Abstract
Objective
Older adults are increasingly likely to have two or more chronic medical conditions (multimorbidity) and are consequently at greater risk of disability. Here we examine the role of inflammation in mediating the relationship between multimorbidity and disability.
Method
Data are from the Survey of Mid-Life in the United States (MIDUS), a national sample of middle-aged and older adults. Structural equation models were used to assess direct relationships between multimorbidity and activities of daily living as well as indirect associations with a latent variable for inflammation (indicated by circulating levels of interleukin-6, C-reactive protein, and fibrinogen) as a mediator.
Results
After adjustment for potential confounds, multimorbidity was positively associated with inflammation (p < .001) and functional limitations (p < .001), and inflammation partially mediated the link between multimorbidity and functional limitations (p < .01).
Discussion
Inflammation may be an important biological mechanism through which chronic medical conditions are linked to disability in later life.
Keywords: multimorbidity, inflammation, disability, MIDUS
Multimorbidity—the co-existence of two or more chronic medical conditions within the same individual—is increasingly common in aging adults (Anderson & Horvath, 2004; Marengoni et al., 2011; Ward & Schiller, 2013; Wolff, Starfield, & Anderson, 2002), and it is a principal cause of disability in this population (Anderson & Horvath, 2004; Marengoni et al., 2011; Rothrock et al., 2010; Wolff et al., 2002). The link between multimorbidity and disability is of particular interest because while multimorbidity is known to significantly increase risk of mortality (DuGoff, Canudas-Romo, Buttorff, Leff, & Anderson, 2014), a number of studies have suggested that this greater mortality risk is partially or completely mediated by disability (Landi et al., 2010; Marengoni, von Strauss, Rizzuto, Winblad, & Fratiglioni, 2009; St. John, Tyas, Menec, & Tate, 2014). To date, however, the mechanisms by which multimorbidity increases risk of disability are not clear. In this study, we test the novel hypothesis that associations between multimorbidity and functional limitations will be partially mediated by inflammation.
We estimate multimorbidity in a national sample using self-reports of chronic medical conditions. Studies of multimorbidity are diverse. Epidemiological surveys typically use self-reports of chronic conditions that were either diagnosed by a physician or experienced by the respondent (or both). Other studies have relied on records from clinical samples (e.g., primary care patients). While prevalence estimates for multimorbidity are higher in clinical samples than in the population at large (Fortin, Stewart, Poitras, Almirall, & Maddocks, 2012), in studies where both types of data have been available, self-reports of chronic conditions tend to match data from medical records (Katz, Chang, Sangha, Fossel, & Bates, 1996; Kriegsman, Penninx, van Eijk, Boeke, & Deeg, 1996). Another variant in multimorbidity studies is the types of conditions included in aggregate multimorbidity measures, and there is currently no accepted standard of which conditions to include. The multimorbidity index in the current study is comprised of conditions that are among those most likely to lead to disability and mortality according to the Charlson Comorbidity Index (Charlson, Pompei, Ales, & MacKenzie, 1987), have been widely used in prior epidemiological studies (Anderson & Horvath, 2004; Diederichs, Berger, & Bartels, 2011; Fortin, Hudon, Haggerty, Akker, & Almirall, 2010; Schneider, O'Donnell, & Dean, 2009; Thacker et al., 2006; Tinetti et al., 2011; Wolff et al., 2002), and are among those cited in recent calls for multimorbidity research from the Institute of Medicine (2012).
Higher circulating levels of inflammatory proteins, such as interleukin-6 (IL-6), C-reactive protein (CRP), and fibrinogen, are linked to both multimorbidity and functional limitations. Conditions that are included in the current multimorbidity index, such as heart disease, diabetes, and hypertension, and obesity are individually linked to higher levels of IL-6, CRP, and fibrinogen (Bastard et al., 2006; Bautista, Vera, Arenas, & Gamarra, 2005; Blake & Ridker, 2003; Hu, Meigs, Li, Rifai, & Manson, 2004; Naugler & Karin, 2008). In earlier work with data from the Survey of Mid-Life in the United States (MIDUS), we found that aggregate number of chronic conditions was linearly associated with circulating levels of IL-6 and CRP (Friedman & Ryff, 2012). Systemic inflammation also predicts functional impairment both cross-sectionally (Cohen, Pieper, Harris, Rao, & Currie, 1997; Kuo, Bean, Yen, & Leveille, 2006) and prospectively (Ferrucci et al., 1999). Inflammatory proteins, including IL-6 and CRP, have been linked to sarcopenia in older adults (Peake, Della Gatta, & Cameron-Smith, 2010), and this loss of muscle strength is thought to underlie in part the greater functional limitations in older adults with higher levels of inflammation (Cesari et al., 2004; Ferrucci et al., 2002; Kuo et al., 2006). Thus, while inflammation has been implicated in the onset of many chronic conditions (Howcroft et al., 2013), these findings support the plausibility of an explanatory chain that begins with the development of multiple conditions (multimorbidity) leading to greater inflammatory activity and in turn leading to functional impairment. The current study represents a test of this novel hypothesis.
