Abstract
Impaired corneal wound healing that occurs with ocular surface disease, trauma, systemic disease, or surgical intervention can lead to persistent corneal epithelial defects (PCED), which result in corneal scarring, ulceration, opacification, corneal neovascularization, and, ultimately, visual compromise and vision loss. The current standard of care can include lubricants, ointments, bandage lenses, amniotic membranes, autologous serum eye drops, and corneal transplants. Various inherent problems exist with application and administration of these treatments, which often may not result in a completely healed surface. A topically applicable compound capable of promoting corneal epithelial cell proliferation and/or migration would be ideal to accelerate healing. We hypothesize that human growth hormone (HGH) is such a compound. In a recent study, HGH was shown to activate signal transducer and activators of transcription-5 (STAT5) signaling and promote corneal wound healing by enhancing corneal epithelial migration in a co-culture system of corneal epithelial cells and fibroblasts. These effects require an intact communication between corneal epithelia and fibroblasts. Further, HGH promotes corneal wound healing in a rabbit debridement model, thus demonstrating the effectiveness of HGH in vivo as well. In conclusion, HGH may represent an exciting and effective topical therapeutic to promote corneal wound healing.
Keywords: corneal epithelial defect, growth factors, human growth hormone, insulin-like growth factor-1, persistent corneal epithelial defects (PCED), wound healing
I. INTRODUCTION
Corneal wound healing is a highly regulated process that requires the proliferation and migration of epithelial cells,1 interactions between epithelial cells and stromal fibroblasts, and actions of various growth factors and cytokines.2,3 Rapid re-epithelialization of the injured area is extremely important in reducing the risk of potentially blinding microbial superinfection and corneal opacification. When the process is altered by ocular surface disease, trauma, systemic disease, and/or surgical intervention, corneal epithelial wound healing can be delayed, leading to corneal defects that will not “close.” These persistent corneal epithelial defects (PCED) result in corneal scarring, ulceration, opacification, corneal neovascularization, and, ultimately, visual compromise and loss of vision.1
There is an unmet need for a therapy that could help heal the cornea pharmacotherapeutically. Currently, “bandage” methods are used to help re-epithelialize a cornea. These may include aggressive lubricants, debridement and patching, application of a bandage contact lens,4 human amniotic membrane,5 use of autologous serum6,7 as a supernatant to provide necessary growth factors, and suturing of the lids via a tarsorrhaphy.8 In severe cases, a conjunctival graft may be placed over the cornea.9 Often, PCEDs recur and are costly to the patients and the healthcare provider.
Agents that can accelerate wound closure by increasing the migration and proliferation of corneal epithelial cells are of interest because of their potential benefit for patients with persistent epithelial damage from dry eye, surgical or non-surgical trauma, refractive interventions, corneal abrasion, non-healing corneal ulcers, and neurotrophic corneas secondary to diabetes, cranial nerve palsies, and herpetic keratitis.1 Such patients could benefit significantly from a topical preparation that could stimulate the epithelial cells to migrate and proliferate and thus heal.
II. Characteristics of Persistent Corneal Epithelial Defects
A. Prevalence
PCED can be defined as a loss of the integrity of the corneal surface and or a defect in the epithelium caused by injury or disease, which does not heal within the usual timeframe of several days, but persists for weeks or even months. The condition generally has a duration of less than 1 year, but it can recur years later. Underlying disease states that may result in such defects include exposure keratopathy, limbal stem cell deficiency, previous herpes simplex or herpes zoster infection, diabetic keratopathy, neurotrophic keratopathy, and severe dry eye. The defects can also be associated with corneal transplant surgery or diabetic vitrectomy used to treat these diseases.10,11
The actual incidence of PCED is not known but can be estimated based on assumptions regarding the likely causes of PCED; i.e., the incidences of the underlying conditions can be used to estimate the number of cases of PCED. Overall, the estimated number of PCEDs per year in the United States (U.S.) is roughly 73,434 −99,465 cases, based on a recent U.S. population of approximately 314,037,169 (http://www.census.gov/population/www/popclockus.html). Thus, the total incidence of PCEDs is less than 200,000 in the US and is therefore considered an orphan disease in this region.
B. Causes
1. Exposure Keratopathy
Exposure keratopathy is the result of incomplete lid closure (lagophthalmos) that causes drying of the cornea despite normal tear production.12 Among the causes of exposure keratopathy are cranial nerve palsy, aneurysm, herpes infection, and lid malposition. No data have been identified concerning the prevalence of PCED in patients with exposure keratopathy; however, the number of cases is expected to be quite low.
2. Limbal Stem Cell Deficiency
Limbal stem cell deficiency is a disease in which the stem cell functions of the limbus and the barrier function of the limbus fail. LSCD can result from trauma or disturbance due to autoimmune processes or infectious or neoplastic conditions. The reduced ability of limbal stem cells to divide and repopulate the cornea as epithelial cells leads to an unstable epithelial surface, pain, decreased vision, and stromal scarring.13 Corneal epithelial defects appear and fail to heal normally.14
3. Herpes Simplex
The incidence of ocular herpes simplex in the U.S. is 20.7 per 100,000 person-years, and the prevalence is 149 per 100,000 persons.6,15 Corneal complications in patients with herpes simplex are of two main types: epithelial keratitis involving inflammation of the cells that form the surface layer of the cornea, and stromal keratitis involving inflammation of the middle layer (stroma) of the cornea. The effects of herpes simplex keratitis include scarring, tissue destruction, neovascularization, glaucoma, and PCED. Of the ocular herpes simplex cases in the U.S., 72% had corneal-epithelial involvement.16 No more than 46,804 episodes of herpes simplex virus with corneal-epithelial involvement are estimated to occur per year. The number of cases of PCED is likely overestimated, because not all ocular herpes simplex ocular infections with corneal-epithelial involvement can be identified as PCED.
4. Herpes Zoster
Herpes zoster ophthalmicus, or ocular shingles, is a rash that involves the divisions of the cranial V nerve that innervates the skin around the upper and lower eyelid, forehead, and scalp. Neurotrophic keratitis can be the end result, with decreased corneal sensation from herpes zoster virus-mediated destruction, including susceptibility to mechanical trauma, decreased lacrimation, and delayed epithelial healing due to decreased corneal sensation. The incidence of herpes zoster ophthalmicus in the U.S. is 40,000–60,000 cases per year. Ocular involvement occurs in 25%–50% of these cases, with neurotrophic keratopathy developing in 16%.17 An estimate of corneal involvement is roughly 1,600–4,800 cases of herpes zoster ophthalmicus-related neurotrophic keratopathy per year.
