individual Needs (GAIN)31 version 5.4 was the primary component of the assessment. The GAIN collects detailed information on demographics, mental health (including Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [DSM-IV]32 criteria), environment and living situation, victimization, substance use, and DSM-IV dependence and has established reliabilities. For this study, we supplemented the GAIN with brief standardized instruments to assess HIV diagnosis and treatment history33 and recent ARV adherence and reasons for nonadherence34; newly developed items captured ARV diversion behaviors. Demographic information gathered on study participants included age, race/ethnicity, gender, level of education, and monthly income. We also computed length of time since HIV diagnosis.
For this analysis, the health behaviors of interest included 3 domains. We assessed substance dependence using DSM-IV criteria, which consisted of 7 items measuring past-year drug problem severity. Endorsement of 6 or more items (e.g., using more or longer than intended, withdrawal problems) resulted in a classification of severe dependence. The α reliability coefficient for the DSM-IV dependence scale was 0.83.
Participants self-reported ARV adherence in the past 7 days using an adaptation of the Adult AIDS Clinical Trials Group instrument,34 which has previously been validated against electronic monitoring.35 Although self-reported adherence can be subject to reporting inaccuracies, its association with clinically relevant outcomes (viral load, treatment failure)36–39 supports its utility as an indicator of medication compliance. We used total ARV doses prescribed and total doses missed in this 7-day period to generate an adherence percentage score.
We assessed reasons for ARV nonadherence for participants who had missed at least 1 dose in the 90 days before the interview using an adaptation of the Adult AIDS Clinical Trials Group scale.34 Participants responded to a series of 19 items tapping a range of possible reasons, including forgetting, being away from home, falling asleep, feeling ill or depressed, and not wanting others to notice. One newly added item specifically measured diversion-related nonadherence: “How often did you miss taking your medication(s) because you ran out of pills because you traded or sold them?” Responses were reported on a 5-point Likert scale ranging from never to almost always. For analysis, we dichotomized these responses into never and all other. Diversion-related nonadherence was the outcome variable in this analysis.
We examined information on environmental risk factors at the neighborhood and individual levels. Neighborhood poverty level was examined using residential zip codes reported by study participants. We categorized zip codes by percentage of individuals below the poverty level, using 2008 to 2012 5-year estimates from the American Community Survey.40
In addition, participants provided ratings of perceived neighborhood disorder using a brief, standardized 10-item scale10 that captures elements of social and physical disorder in the neighborhood environment. Twenty-one participants responded “don’t know” to 1 or more neighborhood disorder scale items, resulting in missing data for those variables. Nevertheless, because all 21 participants had valid answers for the majority of the neighborhood items, we retained their data in the analysis. Scores ranged from 8 to 40, with higher scores indicating greater perceived neighborhood disorder. Alpha reliability for the neighborhood disorder scale was 0.94. Perceived neighborhood disorder was significantly correlated with poverty level (r = .296; P ≤ .001), indicating correspondence between this self-report measure and objective neighborhood conditions. Perceived neighborhood disorder was the independent variable of interest in this analysis.
The following item assessed participants’ personal housing stability: “When was the last time you considered yourself to be homeless or had to stay with someone else to avoid being homeless?” For analysis, we dichotomized this variable as within the past 3 months or not within the past 3 months. In addition, participants reported the type of housing they occupied at the time of interview. Finally, we assessed risky social network connections with 1 item: “How many people do you personally know who are involved in selling or trading their HIV medications?” We examined housing status and diverter network connections as mediators.