We test our hypotheses using a structural equation model (SEM) that treats inflammation as a latent factor for which IL-6, CRP, and fibrinogen are indicators. Inflammation has been modeled as a latent variable in earlier studies of links between inflammation and both metabolic syndrome (Marsland, McCaffery, Muldoon, & Manuck, 2010) and allostatic load (McCaffery, Marsland, Strohacker, Muldoon, & Manuck, 2012). There are two rationales for this approach, one methodological and the other conceptual. First, each of these proteins is measured with error, and this error contributes to inaccurate estimates of their relationships to key variables. A latent factor, in contrast, provides estimates that are without measurement error (because such error is modeled and controlled via a measurement model), and thus the estimates of key associations have greater precision. Second, although IL-6, CRP, and fibrinogen are unique proteins with discrete biological roles, they (and many others, including IL-1, tumor necrosis factor, and interferon-gamma) are often treated as indicators of a singular general process—systemic inflammation—that is hypothesized to be related to diverse health outcomes. A latent factor for inflammation captures the relatedness of these different proteins and then assesses the extent to which that shared component predicts and is predicted by key variables. In short, a latent factor for inflammation is better aligned with conceptual thinking about inflammation than is the use of individual proteins as separate predictors or mediators.
Finally, these analyses adjusted for a number of covariates that represent potential confounds or behavioral mechanisms. Increasing age, female sex, non-White racial status, low socioeconomic status, and unhealthy behaviors are all associated with higher rates of multimorbidity, higher levels of inflammatory markers, and greater likelihood of functional impairment (Adler et al., 1994; Ershler & Keller, 2000; Ferrucci et al., 2005; Gruenewald, Cohen, Matthews, Tracy, & Seeman, 2009; Lakoski et al., 2006; Marengoni et al., 2011; Murtagh & Hubert, 2004; Schoeni, Martin, Andreski, & Freedman, 2005; Ward & Schiller, 2013). To avoid potential confounding, continuous (age) or categorical (sex, race, education) variables were included in all models. We also adjust for two health behaviors, smoking and regular exercise. Epidemiological studies have shown that adults who smoke are at increased risk of disability whereas those who exercise regularly have a reduced risk (d'Orsi et al., 2014; Hardy, McGurl, Studenski, & Degenholtz, 2010; Wolinsky et al., 2011). Moreover, smoking is predictive of higher circulating levels of inflammatory proteins whereas physical activity is linked to lower levels (Colbert et al., 2004; Jousilahti, Salomaa, Rasi, Vahtera, & Palosuo, 2003; Krabbe, Pedersen, & Bruunsgaard, 2004). Although a full examination of mediation by these variables is beyond the scope of this study, adjusting for them can illuminate their potential involvement in the pathway linking multimorbidity to disability by way of inflammation.
Method
Data for the current study are from the Survey of Midlife Development in the United States (MIDUS), a national study of the physical and mental health of middle-aged and older adults. MIDUS comprises a national probability sample of non-institutionalized English-speaking adults (N = 3,487) living in the co-terminus United States and recruited by random digit dialing (RDD). The second wave of MIDUS data collection (MIDUS 2) was completed in 2004 to 2006, and all respondents completed telephone interviews and self- administered questionnaires. The majority (87.8%) of African American respondents came from a city-specific sample from Milwaukee, Wisconsin.
A sub-sample of MIDUS 2 respondents (N = 1,255) participated in a detailed clinic-based assessment of health, disease-related biomarkers, and physiologic function (“biomarker sample”). Participation in the biomarker sample was open to all MIDUS 2 respondents who had completed interview and questionnaire components of MIDUS and were willing to travel to a General Clinical Research Center (GCRC) for an overnight stay. This bio-marker sample was not significantly different from the main MIDUS sample on most variables, although they were significantly more educated than the main sample (Love, Seeman, Weinstein, & Ryff, 2010).
Upon arrival at the GCRC, each respondent provided a detailed medical history interview with a GCRC clinician. Participants were asked to bring all current medications to the GCRC, and these were inventoried by project staff. Fasting blood samples were obtained the next morning between 08:00 a.m. and 10:00 a.m. Serum was isolated from all samples, aliquoted, frozen at −80°C, and stored for assay.
Collection of data for MIDUS 2 and analysis of those data for the current study were approved by the Institutional Review Boards at the University of Wisconsin–Madison and Purdue University.
Multimorbidity
A variable comprising 13 chronic conditions was used in the analyses. Information on nine of these conditions came from participant responses to self-administered questionnaire items. Participants were asked to indicate whether they had experienced or received treatment for any of the following conditions in the prior 12 months: asthma, bronchitis, or emphysema; arthritis or other joint conditions; HIV or AIDS; high blood pressure; diabetes; tuberculosis; neurological disorders; stroke; and/or ulcer. Presence of heart problems and cancer were determined from single items in the telephone interview. Participants were asked whether they had had heart trouble suspected or confirmed by a doctor and whether they had ever had cancer. Obesity was determined from participant measurement of height and weight; body mass index (BMI) was calculated from these data, and a dichotomous variable indicating obesity (BMI ≥ 30) was created. Finally, current use of cholesterol medication was used as a marker for high cholesterol. All “yes” responses were summed into a chronic conditions index with possible scores ranging from 0 to 13. A dichotomous variable was then created indicating multimorbidity (0 = 0-1 conditions; 1 = two or more conditions).
