Abstract
A challenge for HIV-1 immunogen design is inducing neutralizing antibodies (NAbs) against neutralization-resistant (Tier-2) viruses that dominate human transmissions. We show that a soluble recombinant HIV-1 envelope glycoprotein trimer that adopts a native conformation (BG505 SOSIP.664) induced NAbs potently against the sequence-matched Tier-2 virus in rabbits and similar but weaker responses in macaques. The trimer also consistently induced cross-reactive NAbs against more sensitive (Tier-1) viruses. Tier-2 NAbs recognized conformational epitopes that differed between animals and in some cases overlapped with those recognized by broadly neutralizing antibodies (bNAbs), whereas Tier-1 responses targeted linear V3 epitopes. A second trimer, B41 SOSIP.664, also induced a strong autologous Tier-2 NAb response in rabbits. Thus, native-like trimers represent a promising starting point for developing HIV-1 vaccines aimed at inducing bNAbs.
A major goal of HIV-1 vaccine development is to identify immunogens capable of inducing protective titers of broadly neutralizing antibodies (bNAbs) against circulating viruses with a Tier-2 or higher resistance profile (1). Viruses with these characteristics are the most commonly transmitted strains of HIV-1, and hence they dominate new infections. The humoral immune response of infected individuals creates antibody-mediated selection pressure on the virus, which can generally only persist and be transmitted if it is antibody resistant. A successful vaccine must, then, be able to induce antibodies that are able to counter the virus’s evolved resistance mechanisms. In addition, the global sequence diversity among HIV-1 strains is so great that vaccine-induced antibodies should target relatively conserved sites and thereby possess breadth of action. A vaccine with the required properties must be based on the envelope glycoprotein (Env) as the gp120-gp41 trimer on the virus surface is the only bNAb target. After two or more years of HIV-1 infection, ~20% of individuals develop bNAbs, which can serve as templates for vaccine design by exposing vulnerabilities in the viral defense mechanisms (1). As bNAbs usually evolve from strain-specific autologous NAbs via multiple cycles of viral escape and antibody affinity maturation (reviewed in (2, 3)), it is unlikely that bNAbs can be raised against any single Env protein of fixed antigenic composition. However, the induction of autologous NAbs to a Tier-2 virus would be an excellent starting point for iterative vaccine design (3–6).
One or more of the bNAb epitopes present on native, virion-associated trimers are also found on various Env-based immunogens, including soluble, monomeric gp120s and multimeric gp140s that contain both the receptor-binding gp120 and fusion-enabling gp41-ectodomain (gp41ECTO) subunits. These various forms of Env are all derived from the viral gp160 precursor protein, which is proteolytically cleaved into the gp120 and gp41ECTO subunits when it is processed within the cell and forms membrane-associated trimers. For practical purposes, all Env-based immunogens are made as soluble proteins by eliminating the membrane-spanning domain of gp160 and creating entities known as gp140s. In some cases, the gp41ECTO domain is also removed to make a monomeric gp120 protein. The soluble gp140s oligomerize via interactions between their gp41ECTO components. However, the oligomers are very unstable unless the construct is stabilized, either by eliminating the cleavage site between gp120 and gp41ECTO to make a standard uncleaved gp140 protein, or by introducing specific trimer-stabilizing changes into the properly cleaved form of gp140. We have favored the latter strategy, by making stabilized, cleaved trimers that are designated SOSIP.664 gp140s; the SOS term denotes an intermolecular disulfide bond engineered to link the gp120 and gp41ECTO subunits, while IP signifies an I559P point substitution that maintains the gp41ECTO components in their pre-fusion form.
Here, we have evaluated the immunogenicity of a SOSIP.664 trimer based on the BG505 clade A virus, which was isolated from a 6-week old infant that later developed a bNAb response within ~2 years of infection (7, 8). We have also tested, in less detail, a second SOSIP.664 trimer based on a clade B adult infection founder virus, B41 (30). The BG505 and B41 SOSIP.664 trimers display multiple bNAb epitopes, but few non-neutralizing Ab (non-NAb) epitopes that may serve as immunological distractions (9,30). The integrity and native-like appearance of the BG505 SOSIP.664 trimer, including its complex quaternary epitopes, was confirmed when high resolution structures were recently generated by cryo-electron microscopy (cryoEM) and X-ray crystallography, the high resolution depictions of the HIV-1 Env trimer (10–12). In this study, we conducted animal immunization experiments to determine which NAb specificities can be induced by two different, native-like SOSIP.664 trimer mimics of the native Env spike, and we performed comparisons with gp120 monomers and standard designs of uncleaved gp140 immunogens.
Immunogenicity of BG505 SOSIP.664 trimers in rabbits
The various immunogens tested in this study are depicted schematically in fig. S1A along with their conformations, which are based on negative-stain EM images that are strongly supported by antigenicity, biophysical and glycan composition data (figs. S2, S3) (9–11, 13,15–19). Five different experiments were performed, 4 in rabbits and 1 in macaques (fig. S1B). In general, the immunization scheme was based on two closely separated initial doses (weeks 0 and 4), followed by a third after a prolonged rest period of, usually 16-weeks (20). The first study, in rabbits, compared the immunogenicity of clade A BG505 SOSIP.664 trimers and gp120 monomers, both of which were produced in 293S GnT−/− cells (9, 11, 19) (fig. S1A). To assess whether trimer glycosylation affected immunogenicity, we immunized three groups of rabbits with BG505 SOSIP.664 trimers produced in 293T cells (natural glycosylation), 293S GnT-/- cells (oligomannose-only glycans (11, 21)), or 293S GnT−/− cells followed by EndoH treatment (glycan-depleted (11, 22)). In rabbit experiment-1, as well as the BG505 gp120 monomer comparator proteins, we also tested a clade-B YU2 uncleaved gp140 protein containing a Foldon trimerization domain that was produced in 293T cells (YU2 gp140-Fd) (15). In rabbit experiment-3, we compared BG505 SOSIP.664 trimers with the uncleaved, non-native BG505 sequence-matched WT.SEKS gp140 protein (13, 16). The goal of the comparisons with the YU2 gp140-Fd and BG505 WT.SEKS proteins was to explore whether a native-like trimer conformation is beneficial for immunogenicity. Proteolytic cleavage of gp120 from gp41ECTO is critical for soluble gp140 trimers to maintain native-like structures; standard uncleaved gp140 proteins based on multiple different sequences, including YU2 gp140-Fd and BG505 WT.SEKS, are now known to predominantly adopt aberrant, non-native conformations that can be clearly distinguished from the native-like, cleaved SOSIP.664 trimers (13–16) (fig.S1A). Thus, when viewed by EM the standard uncleaved gp140s have an irregular and non-native configuration; frequently seen images represent splayed-out, semi-dissociated gp120 subunits linked by the uncleaved inter-subunit strand to a central core comprising the gp41ECTO components (13–16). In contrast, the BG505 and also B41 SOSIP.664 trimers are consistently regular, tri-lobed, propeller-shaped structures (9, 13, 23). Finally, in experiment-4, which will be described in more detail elsewhere, we assessed the ability of SOSIP.664 trimers based on the clade B41 genotype and produced in CHO cells (23) to induce NAb responses agains the autologous Tier-2 B41 virus.
The anti-gp120, anti-gp41 and anti-trimer binding antibody titers induced over time by the various Env proteins are summarized in fig. S4. To quantify the NAb responses two-weeks after the third immunization (i.e., at week-22 or, for some rabbits, week-26), we used the TZM-bl cell assay, which is based on transactivation of a luciferase reporter gene by an infecting virus (Fig. 1; table S1). We were particularly interested in assessing the NAb response to the autologous (i.e., sequence-matched) BG505.T332N virus as it has a neutralization-resistant Tier-2 phenotype. Although almost all Env protein immunogens (e.g., gp120 monomers, uncleaved gp140s) can raise NAbs against various neutralization-sensitive Tier-1 viruses, inducing NAbs that are able to counter a Tier-2 virus, even an autologous one, has been challenging. Could a native-like trimer do better? We found that all 20 sera from the BG505 SOSIP.664 trimer-immunized rabbits neutralized the autologous (i.e., sequence-matched) Tier-2 virus BG505.T332N (9), with titers that ranged from 39 to 7840 (median 570; Fig. 1A). There was no discernible difference in the magnitude of the autologous NAb responses to the three different trimer glycosylation variants (Fig. 1A, groups 4–6). Sera from the BG505 gp120 monomer recipients also neutralized the autologous virus, although with a lower median titer of 270 (Fig. 1A, group 2). BG505 gp120 is unusual as it was selected to bind PG9 and is known to be atypically immunogenic (24). It may also be relevant that BG505 gp120 was purified via a bNAb column (in this case 2G12), which may select for more native-like forms of gp120 compared to other methods. The uncleaved BG505 WT.SEKS gp140 induced no NAbs against the autologous BG505.T332N virus (median titer <20) (Fig. 1A, group 8), while the uncleaved YU2 gp140-Fd induced NAbs against the autologous, clade-B YU2 virus only weakly (median titer 36) (Fig.1D, group 3). The difference between the autologous Tier-2 NAb responses to the native-like BG505 SOSIP.664 trimer and the non-native WT.SEKS gp140 was significant (P = 0.029 for the intra-experiment (n=4 vs. n=4) comparison; P = 0.0002 when all 20 BG505 SOSIP.664 trimer recipients were included; two-tailed Mann-Whitney test) (Fig. 1A). The poor responses to the WT.SEKS and YU2 proteins are consistent with multiple reports that various uncleaved gp140s are not able to induce NAbs that can neutralize autologous Tier-2 viruses consistently in the TZM-bl cell assay (25–29). The lack of an autologous NAb response to the BG505 WT.SEKS proteins implies that the BG505.T332N virus is not an atypically sensitive Tier-2 virus that is vulnerable to any BG505 Env-binding antibodies. The inferiority of WT.SEKS gp140 to gp120 monomers may be because of formation of aberrant intra- and inter-subunit disulfide bonds in uncleaved gp140s that create non-native gp120 moieties (30, 31).