5. Diabetic Keratopathy
Diabetic keratopathy has been estimated to occur in 47%–64% of diabetic patients during the course of their disease,18,19 and people with diabetes have an increased risk of developing epithelial fragility, corneal epithelial defects, recurrent epithelial erosions, decreased sensitivity, abnormal wound healing, increased susceptibility to injury, and non-healing or infected corneal ulceration. Recurrent corneal erosions in patients with diabetes are usually post-traumatic and can be the result of apparently mild epithelial breakdown following cataract or vitreoretinal surgery.20 Neuropathic keratopathy is a possible complication of diabetes, which can cause corneal epithelial defects due to a lack of innervation and sensation.21 No data are available on the prevalence of PCED in patients with diabetic keratopathy.
6. Neurotrophic Keratopathy
Neurotrophic keratopathy is a degenerative disease of the corneal epithelium resulting from impaired corneal innervation. A reduction in corneal sensitivity or complete corneal anesthesia is the hallmark of this disease and is responsible for producing epithelial keratopathy, PCED, ulceration, and perforation.22 It is most commonly due to herpes infection, chemical injury, or trauma. No data are available on the prevalence of PCED in patients with neurotrophic keratopathy.
7. Corneal Transplantation
The number of corneal transplants (also referred to as penetrating keratoplasty or corneal graft) in 2011 was 46,081 (Eye Bank Association of America). The incidence of corneal PCED following corneal transplants is 16.4%.23 Therefore, 7,558 PCEDs per year are due to corneal transplant.
8. Diabetic Vitrectomy
In the U.S., 250,000 vitrectomy surgeries are performed annually,24 with an estimated 25%–53% performed on diabetic patients. The frequency of epithelial debridement during diabetic vitrectomy is estimated at 17.4%.25 Corneal epithelial defects were found in 22.8% of eyes at 2 weeks after corneal epithelial debridement during diabetic vitrectomy.26 A simple calculation of these data would estimate 2,480–5,257 PCEDs resulting from diabetic vitrectomy per year.
9. Severe Dry Eye
PCED can occur in the setting of severe dry eye, such as mucin-deficient dry eye in patients with Stevens-Johnson syndrome or ocular cicatricial pemphigoid, or in patients with lacrimal gland dysfunction (e.g., Sjogren disease). The prevalence of dry eye disease ranges from 8%–15% in the U.S. and 5.5%–34%, when data from Asia and Australia are included.27 In general, about 10% of dry eye cases are classified as severe.28 The prevalence of severe dry eye was reported to be 2.3% among a U.S. Hispanic population.29 About 5 million people over the age of 50 suffer from dry eye in the U.S.27
10. Corneal Burns
The incidence of ocular trauma is 3.1 cases per 1,000 person-years,30 and chemical or thermal burns comprise 7.7%–18% of all ocular traumas.31 Limited data exist on the number of corneal burns that progress to development of PCED; however, chemical and thermal corneal burns are a leading cause of LSCD.13
III. Treatment of Persistent Corneal Epithelial Defects
A. Current Treatments
The current standard of care for PCED is variable and includes various types of “bandage” treatments or growth modulators, such as lubricants, patching, bandage contact lens, autologous serum, amniotic membranes grafts, and even corneal transplants.11,32,33 The goal is to cover and protect the corneal epithelial defect and allow proliferation of limbal stem cells and migration of mature corneal epithelial cells to heal the epithelial defects.
Lubricants alone, although efficacious for mild dry eye and corneal abrasions in healthy eyes, are generally insufficient to treat PCED. Additional therapies can help create an environment conducive to healing by decreasing friction on the cornea from the lids and creating a moist environment via patching, bandage contact lenses, and amniotic membrane (AM). Of these, bandage contact lenses and AM grafts tend to be the most effective.
AM, the innermost layer of the placenta, contains quantities of HGH34 and growth factor proteins (e.g., epidermal growth factor [EGF], keratocyte growth factor [KGF], and basic fibroblast growth factor [bFGF]),35 along with various cytokines and proteinase inhibitors.36 AM may facilitate epithelial migration/healing when used as a temporary biologic dressing (i.e., patch) following corneal wounding, while the growth factors/cytokines could decrease inflammatory response.37,38 However, the contradictory actions of some of the growth factors/cytokines contained within the AM renders a suboptimal approach to encourage healing. Use of human AM is also limited due to cost and to preparation procedures that may attenuate its healing properties. In the U.S., only processed and sterilized/dried AM can be used, and since fragile biologics may be destroyed during preparation, the extent to which these products are able to deliver bioactive cytokines and growth factors is questionable. Reported complications related to the use of AM include potential for post-operative infection, loosening or dislocation of the transplanted membrane due to loosening/breaking of the sutures, hemorrhage under the membrane, and early disintegration of the membrane.39,40
Numerous studies have been published on both preclinical and clinical use of autologous serum, amniotic fluid, and or umbilical cord serum. The efficacy demonstrated with autologous serum is of particular interest. This serum, loaded with cytokines, chemokines, and proteins (i.e., growth factors), has had excellent efficacy in treating numerous ocular surface conditions and, in particular, PCED.6,41,42 However, use of autologous serum is not an approved therapy, and it is costly, time- and resource-intensive, and inconvenient for the patient. For example, autologous serum has inherent collection, logistic and application issues requiring the drawing of blood, its shipment to a registered compounding facility, and then shipment back to the patient a week or more later in a frozen serum form to be defrosted and applied anywhere from 6–10 times/day.32,33
In severe cases of PCED, especially where limbal stem cells are deficient, limbal stem cell transplants and surgical lid closure, i.e., tarsorraphies, and even corneal transplants may be necessary.11 Limbal stem cell transplant is indicated when the ocular surface damage has reduced the number of stem cells at the limbus that are required to repopulate the cornea,13 thus creating persistent defects. Such patients present with ocular pain and PCED. Autologous conjunctival limbal autografts from the healthy eye have been successfully used to treat the contralateral involved eye.43 These confer no risk for rejection; however, the process creates the risk for the contralateral eye to develop a limbal stem cell-deficient state.44
Because of the limitations of the currently available treatments described above, it is of great interest to identify compounds that can accelerate wound closure by activating epithelial stem cell differentiation, increasing corneal and conjunctival epithelial cell migration and proliferation, and inhibiting scarring, fibrosis and adhesions. Such compounds could also benefit patients with epithelial damage other than PCED, such as occurs with dry eye, surgical trauma, refractive interventions, and simple corneal abrasions.
B. Therapies in Development
The fact that human serum contains such proteins as GH, EGF, and IGF-1 may help explain why therapy with autologous serum has been successful in clinical studies. It has been hypothesized that perhaps it is the intrinsic growth factors that are efficacious in these biologic fluids. Several of these growth factors have been examined individually in preclinical and clinical settings of PCED.
