Introduction
Acetaminophen (N-acetyl-p-aminophenol, APAP, or paracetamol, PARA) is widely used for its analgesic and antipyretic properties in many over-the-counter formulations in both adults and children [1,2]. APAP can be synthesized in the body through O-dealkylation of the prodrug phenacetin, a pain-killer that was withdrawn from the market due to nephrotoxicity and carcinogenesis [3]. At the most usual therapeutic adult dose of 1–2 g/day, oral APAP is indicated for fever and for the relief of mild to moderate acute pain [2]. Administration of acetaminophen via the intravenous route has become increasingly widespread and has been used as a safe and effective antipyretic and analgesic agent [4]. The maximum recommended therapeutic dose of APAP is 4 g/day in adults and 50–75 mg/kg/day in children. Consumption of a single dose greater than 7 g in an adult and 150 mg/kg in a child is considered potentially toxic to the liver and kidneys due to the highly active metabolite, N-acetyl-p-benzoquinone imine (NAPQI)[5]. Acetaminophen overdose is one of the most common drug-related toxicities reported to poison centers. APAP is the main cause of acute liver failure in the United States [2,5]. To reduce the risk of hepatotoxicity, the FDA requires that manufacturers limit the amount of acetaminophen in a pill to 325 mg, and that all the formulations containing the drug have a black box warning for potential liver damage [6]. The FDA has also recommended that the healthcare professionals avoid prescribing and dispensing products containing more than 325 mg of APAP per dose [7].
Pharmacokinetics
Acetaminophen has a high oral bioavailability (88%), it is well absorbed and reaches the peak blood concentrations within 90 minutes after ingestion [5]. APAP is not widely bound to plasma proteins, and has a plasma half-life of 1.5–2.5 hours at the recommended doses [8]. However, after an overdose, metabolism is impaired, the half-life is prolonged to 4–8 hours and is directly related to the extent of the liver injury [5].
Metabolism
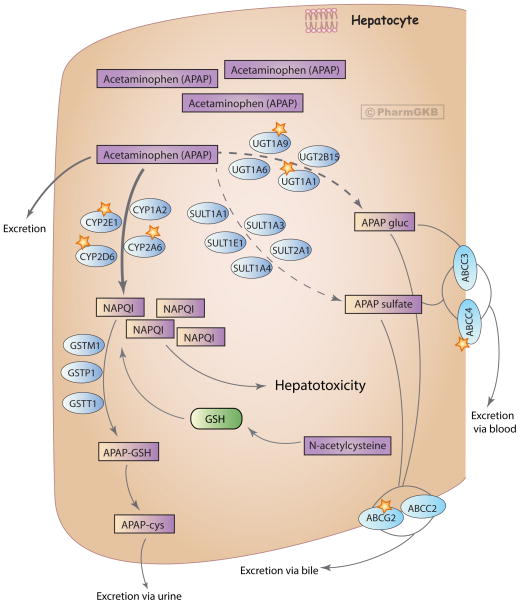
The liver, and to a lesser extent the kidney and intestine, are the major organs implicated in the metabolism of acetaminophen [9]. After a therapeutic dose, APAP is mostly converted to pharmacologically inactive glucuronide (APAP-gluc, 52–57% of urinary metabolites) and sulfate (APAP sulfate, 30–44%) conjugates, with a minor fraction being oxidized to a reactive metabolite NAPQI (5–10%) (Figure 1). Less than 5% of APAP is excreted unchanged [10]. NAPQI is highly reactive and is primarily responsible for acetaminophen-induced hepatotoxicity. Detoxification of NAPQI occurs through its binding to the sulfhydryl group of glutathione (GSH) to form APAP-GSH, which is ultimately excreted in the urine as cysteine and mercapturic acid conjugates (APAP-cys) [5,9]. Acetaminophen disposition involves a complex inter-organ transport of metabolites between the liver, kidney and intestine, through bile and the blood stream, to be ultimately eliminated in urine [9]. From the liver, most of glucuronide and sulfate metabolites get transported into the kidneys through the blood stream, while some APAP-gluc appears in the bile with subsequent transport through the intestines into the blood. The kidney is the main site of the disposition of APAP sulfate, either through direct excretion or through further biotransformation followed by renal excretion. Although most of NAPQI is formed in the liver, the kidney also metabolizes APAP to the toxic metabolite and releases cysteine conjugate of APAP into the bile and blood for further elimination in urine [9].
Figure 1.
Metabolism and transport of acetaminophen in the liver at therapeutic doses. Glucuronidation is the main pathway of acetaminophen metabolism, followed by sulfation and a minor contribution from the oxidation route. Oxidation by CYP isozymes yields a reactive metabolite NAPQI that is detoxified by the glutathione pathway. Phenobarbital and phenytoin inhibit acetaminophen glucuronidation, while ethanol and isoniazid potentiate acetaminophen oxidation. Enzymes playing a major role in the corresponding pathway are denoted with a star. APAP, acetaminophen; APAP gluc, acetaminophen glucuronide; APAP-cys, acetaminophen cysteine; NAPQI, N-acetyl-p-benzoquinone imine. A fully interactive version is available online at http://www.pharmgkb.org/pathway/PA165986279.
At supratherapeutic doses of APAP (more than 4 g/day), the sulfation pathway becomes saturated, while glucuronidation and oxidation increase, and a smaller amount is excreted unchanged. After a highly toxic dose of APAP, glucuronidation gets saturated as well and higher proportions of the drug are eliminated unchanged (~10%) and get oxidized to NAPQI (>15%) (Figure 2). Excess NAPQI eventually depletes GSH stores and starts to form protein adducts through binding to cysteine groups on cellular proteins. NAPQI primarily targets mitochondrial proteins and ion channels leading to the loss of energy production, ion misbalance and cell death [5,9,11]. Following animal studies, N-acetylcysteine (NAC) was shown to be an effective antidote for acetaminophen overdose in humans [12]. NAC replenishes GSH stores, scavenges reactive oxygen species in mitochondria and enhances the sulfation metabolic pathway (Figure 2). If administered within 8–10 hours after an acute overdose, NAC reduces the risk of hepatotoxicity to less than 5%. Overall, NAC prevents liver damage, renal failure and death, and is the treatment of choice for APAP poisoning [5,9,10,13]. Extremely high doses of APAP result in severe liver damage accompanied by dramatically diminished glucuronidation and sulfation capacities [10]. In patients with fatal centrilobular hepatic necrosis, plasma and urinary levels of the glucorinide metabolite are barely detectable [14].
Figure 2.
Metabolism and transport of acetaminophen in the liver at highly toxic doses. After ingestion of highly toxic doses of acetaminophen, glucuronidation and sulfation pathways get saturated and higher portion of the drug gets oxidized and excreted unchanged. Excess NAPQI depletes glutathione stores causing liver injury. Administration of NAC provides an exogenous source of glutathione that will neutralize NAPQI and prevent further hepatotoxicity. Enzymes playing a major role in the corresponding pathway are denoted with a star. APAP, acetaminophen; APAP gluc, acetaminophen glucuronide; APAP-cys, acetaminophen cysteine; NAPQI, N-acetyl-p-benzoquinone imine; NAC, N-acetylcysteine. A fully interactive version is available online at http://www.pharmgkb.org/pathway/PA166117881.
Glucuronidation of acetaminophen is catalyzed by UDP-glucuronosyl transferases (UGT). UGTs make the APAP molecule more water-soluble by transferring the glucuronosyl group from UDP-glucuronic acid [5,8]. Studies in human liver microsomes and cultured hepatocytes indicate that UGT1A1, UGT1A6, UGT1A9 and UGT2B15 are involved in APAP glucuronidation [15–18]. UGT1A6 is important at low APAP concentrations [16], while UGT1A9 and UGT1A1 contribute the most at toxic doses with UGT1A9 catalyzing within a broad range of pharmacologically relevant APAP concentrations [16,17]. Genetic polymorphisms in UGTs have been reported to affect APAP metabolism in healthy subjects [19–21] and in a disease state [22–25], as well as after a specific diet [26] (discussed below).