Functional Limitations
Questionnaire items assessed basic and intermediate activities of daily living (BADL and IADL). Respondents were asked how much health limited their ability to do a number of activities. BADL scores were determined from three items: bathing or dressing yourself, climbing one flight of stairs, and walking one block. IADL scores were determined from seven items: lifting or carrying groceries; climbing several flights of stairs; bending, kneeling, or stooping; walking more than a mile; walking several blocks; vigorous activities (e.g., running, lifting heavy objects); and moderate activities (e.g., bowling, vacuuming). Response options ranged from 1 = not at all to 4 = a lot. Individual participants’ responses to both sets of questions were averaged for analyses. Possible scores on both scales ranged 1 to 4 with higher scores indicating greater impairment. Both were treated as continuous variables in analyses.
Inflammatory Proteins
Serum IL-6 from fasting samples was measured using high-sensitivity enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minnesota). CRP and fibrinogen were measured using particle enhanced immunonephelometric assay (BNII nephelometer, Dade Behring, Inc., Deerfield, IL). The laboratory intra- and inter-assay coefficients of variance for all proteins were in acceptable ranges (<10%). CRP values in excess of 10 mg/L are thought to indicate acute infectious illness (Pearson et al., 2003); we identified and excluded 30 cases with values over this threshold. Due to cases where blood samples were missing or where volume was inadequate for assays, IL-6, CRP, and Fibrinogen data were available for 1,164, 1,156, and 1,156 participants, respectively. As is typically seen, distributions for both IL-6 and CRP were positive skewed, and data were ln-transformed for statistical analyses. Fibrinogen was scaled by a factor of 100 to make values comparable with the other measures (this was necessary for model estimation).
Covariates
A continuous variable was used for age, and dichotomous variables were used for sex (1 = male) and race (1 = non-White). Respondents were asked to indicate their highest level of educational attainment using 12 categories ranging from “no school/some grade school” to “PhD, MD, JD, or other professional degree.” Responses were then aggregated into three categories: high school degree or General Educational Development (GED), some college, and college degree or more; dummy-coded variables were included in all models, with high school degree or GED as the reference category. A categorical variable was used for smoking status (1 = never smoked; 2 = ex-smoker; 3 = current smoker). Respondents were also asked whether they engaged in exercise or physical activity for 20 min or more at least three times a week (1 = yes).
Statistical Analyses
Multivariate models were evaluated using a structural equation modeling framework and Mplus v.7.11 statistical software. The MIDUS sample includes twins and siblings, so a between cluster variance estimator (Binder, 1983) was used to correct for the nesting of individuals within families. Direct maximum likelihood for missing data (aka, FIML) was used to deal with item missing data (Arbuckle, 1996). This method requires less restrictive assumptions about the pattern of missingness than standard case deletion methods and improves power by retaining larger sample sizes. The product of coefficients method is used to obtain mediation effect estimates (MacKinnon, Fairchild, & Fritz, 2007). This method takes the product of the effect of the independent variable on the mediator variable and the effect of the mediator on the outcome. Total effects are estimated as the indirect plus direct effects. An exact standard error estimate for the indirect and total effects is obtained using the delta method (Oehlert, 1992).
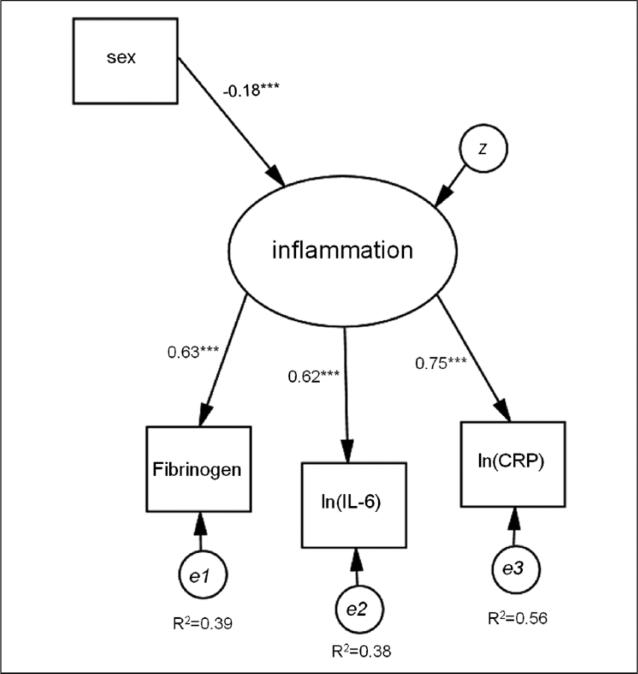
A confirmatory factor model was fit to the three inflammation indicators: IL-6, CRP, and fibrinogen. The latent inflammation variable provided a measure of inflammation as indicated by all three observed inflammation markers. Sex was included as a predictor of the latent variable for the sole purpose of obtaining enough degrees of freedom (an over-identified model) to estimate model fit statistics for the latent inflammation marker. As sex is highly correlated with the latent inflammation variable, this is an ideal variable to use for this purpose. See Figure 1 for a depiction of the latent variable model with standardized coefficient estimates.