1. Epidermal Growth Factor
EGF has been shown to increase the healing rate of traumatic corneal epithelial defects in a randomized, double-blind, placebo-controlled, multicenter clinical trial.45 EGF stimulated proliferation of cultured epithelial cells and stromal fibroblasts, stimulated synthesis of fibronectin by epithelial cells, and was chemotactic for human epithelial and stromal cells.46 Basic research has demonstrated that the mechanism of EGF-stimulated corneal wound healing involves activation of multiple intracellular signaling pathways, including phosphoinositide 3-kinase (PI3K)/AKT, protein kinase C, and mitogen-activated protein kinase cascade, transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), nuclear factor κB subtype-regulated CCCTC binding factor, and suppression of the eye-specific Pax6 expression.47–51
2. Insulin-Like Growth Factor-1
Insulin-like growth factor (IGF)-1 and IGF-1 receptor (IGFR) are both expressed in human ocular surface tissues, including corneal and conjunctival epithelial cells.52,53 Topical IGF has been shown to improve corneal epithelial defects clinically.54–58 In terms of mechanism, IGF-1 may play a role in promoting corneal epithelial cell migration, corneal limbal stem cell differentiation into epithelial like cells, and corneal nerve regeneration.54–56,59,60 When combined with substance P, IGF-1 promotes corneal epithelial migration and has shown efficacy in treating patients with PCED.54–56 Therefore, the role of IGF-1 may be in differentiation, and it may depend on other relevant factors and cellular interactions for corneal epithelial migration.
3. Nerve Growth Factor
NGF and NGFR proteins and mRNAs are present in the human cornea.61 NGF has been shown to provide trophic support after neuronal injuries, to reverse pathologic nerve changes, and to promote healing.62 Topically applied NGF restored corneal integrity in patients with corneal neurotrophic ulcers.63 The mechanism of the beneficial effect of NGF on corneal wound healing is thought to involve increased corneal epithelial cell migration, fibroblastic-keratocyte differentiation into, and increased MMP-9 expression, in addition to its well-known neurotrophic actions on the corneal nerves.64–66
4. Human Growth Hormone
HGH is a hydrophilic anabolic protein with a molecular weight of 22 kDa composed of 191 amino acids.67 It is a member of the somatotropin/prolactin family of hormones. It is produced by the anterior pituitary gland and is required for normal human growth and development. HGH fosters a healthy body composition through various physiological functions: stimulating the expression of IGF, increasing muscle, reducing fat mass, maintaining normal blood glucose levels, and increasing calcium retention and bone mineralization.67
a. Systemic Wound Healing
Recombinant HGH (rHGH) is a manufactured protein that has been shown to activate cells to divide.68,69 rHGH increases muscle mass, promotes lipolysis, augments wound healing, and reduces liver uptake of glucose by activating HGH receptors as well as increasing IGF systemically and locally.70–72 It was first approved by the U.S. Food and Drug Adminstration (FDA) in 1985 for use in the treatment of growth hormone (GH) deficiency, Prader-Willi and Turner syndromes, and other specific medical conditions in children and adults.70 This biologic is now available generically from multiple sources. There is extensive clinical experience with systemic rHGH in adults and children administered for its indicated use in insufficient growth. Furthermore, clinical research has shown evidence for rHGH as an off-label therapy for epidermal wound healing in burns and for its anabolic affects in trauma.72,73 Clinical data suggest that rHGH may be effective in closing cutaneous defects of the epithelium and accelerating wound healing.71,72,74 Animal and cell culture studies have shown that GH activates epidermal and epithelial cell migration and assists wound healing.75–77
HGH exhibits crosstalk with various growth factors, including IGF-1, EGF, bFGF, and NGF, all of which have been shown to have some degree of efficacy in PCED both pre-clinically and clinically.78–83 It is well established that rHGH upregulates insulin and IGF-1 systemically, working through the IGF pathway to induce wound healing.84,85 In addition, GH enhances the transcription of NGFR.86 Consequently, rHGH is attractive as a possible agent to promote corneal epithelial wound healing, as are IGF-1 and NGF, and clinical studies are currently being completed to assess the role of HGH systemically for cutaneous epithelial wound healing (ClinicalTrials.gov identifier: NCT00673309).
b. Ocular Wound Healing
Little research has been conducted on the effect of GH in the cornea. A 1977 study found that GH affected lysosomal glycosidase activity of the sclera and cornea.87 Recently, human studies have shown altered thickness and biomechanics of the cornea in acromegalic patients and GH-deficient children.88–90 These findings suggest a role of GH in the cornea. We hypothesize that HGH can promote corneal wound healing and that it does so by activating phospho-STAT5 (p-STAT5) signaling and promoting corneal epithelial and fibroblast proliferation and/or migration.91 To test this hypothesis, we conducted an in vitro study on cell signaling, proliferation, and migration, using an immortalized human corneal epithelial cell line (abbreviated as epithelial cells) and primary human corneal fibroblasts (abbreviated as fibroblasts).91 We found a dose-dependent increase in p-STAT5 in response to HGH treatment in both the epithelial cells and fibroblasts. Further, we observed increased wound closure rate in a co-culture system of the epithelial cells and fibroblasts with two methods of co-culturing. With one method, a confluent fibroblast layer was grown on an insert with a confluent epithelial cell layer underneath on the well in a transwell system; the two cell types were allowed to exchange soluble factors in real-time with this method. With the second method, a confluent epithelial cell layer was grown directly on top of a confluent fibroblast layer; the two cell types were allowed to physically contact each other. Interestingly, the increased wound closure was seen only when the two types of cells were cultured together, i.e., not alone or using conditioned media, indicating that HGH does not act on corneal epithelial cells directly to stimulate migration, but rather acts through an intact epithelial-fibroblast communication system.91 These effects were also not mediated by IGF-1, as pretreatment of cells with a IGF-1 receptor blocking antibody did not affect HGH’s ability to stimulate epithelial cell migration. Therefore, other soluble factor(s) may mediate the action of HGH on corneal epithelial cell migration. We also demonstrated that neither HGH nor IGF-1 has an effect on corneal epithelial or fibroblast proliferation.91 This is consistent with a report showing no effect of IGF-1 on cornea epithelial cell proliferation in the organ culture,55 but it differs from reports that GH promotes human foreskin fibroblast proliferation via increasing IGF-1.75,92 These differences may be due to the type of epithelia and fibroblasts (cornea versus skin) used.
The beneficial effects of HGH in the in vitro scratch wound test were consistent with findings of a New Zealand rabbit study.93 In this pilot in vivo study (see “Supplementary Information” following references for details of methods), rabbits with corneal epithelial debridement were treated with rHGH delivered from HyStem™, a proprietary crosslinked hyaluronic acid (HA) vehicle, or HyStem™ alone (which was applied as a topical drop), and both groups were treated with dexamethasone to mimic photorefractive keratectomy clinical management in standardized keratorefractive surgery (see Table 1 for experimental plan). This study design was partially based on previous work by Schultz et al, who showed that EGF, HGH, and dexamethasone delivered through a contact lens delivery system, could accelerate wound healing in a New Zealand white rabbit corneal debridement model.94,95 They showed that the molecular structure of EGF and rHGH were not altered upon release from the contact lens hydrogel in the New Zealand white rabbit corneal debridement model. Such a local delivery scheme using a polymer vehicle allows a safe and sustainable release system translatable to clinical applications and is ideal for delivery of a protein.