Figure 1. Induction of autologous Tier-2 and heterologous Tier-1 NAb responses in rabbits and macaques.
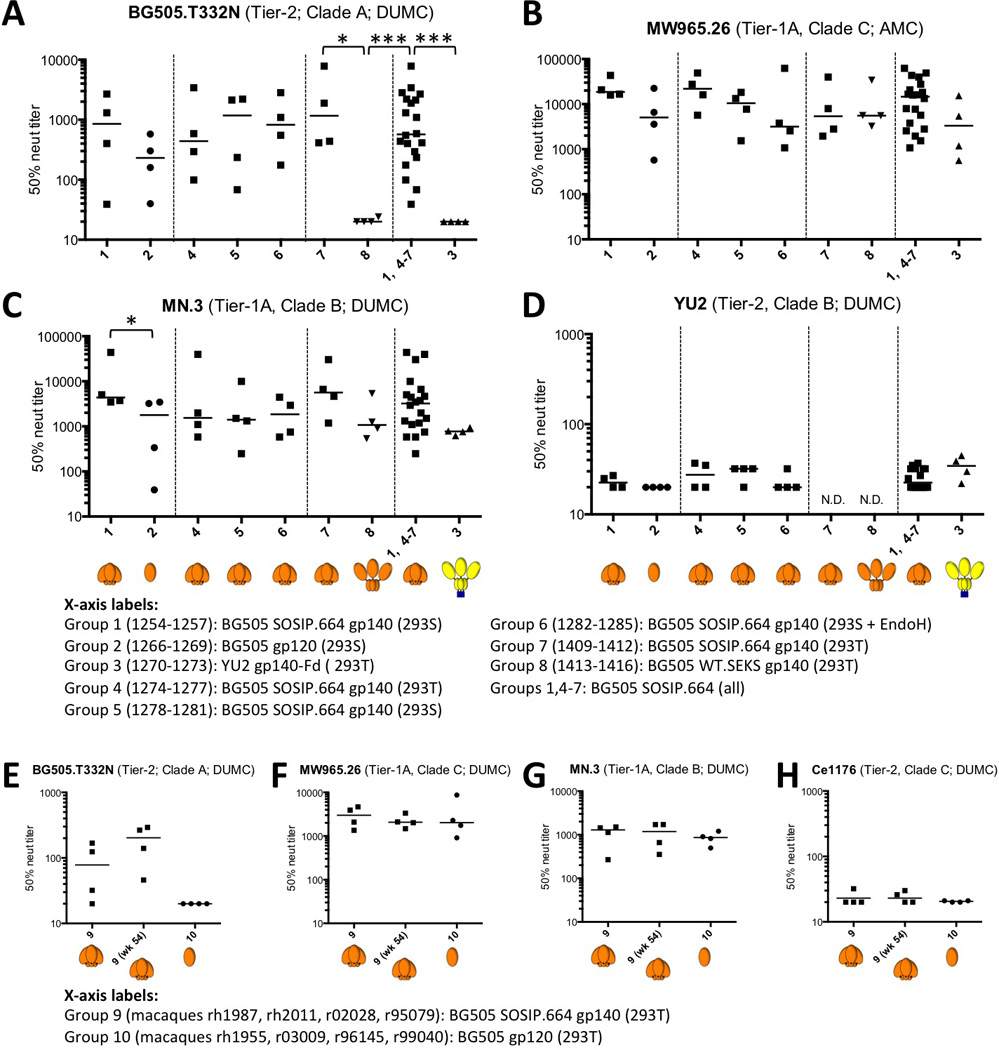
(A-D) Each panel shows the 50% neutralization titers (IC50; TZM-bl cell assay) for sera from every immunized rabbit, arranged in the groups outlined in fig. S1. For convenience, all 20 BG505 SOSIP.664 recipient rabbits (groups 1, 4–7) were also combined into one group. The dotted lines separate groups that were included in the same immunization experiment. The heading on each panel lists the test virus, its Tier classification, and the location of the testing laboratory (41) (see Materials and Methods for performance site abbreviations). For additional neutralization data on rabbit sera, see tables S1 and S2. (E-H) Each panel shows the 50% neutralization titers for individual macaque sera (TZM-bl assay; DUMC) against the specified virus for sera from all 8 animals (organized by group) at week 26, and also from the four trimer-immunized animals (group 9) at week 54. The group-10 animals received no further gp120 immunizations after week 24. For additional neutralization data on macaque sera, see table S4. Note that the scales used for the 50% neutralization titers sometimes vary between panels. Negative stain EM images of the gp140 immunogens are shown in fig. S2 and are the basis for the cartoon depictions shown in brown (BG505 Env) or yellow/blue (YU-2 gp140-Fd). The ISCOMATRIX™ adjuvant had no detectable adverse effects on the antigenicity of the trimers, assessed by ELISA, or on their appearance in negative stain EM images (fig. S3). *: p < 0.05; ***: p < 0.005 by two-tailed Mann-Whitney test.
We conducted an additional rabbit immunogenicity experiment involving a second native-like trimer, B41 SOSIP.664, based on a clade B Tier-2 transmitted/founder virus (fig. S1) (23). Eight of 10 animals given these trimers responded by generating an autologous NAb response, with a median titer of 2535 (Fig. 2A). Heterologous Tier-1, but not Tier-2, NAb responses were induced in all ten rabbits (data not shown). Hence the rabbit response to the B41 trimers is qualitatively similar to what was induced by their BG505 counterparts. The median autologous titers against these two versions of fully native-like SOSIP.664 trimers (BG505, 570; B41, 2535; combined group, 1199) exceeded those reported to confer 50% and 80% protection (105 and 329 respectively) in macaque passive-transfer experiments (32) (Fig.2A). Rabbit immunization experiments were conducted several years ago using earlier generations of SOSIP.681 trimers based on the JR-FL and KNH1144 genotypes (40, 41). Autologous Tier-2 NAbs were only inconsistently induced in those experiments. However, those trimers, although cleaved, were either not fully native-like (JR-FL) or prone to aggregate formation (KNH1144), which may account for their poor immunogenicity.
Figure 2. Comparative depiction of the autologous Tier-2 NAb responses elicited in rabbits or guinea pigs by various Env proteins.
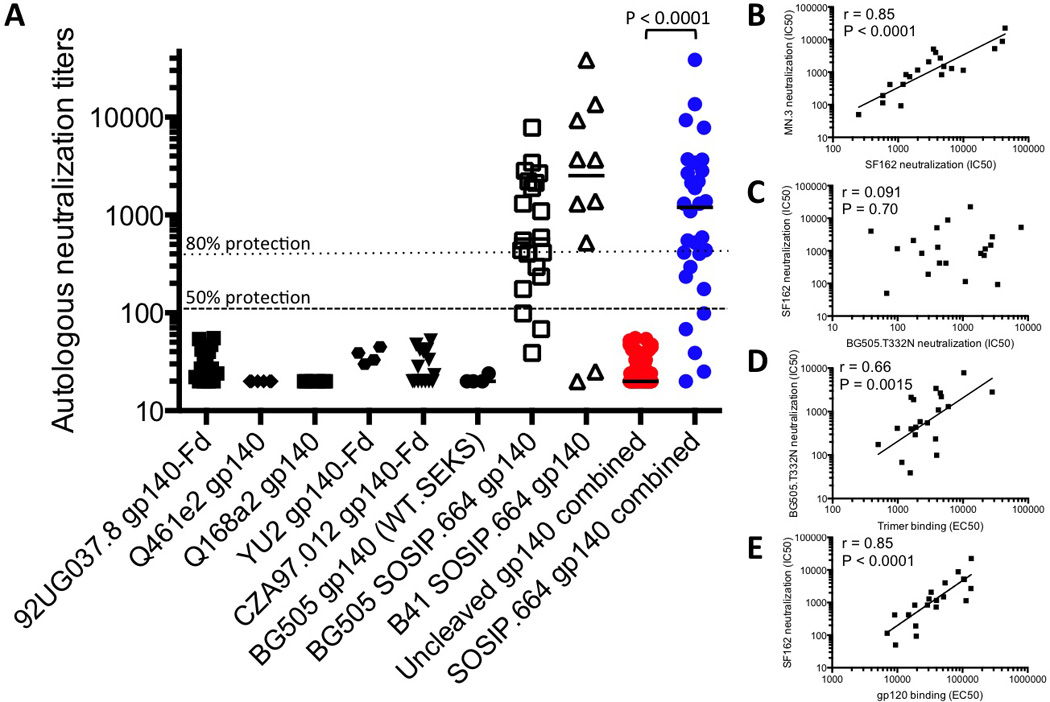
(A) Each data point represents the IC50 neutralization titer for serum derived from an individual animal immunized with the stated gp140 protein (some of which incorporated an Fd trimerization domain) when tested in the TZM-bl cell assay against the sequence-matched (i.e., autologous) Tier-2 virus. The plotted values are taken from the following papers: 92UG037.8 gp140-Fd ((25); n=15), Q461e2 gp140 ((26); n=6), Q168a2 gp140 ((26); n=6), YU2 gp140-Fd (this paper; n=4; table S1), CZA97.012 gp140-Fd ((25); n=15), BG505 gp140 (WT.SEKS; this paper; n=4; table S1), BG505 SOSIP.664 (this paper; n=20; table S1) and B41 SOSIP.664 (this paper; n=10; see SI for more details). To minimize the impact of cross-study variables, we restricted the comparison to immunization of small animals (rabbits and guinea pigs), to Env proteins based on Tier-2 viruses, and to data generated using the TZM-bl assay under broadly similar conditions. In the rightmost two columns, all the data points for each of the uncleaved gp140s (red symbols) are plotted to allow a statistical comparison with the combined BG505 and B41 SOSIP.664 trimer groups (blue symbols). The difference in the median values is highly significant (p < 0.0001 by two-tailed Mann-Whitney test). The dotted and dashed lines denote titers of 105 and 329 that are reported to confer 50% and 80% protection, respectively, to macaques in passive transfer experiments (32). (B-E) Various binding antibody and NAb responses at week 22 (week 26 for experiment 3) from all 20 BG505 trimer-immunized rabbits from experiments 1–3 were cross-compared as follows: (B) SF162 NAbs vs. MN.3 NAbs; (C) BG505.T332N NAbs vs. SF162 NAbs; (D) trimer-binding Abs vs. BG505.T332N NAbs; (E) gp120-binding Abs vs. SF162 NAbs. The NAb data are the reciprocal of the serum dilution giving 50% inhibition (IC50), the binding Ab data are the reciprocal of the serum dilution giving 50% of the maximum signal in ELISA (EC50). The Spearman r and P values for the respective correlations are given. Additional correlation analyses as well as details on the statistics are presented in fig. S5.
