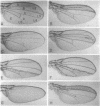
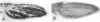Abstract
The vein locus (vn) includes lethal alleles (designated also defective dorsal discs) that prevent growth of dorsal discs and in viable genetic combinations reduce the number of cells of the adult wing. Those effects are prominent in genetic mosaics. Cell proliferation is reduced in all regions of the wing blade in a local autonomous way. These effects are more extreme when mutant clones occupy full intervein regions bordering veins. Clones have, in addition, nonautonomous effects (accommodation) in the proliferation of wild-type cells of the same wing. These effects are more extreme in double mutant vn (ddd) and ve (rhomboid) allelic combinations. Developmental analysis shows that cell proliferation stops earlier in larval development the stronger the vn allele considered. A model is discussed of how cell proliferation is controlled by cellular interactions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basler K., Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994 Mar 17;368(6468):208–214. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- Bryant P. J., Simpson P. Intrinsic and extrinsic control of growth in developing organs. Q Rev Biol. 1984 Dec;59(4):387–415. doi: 10.1086/414040. [DOI] [PubMed] [Google Scholar]
- Cohen B., McGuffin M. E., Pfeifle C., Segal D., Cohen S. M. apterous, a gene required for imaginal disc development in Drosophila encodes a member of the LIM family of developmental regulatory proteins. Genes Dev. 1992 May;6(5):715–729. doi: 10.1101/gad.6.5.715. [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea F. J., Cohen S. M. Interaction between dorsal and ventral cells in the imaginal disc directs wing development in Drosophila. Cell. 1993 Nov 19;75(4):741–752. doi: 10.1016/0092-8674(93)90494-b. [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea F. J., Cohen S. M. wingless acts through the shaggy/zeste-white 3 kinase to direct dorsal-ventral axis formation in the Drosophila leg. Development. 1994 Jun;120(6):1661–1670. doi: 10.1242/dev.120.6.1661. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A., Ripoll P. Cell lineage and differentiation in Drosophila. Results Probl Cell Differ. 1978;9:119–156. doi: 10.1007/978-3-540-35803-9_6. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A., Ripoll P., Morata G. Developmental compartmentalisation of the wing disk of Drosophila. Nat New Biol. 1973 Oct 24;245(147):251–253. doi: 10.1038/newbio245251a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A., de Celis J. F. Developmental genetics of the venation pattern of Drosophila. Annu Rev Genet. 1992;26:277–304. doi: 10.1146/annurev.ge.26.120192.001425. [DOI] [PubMed] [Google Scholar]
- Garrell J., Modolell J. The Drosophila extramacrochaetae locus, an antagonist of proneural genes that, like these genes, encodes a helix-loop-helix protein. Cell. 1990 Apr 6;61(1):39–48. doi: 10.1016/0092-8674(90)90213-x. [DOI] [PubMed] [Google Scholar]
- González-Gaitán M. A., Micol J. L., García-Bellido A. Developmental genetic analysis of Contrabithorax mutations in Drosophila melanogaster. Genetics. 1990 Sep;126(1):139–155. doi: 10.1093/genetics/126.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Gaitán M., Capdevila M. P., García-Bellido A. Cell proliferation patterns in the wing imaginal disc of Drosophila. Mech Dev. 1994 Jun;46(3):183–200. doi: 10.1016/0925-4773(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Morata G., Lawrence P. A. Control of compartment development by the engrailed gene in Drosophila. Nature. 1975 Jun 19;255(5510):614–617. doi: 10.1038/255614a0. [DOI] [PubMed] [Google Scholar]
- Phillips R. G., Roberts I. J., Ingham P. W., Whittle J. R. The Drosophila segment polarity gene patched is involved in a position-signalling mechanism in imaginal discs. Development. 1990 Sep;110(1):105–114. doi: 10.1242/dev.110.1.105. [DOI] [PubMed] [Google Scholar]
- Schubiger M., Palka J. Changing spatial patterns of DNA replication in the developing wing of Drosophila. Dev Biol. 1987 Sep;123(1):145–153. doi: 10.1016/0012-1606(87)90436-2. [DOI] [PubMed] [Google Scholar]
- Simcox A. A., Wurst G., Hersperger E., Shearn A. The defective dorsal discs gene of Drosophila is required for the growth of specific imaginal discs. Dev Biol. 1987 Aug;122(2):559–567. doi: 10.1016/0012-1606(87)90319-8. [DOI] [PubMed] [Google Scholar]
- Simpson P. Parameters of cell competition in the compartments of the wing disc of Drosophila. Dev Biol. 1979 Mar;69(1):182–193. doi: 10.1016/0012-1606(79)90284-7. [DOI] [PubMed] [Google Scholar]
- Struhl G., Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993 Feb 26;72(4):527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Sturtevant M. A., Roark M., Bier E. The Drosophila rhomboid gene mediates the localized formation of wing veins and interacts genetically with components of the EGF-R signaling pathway. Genes Dev. 1993 Jun;7(6):961–973. doi: 10.1101/gad.7.6.961. [DOI] [PubMed] [Google Scholar]
- Wohlwill A. D., Bonner J. J. Genetic analysis of chromosome region 63 of Drosophila melanogaster. Genetics. 1991 Aug;128(4):763–775. doi: 10.1093/genetics/128.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurst G., Hersperger E., Shearn A. Genetic analysis of transdetermination in Drosophila. II. Transdetermination to wing of leg discs from a mutant which lacks wing discs. Dev Biol. 1984 Nov;106(1):147–155. doi: 10.1016/0012-1606(84)90070-8. [DOI] [PubMed] [Google Scholar]