Table 1.
Experimental scheme for rabbit corneal debridement and HGH treatment.
| Corneal debridement surgery in New Zealand white rabbits | |
|---|---|
| Vehicle control (HyStem): 40 µl per eye twice/day; topical 40 ul 0.1 % dexamethasone 4 times/day. Treatment lasted 7 days. | HGH: dissolved in HyStem, with rHGH delivered 4 µg/day; topical 40 ul 0.1 % Dexamethasone 4 times/day. Treatment lasted 7 days. |
Slit-lamp biomicroscopy and fluorescein staining to assess corneal defect twice a day for 4 days and then once a day for days 5–7. Defect size was quantified using Image J.
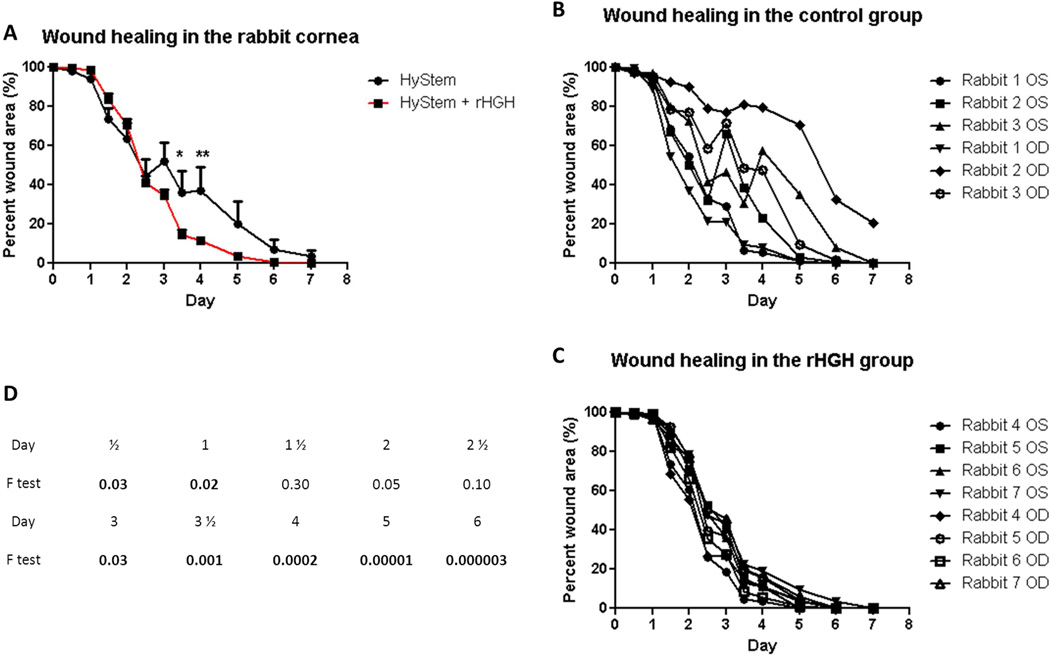
Using a similar delivery scheme (HyStem™ as mentioned above), we found that the rHGH group healed completely (Figure 1C), whereas in the vehicle group (Hystem™ alone), one eye still had a 20% wound area (Figure 1B) by 7 days. The corneal epithelial healing in rHGH and the vehicle groups behaved significantly differently as a function of time (p<.0001 of interaction between treatment and time in repeated measures ANOVA; Figure 1A). The healing was accelerated starting from 3 days, with 3 ½ and 4 days significantly advanced in the rHGH group (Bonferroni multiple comparisons). The trend of this difference was still seen after 5 days and tapered off after 6 days (Figure 1A). Of particular interest, the HA vehicle group showed relatively large variances among individual eyes, especially at later time points (after 2 days; Figure 1B), whereas the rHGH group showed much smaller variances in the percent wound size throughout the study (Figure 1C). In fact, F test statistics showed significant differences in variances between vehicle and rHGH groups at multiple time points, with the differences more pronounced at later time points (Figure 1D). This suggests that rHGH was able to accelerate the epithelial healing in steroid-induced “slow healers,” resulting in similarly ”fast healing” in all eyes versus a wide range of healing speed seen in the control eyes, which received only the vehicle.
Figure 1.
rHGH promotes corneal epithelial wound closure in vivo. A: Percent remaining epithelial defect (baseline being 100%) during 7 days in the control (HyStem alone) and rHGH (HyStem + rHGH) groups. Repeated measures ANOVA revealed p<.0001 for the interaction of time and rHGH treatment, and Bonferroni multiple comparisons showed significant difference between control and rHGH groups after 3 ½ and 4 days (*p<.05, **p<.01). B and C: percent wound area during 7 days in individual rabbit eyes in the HyStem and rHGH groups, respectively. D: F test values between control and rHGH groups at each individual time point, with values <.05 in boldface.
This in vivo work has its limitations. The use of dexamethasone, which served to mimic the clinical PRK setting, delayed corneal wound healing, as was reported previously.96,97 Hence, the healing rate was slow overall (7 days to completely heal instead of the 4 days without dexamethasone that is typically observed). The use of HA in HyStem™, a safe and sustainable delivery system, may complicate the effect of GH. These may be the reasons for the fluctuations in healing in some rabbits of the vehicle group. Nonetheless, this pilot rabbit study suggests that rHGH treatment of the corneal wound is safe and effective, and future studies without the use of dexamethasone and with more controls are warranted.
C. Future Steps in Development
To further verify the efficacy of HGH in promoting corneal wound healing in vivo, it is necessary to develop a local slow-release vehicle to deliver biologics such as HGH to the eye. Ocular drug delivery has been a major challenge to pharmacologists and drug delivery scientists, due to the unique anatomy and physiology of the eye. Static barriers (different tissue layers: cornea, sclera, and retina, including blood-aqueous and blood-retinal barriers) and dynamic barriers (choroidal and conjunctival blood flow, lymphatic clearance, and tear dilution) pose a challenge for delivery of a drug and/or protein alone or in a dosage form. Nevertheless, several novel delivery modifications, such as biodegradable polymers, for overcoming these challenges have been developed that allow the molecules to remain stable in the polymer and be released in its bioactive state.98,99 The HA hydrogel film developed by Jade Therapeutics, Inc., such as was used in the rabbit study presented here, may prove to be a suitable delivery vehicle in the ophthalmic space.