Data Analysis
We conducted all analyses with Stata version 12.0 (StataCorp LLC, College Station, TX). We computed descriptive statistics on the demographic characteristics of the sample, as well as the prevalence of substance dependence, the level of ARV adherence achieved in the previous week, and environmental characteristics of interest, including perceived neighborhood disorder. Using bivariate logistic regression models, we compared these characteristics across the outcome of diversion-related nonadherence. Subsequently, we tested a multiple mediation model to examine the direct and indirect effects of neighborhood disorder on diversion-related nonadherence to ARVs, using the Baron and Kenny41 approach. The mediating variables were past 90-day homelessness (0/no vs 1/yes), and ARV diverter network connections, a continuous variable. Models controlled for age, gender, race/ethnicity, income, and substance dependence. We used a nonparametric bootstrapping technique to examine the significance of the indirect effects.42
RESULTS
Table 1 presents the sociodemographic and environmental characteristics of the sample, compared across diversion-related ARV nonadherence. Approximately 30% of the sample (29.8%) reported nonadherence because of diversion of their ARV medications in the past 90 days. The overall sample had a mean age of 46.1 and had been living with HIV for 13.3 years on average (data not shown). Just more than two thirds identified as African American, followed by Latinos/Latinas at 18.1% and non-Hispanic White at 13.5%. Study participants were economically disadvantaged, with 81% reporting a monthly income below $1000 (data not shown). We found no significant differences on any demographic characteristic by diversion status, with the exception of education. High school completers reported significantly lower odds of diversion-related nonadherence than those with less than a high school education.
TABLE 1—
Individual and Environmental Characteristics of HIV-Positive Substance Abusers by ARV Adherence and Diversion Status: Miami and Fort Lauderdale, FL, 2010–2012
| Missed ARVs Because of Diversion, Past 90 Daysa |
|||
| Characteristic | Yes (n = 150), No. (%) or Mean ±SD | No (n = 353), No. (%) or Mean ±SD | OR (95% CI) |
| Demographics | |||
| Age, y | 46.0 ±7.7 | 46.1 ±7.8 | 1.00 (0.97, 1.02) |
| Male gender (Ref: female) | 96 (64.0) | 203 (57.5) | 1.31 (0.89, 1.95) |
| African American race/ethnicity (Ref: all other) | 106 (70.7) | 234 (66.3) | 1.23 (0.81, 1.86) |
| High school education (Ref: < high school) | 74 (49.3) | 210 (59.5) | 0.66* (0.45, 0.97) |
| Monthly income < $1000 (Ref: ≥ $1000) | 121 (80.7) | 287 (81.3) | 0.96 (0.59, 1.56) |
| Years HIV diagnosisa | 12.8 ±7.4 | 13.4 ±7.2 | 0.99 (0.96, 1.01) |
| Health Behaviors | |||
| Severe substance dependence, past 90 d (Ref: no) | 84 (56.0) | 145 (41.1) | 1.83** (1.24, 2.69) |
| ARV adherence, past week | 0.49 ±0.37 | 0.91 ±0.20 | 0.01*** (0.006, 0.029) |
| Environmental Factors | |||
| Poverty levelb | 0.27 ±0.10 | 0.29 ±0.10 | 0.98* (0.96, 0.99) |
| Neighborhood disorder | 25.8 ±9.7 | 23.3 ±9.7 | 1.03** (1.01, 1.05) |
| Diverters in networkc | 10.4 ±15.5 | 4.3 ±9.4 | 1.04*** (1.03, 1.06) |
| Homeless, past 90 d (Ref: no) | 76 (50.7) | 121 (34.3) | 1.97*** (1.34, 2.90) |
| Current housing type | |||
| Own or rent house or apartment | 43 (28.7) | 137 (38.8) | 1.00 (Ref) |
| Public housing | 26 (17.3) | 88 (24.9) | 0.94 (0.54, 1.64) |
| Residential facility | 4 (2.7) | 29 (8.2) | 0.44 (0.15, 1.32) |
| Staying with friend or relative | 28 (18.7) | 37 (10.5) | 2.41*** (1.33, 4.39) |
| Boarding house or hotel | 4 (2.7) | 7 (2.0) | 1.82 (0.51, 6.52) |
| Shelter | 42 (28.0) | 54 (15.3) | 2.48*** (1.46, 4.21) |
| Street location | 3 (2.0) | 1 (0.3) | 9.56 (0.97, 94.29) |
Note. ARV = antiretroviral; CI = confidence interval; OR = odds ratio. The sample size was n = 503.
n = 502.
n = 495.
n = 500.