To gain an additional perspective on the magnitude of the autologous NAb titers to the BG505 and B41 SOSIP.664 trimers, we compared them with previously reported animal immunization data derived using four uncleaved gp140 proteins based on env sequences from the Tier-2 viruses 92UG037.8, Q461e2, Q168a2 and CZA97.012 (25, 26) (Fig. 2A). We also included the autologous NAb titers for the YU2 gp140-Fd and BG505 WT.SEKS immunogens shown in Fig. 1A. The four additional uncleaved gp140s were of standard designs that are known or expected to resemble the negative stain EM images of the uncleaved YU2 gp140-Fd and BG505 WT.SEKS gp140s shown in fig. S2 (13, 15, 18). The autologous NAb titers induced by the BG505 and B41 SOSIP.664 trimers were greater than the corresponding responses to any other Env protein. When the combined SOSIP.664 trimer groups (median titer 1199, range 20–38598; n = 30 rabbits) were compared with the combined uncleaved gp140 groups (median titer <20, range <20–55; n = 50 animals), the difference was highly significant (P < 0.0001, two-tailed Mann-Whitney test). The magnitude of the difference between the response to the SOSIP.664 trimers and the other Env proteins is so great that immunization protocol variations such as dosing, schedule, the use of priming vectors and the identity of the adjuvant are unlikely to be responsible. Thus, such variables typically influence Tier-1 NAb titers by a few-fold, but do not make the immunogens capable of inducing autologous Tier-2 NAbs.
Sera from BG505 SOSIP.664 trimer-immunized rabbits were usually ineffective against heterologous, neutralization-resistant Tier-2 viruses, although we did see some sporadic neutralization at low IC50 titers of 30–100 (Fig. 1D, tables S1 and S2). The rabbit sera did, however, consistently neutralize more sensitive heterologous, Tier-1 viruses from different clades, including the ultra-sensitive Tier-1A viruses MW965.26 (clade C; Fig. 1B), SF162 and MN (both clade B; Fig. 1C, table S1), and the Tier-1B viruses BZ167, BaL, 6535.3 (all clade B; table S1) and 93IN905 (clade C; table S1). The TZM-bl assay titers in the 1000s and 100s for the Tier-1A and -1B viruses, respectively, are comparable with, or greater than, titers reported from multiple animal studies of various monomeric gp120 or uncleaved gp140 proteins, as are the negligible titers against heterologous Tier-2 viruses (15, 25–29, 33). Overall, the Tier-1 NAb responses to the BG505 SOSIP.664, gp120 and WT.SEKS gp140 immunogens were generally similar (Fig. 1B and C; table S1). Hence, what distinguishes the native-like SOSIP.664 trimers from the other two Env immunogen designs is not their ability to induce an antibody response per se, but rather elicitation of a consistent and strong NAb response against the autologous Tier-2 virus.
Tier-1 and Tier-2 NAb titers do not correlate
To gain an understanding of why the native-like trimers differed from the other Env proteins, we analyzed various aspects of the antibody responses. First, we compared the emergence of heterologous Tier-1 (MN, SF162; clade B) and autologous Tier-2 (BG505.T332N) NAbs with anti-gp120 and anti-trimer binding antibodies (i.e., ELISA titers) in the BG505 SOSIP.664 trimer-immunized rabbits over time (fig. S5A and B). The trimer-binding responses were initially lower than those to gp120, but became comparable after four immunizations. Autologous Tier-2 NAbs were detected only rarely and weakly after two, but strongly and consistently after three immunizations (fig. S5B). The NAb titers then declined with kinetics comparable to the binding antibody responses, but were boosted again after the fourth immunization. The Tier-1 and autologous Tier-2 NAb responses waxed and waned with broadly similar kinetics (fig. S5B).
The NAb titers to the three Tier-1 viruses (MN.3, SF162 and MW965.26) induced in the 20 rabbits two weeks after the third immunization with BG505 SOSIP.664 trimers strongly correlated with one another (Fig. 2B, fig. S5C and D). In contrast, the autologous Tier-2 titers did not correlate significantly with the heterologous Tier-1 titers induced in the same rabbits at the same time (Fig. 2C, fig. S5E and F). The correlation plots suggest that different NAb specificities mediate the Tier-1 and autologous Tier-2 responses, which is confirmed experimentally below. One implication is that inducing a Tier-2 response will not be achieved simply by increasing the titer of Tier-1 NAbs, as entirely different antibody specificities and B-cell subsets are probably involved.
We also sought associations between NAb and binding antibody titers in the same set of 20 rabbits. The autologous Tier-2 NAb titers were correlated with the anti-trimer titers (Fig. 2D; r = 0.66, P =0.0015), but not the anti-gp120 titers (fig. S5K). Conversely, the Tier-1 NAbs were strongly correlated with the anti-gp120 titers, but not at all or only very weakly with the anti-trimer titers (Fig. 2E, fig. S5 H-J, L, M). The anti-gp120 and anti-trimer titers were not significantly correlated (fig. S5G). The data trends suggest that Tier-1 NAb responses are associated with strong binding antibody responses to gp120 monomers. The significant correlation between the BG505.T332N NAb titer and the anti-trimer titer, but not the anti-gp120 titer, again implies that the quaternary structure of a native-like trimer is beneficial for inducing Tier-2 NAbs. The non-native WT.SEKS gp140 induced anti-gp120 antibodies efficiently, but anti-trimer antibodies very poorly (fig. S4A-C).
Autologous Tier-2 and heterologous Tier-1 Nabs target different epitopes
We used multiple techniques to gain insights into the various epitopes targeted by the Tier-1 and Tier-2 NAbs in sera from the BG505 SOSIP.664 trimer-immunized rabbits. First, we performed neutralization assays in the presence of several antigens that could potentially bind and thereby deplete various NAb specificities. These competitors included linear peptides derived from the first, second and third (V1, V2 and V3) variable loops of Env, a V3-Fc construct, a V1V2-scaffold, a gp41 protein, CD4-binding defective gp120-D368R monomers and SOSIP.664-D368R trimers and the resurfaced stabilized core 3 (RSC3) CD4 binding site (CD4bs)-mimetic protein (Table 1). The gp120-D368R monomers and SOSIP.664-D368R trimers absorbed the autologous NAbs comparably and completely, implying that the target epitope(s) is well presented on the isolated gp120 subunit (Table 1; fig. S6). As residue D368 is a key element of the CD4bs, the inhibitory effect of the two Env-D368R mutants, combined with the lack of effect of the RSC3 protein, implies that the autologous NAbs are unlikely to target the CD4bs directly (Table 1). Truncated gp120-D368R variants lacking the V1, V2, V3 or V1V2 domains were effective competitors for the autologous NAbs, but the variable loop-based peptides or protein constructs and the gp41 proteins were generally inactive (Table 1). While the V1/V2 and V3 regions are not generally the targets for autologous NAbs, a noteworthy exception was rabbit 1412. Here, the NAb-depleting effect of the gp120-D368R monomer was completely lost when any of the V1, V2, V1V2 or V3 regions was deleted, but the linear V1, V2 and V3 peptides and the V1V2-scaffold protein were ineffective competitors (Table 1). Hence, the relevant epitope seen by serum 1412 is not present on simple mimics of the V1, V2 and V3 regions (i.e., peptides and scaffolds), but its formation requires that all three variable regions are present on a gp120 monomer or the gp120 subunits of the native trimer.
Table 1.