IV. Conclusion
PCED can lead to corneal ulceration, scarring, perforation, and loss of vision. Although general methods exist to treat PCED (e.g., patching, lubricants, ointments, and, in severe cases, amniotic membrane corneal grafting and lid tarsorraphy), no approved pharmacotherapy is entirely successful.11 The use of topical autologous serum (not an approved therapy) appears to be successful, and it is interesting to speculate that this may be due to the application of its inherent growth factors, including HGH. HGH is upstream to so many of these growth factors and has intrinsic and well-recognized systemic wound healing capabilities, making it an interesting agent for corneal wound healing. We hypothesize that HGH can promote corneal wound healing, and our in vitro and in vivo data suggest an efficacy of HGH in accelerating corneal wound healing. Future studies are needed to investigate the mechanism of HGH action in the corneal wound healing process. The availability of rHGH as an approved drug and as a sustained ocular surface drug delivery system that can be delivered and developed topically make this a viable and exciting potential commercial agent for the treatment of PCED.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01EY05612, 1K99EY023536-01A1 and the Margaret S. Sinon Scholar in Ocular Surface. Dr. Jade has received an unrestricted Technology Commercialization & Innovation Program (TCIP) grant from Utah Governor’s Office of Economic Development (GOED) to help fund this work.
Drs. Ding, Morelli, and Sullivan have no commercial or proprietary interest in any concept or product discussed in this article. Barbara Wirostko is the co-founder and CSO of Jade Therapeutics, Inc. She receives financial support, has personal financial interest, holds patent, and is on the board of Jade, a biotech company working on the development of an rHGH ophthalmic therapeutic agent indicated for corneal wound healing. MaryJane Rafii is the cofounder and COO of Jade Therapeutics, Inc. She receives financial support, has personal financial interest, holds patent, and is on the board of Jade, a biotech start up working on the development of an rHGH ophthalmic therapeutic indicated for corneal wound healing.
Abbreviations
- AM
Amniotic membrane
- bFGF
Basic fibroblast growth factor
- EGF
Epidermal growth factor
- GH
Growth hormone
- HA
Hyaluronic acid
- HGH
Human growth hormone
- IGF
Insulin-like growth factor IGFR IGF-1 receptor
- KGF
Keratocyte growth factor
- LSCD
Limbal stem cell deficiency
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PCED
Persistent corneal epithelial defect
- P13K
Phosphoinositide 3-kinase
- p-STAT5
Phospho--STAT5
- rHGH
Recombinant HGH
- STAT5
Signal transducer and activators of transcription-5
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med (Maywood) 2001;226:653–664. doi: 10.1177/153537020222600711. [DOI] [PubMed] [Google Scholar]
- 2.Wilson SE, Liu JJ, Mohan RR. Stromal-epithelial interactions in the cornea. Prog Retin Eye Res. 1999;18:293–230. doi: 10.1016/s1350-9462(98)00017-2. [DOI] [PubMed] [Google Scholar]
- 3.Zieske JD. Extracellular matrix and wound healing. Curr Opin Ophthalmol. 2001;12:237–241. doi: 10.1097/00055735-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Blackmore SJ. The use of contact lenses in the treatment of persistent epithelial defects. Cont Lens Anterior Eye. 2010;33:239–244. doi: 10.1016/j.clae.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Meller D, Pauklin M, Thomasen H, et al. Amniotic membrane transplantation in the human eye. Deutsches Arzteblatt Int. 2011;108:243–248. doi: 10.3238/arztebl.2011.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young AL, Cheng AC, Ng HK, et al. The use of autologous serum tears in persistent corneal epithelial defects. Eye (Lond) 2004;18:609–614. doi: 10.1038/sj.eye.6700721. [DOI] [PubMed] [Google Scholar]
- 7.Jeng BH. Use of autologous serum in the treatment of ocular surface disorders. Arch Ophthalmol. 2011;129:1610–1612. doi: 10.1001/archophthalmol.2011.336. [DOI] [PubMed] [Google Scholar]
- 8.Pakarinen M, Tervo T, Tarkkanen A. Tarsorraphy in the treatment of persistent corneal lesions. Acta Ophthalmol Suppl. 1987;182:69–73. doi: 10.1111/j.1755-3768.1987.tb02595.x. [DOI] [PubMed] [Google Scholar]
- 9.Thoft RA. Conjunctival transplantation. Arch Ophthalmol. 1977;95:1425–1427. doi: 10.1001/archopht.1977.04450080135017. [DOI] [PubMed] [Google Scholar]
- 10.McCulley JP, Horowitz B, Husseini ZM, Horowitz M. Topical fibronectin therapy of persistent corneal epithelial defects. Fibronectin Study Group. Trans Am Ophthalmol Soc. 1993;91:367–386. discussion 86–90. [PMC free article] [PubMed] [Google Scholar]
- 11.Jeng BH. Treating the nonhealing epithelial defect - an overview of standard and investigational therapies for persistent corneal epithelial defects. Cataract Refractive Surgery Today Europe. 2011:25–28. [Google Scholar]
- 12.Latkany RL, Lock B, Speaker M. Nocturnal lagophthalmos: an overview and classification. Ocul Surf. 2006;4:44–53. doi: 10.1016/s1542-0124(12)70263-x. [DOI] [PubMed] [Google Scholar]
- 13.Lim P, Fuchsluger TA, Jurkunas UV. Limbal stem cell deficiency and corneal neovascularization. Semin Ophthalmol. 2009;24:139–148. doi: 10.1080/08820530902801478. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad S. Concise review: limbal stem cell deficiency, dysfunction, and distress. Transl Med. 2012;1:110–115. doi: 10.5966/sctm.2011-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Liesegang TJ, Melton LJ, 3rd, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1155–1159. doi: 10.1001/archopht.1989.01070020221029. [DOI] [PubMed] [Google Scholar]
- 17.Shaikh S, Ta CN. Evaluation and management of herpes zoster ophthalmicus. Am Fam Physician. 2002;66:1723–1730. [PubMed] [Google Scholar]
- 18.Schultz RO, Van Horn DL, Peters MA, et al. Diabetic keratopathy. Trans Am Ophthalmol Soc. 1981;79:180–199. [PMC free article] [PubMed] [Google Scholar]