A family of cytosolic enzymes, called sulfotransferases (SULT), carries out sulfation of acetaminophen. SULTs transfer a sulfo group from a substrate PAPS to APAP making it more polar and prone to elimination [8]. Using human platelet homogenates as a model for xenobiotic metabolism in the liver, SULT1A1 and SULT1A3/4 were first shown to catalyze APAP sulfation [27]. Human SULT1A3 and SULT1A4 genes are very closely related and code for identical SULT proteins [28]. In addition to SULT1A1 and 1A3/4, sulfation of APAP in the human fetal liver is carried out by SULT1E1 and SULT2A1 [29]. This study showed that in the fetal liver, SULT1A3/4 plays the major role in APAP sulfation; in postnatal development, however, APAP is predominantly sulfated by SULT1A1 and SULT2A1, while SULT1A3/4 activity diminishes [29].
Cytochrome P450 enzymes catalyze oxidation of acetaminophen to the reactive metabolite NAPQI [8,9]. The exact contribution of particular CYP isoforms to APAP bioactivation varies and depends on the concentration of the drug. In human liver microsomes, CYP2E1 and CYP1A2 were first reported to convert high doses of APAP to NAPQI [30]. Later studies, combining purified human proteins or human liver microsomes with specific inhibitors confirm the role of CYP2E1 in bioactivation of toxic levels of APAP, but also report involvement of CYP2A6 [31,32]. Studies with healthy human volunteers pre-treated with the CYP2E1 inhibitor, disulfiram, further confirm the role of CYP2E1 in APAP oxidation [33]. Using human liver microsomes and human recombinant CYP2D6, this enzyme has been reported to oxidize only very high, toxic doses of APAP, when plasma APAP concentration reaches 2 mM [34,35]. The role of CYP3A4 in APAP metabolism is controversial, with findings ranging from no significant contribution to it playing the primary role in APAP oxidation [33,35–37]. Studies with human recombinant CYP enzymes and experiments with human CYP3A4 expressed in a hepatoma cell line suggest a major involvement of CYP3A4 in APAP oxidation [35,37]. Conversely, incubation of human liver microsomes with the CYP3A4 inhibitor, troleandomycin and therapeutic doses of APAP reduced NAPQI formation by 10%; at toxic doses, APAP oxidation was reduced only by 5% [32,36]. In vivo human studies further indicate that the contribution of CYP3A4 to acetaminophen oxidative metabolism is negligible. Healthy volunteers pretreated with the CYP3A4 inducer, rifampin, exhibited insignificant changes in APAP plasma clearance or NAPQI formation [33]. Taken together, human in vitro and in vivo studies suggest that CYP3A4 plays a minor role in the bioactivation of low dose APAP. In addition to CYP450 isoforms, other enzymes might contribute to acetaminophen oxidation. In vitro experiments have shown the formation of the reactive metabolite NAPQI and N-acetyl-p-benzosemiquinone imine (NAPSQI) by prostaglandin H2 synthases (PTGS) [38,39]. This additional pathway is suggested to be secondary and found in tissues with lower cytochrome P450 activity, such as kidneys [9,40]. It should be noted, however, that these observations were made using animal microsomes and thus their relevance to acetaminophen metabolism in humans still needs to be investigated.
Acetaminophen metabolism may change under conditions that affect glutathione stores. Obesity, liver steatosis, starvation and fasting lead to GSH depletion and can be considered as risk factors for acetaminophen-induced hepatotoxicity [41,42]. Prolonged fasting results in redirection of acetaminophen metabolism from glucuronidation to the oxidation pathway. Under conditions of fasting, hepatic metabolism is shunted toward gluconeogenesis, making fewer glucose precursors available for glucuronidation. Increased oxidation of acetaminophen after starvation is also due to induction of CYP450 isoforms that start to convert more APAP to the toxic metabolite NAPQI [43]. Fasting was reported to enhance acetaminophen hepatotoxicity after an overdose and after repeated, low doses of the drug [43,44].
Conjugation of NAPQI to GSH occurs via both a spontaneous process and an enzymatic reaction catalyzed by glutathione-S-transferases (GSTs) [45]. A non-enzymatic reaction yields a GSH conjugate, 3-(glutathione-S-yl)-acetaminophen (APAP-GSH); a reduction product, free APAP; and an oxidation product, glutathione disulfide (GSSG). GST reaction yields APAP-GSH and free APAP. The human cytosolic GST family is comprised of seven distinct classes of enzymes with numerous genetic variants within each class [46]. Human in vitro studies with isolated liver and placenta GSTs have shown that GSTP1 is the most effective catalyst of NAPQI conjugation with GSH, followed by GSTT1 and GSTM1 [45]. In the NAPQI reduction reaction, the most efficient human transferase is GSTT1, followed by GSTM1 and GSTP1. Elevated plasma GST has been correlated with acetaminophen-induced hepatotoxicity and is proposed as a sensitive and early biomarker of acute liver damage [47,48]. Unlike alanine and aspartate aminotransferases, GSTs are quickly and robustly released from cetrilobular and periportal hepatocytes after APAP overdose. As early as 4 hours after APAP poisoning, patients exhibit abnormal plasma GST levels that remain elevated 12 hours after ingestion of the drug [48]. Intravenous administration of NAC results in a significant reduction in plasma GST levels beginning at 4 hours after the treatment [47,48]. If NAC is not provided within 8 hours after APAP intoxication, plasma GST levels will keep increasing and at 40–50 hours will be correlated with the time when the major liver damage occurs [48].
In addition to the prevailing pathways of acetaminophen metabolism – glucuronidation, sulfation and oxidation - acetaminophen might undergo deacetylation. Animal studies showed that deacetylation of APAP by the liver enzyme N-deacetylase yields a minor metabolite p-aminophenol [49]. P-aminophenol was reported to cause nephrotoxicity in rodent models [50,51]; however, its clinical relevance in relation to acetaminophen metabolism by humans is still to be determined. In the brain and the spinal cord, p-aminophenol is conjugated with arachidonic acid by Fatty Acid Amide Hydrolase (FAAH) enzyme to form an active metabolite N-arachidonoylphenolamine (AM404) [49]. In animal studies, AM404 is a potent agonist at the TRPV1 receptor that mediates pro-inflammatory and painful stimuli [49,52].
Transport
Disposition and elimination of acetaminophen depend on its transport through different cell types. Unlike the parent drug, movement of acetaminophen metabolites requires transporters. Interaction of acetaminophen with common drug carriers has been addressed in the context of two superfamilies of transporters, solute carrier transporters (SLC) and ATP-binding cassette (ABC) transporters [53–56]. ABC transporters mediate efflux of substrates from cells, while SLC transporters are responsible for uptake of substrates into cells [57,58]. Excretion of APAP-gluc and sulfate into the bile involves ABCC2 and ABCG2 carriers found in the canalicular membrane of hepatocytes. Movement of APAP-gluc into the blood depends on ABCC3 transporter, while the sulfate metabolite relies on ABCC3 and ABCC4, both located on the sinusoidal side of liver cells [8]. In addition, ABCB1, ABCC1 and ABCC5 transporters might be involved in acetaminophen excretion in humans, as evident by changes in their expression after toxic acetaminophen ingestion [53]. Livers from patients, who overdosed on acetaminophen, exhibit upregulation of ABCC1 and ABCC4 mRNAs and elevated protein levels of ABCB1, ABCG2, ABCC4 and ABCC5. Increased expression of efflux transporters might be an adaptive change to stop accumulation of toxic metabolites in cells and to prevent additional liver damage. Consistent with this hypothesis is increased hepatocyte proliferation and co-localization of upregulated transporters with the regions of rapidly replicating liver cells [53]. These adaptive responses to toxic levels of acetaminophen result in acquired resistance to a repetitive insult on the liver. This phenomenon is reminiscent of autoprotection observed in experimental animals, where initial exposure to the sub-toxic doses of acetaminophen protects rodents from subsequent lethal doses of the drug [59,60]. Individual case reports suggest that human subjects can develop tolerance to repeated and high doses of acetaminophen without any liver injury [61,62]. While the mechanism of such resistance to hepatotoxicity from acetaminophen overdose was not fully elucidated in these patients, autoprotection through upregulation of efflux transporters might be responsible for the development of tolerance to chronic and lethal doses of this drug.