Figure 1.
Confirmatory factor model used to test the fit of the latent inflammation variable.
Note. IL-6 = interleukin-6; CRP = C-reactive protein. ***p < .001.
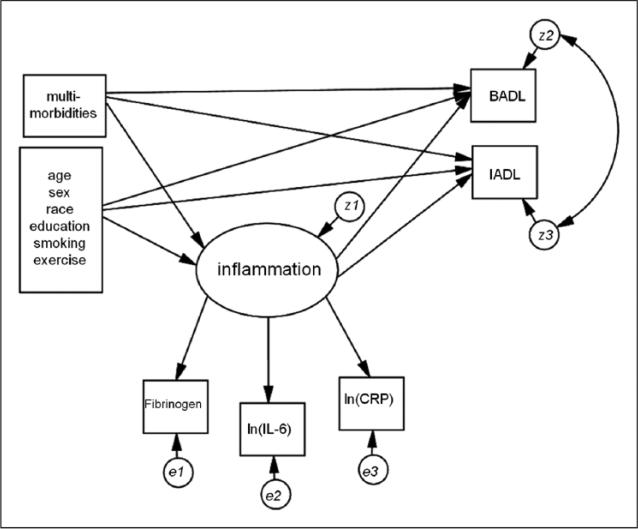
A multivariate mediation model was estimated that included the latent inflammation variable as a mediator between independent variables, including multimorbidities and covariates, and the outcome measures (BADLs and IADLs). BADL and IADL residual errors were allowed to correlate. All independent variables affected the BADL and IADL outcomes both directly and through the inflammation latent variable. See Figure 2 for a depiction of the full mediation model. The inflammation latent variable was tested for measurement invariance (invariance in factor loading estimates) across biological sex using multiple group analysis and the Wald statistic.
Figure 2.
Mediation model used to test indirect effects on ADLs.
Note. ADLs = activities of daily living; BADL = basic ADL; IADL = intermediate ADL; IL-6 = interleukin-6; CRP = C-reactive protein.
Results
Descriptive statistics for the sample are shown in Table 1.
Table 1.
Descriptive Statistics for Sample (N = 1,229).
| Variable | M (SD) | Range | % |
|---|---|---|---|
| Age | 54.5 (0.4) | 34-84 | |
| Sex (% male) | 43.0 | ||
| Race (% non-White) | 22.0 | ||
| Educational attainment | |||
| High school or GED | 7.4 | ||
| Some college | 50.9 | ||
| College or more | 41.7 | ||
| Number of chronic conditions | 1.7 (0.05) | 0-10 | |
| Multimorbidity (2+ chronic conditions) | 54.4 | ||
| BADL | 1.3 (0.02) | 1-4 | |
| IADL | 1.8 (0.03) | 1-4 | |
| IL-6 (pg/mL) | 2.1a | 0.2-23.0 | |
| CRP (μg/mL) | 1.4a | 0.1-10.0 | |
| Fibrinogen (mg/dL)/100 | 3.4 (0.02) | 0.5-7.9 | |
| Smoking status | |||
| Never smoked | 52.5 | ||
| Ex-smoker | 32.6 | ||
| Current smoker | 14.9 | ||
| Regular physical activity (% yes) | 76.5 |
Note. GED = General Educational Development; BADL = basic activities of daily living; IADL = intermediate activities of daily living; IL-6 = interleukin-6; CRP = C-reactive protein.
Geometric mean.
The confirmatory factor model is shown in Figure 1. Model fit was good: χ 2(3) = 16.2, p < .01; confirmatory factor analysis (CFI) = 0.977; Tucker– Lewis index (TLI) = 0.954; root mean square error of approximation (RMSEA) = 0.06 (95% confidence interval [CI] = [0.03, 0.09]). All factor loadings were statistically significant, and while women had higher levels of inflammation overall than men, the factor loadings for each of the inflammatory proteins on the latent inflammation variable did not differ across sexes, Wald χ2 (4)= 1.192, p = .88. Standardized factor loading estimates were similar in size: 0.62, 0.75, and 0.63 for ln(IL-6), ln(CRP), and Fibrinogen/100, respectively.