- 19.Abdelkader H, Patel DV, McGhee C, Alany RG. New therapeutic approaches in the treatment of diabetic keratopathy: a review. Clin Experiment Ophthalmol. 2011;39:259–270. doi: 10.1111/j.1442-9071.2010.02435.x. [DOI] [PubMed] [Google Scholar]
- 20.Jeganathan VS, Wang JJ, Wong TY. Ocular associations of diabetes other than diabetic retinopathy. Diabetes Care. 2008;31:1905–1912. doi: 10.2337/dc08-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lockwood A, Hope-Ross M, Chell P. Neurotrophic keratopathy and diabetes mellitus. Eye (Lond) 2006;20:837–839. doi: 10.1038/sj.eye.6702053. [DOI] [PubMed] [Google Scholar]
- 22.Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye (Lond) 2003;17:989–995. doi: 10.1038/sj.eye.6700616. [DOI] [PubMed] [Google Scholar]
- 23.Rumelt S, Bersudsky V, Blum-Hareuveni T, Rehany U. Persistent epithelial defects and ulcers in repeated corneal transplantation: incidence, causative agents, predisposing factors and treatment outcomes. Graefes Arch Clin Exp Ophthalmol. 2008;246:1139–1145. doi: 10.1007/s00417-008-0797-4. [DOI] [PubMed] [Google Scholar]
- 24.Virata SR, Kylstra JA, Singh HT. Corneal epithelial defects following vitrectomy surgery using hand-held, sew-on, and noncontact viewing lenses. Retina. 1999;19:287–290. doi: 10.1097/00006982-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Friberg TR, Ohji M, Scherer JJ, Tano Y. Frequency of epithelial debridement during diabetic vitrectomy. Am J Ophthalmol. 2003;135:553–554. doi: 10.1016/s0002-9394(02)02014-7. [DOI] [PubMed] [Google Scholar]
- 26.Chen WL, Lin CT, Ko PS, et al. In vivo confocal microscopic findings of corneal wound healing after corneal epithelial debridement in diabetic vitrectomy. Ophthalmology. 2009;116:1038–1047. doi: 10.1016/j.ophtha.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 27.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. (No authors listed). [DOI] [PubMed] [Google Scholar]
- 28.Nichols JJ. 2010 Annual Report on Dry Eye Diseases. A review of current findings and trends in dry eye frequency, diagnosis, and management strategies. Cont Lens Spectrum. 2010 Jan [Google Scholar]
- 29.Hom M, De Land P. Prevalence and severity of symptomatic dry eyes in Hispanics. Optom Vis Sci. 2005;82:206–208. doi: 10.1097/01.opx.0000156310.45736.fa. [DOI] [PubMed] [Google Scholar]
- 30.Wong TY, Klein BE, Klein R. The prevalence and 5-year incidence of ocular trauma. The Beaver Dam Eye Study. Ophthalmology. 2000;107:2196–2202. doi: 10.1016/s0161-6420(00)00390-0. [DOI] [PubMed] [Google Scholar]
- 31.Kuckelkorn R, Schrage N, Keller G, Redbrake C. Emergency treatment of chemical and thermal eye burns. Acta Ophthalmol Scand. 2002;80:4–10. doi: 10.1034/j.1600-0420.2002.800102.x. [DOI] [PubMed] [Google Scholar]
- 32.Sharma N, Goel M, Velpandian T, et al. Evaluation of umbilical cord serum therapy in acute ocular chemical burns. Invest Ophthalmol Vis Sci. 2011;52:1087–1092. doi: 10.1167/iovs.09-4170. [DOI] [PubMed] [Google Scholar]
- 33.Seitz B, Das S, Sauer R, et al. Amniotic membrane transplantation for persistent corneal epithelial defects in eyes after penetrating keratoplasty. Eye (Lond) 2009;23:840–848. doi: 10.1038/eye.2008.140. [DOI] [PubMed] [Google Scholar]
- 34.Chochinov RH, Ketupanya A, Mariz IK, et al. Amniotic fluid reactivity detected by somatomedin C radioreceptor assay: correlation with growth hormone, prolactin and fetal renal maturation. J Clin Endocrinol Metab. 1976;42:983–986. doi: 10.1210/jcem-42-5-983. [DOI] [PubMed] [Google Scholar]
- 35.Koizumi NJ, Inatomi TJ, Sotozono CJ, et al. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–177. [PubMed] [Google Scholar]
- 36.Kim JS, Kim JC, Na BK, et al. Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp Eye Res. 2000;70:329–337. doi: 10.1006/exer.1999.0794. [DOI] [PubMed] [Google Scholar]
- 37.Kheirkhah A, Johnson DA, Paranjpe DR, et al. Temporary sutureless amniotic membrane patch for acute alkaline burns. Arch Ophthalmol. 2008;126:1059–1066. doi: 10.1001/archopht.126.8.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Rahman I, Said DG, Maharajan VS, Dua HS. Amniotic membrane in ophthalmology: indications and limitations. Eye (Lond) 2009;23:1954–1961. doi: 10.1038/eye.2008.410. [DOI] [PubMed] [Google Scholar]
- 40.Dua H, Maharajan VS, Hopkinson A. Controversies and limitations of amniotic membrane in ophthalmic surgery. In: Reinhard T, Larkin DFP, editors. Cornea and External Eye Disease. Berlin Heidelberg: Springer; 2006. pp. 21–33. [Google Scholar]
- 41.Tsubota K, Goto E, Shimmura S, Shimazaki J. Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology. 1999;106:1984–1989. doi: 10.1016/S0161-6420(99)90412-8. [DOI] [PubMed] [Google Scholar]
- 42.Jeng BH, Dupps WJ., Jr Autologous serum 50% eyedrops in the treatment of persistent corneal epithelial defects. Cornea. 2009;28:1104–1108. doi: 10.1097/ICO.0b013e3181a2a7f6. [DOI] [PubMed] [Google Scholar]
- 43.Liang L, Sheha H, Li J, Tseng SC. Limbal stem cell transplantation: new progresses and challenges. Eye (Lond) 2009;23:1946–1953. doi: 10.1038/eye.2008.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dua HS, Saini JS, Azuara-Blanco A, Gupta P. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol. 2000;48:83–92. [PubMed] [Google Scholar]
- 45.Pastor JC, Calonge M. Epidermal growth factor and corneal wound healing. A multicenter study. Cornea. 1992;11:311–314. doi: 10.1097/00003226-199207000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Schultz G, Chegini N, Grant M, et al. Effects of growth factors on corneal wound healing. Acta Ophthalmol Suppl. 1992:60–66. doi: 10.1111/j.1755-3768.1992.tb02170.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Akhtar RA. Epidermal growth factor stimulation of phosphatidylinositol 3-kinase during wound closure in rabbit corneal epithelial cells. Invest Ophthalmol Vis Sci. 1997;38:1139–1148. [PubMed] [Google Scholar]
- 48.Zhang Y, Akhtar RA. Epidermal growth factor stimulates phospholipase D independent of phospholipase C, or protein kinase C or phosphatidylinositol-3 kinase activation in immortalized rabbit corneal epithelial cells. Curr Eye Res. 1998;17:294–300. doi: 10.1076/ceyr.17.3.294.5223. [DOI] [PubMed] [Google Scholar]