Depletion of autologous Tier-2 NAbs in sera from rabbits and macaques immunized with BG505 SOSIP.664 trimers or gp120 monomers by a variety of competitor ligands
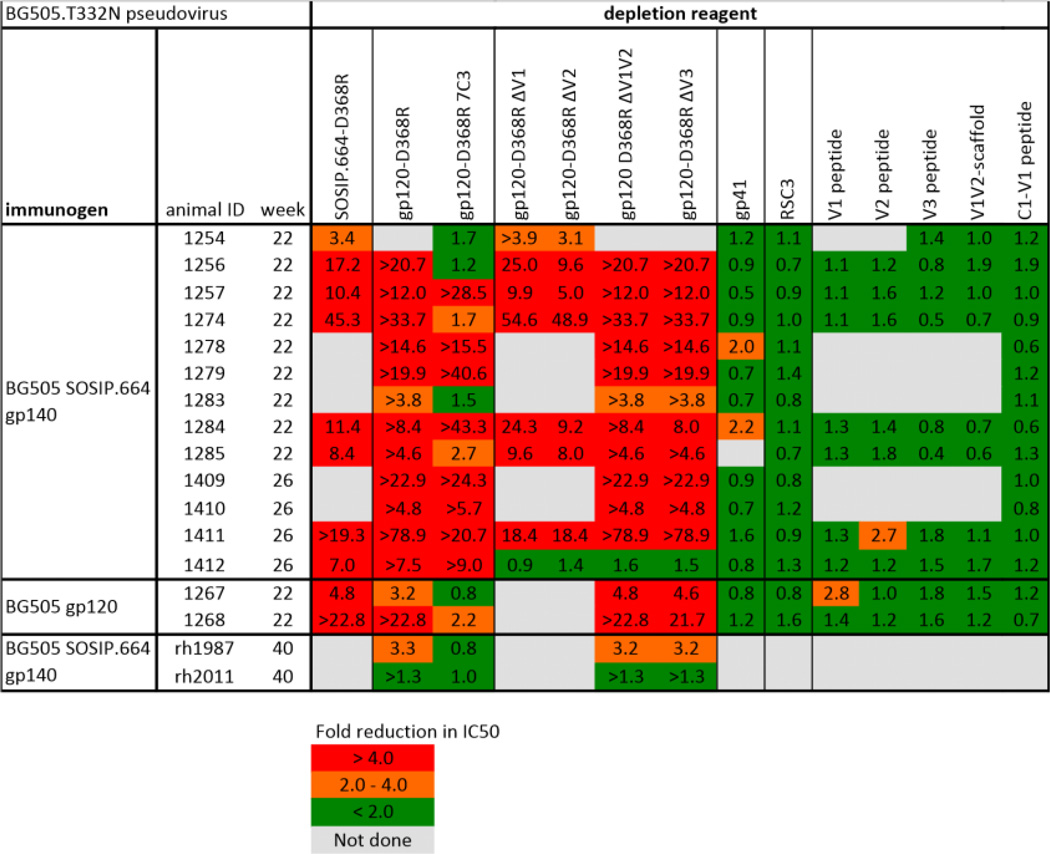 |
Sera from 13 BG505 SOSIP.664 trimer-immunized and two BG505 gp120-immunized rabbits, taken two weeks after the third immunization (i.e., at week-22 or week-26), as well as sera from two BG505 SOSIP.664 trimer-immunized macaques, taken two weeks after the fifth immunization (i.e. week-40), were tested for their ability to neutralize the BG505.T332N virus in the presence of a variety of competitor proteins and peptides, added in excess. The TZM-bl cell assay was conducted at AMC. The data show the fold-reduction in neutralization (IC50) in the presence of the specified test reagents. Strong (>4-fold) and moderate (2–4-fold) inhibitory effects are colored in red and orange, respectively, with no or minimal (<2-fold) inhibition in green. Fold-reduction values preceded by > indicate that neutralization was depleted completely (i.e. titer of <20). Gray boxes indicate tests not performed.
Peptide serology confirmed that some anti-trimer antibodies recognized the V3 region (fig. S7A). Env trimers undergo conformational transitions that expose V3 both on the virus and on their engineered SOSIP.664 counterparts in vitro (9, 23, 34). Such “breathing” events are likely to render V3 immunogenic in vaccinated animals. The linear BG505 V3-peptide consistently reduced (by >10-fold) titers against the Tier-1 viruses MN.3, SF162 and MW965.26 when tested against eight sera from trimer-immunized rabbits (fig. S7B,C,D) (33). This peptide also depleted the Tier-1 NAbs induced by the WT.SEKS protein (fig. S7C,D), consistent with reports that such responses to uncleaved gp140s are typically V3-dominated (33, 35). Deleting the V3 region consistently reduced the ability of the gp120-D368R protein to deplete trimer-induced Tier-1 Nabs, but not autologous NAbs (fig. S7B,C,D; Table 1). We conclude that V3 peptide-reactive antibodies raised against the clade-A trimer cross-neutralize Tier-1 viruses, but do not neutralize the autologous Tier-2 virus, which is consistent with the lack of correlation between Tier-1 and Tier-2 NAb titers (Fig. 2B; fig. S5C and D) and the resistance of BG505.T332N to V3 MAbs that neutralize Tier-1 viruses (9).
To gain further insights into the autologous NAb response, sera from the nine trimer-immunized rabbits with the highest titers against BG505.T332N were tested against 109 mutants of this virus with single alanine point substitutions of gp120 residues. We sought variants with reduced neutralization sensitivities as a result of sequence changes that affected key epitope(s). The neutralization profile across the virus panel was unique for each serum, showing that the autologous response was different, wholly or in part, in each animal (table S3). The data derived from the mutant panel for all nine rabbits were mapped onto the BG505 SOSIP.664 trimer structure (Fig. 3). Multiple alanine substitutions affected BG505.T332N neutralization. In general, the substitutions with the most impact were clustered at the trimer apex and the gp120 outer domain (OD), including the periphery of the CD4bs, an exception being that serum 1257 was sensitive to the loss of the glycan at C1 residue N88 (Fig. 3, side views). Among individual rabbits, 1274 was affected by mutations in V1, V2, C3 and V5; 1410 by C2, C3, V4 and C5 substitutions; and 1412 by V1, V2 and C5 changes. Among the most frequent and largest effects (>5-fold titer reduction for >5 sera) were changes at L125 (C1); R166 and K168 (V2); P299, R304 and K305 (V3 base); and I420, K421 and Q422 (C4). Autologous NAbs present in seven of nine rabbit sera were sensitive to changes in one or both of the basic R166 and K168 residues in V2 β–strand C. These residues are important for forming the epitopes targeted by the bNAbs PG9, PG16 and VRC26 (6, 36), but it is possible they have more distant effects on the conformation of other epitopes. In rabbit 1412, the autologous response was directed against a conformational epitope involving all three of the V1, V2 and V3 regions and also influenced by N137, N156 and K168 (Tables 1 and 3; table S3). This sensitivity pattern is akin to a PG9/16-like response, except for the lack of dependency on glycan N160 at the trimer apex. Hence the autologous NAb response in rabbit 1412 may involve narrow-specificity, PG9/16-like or VRC26-like antibodies. The gp120 OD, particularly residues in C3 and C4, was targeted in several rabbits. The depletion experiments with the 7C3 gp120-D368R protein also implicate C3 residues 354–363 as direct or indirect influences on the epitope(s) recognized by the autologous NAbs in rabbit sera 1254, 1256, 1274, 1283 and 1285 (Table 1Fig. 3). Of note is that BG505.T332N neutralization was adversely affected by mutations that eliminated various OD glycan sites including N185, N301 and N462 (rabbit 1274); N332, N392 and N398 (rabbit 1410); N137, N156 and N386 (rabbit 1412). We are not aware of the induction of NAbs to glycan-dependent epitopes by gp120 monomers or non-native gp140 proteins.
Figure 3. Autologous Tier-2 NAb responses in sera from BG505 SOSIP.664 trimer-immunized rabbits mapped onto the trimer structure.
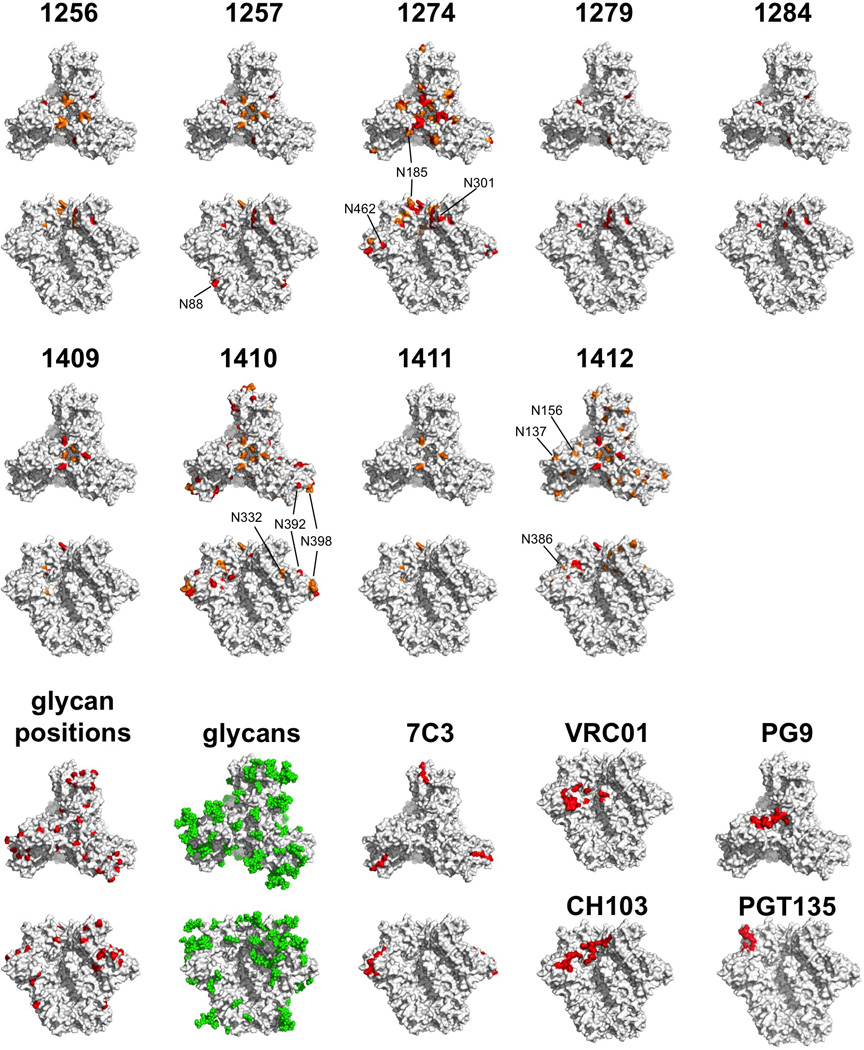
Neutralization data derived when sera from the indicated rabbits were tested against a panel of BG505.T332N virus mutants are shown in table S3. Residues in gp120 where Ala substitutions reduce neutralization by 5–10 fold and >10-fold are colored orange and red, respectively, on the BG505 SOSIP.664 crystal structure (PDB: 4TVP). Each rabbit serum, denoted by a 4-digit number, is mapped individually. In each case, the first row is a top view of the trimer, the second row a side view. The glycosylation sites that affect neutralization by rabbit sera 1257, 1274, 1410 and 1412 are labeled. For comparison, the bottom two panels illustrate the positions of glycans (red), the glycans resolved in the crystal structure (green), the 7 amino acid substitutions that were under selection pressure in the BG505 infant (7C3, red), and the footprints (within 3 Å) of the bNAbs VRC01 and CH103 (both to the CD4bs), PG9 and PGT135 (all side views, except for PG9, top view).