The full SEM mediation model was then fit testing the direct and indirect effects of multimorbidity (2+ chronic conditions), inflammation, and functional limitations (Figure 2). Model fit was acceptable: χ2(27) = 118.5, p < .000; CFI = 0.961; TLI = 0.928; RMSEA = 0.05 (95% CI = [0.04, 0.06]). Unstandardized and standardized model coefficients for direct effects are shown in Table 2. Older age, being female, being non-White, and smoking were all associated with higher levels of inflammation, whereas regular physical activity was associated with lower levels. Having two or more chronic conditions relative to fewer than two conditions increased the inflammation factor by 0.22 units (p < .001); this is equivalent to 0.23 standard deviation units. Older age, being female, being non-White, smoking, and having less than a high school education relative to a college education were associated with higher IADL scores whereas regular physical activity was linked to lower scores. Fewer covariates were related to higher BADLs; these included being female, having lower educational attainment, and not engaging in regular physical activity. Compared with having none or one chronic condition, multimorbidity was associated with greater IADLs (βstd = .32; p < .001) and BADLs (βstd = .25; p < .001). The latent inflammation factor was significantly associated with both BADLs and IADLs (βstd = .12; p < .001 for IADLs and βstd = .13; p < .001 for BADLs).
Table 2.
Mediation Model Direct Effects (N = 1,229).
| Inflammation | IADL | BADL | |
|---|---|---|---|
| Article I. Unstandardized results | |||
| Age | 0.01** | 0.01*** | 0.00 |
| Male | –0.14*** | –0.20*** | –0.09* |
| Black | 0.19*** | 0.09 | 0.05 |
| Some college | 0.03 | –0.12 | –0.11 |
| College | –0.08 | –0.24* | –0.22* |
| Multimorbidities | 0.21*** | 0.52*** | 0.27*** |
| Smoking | 0.05* | 0.06* | 0.02 |
| Exercise | –0.19*** | –0.27*** | –0.17*** |
| Inflammation | 0.24*** | 0.16*** | |
| Article II. Standardized results | |||
| Age | 0.11** | 0.12*** | 0.03 |
| Male | –0.15*** | –0.11*** | –0.07** |
| Black | 0.16*** | 0.04 | 0.04 |
| Some college | 0.03 | –0.07 | –0.08 |
| College | –0.08 | –0.14* | –0.17* |
| Multimorbidities | 0.23*** | 0.32*** | 0.25*** |
| Smoking | 0.08* | 0.05* | 0.02 |
| Exercise | –0.17*** | –0.13*** | –0.11*** |
| Inflammation | 0.13*** | 0.12*** | |
Note. IADL = intermediate activities of daily living; BADL = basic activities of daily living.
p < .05.
p < .01.
p < .001.
Unstandardized and standardized model coefficients for indirect effects are shown in Table 3. There were small but statistically significant indirect pathways linking age, sex, race, regular physical activity, and chronic conditions to both BADLs and IADLs by way of the latent inflammation factor. The largest indirect pathway coefficients were for multimorbidity. These indicate a 0.03 standard deviation increase in BADLs and IADLs for a one standard deviation unit increase in inflammation (p < .01). The total (standardized) effect of multimorbidity on functional limitations both directly and through increased inflammation was 0.27 (p < .001) for BADLs and 0.35 (p < .001) for IADLs. These results are not presented in the table.
Table 3.
Mediation Model Indirect Effects (N = 1,229).
| IADL | BADL | |
|---|---|---|
| Article III. Unstandardized results | ||
| Age | 0.00** | 0.00* |
| Male | –0.04** | –0.03** |
| Black | 0.08*** | 0.05** |
| Some college | 0.00 | 0.00 |
| College | –0.04 | –0.02 |
| Multimorbidities | 0.07*** | 0.05** |
| Smoking | 0.01 | 0.01 |
| Exercise | –0.05** | –0.03* |
| Article IV. Standardized results | ||
| Age | 0.02** | 0.02* |
| Male | –0.03** | –0.02** |
| Black | 0.04*** | 0.03** |
| Some college | 0.00 | 0.00 |
| College | –0.02 | –0.02 |
| Multimorbidities | 0.04*** | 0.04** |
Note. IADL = intermediate activities of daily living; BADL = basic activities of daily living.
p < .05.
p < .01.
p < .001.
Supplemental Analyses
We conducted three sets of additional analyses to aid interpretation of the results. First, we examined a mediation model with each inflammatory protein treated as a measured variable rather than indicators of a single latent factor. The path model fit was excellent: χ2(7) = 0; p = 1.0; CFI = 1.00; TLI = 1.02; RMSEA = 0.00 (95% CI = [0.00, 0.00]). The perfect fit of the model indicates possible over-fitting. The results showed that CRP mediated the association of multimorbidity and BADLs. The total indirect effect of all three protein mediators taken together was statistically significant and the magnitude of the total indirect effect was slightly smaller than the original SEM model (data not shown). For IADLs, the individual IL-6 and CRP mediation pathways were statistically significant, and the magnitude of the total indirect effect was slightly smaller than the original SEM model (data not shown).