*P ≤ .05; **P ≤ .01; ***P ≤ .001.
In terms of health behaviors, 45.5% reported symptoms of severe substance dependence in the 90 days before the interview (data not shown). Past-week ARV adherence was modest, with participants reporting that, on average, they took 78% of their prescribed medication doses. Both of these health behaviors differed significantly by diversion status. Participants with severe substance dependence had 1.83 higher odds of nonadherence resulting from diversion than did those with fewer substance dependence symptoms (95% confidence interval [CI] = 1.24, 2.69). Similarly, participants who reported better overall ARV adherence in the previous week had significantly lower odds of diversion-related missed ARV doses in the past 90 days (95% CI = 0.006, 0.029).
Participants reported challenging environmental circumstances; on average, their residential neighborhoods were characterized by poverty levels of 28%, and more than one third (39.2%) had recently been homeless (data not shown). Self-reported neighborhood disorder had a mean score of 24.0 on a 40-point scale. On average, study participants reported knowing 6.1 individuals who diverted their personal ARV medications. Comparative analyses revealed several significant differences in the environmental characteristics of those who had recently engaged in diversion and those who had not. Of note, participants reporting higher neighborhood disorder had higher odds of diversion-related nonadherence (95% CI = 1.01, 1.05), as did those with larger diverter networks (95% CI = 1.03, 1.06) and those who were recently homeless (95% CI = 1.34, 2.90). Staying in emergency shelter or with friends also conferred higher odds of diversion-related nonadherence, relative to those with their own housing.
Table 2 provides odds ratios, corresponding P values, and goodness-of-fit statistics for the 3 logistic regression models tested. Model 1 included only neighborhood disorder as a predictor of diversion-related nonadherence, model 2 added the mediator homelessness, and model 3 added a second mediator—diverters in network—to the model. Model 3 indicates that both recent homelessness and number of diverters in network are statistically significant predictors of diversion-related nonadherence; also, the coefficient of neighborhood disorder lost its significance with the mediators included. Goodness-of-fit statistics indicate that model 3 significantly improved model fit.
TABLE 2—
Diversion-Related Nonadherence to Antiretroviral Medication Regressed on Neighborhood Disorder, Recent Homelessness, and Diverters in Network: Miami and Fort Lauderdale, FL, 2010–2012
| Model 1a (No Mediation) |
Model 2a (1 Mediator) |
Model 3a (2 Mediators) |
||||
| Variable | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Neighborhood disorder | 1.02 (1.00, 1.04) | .026 | 1.02 (1.00, 1.04) | .098 | 1.01 (0.99, 1.03) | .288 |
| Recent homelessness | 1.74 (1.15, 2.63) | .008 | 1.72 (1.13, 2.63) | .011 | ||
| Diverters in network | 1.04 (1.02, 1.06) | < .001 | ||||
| Goodness of fitb | ||||||
| Parameters | 6 | 7 | 8 | |||
| Raw likelihood (–2LL) | 589.60 | 582.60 | 562.16 | |||
| χ2 | 6.99** | 20.45*** | ||||
Note. CI = confidence interval; LL = log-likelihood; OR = odds ratio. The sample size was n = 503.
All models controlled for age, gender, race/ethnicity, income, and substance dependence.
Both models were compared with previous model; model 2 compared with model 1, and model 3 compared with model 2.
**P < .01; ***P < .001.
Figure 1 displays the results of the mediation analysis, controlling for the covariates age, gender, race/ethnicity, income, and substance dependence described in Table 1. Neighborhood disorder significantly predicted the binary outcome variable, diversion-related nonadherence (P < .05), and both of the mediators, recent homelessness (P < .001) and higher number of social network diverters (P < .01). In the regression model that included neighborhood disorder and both mediators as potential predictors of diversion-related nonadherence, the direct effect of neighborhood disorder was substantially reduced and was statistically nonsignificant; on the basis of the resampling bootstrap estimation, both the indirect effect of homelessness and larger number of social network diverters as mediators were significant (P = .011 and .01, respectively). The total indirect effect was also statistically significant (P < .001). The proportion of the total effect mediated was 53%, which demonstrates that recent homelessness and diverter network connections carry a substantial part of the influence of neighborhood disorder to diversion-related nonadherence.