Table 3.
Comparison of NAb responses induced by BG505 SOSIP.664 trimers in rabbits and by natural infection of an infant with HIV-1 BG505 virus
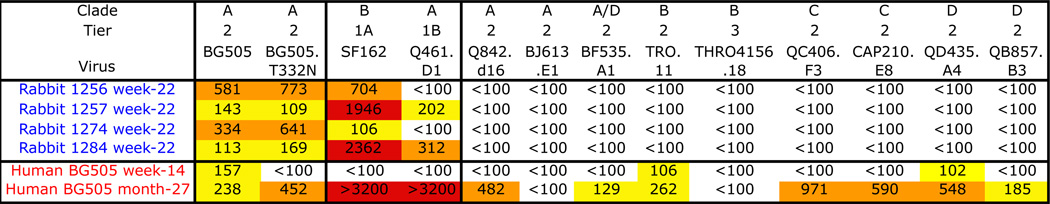 |
Sera from rabbits 1256, 1257, 1274 and 1284 after three immunizations (i.e., at week-22) with BG505 SOSIP.664 trimers were compared with sera from infant BG505 taken at week-14 and month-27 post-infection. The trimers are based on a sequence derived from the infant at week-6. The TZM-bl cell assay was conducted at FHCRC. The clades and the Tier-classifications of the test viruses are indicated. The BG505 and BG505.T32N viruses are described in SOM Methods. The NAb titers are color coded as follows: white <100; yellow 100–300; orange 300–1000; red >1000.
We used a competition ELISA to study whether the BG505 SOSIP.664, WT.SEKS gp140 or gp120 proteins had induced Abs that could block the trimer binding of various bNAbs (37). Several sera from SOSIP.664 or gp120 recipients reduced the binding of CD4bs bNAbs CH103 and VRC01, and also CD4-IgG2, by >50% whereas sera from WT.SEKS gp140 recipients had no such effect (fig. S8A). The inhibition of CH103 and VRC01 binding correlated with BG505.T332N neutralization, suggesting that at least some serum antibodies mediating the autologous response do so by impeding access to the CD4bs (fig. S8B). However, taken together, the virus mutant data and neutralization-depletion experiments with the RSC3 and gp120-D368R proteins imply that such antibodies do not target the CD4bs directly (Table 1). We note that when the PGT135 bNAb binds to its V3-glycan dependent epitope on the trimer, it reorients other glycans so that they now occlude the CD4bs (37, 38). The PGT135 epitope on the trimer is proximal to residues implicated in the autologous NAb responses for rabbits 1274, 1410 and 1412 (Fig. 3, fig. S8A). Narrow-specificity NAbs with broadly similar properties to PGT135, including glycan dependency, that indirectly impede access to the CD4bs may be present in some rabbit sera.
The competition ELISA also showed that sera from some trimer-immunized rabbits inhibited the binding of bNAbs 35022 and 3BC315 to their trimer epitopes at the gp120-gp41 interface or gp41, while sera from gp120- or WT.SEKS-immunized rabbits did not (fig. S8A). Some autologous NAbs may therefore directly or indirectly occlude epitopes near the bottom of the trimer. Overall, the competition ELISA data confirm that multiple epitopes on the BG505 SOSIP.664 trimer are immunogenic in rabbits, and imply that the trimers were generally more efficient than gp120 and WT.SEKS gp140 proteins at inducing Abs capable of inhibiting bNAb-trimer interactions (fig. S8A).
Comparison with the NAb response induced by the BG505 virus in the infected infant
As our long-term goal is to devise an immunization regimen that can induce bNAbs, we compared the autologous NAb response against the BG505 SOSIP.664 trimers in rabbits with the much broader response that developed in the infant from whom the BG505 virus was isolated (8) (see SOM Methods). The infant’s week-14 serum weakly neutralized the BG505 virus, but not BG505.T332N, and completely lacked heterologous neutralization activity even against the Tier-1A virus SF162 (Table 3). However, a strong cross-neutralization response against Tier-1 and Tier-2 viruses, including BG505 and BG505.T332N, had developed by month-27. In a direct comparison, week-22 sera from four BG505 SOSIP.664 trimer-immunized rabbits (1256, 1257, 1274, 1284) neutralized BG505 and BG505.T332N viruses more strongly than the early infant sera, but lacked the neutralization breadth present in the month-27 serum (Table 3). Thus, the recombinant trimers induced NAb responses in rabbits that are similar, but not identical to, the primary infection response of the human infant; autologous NAbs were present in both species.
To guide immunogen design, we studied how BG505 Env sequences evolved in the infant. As expected, the month-27 sequences were highly divergent (9%) from week-6. In particular, multiple changes within a 10-residue stretch of C3 (residues 354–363) imply a strong selection pressure on this region (fig. S9). The predominant month-27 sequence had seven C3 changes: G354E, Δ356, T357K, I358T, R360I, A362T and N363K (which removed a glycan site). A gp120-D368R variant containing all seven changes (termed 7C3) was unable to deplete autologous NAbs from sera of five of the 13 test rabbits (numbers 1254, 1256, 1274, 1283, 1285), but was active against the other eight (Table 1). This variant also did not deplete autologous NAbs from gp120-immunized rabbit sera 1267 and 1268. These findings underscore similarities in how the humoral immune systems of the two species respond to BG505 Env.
Immunogenicity of BG505 SOSIP.664 trimers in rhesus macaques
In a pilot study, we compared the immunogenicity of BG505 SOSIP.664 trimers and gp120 monomers in rhesus macaques (fig. S1). Three of four sera neutralized the autologous BG505.T332N virus at week-26 (median titer, 78), while none of four sera from gp120-immunized animals did so (Fig. 1E; table S4). By week-54, the median autologous titer in the macaques approached that in the rabbits at week 22–26, i.e., 203 vs. 570 (Fig. 1E; table S4, cf. Fig. 1A). As the anti-gp120 and anti-trimer ELISA titers in the macaques were ~5-fold lower than in the rabbits (fig. S10,A and B, cf. fig. S4A), a stronger adjuvant might be advantageous. NAb titers against Tier-1 viruses MW965.26 and MN.3 were similar at week 26 and 54 time points, and did not differ markedly between trimer and monomer groups at week-26 (Fig. 1F and G). None of the eight sera neutralized the heterologous Tier-2 clade C virus Ce1176 (Fig. 1H).
In the competition ELISA, sera from trimer-immunized macaques induced stronger bNAb-blocking responses than gp120 recipients, including Abs that reduced access of CH103 and VRC01 to the CD4bs and, to a lesser extent, of PGT145 to the trimer apex (fig. S8A). In addition, the BG505 gp120-D368R protein but not its 7C3 variant depleted BG505.T332N NAbs from rh1987 sera (Table 1) suggesting that, as in some rabbits, the C3 region is involved in this autologous response.
Conclusions
Inducing high titers of autologous Tier-2 NAbs may be a necessary first step in the elicitation of bNAbs (3–6). The strong and consistent autologous response to the BG505 and B41 SOSIP.664 trimers reflects their native-like structure, homogeneity, stability and antigenicity, as well as the immunogenicity of the BG505 virus in the infected infant (8–11, 13, 16, 23). Taken together, the mapping studies show that multiple specificities contribute to the autologous NAb responses against the BG505 trimers, including antibodies that recognize glycan-influenced epitopes. If a polyspecific response could be achieved in humans, it might provide broader coverage against circulating viruses, but a human vaccine must generate a more broadly neutralizing response than we report here. The native structure of SOSIP.664 trimers allows candidate immunogens to be rationally redesigned to try to improve immunogenicity. Among relevant but not mutually exclusive strategies include: introducing sequence changes to increase the stability of the trimer apex and associated bNAb epitopes while reducing the antigenicity of V3 and other non-NAb epitopes that may be immunologic distractions; immunizing with sequential SOSIP.664 trimers based on later-arising BG505 sequences or cocktails of different trimers (e.g., from clades A, B and C, etc.) (23); priming with trimer variants that trigger germline responses (4, 5, 39, 40). Overall, our results strongly validate the concept of using native-like trimers and structural information to create an HIV-1 vaccine that induces bNAbs.