Second, we tested the possibility that the observed direct and indirect effects associated with multimorbidity might be attributable to particularly adverse single conditions rather than aggregate multimorbidity. To do this, we created 13 new multimorbidity index variables, each omitting one of the conditions, and then re-estimated the SEM model using each of these new variables. A recent article took this approach to assessing the impact of inflammation and changes in inflammation on multimorbidity (Fabbri et al., 2014). The results showed that omitting obesity weakened the association between multimorbidity and inflammation (b = 0.22 for full model and b = 0.13 without obesity). Direct associations between the new multimorbidity variables and both measures of functional limitations were largely no different than those for the full multimorbidity variable (data not shown). However, removing joint pain reduced the direct effect of multimorbidities on IADLs from 0.56 to 0.44. Finally, the coefficients for the indirect pathways linking multimorbidity to functional limitations by way of inflammation from models using these new variables did not differ from the model based on the complete set of conditions (data not shown).
Third, given regional differences between the Milwaukee sample and the rest of the MIDUS sample, we repeated the SEM model with Milwaukee participants excluded. The fit of the overall SEM model was slightly better than for the full sample: χ2(21) = 80.0, p < .000, CFI = 0.966; TLI = 0.934; RMSEA = 0.05 (95% CI = [0.04, 0.07]). With the exception of race, the direct and indirect effects were virtually identical to those from the model including the full sample (data not shown).
Discussion
The current study examined the extent to which three inflammatory proteins—IL-6, CRP, and fibrinogen—mediate the link between multimorbidity, on one hand, and functional limitations, on the other. The results indicated partial mediation, suggesting that higher levels of these markers of inflammation in the context of multimorbidity predict greater functional limitation. All analyses adjusted for a set of covariates—age, sex, race, and educational background—that represent potential confounds, and the observed mediation effects persisted in these fully adjusted models. We also controlled for two health behaviors—smoking and physical activity—that have been linked both to inflammation and functional impairments. Smoking was associated with higher levels of inflammation and higher BADL and IADL scores, and physical activity was associated with lower levels of inflammation and functional limitations; physical activity also partially mediated the association of multimorbidity and both BADL and IADL scores. However, the coefficients for the indirect associations of multimorbidity and the functional limitation measures by way of the inflammation latent factor remained robust to these additional model adjustments. Indeed these coefficients were the strongest indirect effects in the full model. To the best of our knowledge, this specific role for inflammation has not been examined previously. These results suggest that inflammation may be an important pathway linking chronic conditions to functional impairment in middle and later life.
We modeled inflammation as a latent factor indicated by IL-6, CRP, and fibrinogen. The latent factor provided a good model fit in confirmatory factor analyses, and the full SEM model compared well with the path model that treated the same three proteins as measured variables. This is not the first study to use a latent factor approach to modeling inflammation. Two earlier studies modeled a latent factor indicated by IL-6 and CRP in the context of allostatic load and metabolic syndrome, two leading indicators of multi- system dysregulation (Marsland et al., 2010; McCaffery et al., 2012). Collectively, these efforts support the statistical validity of using a latent factor to assess the underlying construct of inflammation. Methodologically, the latent variable approach provides measurement of inflammation independent of the random measurement error in the three observed clinical protein measures, and this precision increases the reliability of the measure as well as the statistical power to detect associations between the latent variable and other variables (Rigdon, 1994). Beyond statistical validity, the latent factor approach is aligned with prevailing ideas about systemic inflammation as a dysregulated biological process that is linked to health outcomes and can be indicated by diverse markers.
The possibility that inflammation might act as a mechanism linking multimorbidity and disability is supported by diverse lines of research. Population-based studies have shown that circulating levels of inflammatory proteins are higher in adults with single chronic medical conditions (Bastard et al., 2006; Bautista et al., 2005; Blake & Ridker, 2003; Hu et al., 2004) and increase with each additional chronic condition in adults with multimorbidity (Fabbri et al., 2014; Friedman & Ryff, 2012). Higher levels of inflammation are also associated with greater disability cross-sectionally (Brinkley et al., 2009; Cesari et al., 2004; Kuo et al., 2006; Taaffe, Harris, Ferrucci, Rowe, & Seeman, 2000) and with greater risk of disability over time (Ferrucci et al., 1999). Finally, muscle strength is thought to be a key component of age-related or disease-related disability (Cesari, Landi, Vellas, Bernabei, & Marzetti, 2014), and higher levels of inflammatory proteins are associated with decreased muscle strength in population-based studies (Cesari et al., 2004; Ferrucci et al., 2002; Peake et al., 2010). In addition, laboratory studies have shown that inflammatory proteins reduce the production of muscle cells and increase muscle catabolism (Michaud et al., 2013), suggesting that even low-grade inflammation may act directly on muscle tissue and muscle-related genes to impair function (Michaud et al., 2013; Peake et al., 2010). Collectively, these lines of research suggest that the increased levels of inflammation associated with multimorbidity may put older adults at risk of disability in part because of reduced muscle strength.