FIGURE 1—
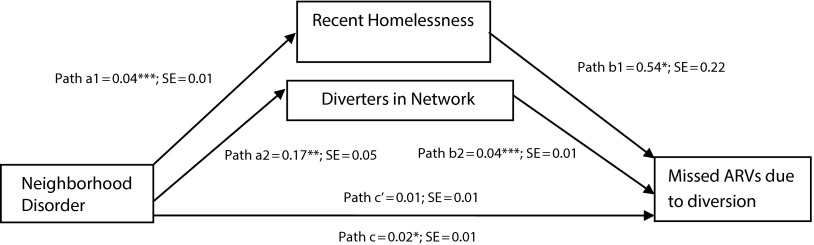
Recent homelessness and diverters in network mediating the effect of neighborhood disorder on nonadherence to antiretrovirals (ARVs): Miami and Fort Lauderdale, FL, 2010–2012.
Note. All models controlled for age, gender, race/ethnicity, income, and substance dependence; path c′ coefficient obtained with 2 mediators in the model.
*P < .05; **P < .01; ***P < .001.
DISCUSSION
We examined the effects of neighborhood disorder on diversion-related ARV nonadherence among a sample of socioeconomically disadvantaged HIV-positive individuals in South Florida. Although ARV medication compliance clearly falls within the realm of an individual-level health behavior, the diversion of ARVs is an organized profit-making activity in the illicit market that appears to exert significant environmental pressure on nonadherence among highly vulnerable substance-abusing HIV-positive patients.21,22 ARV diversion represents a somewhat unique phenomenon in the scientific literature on health behaviors, in the sense that there is tangible financial incentive offered to patients by outside parties to engage in a behavior that is potentially detrimental to the individual’s viral suppression and health outcomes21,43,44 and creates risk for transmitting resistant virus to others. The existence of such external ARV market pressures engendered this examination of environmental exposure to disorder and the mechanisms by which it may influence HIV disease management.
Our findings indicate that higher neighborhood disorder significantly reduced HIV medication adherence among the most vulnerable individuals, through exposures to environmental risks. Our hypothesis related to recent homelessness was fully supported by the data, demonstrating a strong indirect effect on diversion-related nonadherence. These findings resonate with previous research that has indicated that those who are unstably housed face particularly difficult challenges related to HIV disease management and ARV adherence,28,29 but they add to our understanding of this vulnerability. Homeless individuals have few, if any, buffers or protections from a disordered neighborhood environment, which leaves them especially vulnerable to a variety of environmental threats, in this case exploitation by ARV pill brokers21,22 that ultimately reduces adherence.
Social network exposure to ARV diverters also displayed statistical significance in the mediation analysis, demonstrating a strong indirect effect on diversion-related nonadherence. This finding indicates that the concentration of diverters in one’s social network increases individual-level diversion risk, which is consistent with previous research illustrating the influence of risky social norms on network members.23,24 This network effect may warrant examination in future research. A more detailed examination of social network structure, characteristics, and influences on diversion-related nonadherence may yield important results with possible implications for network-based intervention strategies.
Limitations
This study had limitations that are important to consider. First, our analysis used cross-sectional data gathered from a single interview. The absence of longitudinal data limits our ability to delineate causal relationships among our key variables; as such, the associations we identified between perceived neighborhood disorder and the mediating variables could be interpreted as bidirectional. Our model examined disorder acting to increase individual exposure to risk, yet exposure could also plausibly operate on perceptions of neighborhood disorder.