The SLC transporters are comprised of two gene superfamilies, the SLC22A superfamily, which contains the organic cation transporters (OCTs) and the organic anion transporters (OATs), and the SLCO superfamily, which includes the organic anion transporting polypeptides (OATPs). OATPs mostly transport large, hydrophobic organic anions, while OATs transport small and hydrophilic molecules; OCTs mediate cation movement [57]. Using stable cell lines expressing human transporters, interaction of acetaminophen with hOATs and hOCTs was assessed [63]. Acetaminophen inhibited organic anion uptake mediated by hOAT1 (SLC22A6), 2 (SLC22A7), 3 (SLC22A8), and 4 (SLC22A9). OCT1 (SLC22A1) and 2 (SLC22A2) did not mediate the uptake of acetaminophen, but could be inhibited by it, suggesting that acetaminophen could potentially interfere with removal of other drugs relying on these transporters [63]. With regards to the OATP family, in vitro assays demonstrated that acetaminophen did not interact with OATP1B1 (SLCO1B1) or OATP1B3 (SLCO1B3) transporters [55].
Drug-drug interactions
Numerous drugs have been reported to interact with acetaminophen leading to exacerbation of its toxicity [18,64–68]. Several case reports suggested that epileptic patients on long-term anticonvulsant therapy exhibited increased acetaminophen-induced hepatotoxicity [68–71]. In most cases, chronic use of phenytoin or phenobarbital enhanced clinical features of toxicity after acetaminophen overdose [68–70]. It was suggested that epileptic patients exhibit lower bioavailability of acetaminophen due to increased first-pass metabolism of the drug [69]. In vitro studies with human hepatocytes showed that phenytoin and phenobarbital inhibit acetaminophen glucuronidation, suggesting that other pathways of the drug metabolism, like oxidation to toxic NAPQI, may be potentiated [17,18]. Each drug alone or in combination directly blocked UGT1A6, UGT1A9, and UGT2B15 when co-incubated with acetaminophen. Treatment of hepatocytes with phenytoin or phenobarbital increased acetaminophen-induced toxicity in these cells [17,18]. However, controlled studies with human subjects showed that co-administration with anticonvulsants increases acetaminophen glucuronidation suggesting a protective role of anticonvulsant therapy in APAP-induced toxicity [72,73]. Compared to the healthy controls (n=20), epileptic patients on chronic phenytoin therapy (n=6) exhibited a significant elevation in the glucuronide metabolites of APAP, while mercapturic acid, sulfate and cysteine metabolites were reduced [73]. Similarly, patients on long-term therapy with various anticonvulsants (n=15) had a significantly lower urinary recovery of the sulfate conjugate and unchanged drug but a higher recovery of glucuronide metabolites of APAP relative to the healthy subjects (n=12) [72]. In light of the contradictory evidence for an acetaminophen-anticonvulsant drugs interaction from case reports and in vitro studies, on the one hand, and small human studies, on the other, the safety of co-administration of these drugs should be further investigated. To address this question, a large-scale, controlled human study with patients on chronic anticonvulsant therapy receiving different doses of acetaminophen is warranted.
Many agents, including ethanol and isoniazid, induce CYP450 isozymes during their metabolism [74,75]. The antituberculosis drug isoniazid induces CYP2E1, which is crucial for acetaminophen metabolism through an oxidation pathway. Co-administration of isoniazid with acetaminophen was reported to increase acetaminophen oxidation, promote GSH depletion and NAPQI formation and ultimately lead to increased hepatotoxicity [65,66,76]. CYP2E1 is also dramatically upregulated by ethanol and acetaminophen hepatotoxicity in alcoholics is well documented [64]. Low to moderate doses of acetaminophen combined with a heavy consumption of alcohol interact to result in an abnormal liver enzyme profile, jaundice and coagulopathy. Taken together, subjects receiving isoniazid therapy or consuming excessive amounts of alcohol should take particular care when considering acetaminophen to avoid hepatotoxicity due to CYP2E1 induction.
Pharmacodynamics
There is no consensus on the mechanism of action of acetaminophen, with the eicosanoid, endocannabinoid, serotonergic, and nitric oxide pathways implicated in the drug’s analgesic effect [1,77]. APAP’s main mechanism of action is linked to its inhibitory effect on the synthesis of prostaglandins (PGs) [77]. PGs are lipids derived from the arachidonic acid pathway that act as mediators of inflammation, fever and pain [78]. PGs are synthesized upon oxidation of arachidonic acid (AA) by PTGS enzymes that contain both the cyclooxygenase and peroxidase functions. The more constitutively expressed PTGS1 and the more readily inducible PTGS2 (by cytokines and growth factors particularly) are commonly referred to as cyclooxygenase-1 (COX-1) and -2 (COX-2), respectively [78]. Both traditional non-steroidal anti-inflammatory drugs (tNSAIDs) and those designed purposefully to inhibit selectively COX-2 block only the cyclooxygenase activity of the enzymes [79]. However, acetaminophen inhibits both COX isoforms by acting on the peroxide site and reducing the amount of the PTGS oxidized form required for AA conversion [2,80,81]. Acetaminophen is often preferred to other NSAIDs as it is thought less likely to cause enteropathy. However, this may reflect no more than its relative potency as a prostaglandin inhibitor: common therapeutic doses of 1–2gm/day reduce prostaglandin formation by ~50% in comparison to the more complete suppression by other tNSAIDs like ibuprofen [82]. Acetaminophen readily crosses the blood-brain barrier, and the central nervous system (CNS) is considered to be the primary site of action of the drug [1]. The CNS is characterized by low peroxide tone and thus provides the optimal environment for APAP action [1,78]. Unlike NSAIDs, acetaminophen has only mild anti-inflammatory effect due to its ability to inhibit prostaglandin synthesis only in the presence of low levels of arachidonic acid and peroxides. Thus, it is efficient in suppressing the mild inflammation evoked by extraction of teeth but has little activity in reducing the severe chronic inflammation of rheumatoid arthritis or gout [2]. In contrast to NSAIDs, acetaminophen blocks other peroxidase enzymes, such as myeloperoxidase, inhibition of which results in reduced levels of halogenating oxidants associated with various inflammatory conditions.
Pharmacometabolomics
Pharmacometabolomics, also known as pharmacometabonomics, identifies nongenetic, environmental factors (e.g. age, gender, diet, gut microbiome, disease subtype, concurrent medications) that determine the metabolic state of a patient and affect the overall drug response [83,84]. Analysis of patient’s biological fluids by Mass Spectrometry (MS) or NMR spectroscopy helps identify pre-drug metabolomics signatures that can predict the post-drug exposure effects and provides molecular basis for variability in drug response. Metabolomics captures complex aspects of human biology, reflective of individual genomics and environmental exposures and, together with direct pharmacogenomic approaches, brings closer the prospect of precision medicine [84,85].
The first pharmacometabolomics studies on acetaminophen aimed to identify the biomarkers of drug-induced liver injury (DILI) [86,87]. Using NMR-based analysis and mathematical models, drug metabolism and toxicity were predicted after rats were treated with a single, toxic dose of acetaminophen [86]. Based on the pre-drug urine metabolome, the mole ratio of acetaminophen glucuronide to parent drug was predicted, while the pre-drug urine metabolites strongly associated with the extent of acetaminophen-induced liver injury. A higher pre-drug urinary level of the compound taurine was associated with a lower degree of hepatotoxicity, whereas a higher pre-drug level of trimethylamine-N-oxide and betaine was associated with a greater severity of liver damage. This study demonstrated that metabolomics analysis before drug exposure could shed light on the degree of drug-induced hepatotoxicity, a common side effect of acetaminophen [86]. In a follow-up human study, a pharmacometabolomic approach has been used to identify individuals susceptible to acetaminophen-induced liver injury [87]. Human subjects were dosed with acetaminophen for a week followed by urine and serum collection for metabolomics analysis. Urinary metabolomics after acetaminophen treatment distinguished individuals susceptible to mild acetaminophen-induced hepatotoxicity from those who were not. Unlike the study with rats, the human study could not differentiate subjects prone to liver injury based on the pre-drug urinary metabolome [87]. NMR spectroscopy is further used to identify p-cresol as a pre-dose urinary biomarker of acetaminophen metabolism [88]. Human subjects with a high pre-dose level of p-cresol had low post-dose urinary ratios of acetaminophen sulfate to acetaminophen glucuronide. Bacterially derived p-cresol competes for sulfation with phenolic drugs, including acetaminophen; therefore, individuals with high levels of p-cresol will have less efficient capacity to metabolize acetaminophen through the sulfation pathway. Most importantly, competition for limited sulfur pools will affect other pathways, such as glutathione production. Elevated excretion of p-cresol sulfate was accompanied by a reduced production of N-acetylcysteinyl conjugates of acetaminophen suggesting an impaired ability to detoxify APAP reactive metabolites. Thus, individuals with a gut microbiome high in p-cresol-producing bacteria and ingesting a diet low in sulfur-containing amino acids may be more prone to acetaminophen toxicity; whereas those exposed to the same doses of the drug but having low p-cresol content in the gut may not experience the same adverse reactions to acetaminophen[88].