Supplemental analyses tested the possibility that single conditions accounted for the associations observed for aggregate multimorbidity. Specifically, we re-estimated the SEM model using 13 different multimorbidity indices, each version of the index omitting a different chronic condition. The results showed that the omissions of hypertension and obesity reduced the magnitude of direct associations between the multimorbidity variable and inflammation specifically. However, the direct associations between multi-morbidity and the measures of functional limitations and the indirect associations with functional limitations by way of inflammation were virtually identical whether the multimorbidity measure included all 13 conditions or omitted any of them. This is similar to the approach taken by Fabbri et al. (2014) in a recent paper linking changes in inflammation to longitudinal multimorbidity in the InCHIANTI study, and the results support the conclusion that the mediating role for inflammation applies to multimorbidity generally.
These results help to illuminate the conceptual relationship between multimorbidity and functional limitations. In many instances, the severity of any given chronic medical condition is defined in part by its ability to cause functional impairment (Charlson et al., 1987). In this study, we take the perspective that functional impairment is not an inevitable result of chronic conditions or multimorbidity. This perspective is consistent with the disablement process model, which allows for the possibility that biological, psychological, and social factors can affect the course of disablement in the context of disease (Verbrugge & Jette, 1994). Here, we find that variability in inflammation contributes to the extent to which chronic conditions are disabling. In other words, conditions that are considered severe because of their potential to cause functional impairments may be rendered less severe in people with relatively low circulating levels of inflammatory proteins. Many older adults with multiple conditions report good quality of life. Lower levels of inflammation may be one mechanism that facilitates good quality of life in the context of chronic conditions. Future research needs to identify factors, especially social or psychological factors, that may act as moderators, weakening the link between multimorbidity and functioning limitations by reducing inflammation. Individuals with multimorbidity who are also possessed of such social or psychological resources may be able to stave off functional impairment, and may in some ways be considered resilient in the face of disease, especially multimorbid disease.
Interpretations of the current results are tempered by some notable limitations. Above all, these data are cross-sectional, meaning that causal associations are not clear. While we hypothesize, based on existing literature, that chronic illness leads to functional decline, it may be that functional limitations increase risk of chronic illness. For example, functional limitations can lead to reduced physical activity (Hardy et al., 2010), and reduced activity increases the risk of multiple chronic conditions (Pate et al., 1995) and overall mortality in older adults (Buchman, Yu, Boyle, Shah, & Bennett, 2012). While this is a valid limitation, the aim of the present study was to test a mediating role for inflammation, and the results support such a role no matter which way the causal arrows point. Longitudinal data will be critical for illuminating the causal relationships between multimorbidity and functional limitations. Another limitation is the relatively small group of inflammatory proteins used to indicate the latent factor. Although these are the primary inflammatory proteins available in the MIDUS dataset, we regard this less as a limitation than an invitation to further empirical examination. Other blood-borne markers of inflammation (e.g., IL-1, TNF-α, IFN-γ) may also cluster with markers, such as IL-6, CRP, and fibrinogen, supporting the existence of a single latent factor for inflammation. They may, however, form separate clusters that may relate to variables of interest in different ways. Use of cluster analyses would thus put prevailing conceptual ideas of inflammation as a unitary phenomenon to the empirical test.
In spite of these limitations, the current results highlight a biological mechanism, inflammation, that appears to partially explain the link between age-related chronic illness, especially multimorbidity, and declines in functional abilities. The aging of the population and the attendant rise in chronic disease burden makes understanding the mechanisms whereby chronic conditions result in disability in later years a pressing issue. The present results suggest that inflammation may be one such mechanism.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Grant R01-AG041750 (to EMF) from the National Institute on Aging. The MIDUS I study (Midlife in the U.S.) was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. The MIDUS II research was supported by a grant from the National Institute on Aging (P01-AG020166) to conduct a longitudinal follow-up of the MIDUS I investigation. The research was further supported by the following grants: M01-RR023942 (Georgetown), M01-RR00865 (UCLA) from the General Clinical Research Centers Program, and 1UL1RR025011 (UW) from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL. Socioeconomic status and health: The challenge of the gradient. American Psychologist. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Anderson G, Horvath J. The growing burden of chronic disease in America. Public Health Reports. 2004;119:263–270. doi: 10.1016/j.phr.2004.04.005. doi:10.1016/j.phr.2004.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle JL. Full information estimation in the presence of incomplete data. Lawrence Erlbaum; Mahwah, NJ: 1996. [Google Scholar]
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. European Cytokine Network. 2006;17(1):4–12. [PubMed] [Google Scholar]
- Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. Journal of Human Hypertension. 2005;19:149–154. doi: 10.1038/sj.jhh.1001785. doi:10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- Binder D. On the variance of asymptotically normal estimators from complex surveys. International Statistics Review. 1983;51:279–292. [Google Scholar]