Materials and Methods
Immunogens and immunizations
The BG505 SOSIP.664 trimers, gp120 monomers and WT.SEKS uncleaved gp140 proteins were all produced and purified as reported previously (9, 13, 19). Unless specified, the proteins were expressed in HEK293T cells by transient transfection and purified via a 2G12 MAb-affinity column followed by size-exclusion chromatography (SEC). Protein purities and properties were comparable to those described elsewhere (9, 13, 19). BG505 SOSIP.664 trimers were also expressed in HEK293S cells that lack N-acetylglucosaminyltransferase I (GnTI−/−). The resulting Env proteins bear glycans that are not fully processed and remain in oligomannose form (21, 22). Samples of the 293S cell-derived trimers were treated with the EndoH glycosidase, as previously described, to reduce their total glycan content (22). The clade B B41 SOSIP.664 and B41 SOSIP.664-D7324 trimers were produced from stable CHO cell lines that were cultured in 0.5% serum (23). The trimers were purified by 2G12 affinity chromatography followed by SEC, as described elsewhere; the extent of V3-clipping was negligible (23). The YU2 gp140-Fd protein was made in HEK293T cells under contract for IAVI by Dr. Guillaume Stewart-Jones (University of Oxford, Oxford, UK), as described elsewhere (44).
Rabbit immunizations and blood sampling were carried out under subcontract at Covance (Denver, PA) according to the schedule presented in fig. S1B. Female New Zealand White rabbits (usually 4 per group) were immunized intramuscularly with 30 µg of the various Env proteins (40 µg in experiment 3). The proteins were formulated in 75 Units of ISCOMATRIX™, a saponin-based adjuvant obtained from CSL Ltd. (Parkville, Victoria, Australia) (45). Macaque immunizations and blood sampling were carried out at the Wisconsin Primate Center according to the schedule in fig. S1B. Rhesus macaques (4 per group) were immunized intramuscularly with 100 µg of BG505 SOSIP.664 or gp120 proteins formulated in 75 Units of ISCOMATRIX™.
MAbs and human sera
The MAbs used here were provided by the following individuals: CH103 and CH31, Dr. Barton Haynes (Duke University, Durham, NC); VRC01, Drs. Peter Kwong and John Mascola (NIH/VRC, Bethesda, MD); NIH45–46, NIH45–46W, 3BNC60, 3BNC117, 12A12, 12A21, Dr. Michel Nussenzweig (Rockefeller University, New York); various MAbs used for tier classification, Dr. Susan Zolla-Pazner (New York University School of Medicine, New York). MAbs 2G12 and 2F5 were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH from Dr. Hermann Katinger. The serum samples used for the neutralization sensitivity (i.e., Tier) classification of BG505.T332N have been described elsewhere (46). Sera obtained from individuals chronically infected with clade-A HIV-1 strains were gifts from Dr. Barton Haynes and Dr. Aine McKnight (Barts & The London Medical School, London, UK). MAb ARP3119 used for western blotting was acquired from the Programme EVA Centre for AIDS Reagents.
BG505 viruses and serum
The BG505 infant was HIV-1 DNA-negative at birth, but DNA-positive six weeks later, suggesting that infection occurred within this window (8). The sequence of a week-6 clone is the basis of the BG505 SOSIP.664 protein construct, in which a T332N substitution was made to restore bNAb epitopes that require the N332 glycan (7–9, 24). The same change was made to create the BG505.T332N variant of the week-6 BG505 virus (9). Serum samples were available from week-6, week-14 and month-27.
ELISA reagents and procedures
The D7324-epitope-tagged version of BG505 SOSIP.664, referred to as SOSIP.664-D7324, and BG505 gp120 with a reconstructed D7324 epitope in C5, were made as described previously (9). JR-CSF gp120 was prepared by Diane Kubitz at the Scripps Center for Antibody Development and Production (La Jolla, CA) using transient transfection of HEK293F cells, and purified by Galanthus nivalis lectin (Vector Labs, Burlingame, CA) affinity chromatography followed by SEC using a Sephacryl S200HR column. Anti-gp120 and anti-trimer ELISAs using the above proteins as antigens were performed as described previously (9). The C-terminal His-tagged BG505 SOSIP.664-His trimer was prepared as described previously (16, 37). His-tagged BG505 gp41 (gp41-His) was produced as follows: A His-tagged version of the BG505 IP.664 gp140 protein, which is based on the SOSIP.664 construct but with the SOS disulfide bond omitted (13), was expressed in the presence of excess furin in 293F cells. The protein was purified via the His-tag using Ni-NTA chromatography with elution using 250 mM imidazole, followed by three rounds of negative selection using a 2G12 bNAb column to remove any residual gp120 or uncleaved gp140 proteins. The purified gp41 protein bound the gp41-specific non-NAb F240 efficiently, but did not bind 2G12 or VRC01, indicating that contaminant gp120 or gp140 proteins were not present (data not shown). The gp41 protein also did not bind the PGT151 or 3BC315 bNAbs, suggesting that it was not in a pre-fusion conformation (not shown; (37, 47). Ni-NTA ELISAs using His-tagged trimers and the gp41 protein were performed as described elsewhere (37).
For bNAb competition ELISA experiments, rabbit or macaque sera (1:100 dilution) were incubated with D7324-captured BG505 SOSIP.664-D7324 trimers for 1 h. A biotinylated bNAb was then added at a concentration sufficient to give ~80% of the maximum binding signal, as assessed in a prior titration experiment (i.e., with no competitor present). The bound bNAb was detected using horseradish peroxidase (HRP)-labeled streptavidin. As the PGT151, 35O22 and 3BC315 bNAbs, and also CD4-IgG2, could not be biotinylated without impairing their binding activity (37) we detected unlabeled human bNAbs using an HRP-labeled donkey anti-human IgG conjugate that was minimally cross-reactive with rabbit IgG (Jackson Immunoresearch, Westgrove, PA). The latter assay format was unsuitable for macaque sera because the anti-human antibody cross-reacted with macaque IgG. As a result, it was not possible to test the macaque sera for inhibition of PGT151, 35O22, 3BC315 or CD4-IgG2 binding to the trimer.
To analyze the relative titers for serum antibody binding to conformational vs. linear epitopes, trimers (0.1 μg/ml) or monomers (0.03 μg/ml) were denatured by heating for 5 min at 99°C in 50 μl of TBS/10% FCS/1% SDS/50 mM DTT (sodium dodecyl sulfate, SDS; dithiothreitol, DTT). The samples were then diluted 140-fold in TBS/10% FCS to prevent SDS and DTT from interfering with the ELISA. The use of both SDS and DTT ensures that the Env proteins are fully denatured by heat treatment (48). The native or denatured proteins were then used in a D7324-capture ELISA, essentially as described elsewhere (9, 48). The relative reduction of binding to the denatured vs. native Env proteins was calculated using the half maximal binding values (EC50).
Neutralization assays
TZM-bl cell neutralization assays using Env-pseudotyped viruses were performed at six sites. For additional information on the assay and all supporting protocols see: http://www.hiv.lanl.gov/content/nab-reference-strains/html/home.htm. The performance sites were as follows: HMS, Harvard Medical School, Boston, MA and DUMC, Duke University Medical Center, Durham, NC (for methodology see (49)); IAVI, International AIDS Vaccine Initiative, Brooklyn, NY (for methodology see (24)); AMC, Academic Medical Center, Amsterdam (methodology see (9)); WCMC, Weill Cornell Medical College, New York, NY (methodology see (50)); FHCRC, Fred Hutchinson Cancer Research Center, Seattle, WA (methodology see (8)); TSRI, The Scripps Research Institute (methodology see (51)). The Env-pseudotyped viruses and their Tier classifications have been described elsewhere (8, 52–55), as have the BG505.T332N and BG505 Env-pseudotyped viruses (8, 9, 24). The BG505.T332N Env-pseudotyped virus was used except when the test virus is specifically stated to be BG505 (i.e., without the T332N substitution). Also note that the MN Env-pseudotyped virus used at DUMC is designated MN.3. We did not use the A3R5 cell assay because of our concerns that it produces false-positive, and hence misleading, detection of NAbs to Tier-2 viruses.
Tier categorization of the BG505.T332N and B41 Env-pseudotyped viruses was based on the neutralization sensitivity to a panel of MAbs directed against various epitopes as well as a panel of sera from humans infected with clade-A viruses, in comparison with previously tiered viruses (52). Data on BG505.T332N sensitivity to another panel of 50 MAbs can be found elsewhere (9). For both viruses, the tier classification experiments were performed at DUMC. We constructed a set of BG505.T332N alanine mutants for mapping NAb responses (table S3).
Neutralization depletion experiments
Proteins for neutralization depletion experiments (BG505 gp120-D368R and variants; BG505 SOSIP.664-D368R; RSC3 (56)) were expressed transiently in HEK293F cells and purified by 2G12-affinity chromatography. All reagents were based on the BG505 sequence, except for the RSC3 protein. The D368R change was introduced to ensure that the gp120 or SOSIP.664 gp140 proteins do not bind to CD4 on the cell surface and thereby inhibit HIV-1 infection competitively. ELISA experiments confirmed that the D368R substitution strongly reduced the binding of CD4 and several CD4bs bNAbs to BG505 gp120 (data not shown). The gp120-D368R 7C3 reagent contains 7 amino acid changes in C3 (G354E, Δ356, T357K, I358T, R360I, A362T and N363K) based on the month-27 sequences from infant BG505 (fig. S9). Substitutions and deletions were made using the Quickchange mutagenesis kit (Agilent, Santa Clara, CA). The plasmid expressing RSC3 (donated by Drs. Kwong and Mascola; NIH/VRC, Bethesda, MD) has been described elsewhere (56). The basis for the design of the BG505 V1V2-scaffold protein has also been described (57). The protein was expressed in HEK293S GnTI−/− cells and purified via its C-terminal 6xHis tag using Ni-NTA chromatography and NiCl2 elution, followed by SEC on a Superdex 200 column. The theoretical MW of the scaffold, including glycans, is ~25 kDa.