Pharmacogenomics
Genetic polymorphisms in the drug metabolizing enzymes may be an important factor in the differential therapeutic and toxic responses in humans. While polymorphisms in UGT, CYP, SULT and GST genes are well established [22,23,89–92] and might affect the response to acetaminophen, only polymorphisms in UGT genes have been widely studied in relation to APAP pharmacokinetics in humans [20,23,26,93].
Numerous studies have investigated the effect of genetic variation in UGT genes on acetaminophen glucuronidation due to the key role of this pathway in acetaminophen metabolism. UGT1A6 and UGT1A9 are the main UGT isoforms responsible for acetaminophen glucuronidation in humans (Figure 1, see Pharmacokinetics: Metabolism). In vitro studies with HEK cells stably transfected with various UGT1A6 amino acid variants indicate that the UGT1A6*2 genotype had a 60% higher glucuronidation activity than the UGT1A6*1 variant [21]. A repetition of two nucleotides (TA) in the promoter region of UGT1A1 gene results in the mutated sequence, referred to as UGT1A1*28 [94], and leads to a reduction in the UGT enzyme activity [95]. However, when assessed in healthy subjects or β-Thalassemia patients, selected for UGT1A1 genotype, the UGT1A1*28 variant had no effect on acetaminophen glucuronidation [23,93]. This suggests that enzymatic activity of other UGTs involved in APAP metabolism – UGT1A9, UGT1A6, and UGT2B15 - might compensate for deficiency in UGT1A1 function. Using liver microsomes from human liver bank samples, three linked single nucleotide polymorphisms (SNPs) rs10929303, rs1042640, and rs8330 in the UGT1A-3′UTR region were associated with acetaminophen glucuronidation [20]. Of the three SNPs, rs8330 is consistently associated with glucuronidation of acetaminophen at various concentrations of the drug. This suggests that rs8330 could serve as a biomarker of acetaminophen glucuronidation at a wide range of therapeutic and toxic doses of the drug. Moreover, rs8330 demonstrated a lower risk of hepatotoxicity due to acetaminophen glucuronidation in patients with acute liver failure. Investigations into other genotypes, i.e. UGT1A1*28, UGT1A6*2, UGT1A9 (rs6714486 and rs45625337) and UGT2B15*2, did not yield any associations [20]. Finally, UGT1A6 and UGT2B15 genotypes were compared in their contribution to acetaminophen glucuronidation [26]. After a single therapeutic dose of acetaminophen, APAP glucuronidation was significantly influenced by the UGT2B15*2 polymorphism and very modestly by the UGT1A6*2 genotype. For UGT2B15, the percentage of APAP-glucuronide metabolite and the ratio of APAP-gluc to free APAP is diminished, while that of APAP-sulfate increased across genotypes from *1/*1 to *2/*2 [26].
Several studies have examined the effect of genetic polymorphisms on acetaminophen metabolism under pathological conditions. Metabolism of acetaminophen is affected in patients with Gilbert’s syndrome, a chronic unconjugated hyperbilirubinemia [25,96]. The underlying cause of this disorder is a polymorphism in the promoter region of the UGT isoform 1A1 gene (UGT1A1*28) that increases the length of the promoter [97]. This compromises the UGT enzyme activity and therefore leads to increased serum levels of unconjugated bilirubin. Patients with Gilbert’s syndrome might be more susceptible to acetaminophen-induced hepatotoxicity due to increased availability of the free drug for the oxidation pathway of metabolism [25,96]. While contradictory results have been published for acetaminophen glucuronidation, a subgroup of subjects with Gilbert’s syndrome shows a reduction in excretion of APAP glucuronide and a concomitant increase in the elimination of the CYP450 APAP metabolites [22,25,96,98,99]. A study with a few β-Thalassemia/HbE patients aimed at elucidating the effect of combined UGT1A6*2 and UGT1A1*28 polymorphisms on acetaminophen pharmacokinetics [23]. As compared to the wild-type β-Thalassemia/HbE patients, the heterozygous UGT1A6*2 without UGT1A1*28 genotype exhibited a reduction in AUC of the free drug and of APAP glucuronide which might be due to UGT1A6*2 polymorphism. The same group of patients exhibited elevated ALT but reduced APAP glucuronide levels, suggesting that UGT1A6*2 polymorphism is a modifier of acetaminophen glucuronidation in patients with abnormal liver function. Beta-Thalassemia/HbE patients with both UGT1A1*28 and UGT1A6*2 polymorphisms have not demonstrated a significant difference in pharmacokinetics of acetaminophen [23].
Despite a major role of CYP450 enzymes in acetaminophen-induced toxicity, very few studies attempted to address the relationship between CYP gene polymorphisms and APAP metabolism [90,100]. In a small cohort study, a non-significant association between CYP2E1 promoter RsaI restriction fragment length polymorphism and a shorter half-life and elimination rate of acetaminophen is reported [90]. In the acute liver failure study, genotype frequency differences were evaluated in patients who intentionally consumed a single overdose of acetaminophen and those who unintentionally consumed high doses of the drug over a long period of time. Thus, it should be noted that although both groups were exposed to the same total amount of acetaminophen, the daily dose in the unintentional group was lower than that normally causing liver failure, and there might have been adaptive changes over time. The carriers of CYP3A5 rs776746 A allele were overrepresented in the intentionally overdosed group and were more predisposed to acetaminophen-induced hepatotoxicity than individuals with the G allele, that rendered CYP3A5 enzyme inactive due to aberrant gene splicing [100]. CYP3A5 rs776746 A allele polymorphism is associated with increased formation of NAPQI; however, the involvement of CYP3A5 in acetaminophen oxidation has not been reported and if occurs, it might be due to a big overlap in substrate selectivity between CYP3A enzymes. Associations with polymorphisms in genes encoding UGT1A1, UGT1A6, UGT1A9, UGT2B15, and SULT1A1 were not detected in the same patient populations [100].
Genetic variability in SULT and GST genes are not well established, and only a few studies have been conducted in relation to GST polymorphisms and acetaminophen detoxification [91,101,102]. In a study investigating associations between polymorphisms in the glutathione-S-transferase genes GSTT1, GSTM1, GSTP1 and an increased risk of acetaminophen poisoning, prothrombin time was used as a marker of survival in poisoned patients [91]. A borderline association between a high prothrombin time, as an index of a good prognosis, and GSTT1 homozygous deletion was established, indicating that patients with this polymorphism are more likely to survive after NAC treatment for APAP poisoning. The frequency of GSTP1 homozygous variant (Val/Val) was lower in APAP poisoned patients than in healthy individuals, suggesting that this genotype may reduce the risk of being poisoned. However, the GSTP1 genotype was not associated with prothrombin time, which might have been due to a small sample size in this group (n=5) [91]. A couple of studies address the relationship between prenatal and infant acetaminophen exposure, GST polymorphisms in mothers and children, and the risk of developing asthma later in life [101,102]. First, numerous studies reported an association between acetaminophen use during pregnancy and an increased risk of wheezing and later asthma development in infancy and/or childhood [102–105]. It was suggested that this association is related to the maternal polymorphisms in APAP detoxification mechanisms, namely in GST genes. Indeed, an increased risk of wheezing is associated with the presence of GSTM1 and GSTT1 genotypes, respectively, in mothers exposed to acetaminophen [101]. Moreover, these risks are further potentiated if both the APAP-consuming mother and her child exhibited GSTM1 polymorphism [101]. In a different study, GSTP1 polymorphism modified the risk of wheeze in children at age 5 years and was common only for the carriers of the GSTP1 minor allele [102]. Taken together, these studies demonstrate an interaction between prenatal acetaminophen use and GST genotype of the mother, and in some cases of the child, with airways disease in children.