An additional limitation relates to the study sampling strategy, which was not designed to yield a representative sample of HIV-positive patients. Recruitment used targeted sampling to enroll disadvantaged substance abusers in high HIV prevalence areas of South Florida in which ARV diversion was thought to be active. This limits our ability to generalize the findings to other HIV-positive patient populations and may reflect unique characteristics of illicit drug markets in South Florida. Finally, our study also relied on self-report data, including our key measures of interest. It is possible, therefore, that there were reporting problems or inaccuracies in participant responses to the interview items; nevertheless, the high levels of substance use, ARV diversion, and low ARV adherence reported suggest that participants did not systematically underreport these behaviors. Our measure of diversion-related ARV nonadherence was a newly developed self-report item; future studies may benefit from the development of a more robust measure. Although our primary measure of neighborhood disorder was also obtained by self-report, the data correlated significantly with an objective measure of poverty level in the community, which resonates with prior research supporting the validity of self-report data as an indicator of neighborhood conditions.45
Conclusions
Our findings provide important support for the notion of the HIV risk environment, which operates to enable or constrain individual-level behaviors and contributes to HIV-related vulnerabilities.1,18 Although we recognize that many other factors, such as psychological distress, addiction severity, social exclusion, disempowerment, and inaccessibility of medical care also have important roles to play in ARV diversion and adherence behaviors,22 we sought to understand exposure to a disordered environment as a contributor to poor HIV disease management. In this regard, we demonstrated a substantial influence of neighborhood environmental pressures on diversion-related nonadherence to ARVs.
In light of these findings, what can be done to better support ARV adherence among HIV-positive individuals who are confronted by such serious environmental pressures? It is worth noting that, in spite of numerous environmental threats and high levels of disorder, 70% of this highly vulnerable sample did not report ARV adherence problems related to diversion. Our modeling suggests that unstably housed individuals are an especially vulnerable subset of HIV-positive patients who require additional attention to improve medication compliance, yet addressing the breadth of environmental pressures faced by these individuals is a daunting task.
One approach to reducing diversion and associated nonadherence would be to implement measures that address demand for ARV medications in the illicit market. Given that demand originates from multiple sources, which are both need and profit based, a number of approaches warrant consideration in addressing the issues. In terms of need factors, our previous research indicates that substantial ARV demand originates from medically underserved HIV-positive patients who experience inconsistent formal HIV care or medication insurance coverage because of missed appointments, waiting lists, or other urgent circumstances.22 We have argued that public insurance and prescription programs be mandated to establish provisions for emergency access to short-term supplies of ARVs, which could reduce patient-level demand for illicit ARVs in highly vulnerable populations.22 Reducing profit-based ARV demand from pill brokers will require a wholly different approach, namely wide-ranging structural changes to ARV pricing and reimbursement, as well as changes in medication distribution policies of insurance programs that may facilitate diversion.21,22 These strategies would be key in reducing the economic motivations of pill brokers as well as of rogue pharmacies that increasingly participate in fraudulent ARV medication diversion practices.46,47
A second related strategy involves reducing vulnerability to diversion among disadvantaged patients who supply the illicit market with ARVs. Long-term reductions in vulnerability to ARV diversion will necessitate commitment to increase funding for accessible housing, substance abuse treatment, and other supportive services that have been shown to successfully improve medication adherence and HIV-related health status among unstably housed and substance-dependent individuals.48–50 Although acquisition of such services may not wholly eliminate ARV diversion, our findings suggest that reducing exposure to street-based drug markets and networks may act as a significant protective factor in mitigating diversion behaviors.
In the current resource-limited environment, it would appear reasonable to consider the utility of testing novel short-term interventions among unstably housed and substance-dependent individuals that offer incentives for ARV medication compliance, therein reducing diversion as well. Contingency management approaches have shown short-term positive effects in increasing ARV adherence and lowering viral load among substance-using HIV-positive patients50–52 and have been recommended as a priority for research on HIV medical care linkage and retention.53 For economically disadvantaged HIV-positive individuals, short-term monetary incentives for adherence may prove to be a useful element in offsetting vulnerability to the environmental pressures of ARV street markets.