- 49.Kang SS, Li T, Xu D, et al. Inhibitory effect of PGE2 on EGF-induced MAP kinase activity and rabbit corneal epithelial proliferation. Invest Ophthalmol Vis Sci. 2000;41:2164–2219. [PubMed] [Google Scholar]
- 50.Wang L, Wu X, Shi T, Lu L. Epidermal growth factor (EGF)-induced corneal epithelial wound healing through nuclear factor κB subtype-regulated CCCTC binding factor (CTCF) activation. J Biol Chem. 2013;288:24363–24371. doi: 10.1074/jbc.M113.458141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li T, Lu L. Epidermal growth factor-induced proliferation requires down-regulation of Pax6 in corneal epithelial cells. J Biol Chem. 2005;280:12988–12995. doi: 10.1074/jbc.M412458200. [DOI] [PubMed] [Google Scholar]
- 52.Rocha EM, Cunha DA, Carneiro EM, et al. Identification of insulin in the tear film and insulin receptor and IGF-1 receptor on the human ocular surface. Invest Ophthalmol Vis Sci. 2002;43:963–967. [PubMed] [Google Scholar]
- 53.Wu YC, Buckner BR, Zhu M, et al. Elevated IGFBP3 levels in diabetic tears: a negative regulator of IGF-1 signaling in the corneal epithelium. Ocul Surf. 2012;10:100–107. doi: 10.1016/j.jtos.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishida T, Chikama T, Morishige N, et al. Persistent epithelial defects due to neurotrophic keratopathy treated with a substance p-derived peptide and insulin-like growth factor 1. Jpn J Ophthalmol. 2007;51:442–447. doi: 10.1007/s10384-007-0480-z. [DOI] [PubMed] [Google Scholar]
- 55.Nishida T, Nakamura M, Ofuji K, et al. Synergistic effects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. J Cell Physiol. 1996;169:159–166. doi: 10.1002/(SICI)1097-4652(199610)169:1<159::AID-JCP16>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 56.Yamada N, Matsuda R, Morishige N, et al. Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br J Ophthalmol. 2008;92:896–900. doi: 10.1136/bjo.2007.130013. [DOI] [PubMed] [Google Scholar]
- 57.Nakamura M, Kawahara M, Morishige N, et al. Promotion of corneal epithelial wound healing in diabetic rats by the combination of a substance P-derived peptide (FGLM-NH2) and insulin-like growth factor-1. Diabetologia. 2003;46:839–842. doi: 10.1007/s00125-003-1105-9. [DOI] [PubMed] [Google Scholar]
- 58.Nakamura M, Kawahara M, Nakata K, Nishida T. Restoration of corneal epithelial barrier function and wound healing by substance P and IGF-1 in rats with capsaicin-induced neurotrophic keratopathy. Invest Ophthalmol Vis Sci. 2003;44:2937–2940. doi: 10.1167/iovs.02-0868. [DOI] [PubMed] [Google Scholar]
- 59.Lee HK, Lee JH, Kim M, et al. Insulin-like growth factor-1 induces migration and expression of laminin-5 in cultured human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:873–882. doi: 10.1167/iovs.05-0826. [DOI] [PubMed] [Google Scholar]
- 60.Trosan P, Svobodova E, Chudickova M, et al. The key role of insulin-like growth factor I in limbal stem cell differentiation and the corneal wound-healing process. Stem Cells Dev. 2012;21:3341–3350. doi: 10.1089/scd.2012.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lambiase A, Manni L, Bonini S, et al. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest Ophthalmol Vis Sci. 2000;41:1063–1069. [PubMed] [Google Scholar]
- 62.Landi F, Aloe L, Russo A, et al. Topical treatment of pressure ulcers with nerve growth factor: a randomized clinical trial. Ann Intern Med. 2003;139:635–641. doi: 10.7326/0003-4819-139-8-200310210-00006. [DOI] [PubMed] [Google Scholar]
- 63.Lambiase A, Rama P, Bonini S, et al. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. 1998;338:1174–1180. doi: 10.1056/NEJM199804233381702. [DOI] [PubMed] [Google Scholar]
- 64.Blanco-Mezquita T, Martinez-Garcia C, Proenca R, et al. Nerve growth factor promotes corneal epithelial migration by enhancing expression of matrix metalloprotease-9. Invest Ophthalmol Vis Sci. 2013;54:3880–3890. doi: 10.1167/iovs.12-10816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Micera A, Lambiase A, Puxeddu I, et al. Nerve growth factor effect on human primary fibroblastic-keratocytes: possible mechanism during corneal healing. Exp Eye Res. 2006;83:747–757. doi: 10.1016/j.exer.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 66.Lambiase A, Aloe L, Mantelli F, et al. Capsaicin-induced corneal sensory denervation and healing impairment are reversed by NGF treatment. Invest Ophthalmol Vis Sci. 2012;53:8280–8287. doi: 10.1167/iovs.12-10593. [DOI] [PubMed] [Google Scholar]
- 67.Kopchick JJ, Andry JM. Growth hormone (GH), GH receptor, and signal transduction. Mol Genet Metab. 2000;71:293–314. doi: 10.1006/mgme.2000.3068. [DOI] [PubMed] [Google Scholar]
- 68.Miquet JG, Gonzalez L, Matos MN, et al. Transgenic mice overexpressing GH exhibit hepatic upregulation of GH-signaling mediators involved in cell proliferation. J Endocrinol. 2008;198:317–330. doi: 10.1677/JOE-08-0002. [DOI] [PubMed] [Google Scholar]
- 69.Gallagher EJ, LeRoith D. Minireview: IGF, insulin, and cancer. Endocrinology. 2011;152:2546–2551. doi: 10.1210/en.2011-0231. [DOI] [PubMed] [Google Scholar]
- 70.Lanes R. Growth velocity, final height and bone mineral metabolism of short children treated long term with growth hormone. Curr Pharmaceut Biotech. 2000;1:33–46. doi: 10.2174/1389201003378997. [DOI] [PubMed] [Google Scholar]
- 71.Herndon DN, Hawkins HK, Nguyen TT, et al. Characterization of growth hormone enhanced donor site healing in patients with large cutaneous burns. Ann Surg. 1995;221:649–656. doi: 10.1097/00000658-199506000-00004. discussion: 56-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gilpin DA, Barrow RE, Rutan RL, et al. Recombinant human growth hormone accelerates wound healing in children with large cutaneous burns. Ann Surg. 1994;220:19–24. doi: 10.1097/00000658-199407000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jeevanandam M, Holaday NJ, Petersen SR. Integrated nutritional, hormonal, and metabolic effects of recombinant human growth hormone (rhGH) supplementation in trauma patients. Nutrition. 1996;12:777–787. doi: 10.1016/s0899-9007(96)00220-1. [DOI] [PubMed] [Google Scholar]