Finally, two studies reported that genetic variability in CD44 antigen might predispose patients to acetaminophen-induced liver injury at supra-therapeutic doses [106] or to acute liver failure after the drug overdose [100]. Evaluation of two independent cohorts of patients, who received 4g/day APAP for 1–2 weeks, revealed an association between CD44 rs1467558 polymorphism and elevated serum ALT levels, a biomarker of hepatocellular injury [106]. Similarly, the same polymorphism was associated with unintentional acetaminophen-induced acute liver failure [100]. These are the first reports demonstrating that a polymorphism in an immune response gene may predispose to increased acetaminophen-induced hepatotoxicity. However, considering a multitude of physiological and pathological roles of CD44 [107,108], the mechanism of CD44-driven increase in susceptibility to APAP toxicity may be multifactorial and requires further investigation to determine.
Interestingly, polymorphisms in genes encoding acetaminophen-metabolizing enzymes might be responsible for dramatic ethnic and racial differences in APAP metabolism and toxicity [109–113]. In comparison with Caucasians, Hong Kong Chinese were reported to have more rapid absorption, a longer half-life and a lower clearance of acetaminophen, and exhibited an increased capacity for sulfation but lower glucuronidation and oxidation of the drug [109,110,114]. Individuals of African descent were shown to have a greater clearance of acetaminophen relative to Caucasians [111]. In terms of hepatotoxicity, metabolic activation of acetaminophen is much lower in the Africans than Caucasians [112], and the rate of acetaminophen-induced hepatotoxicity is low in Asian populations as compared with patients from Western countries [113]. It should be noted, however, that further studies are required to determine if these associated polymorphisms account for the ethnic differences in acetaminophen pharmacokinetics.
Conclusions
To date, our understanding of the role of genetic polymorphisms in acetaminophen metabolism and toxicity is quite limited and has been primarily studied for UGT genes. Considering a high contribution of sulfation in acetaminophen metabolism, the importance of oxidation in APAP toxicity and of glutathione in APAP detoxification, more studies are needed to establish the relationship between polymorphisms in SULT, GST and CYP genes, and interindividual variability in response to acetaminophen. Finally, clinically relevant biomarkers of acetaminophen-induced toxicity are yet to be determined.
Acknowledgments
The authors thank Feng Liu for assistance with the graphics. This study is supported by the NIH/NIGMS R24 GM61374, The Personalized NSAID Therapeutics Consortium (PENTACON: HL117798) and by T32 H107971.
Footnotes
Conflicts of interest
RBA and TEK are stockholders in Personalis Inc. The other authors declare no conflicts of interest. GAF is the McNeil Professor of Translational Medicine and Therapeutics.
References
- 1.Toussaint K, Yang XC, Zielinski MA, Reigle KL, Sacavage SD, et al. What do we (not) know about how paracetamol (acetaminophen) works? J Clin Pharm Ther. 2010;35:617–638. doi: 10.1111/j.1365-2710.2009.01143.x. [DOI] [PubMed] [Google Scholar]
- 2.Graham GG, Davies MJ, Day RO, Mohamudally A, Scott KF. The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology. 2013;21:201–232. doi: 10.1007/s10787-013-0172-x. [DOI] [PubMed] [Google Scholar]
- 3.Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980;10(Suppl 2):291S–298S. doi: 10.1111/j.1365-2125.1980.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ubaldo CD, Hall NS, Le B. Postmarketing review of intravenous acetaminophen dosing based on food and drug administration prescribing guidelines. Pharmacotherapy. 2014;34(Suppl 1):34S–39S. doi: 10.1002/phar.1511. [DOI] [PubMed] [Google Scholar]
- 5.Hodgman MJ, Garrard AR. A review of acetaminophen poisoning. Crit Care Clin. 2012;28:499–516. doi: 10.1016/j.ccc.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Thompson CA. Spell out ‘acetaminophen’ for patients’ sake, group says. Am J Health Syst Pharm. 2011;68:1768. doi: 10.2146/news110069. [DOI] [PubMed] [Google Scholar]
- 7.Mitka M. FDA asks physicians to stop prescribing high-dose acetaminophen products. JAMA. 2014;311:563. doi: 10.1001/jama.2014.716. [DOI] [PubMed] [Google Scholar]
- 8.McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013;30:2174–2187. doi: 10.1007/s11095-013-1007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bessems JG, Vermeulen NP. Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol. 2001;31:55–138. doi: 10.1080/20014091111677. [DOI] [PubMed] [Google Scholar]
- 10.Prescott LF. Paracetamol overdosage. Pharmacological considerations and clinical management. Drugs. 1983;25:290–314. doi: 10.2165/00003495-198325030-00002. [DOI] [PubMed] [Google Scholar]
- 11.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31:1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 12.Prescott LF. Treatment of severe acetaminophen poisoning with intravenous acetylcysteine. Arch Intern Med. 1981;141:386–389. doi: 10.1001/archinte.141.3.386. [DOI] [PubMed] [Google Scholar]
- 13.Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319:1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 14.Prescott LF, Wright N. The effects of hepatic and renal damage on paracetamol metabolism and excretion following overdosage. A pharmacokinetic study. Br J Pharmacol. 1973;49:602–613. doi: 10.1111/j.1476-5381.1973.tb08536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bock KW, Forster A, Gschaidmeier H, Bruck M, Munzel P, et al. Paracetamol glucuronidation by recombinant rat and human phenol UDP-glucuronosyltransferases. Biochem Pharmacol. 1993;45:1809–1814. doi: 10.1016/0006-2952(93)90437-2. [DOI] [PubMed] [Google Scholar]
- 16.Court MH, Duan SX, von Moltke LL, Greenblatt DJ, Patten CJ, et al. Interindividual variability in acetaminophen glucuronidation by human liver microsomes: identification of relevant acetaminophen UDP-glucuronosyltransferase isoforms. J Pharmacol Exp Ther. 2001;299:998–1006. [PubMed] [Google Scholar]
- 17.Mutlib AE, Goosen TC, Bauman JN, Williams JA, Kulkarni S, et al. Kinetics of acetaminophen glucuronidation by UDP-glucuronosyltransferases 1A1, 1A6, 1A9 and 2B15. Potential implications in acetaminophen-induced hepatotoxicity. Chem Res Toxicol. 2006;19:701–709. doi: 10.1021/tx050317i. [DOI] [PubMed] [Google Scholar]
- 18.Kostrubsky SE, Sinclair JF, Strom SC, Wood S, Urda E, et al. Phenobarbital and phenytoin increased acetaminophen hepatotoxicity due to inhibition of UDP-glucuronosyltransferases in cultured human hepatocytes. Toxicol Sci. 2005;87:146–155. doi: 10.1093/toxsci/kfi211. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, Pickering G. Paracetamol metabolism and related genetic differences. Drug Metab Rev. 2011;43:41–52. doi: 10.3109/03602532.2010.527984. [DOI] [PubMed] [Google Scholar]