Acknowledgments
This research was supported by the National Institute on Drug Abuse (R01DA023157).
Note. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Human Participant Protection
The study was approved by the institutional review board of Nova Southeastern University. All project staff completed the requirements for National Institutes of Health Web-based certification for protection of human participants. A Certificate of Confidentiality from the National Institutes of Health was also obtained, and a copy was offered to participants.
References
- 1.Milloy M-J, Marshall BD, Kerr T et al. Social and structural factors associated with HIV disease progression among illicit drug users: a systematic review. AIDS. 2012;26(9):1049–1063. doi: 10.1097/QAD.0b013e32835221cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marmot M. Social determinants of health inequalities. Lancet. 2005;365(9464):1099–1104. doi: 10.1016/S0140-6736(05)71146-6. [DOI] [PubMed] [Google Scholar]
- 3.Dean HD, Williams KM, Fenton KA. From theory to action: applying social determinants of health to public health practice. Public Health Rep. 2013;128(suppl 3):1–4. doi: 10.1177/00333549131286S301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health. 2001;91(11):1783–1789. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riley ED, Gandhi M. Bradley Hare C, Cohen J, Hwang SW. Poverty, unstable housing, and HIV infection among women living in the United States. Curr HIV/AIDS Rep. 2007;4(4):181–186. doi: 10.1007/s11904-007-0026-5. [DOI] [PubMed] [Google Scholar]
- 6.Hixson BA, Omer SB, del Rio C, Frew PM. Spatial clustering of HIV prevalence in Atlanta, Georgia and population characteristics associated with case concentrations. J Urban Health. 2011;88(1):129–141. doi: 10.1007/s11524-010-9510-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold M, Hsu L, Pipkin S, McFarland W, Rutherford GW. Race, place and AIDS: The role of socioeconomic context on racial disparities in treatment and survival in San Francisco. Soc Sci Med. 2009;69(1):121–128. doi: 10.1016/j.socscimed.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shacham E, Lian M, Onon NF, Donovan M, Overton ET. Are neighborhood conditions associated with HIV management? HIV Med. 2013;14(10):624–632. doi: 10.1111/hiv.12067. [DOI] [PubMed] [Google Scholar]
- 9.Latkin CA, German D, Vlahov D, Galea S. Neighborhoods and HIV: a social ecological approach to prevention and care. Am Psychol. 2013;68(4):210–224. doi: 10.1037/a0032704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross CE, Mirowsky J. Disorder and decay: the concept and measurement of perceived neighborhood disorder. Urban Aff Rev. 1999;34(3):412–432. [Google Scholar]
- 11.Ross CE, Mirowsky J. Neighborhood disadvantage, disorder and health. J Health Soc Behav. 2001;42(3):258–276. [PubMed] [Google Scholar]
- 12.Hill TD, Ross CE, Angel RJ. Neighborhood disorder, psychophysiological distress, and health. J Health Soc Behav. 2005;46(2):170–186. doi: 10.1177/002214650504600204. [DOI] [PubMed] [Google Scholar]
- 13.Hill TD, Burdette AM, Hale L. Neighborhood disorder, sleep quality, and psychological distress: testing a model of structural amplification. Health Place. 2009;15(4):1006–1013. doi: 10.1016/j.healthplace.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Kim J. Neighborhood disadvantage and mental health: the role of neighborhood disorder and social relationships. Soc Sci Res. 2010;39(2):260–271. [Google Scholar]
- 15.Latkin CA, Knowlton AR. Micro-social structural approaches to HIV prevention: a social ecological perspective. AIDS Care. 2005;17(suppl 1):102–113. doi: 10.1080/09540120500121185. [DOI] [PubMed] [Google Scholar]
- 16.Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;277(5328):918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 17.Latkin CA, German D, Hua W, Curry AD. Individual-level influences on perceptions of neighborhood disorder: a multilevel analysis. J Community Psychol. 2009;37(1):122–133. doi: 10.1002/jcop.20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhodes T. Risk environments and drug harms: a social science for harm reduction approach. Int J Drug Policy. 2009;20:193–201. doi: 10.1016/j.drugpo.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Latkin CA, Williams CT, Wang J, Curry AD. Neighborhood social disorder as a determinant of drug injection behaviors: a structural equation modeling approach. Health Psychol. 2005;24(1):96–100. doi: 10.1037/0278-6133.24.1.96. [DOI] [PubMed] [Google Scholar]