BG505-derived peptides with the following sequences were purchased from Genscript (Piscataway, NJ):
V1: TNVTNNITDDMRGELKN;
V2 (5 overlapping peptides): MTTELRDKKQKVYSL, DKKQKVYSLFYRLDV, YSLFYRLDVVQINEN, LDVVQINENQGNRSN, ENQGNRSNNSNKEYR;
V3: TRPNNNTRKSIRIGPGQAFYATGDIIGDIRQAH;
C1-V1: VKLTPLCVTLQCTNVTNNITDDMRGELKN.
To characterize the specificities of NAb responses induced in the rabbits, competitor Env proteins or peptides were incubated with appropriately diluted sera (total volume 25 µl) for 1 h at 37 °C. The competitor Env proteins were present at a concentration of 40 µg/ml except for the V1V2-scaffold protein (20 µg/ml). The competitor peptides were also used at 40 µg/ml, except that each individual component of a cocktail of 5 overlapping V2 peptides was present at 20 µg/ml. The Env-pseudotyped virus was then added to the serum-competitor mixture for 1 h before infection of TZM-bl target cells was initiated. The rest of the assay was carried out as described above and elsewhere (9). Neutralization titers were expressed as the reciprocal serum dilution that caused 50% inhibition of virus infection (IC50). The extent of neutralization depletion by the added competitor was expressed as the fold-reduction in the IC50 value.
Pepscan analysis
15-mer peptides, overlapping by 14 residues, from the BG505 SOSIP.664 as well as the unmodified BG505 gp140 sequences, were synthesized by Fmoc coupling on the solid support of a Pepscan hydrogel (58). The peptide libraries were probed with heat-inactivated human sera, at a 1:1000 dilution. After extensive washing, a goat anti-human HRP conjugated secondary antibody was added, followed by color development using 2,2'-azino-bis(3–ethylbenzothiazoline-6-sulphonic acid). A charge-coupled device camera was used to quantify the absorbance at 405 nm. For every individual Pepscan dataset, the data were normalized to the average signal intensity derived from the overall analysis.
Negative stain electron microscopy
The BG505 WT.SEKS, BG505 SOSIP.664 and YU2 gp140-Fd proteins, as well as ISCOMATRIX™ adjuvant-formulated BG505 SOSIP.664 trimers, were analyzed by negative stain electron microscopy (EM). Samples were prepared for analysis as previously described (9, 13). Briefly, a 3 µL aliquot containing ~0.01 mg/mL of protein was applied for 5 s onto a carbon-coated 400 Cu mesh grid that had been glow-discharged at 20 mA for 30 s, then negatively stained with 2% (w/v) uranyl formate for 60 s. Data were collected using an FEI Tecnai T12 electron microscope operating at 120 keV, with an electron dose of ~25 e−/Å2 and a magnification of 52,000x that resulted in a pixel size of 2.05Å at the specimen plane. Images were acquired with a Tietz TemCam-F416 CMOS camera using a nominal defocus range of 900 to 1300 nm.
Data processing methods were adapted from those used previously (9, 13). Particles were picked automatically using DoG Picker and put into a particle stack using the Appion software package (59). Initial, reference-free, two-dimensional (2D) class averages were calculated using particles binned by two via Iterative Multivariate Statistical Analysis (MSA)/Multi-reference Alignment (MRA) and sorted into classes (60). Particles corresponding to trimers were selected into a substack and binned by two before another round of reference-free alignment was carried out using Iterative MSA/MRA and Xmipp Clustering and 2D alignment algorithms (61).
Supplementary Material
Table 2.
Summary of autologous NAb specificities in sera from rabbits and macaques immunized with BG505 SOSIP.664 trimers or gp120 monomers when analyzed in different assays.
| Immunogen | producer cell | Animal ID | Neutralization mutants (glycan) |
Neutralization mutants | Neutralization depletion |
CD4/bNAb block |
|---|---|---|---|---|---|---|
| BG505 SOSIP.664 gp140 |
293S | 1254 | ND | ND | C3 | CD4, CH103, PGT151, 35O22, 3BC315 |
| 293S | 1256 | C1, V2, V3, C4 | C3 | CD4, CH103, VRC01, PGT151, 35O22, 3BC315 | ||
| 293S | 1257 | N88 | C1, V2, V3, C4 | CD4, 35O22, 3BC315 | ||
| 293T | 1274 | N185, N301, N462 | C1, V2, V3, C3, C4, V5 | C3 | CH103, VRC01 | |
| 293S | 1278 | ND | ND | CD4, CH103, PGT151, 35O22, 3BC315 | ||
| 293S | 1279 | C1, V3, C4 | CD4, CH103, VRC01, PGT121, PGT126, PGT151, 35O22, 3BC315 | |||
| 293S + EndoH | 1283 | ND | ND | C3 | PGT126 | |
| 293S + EndoH | 1284 | C1, V3, C4 | CH103, 35O22, 3BC315 | |||
| 293S + EndoH | 1285 | ND | ND | C3 | VRC01, 35O22, 3BC315 | |
| 293T | 1409 | V2, C4, C5 | CH103, VRC01, 3BC315 | |||
| 293T | 1410 | N332, N392, N398 | V2, C2, V3, C3, V4, C4, C5 | CH103, VRC01, 3BC315 | ||
| 293T | 1411 | V2, C4, C5 | VRC01, 3BC315 | |||
| 293T | 1412 | N137, N156, N386 | C1, V1, V2, C2, V3, C3, C4, C5 | V1, V2, V3 | CH103, VRC01, PGT126, 3BC315 | |
| BG505 gp120 | 293S | 1267 | ND | ND | C3 | CH103, VRC01 |
| 293S | 1268 | ND | ND | C3 | CD4, CH103, VRC01 | |
| BG505 SOSIP.664 gp140 |
293T | rh1987 | ND | ND | C3 | CH103, VRC01, PGT126, PGT135 |
| 293T | rh2011 | ND | ND | CH103, VRC01, PGT145, PGT126, PGT135 | ||
The immunogen, producer cell/treatment (i.e., influence on glycosylation profile) and rabbit ID number are listed in the first three columns. The fourth column lists N-linked glycosylation sites that affect autologous BG505.T332N neutralization (>5-fold decrease in IC50; see table S3). The fifth column lists gp120 domains where substitutions decrease neutralization by >5-fold (table S3). The sixth column lists the domains that, when mutated or deleted from the BG505 gp120-D368R protein, impair its neutralization-depletion capacity (Table 1). The seventh column list the bNAbs for which binding to D7324-tagged BG505 SOSIP.664 trimers in ELISA is inhibited by >50% by the test serum (fig. S9). In columns 4 and 5, ND = Not Done (the serum was not tested against the mutant virus panel). In columns 4, 6 and 7, a blank entry indicates that the assay was performed, but the outcome was uninformative.
Acknowledgements
This work was supported by National Institutes of Health Grants P01 AI082362, R37 AI036082, R01 AI084817, R01 AI076105, NIAID-NIH Contract HHSN27201100016C, by Scripps CHAVI-ID (UM1 AI100663), by grant P51OD011106, and by the Aids Fonds Netherlands, grants #2011032 and 2012041. J.-P.J. is a recipient of a Canadian Institutes of Health Research (CIHR) Fellowship. R.W.S. is a recipient of a Vidi grant from the Netherlands Organization for Scientific Research (NWO) and a Starting Investigator Grant from the European Research Council (ERC-StG-2011–280829-SHEV). This work was partially funded by IAVI with the generous support of USAID and the Bill & Melinda Gates Foundation; a full list of IAVI donors is available at www.iavi.org. The contents of this manuscript are the responsibility of the authors and do not necessarily reflect the views of USAID or the US Government. We thank the following individuals for technical support: K. Sliepen, J. Korzun, M. Golabek, K. de los Reyes, L. Kong, K. Weisgrau, N. Pomplun, J. Hsueh, K. L. Saye-Francisco, A. Ramos; for advice: S. Schmidt, J. Gorman and N. Doria-Rose; or for reagents: G. Stewart-Jones, D. Kubitz, P. Kwong, J. Mascola, B. Haynes, A. McKnight, H. Katinger, S. Zolla-Pazner, P. Poignard and M. Nussenzweig. The International AIDS Vaccine Initiative has previously filed a patent relating to the BG505 SOSIP.664 trimer: U.S. Provisional Application no. 61/772,739, titled “HIV-1 envelope glycoprotein,” with inventors M. Caulfield, A.C., H. D., S. Hoffenberg, C. R. King, P.J.K., A. Marozsan, J.P.M., R.S., A.B.W., I.A.W., J.-P.J. This does not alter our adherence to all Science policies on sharing data and materials.