- 20.Court MH, Freytsis M, Wang X, Peter I, Guillemette C, et al. The UDP-glucuronosyltransferase (UGT) 1A polymorphism c.2042C>G (rs8330) is associated with increased human liver acetaminophen glucuronidation, increased UGT1A exon 5a/5b splice variant mRNA ratio, and decreased risk of unintentional acetaminophen-induced acute liver failure. J Pharmacol Exp Ther. 2013;345:297–307. doi: 10.1124/jpet.112.202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnaswamy S, Hao Q, Al-Rohaimi A, Hesse LM, von Moltke LL, et al. UDP glucuronosyltransferase (UGT) 1A6 pharmacogenetics: II. Functional impact of the three most common nonsynonymous UGT1A6 polymorphisms (S7A, T181A, and R184S) J Pharmacol Exp Ther. 2005;313:1340–1346. doi: 10.1124/jpet.104.081968. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa T, Mure T, Yusoff S, Ono E, Harahap IS, et al. Acetaminophen administration in a patient with Gilbert’s syndrome. Pediatr Int. 2012;54:934–936. doi: 10.1111/j.1442-200X.2012.03602.x. [DOI] [PubMed] [Google Scholar]
- 23.Tankanitlert J, Morales NP, Howard TA, Fucharoen P, Ware RE, et al. Effects of combined UDP-glucuronosyltransferase (UGT) 1A1*28 and 1A6*2 on paracetamol pharmacokinetics in beta-thalassemia/HbE. Pharmacology. 2007;79:97–103. doi: 10.1159/000097908. [DOI] [PubMed] [Google Scholar]
- 24.de Morais SM, Wells PG. Deficiency in bilirubin UDP-glucuronyl transferase as a genetic determinant of acetaminophen toxicity. J Pharmacol Exp Ther. 1988;247:323–331. [PubMed] [Google Scholar]
- 25.de Morais SM, Uetrecht JP, Wells PG. Decreased glucuronidation and increased bioactivation of acetaminophen in Gilbert’s syndrome. Gastroenterology. 1992;102:577–586. doi: 10.1016/0016-5085(92)90106-9. [DOI] [PubMed] [Google Scholar]
- 26.Navarro SL, Chen Y, Li L, Li SS, Chang JL, et al. UGT1A6 and UGT2B15 polymorphisms and acetaminophen conjugation in response to a randomized, controlled diet of select fruits and vegetables. Drug Metab Dispos. 2011;39:1650–1657. doi: 10.1124/dmd.111.039149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiter C, Weinshilboum RM. Acetaminophen and phenol: substrates for both a thermostable and a thermolabile form of human platelet phenol sulfotransferase. J Pharmacol Exp Ther. 1982;221:43–51. [PubMed] [Google Scholar]
- 28.Freimuth RR, Wiepert M, Chute CG, Wieben ED, Weinshilboum RM. Human cytosolic sulfotransferase database mining: identification of seven novel genes and pseudogenes. Pharmacogenomics J. 2004;4:54–65. doi: 10.1038/sj.tpj.6500223. [DOI] [PubMed] [Google Scholar]
- 29.Adjei AA, Gaedigk A, Simon SD, Weinshilboum RM, Leeder JS. Interindividual variability in acetaminophen sulfation by human fetal liver: implications for pharmacogenetic investigations of drug-induced birth defects. Birth Defects Res A Clin Mol Teratol. 2008;82:155–165. doi: 10.1002/bdra.20535. [DOI] [PubMed] [Google Scholar]
- 30.Raucy JL, Lasker JM, Lieber CS, Black M. Acetaminophen activation by human liver cytochromes P450IIE1 and P450IA2. Arch Biochem Biophys. 1989;271:270–283. doi: 10.1016/0003-9861(89)90278-6. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Koenigs LL, Thompson SJ, Peter RM, Rettie AE, et al. Oxidation of acetaminophen to its toxic quinone imine and nontoxic catechol metabolites by baculovirus-expressed and purified human cytochromes P450 2E1 and 2A6. Chem Res Toxicol. 1998;11:295–301. doi: 10.1021/tx9701687. [DOI] [PubMed] [Google Scholar]
- 32.Hazai E, Vereczkey L, Monostory K. Reduction of toxic metabolite formation of acetaminophen. Biochem Biophys Res Commun. 2002;291:1089–1094. doi: 10.1006/bbrc.2002.6541. [DOI] [PubMed] [Google Scholar]
- 33.Manyike PT, Kharasch ED, Kalhorn TF, Slattery JT. Contribution of CYP2E1 and CYP3A to acetaminophen reactive metabolite formation. Clin Pharmacol Ther. 2000;67:275–282. doi: 10.1067/mcp.2000.104736. [DOI] [PubMed] [Google Scholar]
- 34.Dong H, Haining RL, Thummel KE, Rettie AE, Nelson SD. Involvement of human cytochrome P450 2D6 in the bioactivation of acetaminophen. Drug Metab Dispos. 2000;28:1397–1400. [PubMed] [Google Scholar]
- 35.Laine JE, Auriola S, Pasanen M, Juvonen RO. Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica. 2009;39:11–21. doi: 10.1080/00498250802512830. [DOI] [PubMed] [Google Scholar]
- 36.Thummel KE, Lee CA, Kunze KL, Nelson SD, Slattery JT. Oxidation of acetaminophen to N-acetyl-p-aminobenzoquinone imine by human CYP3A4. Biochem Pharmacol. 1993;45:1563–1569. doi: 10.1016/0006-2952(93)90295-8. [DOI] [PubMed] [Google Scholar]
- 37.Patten CJ, Thomas PE, Guy RL, Lee M, Gonzalez FJ, et al. Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol. 1993;6:511–518. doi: 10.1021/tx00034a019. [DOI] [PubMed] [Google Scholar]
- 38.Potter DW, Hinson JA. The 1- and 2-electron oxidation of acetaminophen catalyzed by prostaglandin H synthase. J Biol Chem. 1987;262:974–980. [PubMed] [Google Scholar]
- 39.Moldeus P, Rahimtula A. Metabolism of paracetamol to a glutathione conjugate catalyzed by prostaglandin synthetase. Biochem Biophys Res Commun. 1980;96:469–475. doi: 10.1016/0006-291x(80)91238-3. [DOI] [PubMed] [Google Scholar]
- 40.Pirmohamed M, Madden S, Park BK. Idiosyncratic drug reactions. Metabolic bioactivation as a pathogenic mechanism. Clin Pharmacokinet. 1996;31:215–230. doi: 10.2165/00003088-199631030-00005. [DOI] [PubMed] [Google Scholar]
- 41.Ferner RE, Dear JW, Bateman DN. Management of paracetamol poisoning. BMJ. 2011;342:d2218. doi: 10.1136/bmj.d2218. [DOI] [PubMed] [Google Scholar]
- 42.Amar PJ, Schiff ER. Acetaminophen safety and hepatotoxicity--where do we go from here? Expert Opin Drug Saf. 2007;6:341–355. doi: 10.1517/14740338.6.4.341. [DOI] [PubMed] [Google Scholar]
- 43.Whitcomb DC, Block GD. Association of acetaminophen hepatotoxicity with fasting and ethanol use. JAMA. 1994;272:1845–1850. doi: 10.1001/jama.1994.03520230055038. [DOI] [PubMed] [Google Scholar]
- 44.Eriksson LS, Broome U, Kalin M, Lindholm M. Hepatotoxicity due to repeated intake of low doses of paracetamol. J Intern Med. 1992;231:567–570. doi: 10.1111/j.1365-2796.1992.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 45.Coles B, Wilson I, Wardman P, Hinson JA, Nelson SD, et al. The spontaneous and enzymatic reaction of N-acetyl-p-benzoquinonimine with glutathione: a stopped-flow kinetic study. Arch Biochem Biophys. 1988;264:253–260. doi: 10.1016/0003-9861(88)90592-9. [DOI] [PubMed] [Google Scholar]
- 46.Board PG, Menon D. Glutathione transferases, regulators of cellular metabolism and physiology. Biochim Biophys Acta. 2013;1830:3267–3288. doi: 10.1016/j.bbagen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 47.Beckett GJ, Donovan JW, Hussey AJ, Proudfoot AT, Prescott LF. Intravenous N-acetylcysteine, hepatotoxicity and plasma glutathione S-transferase in patients with paracetamol overdosage. Hum Exp Toxicol. 1990;9:183–186. doi: 10.1177/096032719000900311. [DOI] [PubMed] [Google Scholar]
- 48.Beckett GJ, Chapman BJ, Dyson EH, Hayes JD. Plasma glutathione S-transferase measurements after paracetamol overdose: evidence for early hepatocellular damage. Gut. 1985;26:26–31. doi: 10.1136/gut.26.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hogestatt ED, Jonsson BA, Ermund A, Andersson DA, Bjork H, et al. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J Biol Chem. 2005;280:31405–31412. doi: 10.1074/jbc.M501489200. [DOI] [PubMed] [Google Scholar]
- 50.Gemborys MW, Mudge GH. Formation and disposition of the minor metabolites of acetaminophen in the hamster. Drug Metab Dispos. 1981;9:340–351. [PubMed] [Google Scholar]
- 51.Davis JM, Emslie KR, Sweet RS, Walker LL, Naughton RJ, et al. Early functional and morphological changes in renal tubular necrosis due to p-aminophenol. Kidney Int. 1983;24:740–747. doi: 10.1038/ki.1983.221. [DOI] [PubMed] [Google Scholar]