- 20.Bangsberg DR, Perry S, Charlebois ED et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15(9):1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 21.Surratt HL, Kurtz SP, Cicero TJ, O’Grady C, Levi-Minzi MA. Antiretroviral medication diversion among HIV-positive substance abusers in South Florida. Am J Public Health. 2013;103(6):1026–1028. doi: 10.2105/AJPH.2012.301092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuyuki K, Surratt HL, Levi-Minzi MA, O’Grady C, Kurtz SP. The demand for antiretroviral drugs in the illicit marketplace: implications for HIV disease management among vulnerable populations. AIDS Behav. 2014 doi: 10.1007/s10461-014-0856-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman SR, Aral S. Social networks, risk-potential networks, health, and disease. J Urban Health. 2001;78(3):411–418. doi: 10.1093/jurban/78.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latkin CA, Kuramoto SJ, Davey-Rothwell MA, Tobin KE. Social norms, social networks, and HIV risk behavior among injection drug users. AIDS Behav. 2010;14(5):1159–1168. doi: 10.1007/s10461-009-9576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neblett RC, Davey-Rothwell MA, Chander G, Latkin CA. Social network characteristics and HIV sexual risk behavior among urban African American women. J Urban Health. 2011;88(1):54–65. doi: 10.1007/s11524-010-9513-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milloy M-J, Marshall BDL, Montaner J, Wood E. Housing status and the health of people living with HIV/AIDS. Curr HIV/AIDS Rep. 2012;9(4):364–374. doi: 10.1007/s11904-012-0137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aidala A, Cross JE, Stall R, Harre D, Sumartojo E. Housing status and HIV risk behaviors: implications for prevention and policy. AIDS Behav. 2005;9(3):251–265. doi: 10.1007/s10461-005-9000-7. [DOI] [PubMed] [Google Scholar]
- 28.Kidder DP, Wolitski RJ, Campsmith ML, Nakamura GV. Health status, health care use, medication use, and medication adherence among homeless and housed people living with HIV/AIDS. Am J Public Health. 2007;97(12):2238–2245. doi: 10.2105/AJPH.2006.090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palepu A, Milloy M-J, Kerr T, Zhang R, Wood E. Homelessness and adherence to antiretroviral therapy among a cohort of HIV-infected injection drug users. J Urban Health. 2011;88(3):545–555. doi: 10.1007/s11524-011-9562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watters JK, Biernacki P. Targeted sampling: options for the study of hidden populations. Soc Probl. 1989;36(4):416–430. [Google Scholar]
- 31.Dennis ML, Titus JC, White MK, Unsicker JI, Hodgkins D. Global Appraisal of Individual Needs—Initial (GAIN-I) Bloomington, IL: Chestnut Health Systems; 2002. [Google Scholar]
- 32.Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 33.RAND Corporation. Disparities in Care for HIV Patients. Santa Monica, CA: RAND Health; 2006. [Google Scholar]
- 34.Chesney MA, Ickovics JR, Chambers DB et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. AIDS Care. 2000;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 35.Machtinger EL, Bangsberg DR. Adherence to HIV antiretroviral therapy: related resources. HIV InSite. Available at: http://hivinsite.ucsf.edu/insite?page=kbr-03-02-09. Accessed November 10, 2014. [Google Scholar]
- 36.Arnsten JH, Demas PA, Farzadegan H et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33(8):1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gifford AL, Bormann JE, Shively MJ, Wright BC, Richman DD, Bozzette SA. Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. J Acquir Immune Defic Syndr. 2000;23(5):386–395. doi: 10.1097/00126334-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 38.Haubrich RH, Little SJ, Currier JS et al. The value of patient-reported adherence to antiretroviral therapy in predicting virologic and immunologic response. California Collaborative Treatment Group. AIDS. 1999;13(9):1099–1107. doi: 10.1097/00002030-199906180-00014. [DOI] [PubMed] [Google Scholar]
- 39.Simoni JM, Huh D, Wang Y et al. The validity of self-reported medication adherence as an outcome in clinical trials of adherence-promotion interventions: findings from the MACH14 study. AIDS Behav. 2014;18(12):2285–2290. doi: 10.1007/s10461-014-0905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.US Census Bureau. American Community Survey 5-year estimates, individuals below poverty level. Available at: http://factfinder2.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_12_5YR_S1703&prodType=table. Accessed February 24, 2014.