Footnotes
Supplementary Materials:
Figures S1 to S10
Tables S1 to S4
References (62–63)
References and Notes
- 1.van Gils MJ, Sanders RW. Broadly neutralizing antibodies against HIV-1: templates for a vaccine. Virology. 2013;435:46–56. doi: 10.1016/j.virol.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 2.West AP, et al. Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell. 2014;156:633–648. doi: 10.1016/j.cell.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derdeyn C, Moore PL, Morris L. Development of broadly neutralizing antibodies from autologous neutralizing antibody responses in HIV infection. Curr. Opin. HIV AIDS. 2014;9:210–216. doi: 10.1097/COH.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haynes BF, Kelsoe G, Harrison SC, Kepler TB. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat. Biotechnol. 2012;30:423–433. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao H-X, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doria-Rose N, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509:55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu X, et al. Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant. J. Virol. 2006;80:835–844. doi: 10.1128/JVI.80.2.835-844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goo L, Chohan V, Nduati R, Overbaugh J. Early development of broadly neutralizing antibodies in HIV-1-infected infants. Nat. Med. 2014;20:655–658. doi: 10.1038/nm.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders RW, et al. A next-generation cleaved, soluble HIV-1 Env Trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 2013;9:e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyumkis D, et al. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science. 2013;342:1484–1490. doi: 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Julien J-P, et al. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science. 2013;342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pancera M, et al. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature. 2014;514:455–461. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ringe RP, et al. Cleavage strongly influences whether soluble HIV-1 envelope glycoprotein trimers adopt a native-like conformation. Proc. Natl. Acad. Sci. U. S. A. 2013;110:18256–18261. doi: 10.1073/pnas.1314351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guttman M, Lee KK. A functional interaction between gp41 and gp120 is observed for monomeric but not oligomeric, uncleaved HIV-1 Env gp140. J. Virol. 2013;87:11462–11475. doi: 10.1128/JVI.01681-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran K, et al. Vaccine-elicited primate antibodies use a distinct approach to the HIV-1 primary receptor binding site informing vaccine redesign. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E738–E747. doi: 10.1073/pnas.1319512111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasmeen A, et al. Differential binding of neutralizing and non-neutralizing antibodies to native-like soluble HIV-1 Env trimers, uncleaved Env proteins, and monomeric subunits. Retrovirology. 2014;11:41. doi: 10.1186/1742-4690-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guttman M, et al. CD4-induced activation in a soluble HIV-1 Env trimer. Structure. 2014;22:974–984. doi: 10.1016/j.str.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pritchard LK, et al. Structural constraints determine the glycosylation of HIV-1 envelope trimers. Cell Rep. 2015 doi: 10.1016/j.celrep.2015.05.017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Julien J, et al. Asymmetric recognition of the HIV-1 trimer by broadly neutralizing antibody PG9. Proc. Natl. Acad. Sci. U. S. A. 2013;110:4351–4356. doi: 10.1073/pnas.1217537110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ledgerwood JE, et al. Prime-boost interval matters: A randomized phase 1 study to identify the minimum interval necessary to observe the h5 dna influenza vaccine priming effect. J. Infect. Dis. 2013;208:418–422. doi: 10.1093/infdis/jit180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eggink D, et al. Lack of complex N-glycans on HIV-1 envelope glycoproteins preserves protein conformation and entry function. Virology. 2010;401:236–247. doi: 10.1016/j.virol.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Depetris RS, et al. Partial enzymatic deglycosylation preserves the structure of cleaved recombinant HIV-1 envelope glycoprotein trimers. J. Biol. Chem. 2012;287:24239–24254. doi: 10.1074/jbc.M112.371898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pugach P, et al. A native-like SOSIP.664 trimer based on a HIV-1 subtype B env gene. J. Virol. 2015;89:3380–3395. doi: 10.1128/JVI.03473-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffenberg S, et al. Identification of an HIV-1 clade A envelope that exhibits broad antigenicity and neutralization sensitivity and elicits antibodies targeting three distinct epitopes. J. Virol. 2013;87:5372–5383. doi: 10.1128/JVI.02827-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nkolola JP, et al. Breadth of Neutralizing Antibodies Elicited by Stable, Homogeneous Clade A and Clade C HIV-1 gp140 Envelope Trimers in Guinea Pigs. J. Virol. 2010;84:3270–3279. doi: 10.1128/JVI.02252-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blish Ca, et al. Comparative Immunogenicity of Subtype A Human Immunodeficiency Virus Type 1 Envelope Exhibiting Differential Exposure of Conserved Neutralization Epitopes. J. Virol. 2009;84:2573–2584. doi: 10.1128/JVI.01687-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sundling C, et al. Soluble HIV-1 Env trimers in adjuvant elicit potent and diverse functional B cell responses in primates. J. Exp. Med. 2010;207:2003–2017. doi: 10.1084/jem.20100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grundner C, et al. Analysis of the neutralizing antibody response elicited in rabbits by repeated inoculation with trimeric HIV-1 envelope glycoproteins. Virology. 2005;331:33–46. doi: 10.1016/j.virol.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Forsell MNE, et al. Biochemical and immunogenic characterization of soluble human immunodeficiency virus type 1 envelope glycoprotein trimers expressed by semliki forest virus. J. Virol. 2005;79:10902–10914. doi: 10.1128/JVI.79.17.10902-10914.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Go EP, Zhang Y, Menon S, Desaire H. Analysis of the disulfide bond arrangement of the HIV-1 envelope protein CON-S gp140 ΔCFI shows variability in the V1 and V2 regions. J. Proteome Res. 2011;10:578–591. doi: 10.1021/pr100764a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Go EP, Hua D, Desaire H. Glycosylation and disulfide bond analysis of transiently and stably expressed clade C HIV-1 gp140 trimers in 293T cells identifies disulfide heterogeneity present in both proteins and differences in O-linked glycosylation. J. Proteome Res. 2014;13:4012–4027. doi: 10.1021/pr5003643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shingai M, et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J. Exp. Med. 2014;211:2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malherbe DC, et al. Envelope Variants Circulating as Initial Neutralization Breadth Developed in Two HIV-Infected Subjects Stimulate Multiclade Neutralizing Antibodies in Rabbits. J. Virol. 2014;88:12949–12967. doi: 10.1128/JVI.01812-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munro JB, et al. Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions. Science. 2014;346:759–763. doi: 10.1126/science.1254426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melchers M, et al. Targeting HIV-1 envelope glycoprotein trimers to B cells by using APRIL improves antibody responses. J. Virol. 2012;86:2488–2500. doi: 10.1128/JVI.06259-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ringe R, Phogat S, Bhattacharya J. Subtle alteration of residues including N-linked glycans in V2 loop modulate HIV-1 neutralization by PG9 and PG16 monoclonal antibodies. Virology. 2012;426:34–41. doi: 10.1016/j.virol.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Derking R, et al. Comprehensive Antigenic Map of a Cleaved Soluble HIV-1 Envelope Trimer. PLoS Pathog. 2015;11:e1004767. doi: 10.1371/journal.ppat.1004767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong L, et al. Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat. Struct. Mol. Biol. 2013;20:796–803. doi: 10.1038/nsmb.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jardine J, et al. Rational HIV immunogen design to target specific germline B cell receptors. Science. 2013;340:711–716. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGuire AT, et al. HIV antibodies. Antigen modification regulates competition of broad and narrow neutralizing HIV antibodies. Science. 2014;346:1380–1383. doi: 10.1126/science.1259206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.see Materials and Methods for performance site abbreviations.
- 42.Beddows S, et al. A comparative immunogenicity study in rabbits of disulfide-stabilized, proteolytically cleaved, soluble trimeric human immunodeficiency virus type 1 gp140, trimeric cleavage-defective gp140 and monomeric gp120. Virology. 2007;360:329–340. doi: 10.1016/j.virol.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 43.Kang YK, et al. Structural and immunogenicity studies of a cleaved, stabilized envelope trimer derived from subtype A HIV-1. Vaccine. 2009;27:5120–5132. doi: 10.1016/j.vaccine.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchtenhagen H, et al. Boosting of HIV-1 neutralizing antibody responses by a distally related retroviral envelope protein. J. Immunol. 2014;192:5802–5812. doi: 10.4049/jimmunol.1301898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maraskovsky E, et al. Development of prophylactic and therapeutic vaccines using the ISCOMATRIX adjuvant. Immunol. Cell Biol. 2009;87:371–376. doi: 10.1038/icb.2009.21. [DOI] [PubMed] [Google Scholar]
- 46.Hraber P, et al. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS. 2014;28:163–169. doi: 10.1097/QAD.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blattner C, et al. Structural delineation of a quaternary, cleavage-dependent epitope at the gp41-gp120 interface on intact HIV-1 Env trimers. Immunity. 2014;40:669–680. doi: 10.1016/j.immuni.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore JP, Sattentau QJ, Wyatt R, Sodroski J. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J. Virol. 1994;68:469–484. doi: 10.1128/jvi.68.1.469-484.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol. Biol. 2009;485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- 50.Ketas TJ, Holuigue S, Matthews K, Moore JP, Klasse PJ. Env-glycoprotein heterogeneity as a source of apparent synergy and enhanced cooperativity in inhibition of HIV-1 infection by neutralizing antibodies and entry inhibitors. Virology. 2012;422:22–36. doi: 10.1016/j.virol.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sok D, et al. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc. Natl. Acad. Sci. U. S. A. 2014;111:17624–17629. doi: 10.1073/pnas.1415789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seaman MS, et al. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J. Virol. 2010;84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li M, et al. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J. Virol. 2006;80:11776–11790. doi: 10.1128/JVI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.deCamp A, et al. Global panel of HIV-1 Env reference strains for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 2014;88:2489–2507. doi: 10.1128/JVI.02853-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simek MD, et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J. Virol. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lynch RM, et al. The development of CD4 binding site antibodies during HIV-1 infection. J. Virol. 2012;86:7588–7595. doi: 10.1128/JVI.00734-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McLellan JS, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Langedijk JPM, Zekveld MJ, Ruiter M, Corti D, Back JW. Helical peptide arrays for lead identification and interaction site mapping. Anal. Biochem. 2011;417:149–155. doi: 10.1016/j.ab.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Voss NR, Yoshioka CK, Radermacher M, Potter CS, Carragher B. DoG Picker and TiltPicker: software tools to facilitate particle selection in single particle electron microscopy. J. Struct. Biol. 2009;166:205–213. doi: 10.1016/j.jsb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogura T, Iwasaki K, Sato C. Topology representing network enables highly accurate classification of protein images taken by cryo electron-microscope without masking. J. Struct. Biol. 2003;143:185–200. doi: 10.1016/j.jsb.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Lander GC, et al. Appion: an integrated, database-driven pipeline to facilitate EM image processing. J. Struct. Biol. 2009;166:95–102. doi: 10.1016/j.jsb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang J, et al. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature. 2014;515:138–142. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker LM, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