- 52.Veronesi B, Oortgiesen M. The TRPV1 receptor: target of toxicants and therapeutics. Toxicol Sci. 2006;89:1–3. doi: 10.1093/toxsci/kfj034. [DOI] [PubMed] [Google Scholar]
- 53.Barnes SN, Aleksunes LM, Augustine L, Scheffer GL, Goedken MJ, et al. Induction of hepatobiliary efflux transporters in acetaminophen-induced acute liver failure cases. Drug Metab Dispos. 2007;35:1963–1969. doi: 10.1124/dmd.107.016170. [DOI] [PubMed] [Google Scholar]
- 54.Kidron H, Wissel G, Manevski N, Hakli M, Ketola RA, et al. Impact of probe compound in MRP2 vesicular transport assays. Eur J Pharm Sci. 2012;46:100–105. doi: 10.1016/j.ejps.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 55.Kindla J, Muller F, Mieth M, Fromm MF, Konig J. Influence of non-steroidal anti-inflammatory drugs on organic anion transporting polypeptide (OATP) 1B1- and OATP1B3-mediated drug transport. Drug Metab Dispos. 2011;39:1047–1053. doi: 10.1124/dmd.110.037622. [DOI] [PubMed] [Google Scholar]
- 56.Maeda A, Tsuruoka S, Kanai Y, Endou H, Saito K, et al. Evaluation of the interaction between nonsteroidal anti-inflammatory drugs and methotrexate using human organic anion transporter 3-transfected cells. Eur J Pharmacol. 2008;596:166–172. doi: 10.1016/j.ejphar.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 57.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165:1260–1287. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch. 2007;453:661–673. doi: 10.1007/s00424-006-0054-9. [DOI] [PubMed] [Google Scholar]
- 59.Strubelt O, Siegers CP, Volpel M, Younes M. Studies on the mechanism of paracetamol-induced protection against paracetamol hepatotoxicity. Toxicology. 1979;12:121–133. doi: 10.1016/0300-483x(79)90038-6. [DOI] [PubMed] [Google Scholar]
- 60.Rudraiah S, Rohrer PR, Gurevich I, Goedken MJ, Rasmussen T, et al. Tolerance to Acetaminophen Hepatotoxicity in the Mouse Model of Autoprotection Is Associated with Induction of Flavin-Containing Monooxygenase-3 (FMO3) in Hepatocytes. Toxicol Sci. 2014;141:263–277. doi: 10.1093/toxsci/kfu124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shayiq RM, Roberts DW, Rothstein K, Snawder JE, Benson W, et al. Repeat exposure to incremental doses of acetaminophen provides protection against acetaminophen-induced lethality in mice: an explanation for high acetaminophen dosage in humans without hepatic injury. Hepatology. 1999;29:451–463. doi: 10.1002/hep.510290241. [DOI] [PubMed] [Google Scholar]
- 62.Tredger JM, Thuluvath P, Williams R, Murray-Lyon IM. Metabolic basis for high paracetamol dosage without hepatic injury: a case study. Hum Exp Toxicol. 1995;14:8–12. doi: 10.1177/096032719501400102. [DOI] [PubMed] [Google Scholar]
- 63.Khamdang S, Takeda M, Noshiro R, Narikawa S, Enomoto A, et al. Interactions of human organic anion transporters and human organic cation transporters with nonsteroidal anti-inflammatory drugs. J Pharmacol Exp Ther. 2002;303:534–539. doi: 10.1124/jpet.102.037580. [DOI] [PubMed] [Google Scholar]
- 64.Seeff LB, Cuccherini BA, Zimmerman HJ, Adler E, Benjamin SB. Acetaminophen hepatotoxicity in alcoholics. A therapeutic misadventure. Ann Intern Med. 1986;104:399–404. doi: 10.7326/0003-4819-104-3-399. [DOI] [PubMed] [Google Scholar]
- 65.Crippin JS. Acetaminophen hepatotoxicity: potentiation by isoniazid. Am J Gastroenterol. 1993;88:590–592. [PubMed] [Google Scholar]
- 66.Epstein MM, Nelson SD, Slattery JT, Kalhorn TF, Wall RA, et al. Inhibition of the metabolism of paracetamol by isoniazid. Br J Clin Pharmacol. 1991;31:139–142. doi: 10.1111/j.1365-2125.1991.tb05501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cook MD, Williams SR, Clark RF. Phenytoin-potentiated hepatotoxicity following acetaminophen overdose? A closer look. Dig Dis Sci. 2007;52:208–209. doi: 10.1007/s10620-006-9153-x. [DOI] [PubMed] [Google Scholar]
- 68.Minton NA, Henry JA, Frankel RJ. Fatal paracetamol poisoning in an epileptic. Hum Toxicol. 1988;7:33–34. doi: 10.1177/096032718800700106. [DOI] [PubMed] [Google Scholar]
- 69.Perucca E, Richens A. Paracetamol disposition in normal subjects and in patients treated with antiepileptic drugs. Br J Clin Pharmacol. 1979;7:201–206. doi: 10.1111/j.1365-2125.1979.tb00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bray GP, Harrison PM, O’Grady JG, Tredger JM, Williams R. Long-term anticonvulsant therapy worsens outcome in paracetamol-induced fulminant hepatic failure. Hum Exp Toxicol. 1992;11:265–270. doi: 10.1177/096032719201100405. [DOI] [PubMed] [Google Scholar]
- 71.Pirotte JH. Apparent potentiation of hepatotoxicity from small doses of acetaminophen by phenobarbital. Ann Intern Med. 1984;101:403. doi: 10.7326/0003-4819-101-3-403_1. [DOI] [PubMed] [Google Scholar]
- 72.Prescott LF, Critchley JA, Balali-Mood M, Pentland B. Effects of microsomal enzyme induction on paracetamol metabolism in man. Br J Clin Pharmacol. 1981;12:149–153. doi: 10.1111/j.1365-2125.1981.tb01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tomlinson B, Young RP, Ng MC, Anderson PJ, Kay R, et al. Selective liver enzyme induction by carbamazepine and phenytoin in Chinese epileptics. Eur J Clin Pharmacol. 1996;50:411–415. doi: 10.1007/s002280050132. [DOI] [PubMed] [Google Scholar]
- 74.Zand R, Nelson SD, Slattery JT, Thummel KE, Kalhorn TF, et al. Inhibition and induction of cytochrome P4502E1-catalyzed oxidation by isoniazid in humans. Clin Pharmacol Ther. 1993;54:142–149. doi: 10.1038/clpt.1993.125. [DOI] [PubMed] [Google Scholar]
- 75.Buhler R, Lindros KO, von Boguslawsky K, Karkkainen P, Makinen J, et al. Perivenous expression of ethanol-inducible cytochrome P450 IIE1 in livers from alcoholics and chronically ethanol-fed rats. Alcohol Alcohol Suppl. 1991;1:311–315. [PubMed] [Google Scholar]
- 76.Murphy R, Swartz R, Watkins PB. Severe acetaminophen toxicity in a patient receiving isoniazid. Ann Intern Med. 1990;113:799–800. doi: 10.7326/0003-4819-113-10-799. [DOI] [PubMed] [Google Scholar]
- 77.Smith HS. Potential analgesic mechanisms of acetaminophen. Pain Physician. 2009;12:269–280. [PubMed] [Google Scholar]
- 78.Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res. 2009;50(Suppl):S423–428. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grosser T. The pharmacology of selective inhibition of COX-2. Thromb Haemost. 2006;96:393–400. [PubMed] [Google Scholar]
- 80.Boutaud O, Aronoff DM, Richardson JH, Marnett LJ, Oates JA. Determinants of the cellular specificity of acetaminophen as an inhibitor of prostaglandin H(2) synthases. Proc Natl Acad Sci U S A. 2002;99:7130–7135. doi: 10.1073/pnas.102588199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aronoff DM, Oates JA, Boutaud O. New insights into the mechanism of action of acetaminophen: Its clinical pharmacologic characteristics reflect its inhibition of the two prostaglandin H2 synthases. Clin Pharmacol Ther. 2006;79:9–19. doi: 10.1016/j.clpt.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 82.Catella-Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345:1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 83.James LP. Metabolomics: integration of a new “omics” with clinical pharmacology. Clin Pharmacol Ther. 2013;94:547–551. doi: 10.1038/clpt.2013.166. [DOI] [PubMed] [Google Scholar]
- 84.Kaddurah-Daouk R, Weinshilboum RM Pharmacometabolomics Research N. Pharmacometabolomics: implications for clinical pharmacology and systems pharmacology. Clin Pharmacol Ther. 2014;95:154–167. doi: 10.1038/clpt.2013.217. [DOI] [PubMed] [Google Scholar]