- 41.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 42.Efron B, Tibshirani R. An Introduction to the Bootstrap. Boca Raton, FL: Chapman & Hall/CRC; 1993. [Google Scholar]
- 43.Inciardi JA, Surratt HL, Kurtz SP, Cicero TJ. Mechanisms of prescription drug diversion among drug-involved club- and street-based populations. Pain Med. 2007;8(2):171–183. doi: 10.1111/j.1526-4637.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boseley S, Carroll R. Profiteers resell Africa’s cheap AIDS drugs. The Guardian. October 3, 2002. Available at: http://www.guardian.co.uk/world/2002/oct/04/aids.rorycarroll. Accessed June 15, 2012. [Google Scholar]
- 45.Elo IT, Mykyta L, Margolis R, Culhane JF. Perceptions of neighborhood disorder: the role of individual and neighborhood characteristics. Soc Sci Q. 2009;90(5):1298–1320. doi: 10.1111/j.1540-6237.2009.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bandell B. HIV drug fraud in Medicare plagues Miami. South Florida Business Journal. August 15, 2014. Available at: http://www.bizjournals.com/southflorida/news/2014/08/15/hiv-drug-fraud-in-medicare-plagues-miami.html?page=all. Accessed November 13, 2014. [Google Scholar]
- 47.Gibson WE. Pharmaceutical fraud spreads in Florida. Sun Sentinel. March 26, 2014. Available at: http://articles.sun-sentinel.com/2014-03-26/news/fl-medicare-fraud-florida-20140326_1_medicare-fraud-health-care-fraud-miami-lakes. Accessed November 13, 2014. [Google Scholar]
- 48.Buchanan D, Kee R, Sadowski LS, Garcia D. The health impact of supportive housing for HIV-positive homeless patients: a randomized controlled trial. Am J Public Health. 2009;99(suppl 3):S675–S680. doi: 10.2105/AJPH.2008.137810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolitski RJ, Kidder DP, Pals SL et al. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless unstably housed people living with HIV. AIDS Behav. 2010;14(3):493–503. doi: 10.1007/s10461-009-9643-x. [DOI] [PubMed] [Google Scholar]
- 50.Lucas GM. Substance abuse, adherence with antiretroviral therapy, and clinical outcomes among HIV-infected individuals. Life Sci. 2011;88(21–22):948–952. doi: 10.1016/j.lfs.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosen MI, Dieckhaus K, McMahon TJ et al. Improved adherence with contingency management. AIDS Patient Care STDS. 2007;21(1):30–40. doi: 10.1089/apc.2006.0028. [DOI] [PubMed] [Google Scholar]
- 52.Rigsby MO, Rosen MI, Beauvais JE et al. Cue-dose training with monetary reinforcement: a pilot study of an antiretroviral adherence intervention. J Gen Intern Med. 2000;15(12):841–847. doi: 10.1046/j.1525-1497.2000.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson MA, Mugavero MJ, Amico KR et al. Guidelines for improving entry and retention into care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS care panel. Ann Intern Med. 2012;156(11):817–833. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]