- 74.Decker D, Springer W, Tolba R, et al. Perioperative treatment with human growth hormone down-regulates apoptosis and increases superoxide production in PMN from patients undergoing infrarenal abdominal aortic aneurysm repair. Growth Horm IGF Res. 2005;15:193–199. doi: 10.1016/j.ghir.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 75.Lee SW, Kim SH, Kim JY, Lee Y. The effect of growth hormone on fibroblast proliferation and keratinocyte migration. J Plast Reconstr Aesthet Surg. 2010;63:e364–e369. doi: 10.1016/j.bjps.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 76.Garcia-Esteo F, Pascual G, Garcia-Honduvilla N, et al. Histological evaluation of scar tissue inflammatory response: the role of hGH in diabetic rats. Histol Histopathol. 2005;20:53–57. doi: 10.14670/HH-20.53. [DOI] [PubMed] [Google Scholar]
- 77.Garcia-Esteo F, Pascual G, Gallardo A, et al. A biodegradable copolymer for the slow release of growth hormone expedites scarring in diabetic rats. J Biomed Mater Res B Appl Biomater. 2007;81:291–304. doi: 10.1002/jbm.b.30665. [DOI] [PubMed] [Google Scholar]
- 78.Brazzell RK, Stern ME, Aquavella JV, et al. Human recombinant epidermal growth factor in experimental corneal wound healing. Invest Ophthalmol Vis Sci. 1991;32:336–340. [PubMed] [Google Scholar]
- 79.Leibowitz HM, Morello S, Jr, Stern M, Kupferman A. Effect of topically administered epidermal growth factor on corneal wound strength. Arch Ophthalmol. 1990;108:734–737. doi: 10.1001/archopht.1990.01070070120048. [DOI] [PubMed] [Google Scholar]
- 80.Baldwin HC, Marshall J. Growth factors in corneal wound healing following refractive surgery: a review. Acta Ophthalmol Scand. 2002;80:238–247. doi: 10.1034/j.1600-0420.2002.800303.x. [DOI] [PubMed] [Google Scholar]
- 81.Nagano T, Nakamura M, Nakata K, et al. Effects of substance P and IGF-1 in corneal epithelial barrier function and wound healing in a rat model of neurotrophic keratopathy. Invest Ophthalmol Vis Sci. 2003;44:3810–3815. doi: 10.1167/iovs.03-0189. [DOI] [PubMed] [Google Scholar]
- 82.Aloe L, Tirassa P, Lambiase A. The topical application of nerve growth factor as a pharmacological tool for human corneal and skin ulcers. Pharmacol Res. 2008;57:253–258. doi: 10.1016/j.phrs.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 83.Chen W, Hoo RL, Konishi M, et al. Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J Biol Chem. 2011;286:34559–34566. doi: 10.1074/jbc.M111.285965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Demling RH. The role of anabolic hormones for wound healing in catabolic states. J Burns Wounds. 2005;4:e2. [PMC free article] [PubMed] [Google Scholar]
- 85.Huang Y, Kim SO, Yang N, et al. Physical and functional interaction of growth hormone and insulin-like growth factor-I signaling elements. Mol Endocrinol. 2004;18:1471–1485. doi: 10.1210/me.2003-0418. [DOI] [PubMed] [Google Scholar]
- 86.Scharfmann R, Atouf F, Tazi A, Czernichow P. Growth hormone and prolactin regulate the expression of nerve growth factor receptors in INS-1 cells. Endocrinology. 1994;134:2321–2328. doi: 10.1210/endo.134.6.8194458. [DOI] [PubMed] [Google Scholar]
- 87.Kasavina BS, Torbenko VP, Ukhina TV. Biull Eksp Biol Med. Vol. 84. Russian: 1977. Responses of different types of connective tissue to hormone administration; pp. 38–41. [PubMed] [Google Scholar]
- 88.Ciresi A, Amato MC, Morreale D, et al. Cornea in acromegalic patients as a possible target of growth hormone action. J Endocrinol Invest. 2011;34:e30–e35. doi: 10.1007/BF03347058. [DOI] [PubMed] [Google Scholar]
- 89.Ozkok A, Hatipoglu E, Tamcelik N, et al. Corneal biomechanical properties of patients with acromegaly. Br J Ophthalmol. 2014;98:651–657. doi: 10.1136/bjophthalmol-2013-304277. [DOI] [PubMed] [Google Scholar]
- 90.Parentin F, Pensiero S. Central corneal thickness in children with growth hormone deficiency. Acta Ophthalmol. 2010;88:692–694. doi: 10.1111/j.1755-3768.2009.01519.x. [DOI] [PubMed] [Google Scholar]
- 91.Ding J, Wirostko BM, Sullivan DA. Human growth hormone promotes corneal epithelial cell migration in vitro. Cornea. 2015 doi: 10.1097/ICO.0000000000000418. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dykstra KD, Payne AM, Abdelrahim M, Francis GL. Insulin-like growth factor 1, but not growth hormone, has in vitro proliferative effects on neonatal foreskin fibroblasts without affecting 5-alpha-reductase or androgen receptor activity. J Androl. 1993;14:73–78. [PubMed] [Google Scholar]
- 93.Rafii MJ, Wirostko B, Werner L, et al. Hystem, a bio-absorbable protein delivery polymer: safety, tolerability and efficacy in a rabbit corneal debridement model. Invest Ophthalmol Vis Sci. 2013;54 E-Abstract 5048. [Google Scholar]
- 94.Schultz CL, Morck DW. Contact lenses as a drug delivery device for epidermal growth factor in the treatment of ocular wounds. Clin Exp Optom. 2010;93:61–65. doi: 10.1111/j.1444-0938.2010.00459.x. [DOI] [PubMed] [Google Scholar]
- 95.Schultz C, Breaux J, Schentag J, Morck D. Drug delivery to the posterior segment of the eye through hydrogel contact lenses. Clin Exp Optom. 2011;94:212–218. doi: 10.1111/j.1444-0938.2010.00553.x. [DOI] [PubMed] [Google Scholar]
- 96.Chung JH, Kang YG, Kim HJ. Effect of 0.1% dexamethasone on epithelial healing in experimental corneal alkali wounds: morphological changes during the repair process. Graefes Arch Clin Exp Ophthalmol. 1998;236:537–545. doi: 10.1007/s004170050118. [DOI] [PubMed] [Google Scholar]
- 97.Woost PG, Brightwell J, Eiferman RA, Schultz GS. Effect of growth factors with dexamethasone on healing of rabbit corneal stromal incisions. Exp Eye Res. 1985;40:47–60. doi: 10.1016/0014-4835(85)90107-1. [DOI] [PubMed] [Google Scholar]
- 98.Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12:348–346. doi: 10.1208/s12248-010-9183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pisal DS, Kosloski MP, Balu-Iyer SV. Delivery of therapeutic proteins. J Pharm Sci. 2010;99:2557–2575. doi: 10.1002/jps.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.