- 85.Jayachandran D, Ramkrishna U, Skiles J, Renbarger J, Ramkrishna D. Revitalizing personalized medicine: respecting biomolecular complexities beyond gene expression. CPT Pharmacometrics Syst Pharmacol. 2014;3:e110. doi: 10.1038/psp.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clayton TA, Lindon JC, Cloarec O, Antti H, Charuel C, et al. Pharmaco-metabonomic phenotyping and personalized drug treatment. Nature. 2006;440:1073–1077. doi: 10.1038/nature04648. [DOI] [PubMed] [Google Scholar]
- 87.Winnike JH, Li Z, Wright FA, Macdonald JM, O’Connell TM, et al. Use of pharmaco-metabonomics for early prediction of acetaminophen-induced hepatotoxicity in humans. Clin Pharmacol Ther. 2010;88:45–51. doi: 10.1038/clpt.2009.240. [DOI] [PubMed] [Google Scholar]
- 88.Clayton TA, Baker D, Lindon JC, Everett JR, Nicholson JK. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc Natl Acad Sci U S A. 2009;106:14728–14733. doi: 10.1073/pnas.0904489106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheung C, Yu AM, Ward JM, Krausz KW, Akiyama TE, et al. The cyp2e1-humanized transgenic mouse: role of cyp2e1 in acetaminophen hepatotoxicity. Drug Metab Dispos. 2005;33:449–457. doi: 10.1124/dmd.104.002402. [DOI] [PubMed] [Google Scholar]
- 90.Ueshima Y, Tsutsumi M, Takase S, Matsuda Y, Kawahara H. Acetaminophen metabolism in patients with different cytochrome P-4502E1 genotypes. Alcohol Clin Exp Res. 1996;20:25A–28A. doi: 10.1111/j.1530-0277.1996.tb01722.x. [DOI] [PubMed] [Google Scholar]
- 91.Buchard A, Eefsen M, Semb S, Andersen SE, Morling N, et al. The role of the glutathione S-transferase genes GSTT1, GSTM1, and GSTP1 in acetaminophen-poisoned patients. Clin Toxicol (Phila) 2012;50:27–33. doi: 10.3109/15563650.2011.639713. [DOI] [PubMed] [Google Scholar]
- 92.Nagar S, Walther S, Blanchard RL. Sulfotransferase (SULT) 1A1 polymorphic variants *1, *2, and *3 are associated with altered enzymatic activity, cellular phenotype, and protein degradation. Mol Pharmacol. 2006;69:2084–2092. doi: 10.1124/mol.105.019240. [DOI] [PubMed] [Google Scholar]
- 93.Rauchschwalbe SK, Zuhlsdorf MT, Wensing G, Kuhlmann J. Glucuronidation of acetaminophen is independent of UGT1A1 promotor genotype. Int J Clin Pharmacol Ther. 2004;42:73–77. doi: 10.5414/cpp42073. [DOI] [PubMed] [Google Scholar]
- 94.Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med. 1995;333:1171–1175. doi: 10.1056/NEJM199511023331802. [DOI] [PubMed] [Google Scholar]
- 95.Beutler E, Gelbart T, Demina A. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism? Proc Natl Acad Sci U S A. 1998;95:8170–8174. doi: 10.1073/pnas.95.14.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Esteban A, Perez-Mateo M. Gilbert’s disease: a risk factor for paracetamol overdosage? J Hepatol. 1993;18:257–258. doi: 10.1016/s0168-8278(05)80256-9. [DOI] [PubMed] [Google Scholar]
- 97.Monaghan G, Ryan M, Seddon R, Hume R, Burchell B. Genetic variation in bilirubin UPD-glucuronosyltransferase gene promoter and Gilbert’s syndrome. Lancet. 1996;347:578–581. doi: 10.1016/s0140-6736(96)91273-8. [DOI] [PubMed] [Google Scholar]
- 98.Peters WH, te Morsche RH, Roelofs HM. Combined polymorphisms in UDP-glucuronosyltransferases 1A1 and 1A6: implications for patients with Gilbert’s syndrome. J Hepatol. 2003;38:3–8. doi: 10.1016/s0168-8278(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 99.Ullrich D, Sieg A, Blume R, Bock KW, Schroter W, et al. Normal pathways for glucuronidation, sulphation and oxidation of paracetamol in Gilbert’s syndrome. Eur J Clin Invest. 1987;17:237–240. doi: 10.1111/j.1365-2362.1987.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 100.Court MH, Peter I, Hazarika S, Vasiadi M, Greenblatt DJ, et al. Candidate gene polymorphisms in patients with acetaminophen-induced acute liver failure. Drug Metab Dispos. 2014;42:28–32. doi: 10.1124/dmd.113.053546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shaheen SO, Newson RB, Ring SM, Rose-Zerilli MJ, Holloway JW, et al. Prenatal and infant acetaminophen exposure, antioxidant gene polymorphisms, and childhood asthma. J Allergy Clin Immunol. 2010;126:1141–1148. e1147. doi: 10.1016/j.jaci.2010.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perzanowski MS, Miller RL, Tang D, Ali D, Garfinkel RS, et al. Prenatal acetaminophen exposure and risk of wheeze at age 5 years in an urban low-income cohort. Thorax. 2010;65:118–123. doi: 10.1136/thx.2009.121459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Persky V, Piorkowski J, Hernandez E, Chavez N, Wagner-Cassanova C, et al. Prenatal exposure to acetaminophen and respiratory symptoms in the first year of life. Ann Allergy Asthma Immunol. 2008;101:271–278. doi: 10.1016/S1081-1206(10)60492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rebordosa C, Kogevinas M, Sorensen HT, Olsen J. Pre-natal exposure to paracetamol and risk of wheezing and asthma in children: a birth cohort study. Int J Epidemiol. 2008;37:583–590. doi: 10.1093/ije/dyn070. [DOI] [PubMed] [Google Scholar]
- 105.Shaheen SO, Newson RB, Sherriff A, Henderson AJ, Heron JE, et al. Paracetamol use in pregnancy and wheezing in early childhood. Thorax. 2002;57:958–963. doi: 10.1136/thorax.57.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Harrill AH, Watkins PB, Su S, Ross PK, Harbourt DE, et al. Mouse population-guided resequencing reveals that variants in CD44 contribute to acetaminophen-induced liver injury in humans. Genome Res. 2009;19:1507–1515. doi: 10.1101/gr.090241.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Louderbough JM, Schroeder JA. Understanding the dual nature of CD44 in breast cancer progression. Mol Cancer Res. 2011;9:1573–1586. doi: 10.1158/1541-7786.MCR-11-0156. [DOI] [PubMed] [Google Scholar]
- 108.Krettek A, Sjoberg S. CD44 - a new cardiovascular drug target or merely an innocent bystander? Cardiovasc Hematol Disord Drug Targets. 2009;9:293–302. doi: 10.2174/1871529x10909040293. [DOI] [PubMed] [Google Scholar]
- 109.Critchley JA, Critchley LA, Anderson PJ, Tomlinson B. Differences in the single-oral-dose pharmacokinetics and urinary excretion of paracetamol and its conjugates between Hong Kong Chinese and Caucasian subjects. J Clin Pharm Ther. 2005;30:179–184. doi: 10.1111/j.1365-2710.2004.00626.x. [DOI] [PubMed] [Google Scholar]
- 110.Lee HS, Ti TY, Koh YK, Prescott LF. Paracetamol elimination in Chinese and Indians in Singapore. Eur J Clin Pharmacol. 1992;43:81–84. doi: 10.1007/BF02280759. [DOI] [PubMed] [Google Scholar]
- 111.Sommers DK, van Staden DA, Moncrieff J, Schoeman HS. Paracetamol metabolism in African villagers. Hum Toxicol. 1985;4:385–389. doi: 10.1177/096032718500400404. [DOI] [PubMed] [Google Scholar]
- 112.Critchley JA, Nimmo GR, Gregson CA, Woolhouse NM, Prescott LF. Inter-subject and ethnic differences in paracetamol metabolism. Br J Clin Pharmacol. 1986;22:649–657. doi: 10.1111/j.1365-2125.1986.tb02953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marzilawati AR, Ngau YY, Mahadeva S. Low rates of hepatotoxicity among Asian patients with paracetamol overdose: a review of 1024 cases. BMC Pharmacol Toxicol. 2012;13:8. doi: 10.1186/2050-6511-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yin OQ, Tomlinson B, Chow AH, Chow MS. Pharmacokinetics of acetaminophen in Hong Kong Chinese subjects. Int J Pharm. 2001;222:305–308. doi: 10.1016/s0378-5173(01)00712-8. [DOI] [PubMed] [Google Scholar]