- Blake GJ, Ridker PM. C-reactive protein and other inflammatory risk markers in acute coronary syndromes. Journal of the American College of Cardiology. 2003;41(4 Suppl. 1):S37–S42. doi: 10.1016/s0735-1097(02)02953-4. [DOI] [PubMed] [Google Scholar]
- Brinkley TE, Leng X, Miller ME, Kitzman DW, Pahor M, Berry MJ, Nicklas BJ. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences. 2009;64:455–461. doi: 10.1093/gerona/gln038. doi:10.1093/ gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Yu L, Boyle PA, Shah RC, Bennett DA. Total daily physical activity and longevity in old age. Archives of Internal Medicine. 2012;172:444–446. doi: 10.1001/archinternmed.2011.1477. doi:10.1001/archinternmed.2011.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: Two sides of the same coin. Frontiers in Aging Neuroscience. 2014;6:192. doi: 10.3389/fnagi.2014.00192. doi:10.3389/fnagi.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, Ferrucci L. Inflammatory markers and physical performance in older persons: The InCHIANTI study. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Disease. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences. 1997;52(4):M201–M208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Harris TB. Physical activity, exercise, and inflammatory markers in older adults: Findings from the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2004;52:1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. doi:10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases—A systematic review on existing multimorbidity indices. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences. 2011;66:301–311. doi: 10.1093/gerona/glq208. doi:10.1093/gerona/glq208. [DOI] [PubMed] [Google Scholar]
- d'Orsi E, Xavier AJ, Steptoe A, de Oliveira C, Ramos LR, Orrell M, Marmot MG. Socioeconomic and lifestyle factors related to instrumental activity of daily living dynamics: Results from the English longitudinal study of ageing. Journal of the American Geriatrics Society. 2014;62:1630–1639. doi: 10.1111/jgs.12990. doi:10.1111/jgs.12990. [DOI] [PubMed] [Google Scholar]
- DuGoff EH, Canudas-Romo V, Buttorff C, Leff B, Anderson GF. Multiple chronic conditions and life expectancy: A life table analysis. Medical Care. 2014;52:688–694. doi: 10.1097/MLR.0000000000000166. doi:10.1097/mlr.0000000000000166. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annual Review of Medicine. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Fabbri E, An Y, Zoli M, Simonsick EM, Guralnik JM, Bandinelli S, Ferrucci L. Aging and the burden of multimorbidity: Associations with inflammatory and anabolic hormonal biomarkers. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences. 2014;70:63–70. doi: 10.1093/gerona/glu127. doi:10.1093/gerona/glu127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Havlik RJ. Serum IL-6 level and the development of disability in older persons. Journal of the American Geriatrics Society. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, Guralnik JM. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. Journal of the American Geriatrics Society. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- Fortin M, Hudon C, Haggerty J, Akker M, Almirall J. Prevalence estimates of multimorbidity: A comparative study of two sources. BMC Health Services Research. 2010;10 doi: 10.1186/1472-6963-10-111. Article 111. doi:10.1186/1472-6963-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M, Stewart M, Poitras ME, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: Toward a more uniform methodology. Annals of Family Medicine. 2012;10:142–151. doi: 10.1370/afm.1337. doi:10.1370/afm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Ryff CD. Living well with medical comorbidities: A bio-psychosocial perspective. The Journals of Gerontology, Series B: Psychological Sciences & Social Sciences. 2012;67:535–544. doi: 10.1093/geronb/gbr152. doi:10.1093/geronb/gbr152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Cohen S, Matthews KA, Tracy R, Seeman TE. Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Social Science & Medicine. 2009;69:451–459. doi: 10.1016/j.socscimed.2009.05.018. doi:10.1016/j. socscimed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy SE, McGurl DJ, Studenski SA, Degenholtz HB. Biopsychosocial characteristics of community-dwelling older adults with limited ability to walk one-quarter of a mile. Journal of the American Geriatrics Society. 2010;58:539–544. doi: 10.1111/j.1532-5415.2010.02727.x. doi:10.1111/j.1532-5415.2010.02727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howcroft TK, Campisi J, Louis GB, Smith MT, Wise B, Wyss-Coray T, Sierra F. The role of inflammation in age-related disease. Aging. 2013;5:84–93. doi: 10.18632/aging.100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine . Living well with chronic illness: A call for public health action. National Academies Press; Washington, DC: 2012. [Google Scholar]
- Jousilahti P, Salomaa V, Rasi V, Vahtera E, Palosuo T. Association of markers of systemic inflammation, C reactive protein, serum amyloid A, and fibrinogen, with socioeconomic status. Journal of Epidemiology & Community Health. 2003;57:730–733. doi: 10.1136/jech.57.9.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Medical Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Experimental Gerontology. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Kriegsman DM, Penninx BW, van Eijk JT, Boeke AJ, Deeg DJ. Self-reports and general practitioner information on the presence of chronic diseases in community dwelling elderly. A study on the accuracy of patients’ self-reports and on determinants of inaccuracy. Journal of Clinical Epidemiology. 1996;49:1407–1417. doi: 10.1016/s0895-4356(96)00274-0. doi:10.1016/S0895-4356(96)00274-0. [DOI] [PubMed] [Google Scholar]
- Kuo HK, Bean JF, Yen CJ, Leveille SG. Linking C-reactive protein to late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999-2002. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences. 2006;61:380–387. doi: 10.1093/gerona/61.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakoski SG, Cushman M, Criqui M, Rundek T, Blumenthal RS, D'Agostino RB, Jr., Herrington DM. Gender and C-reactive protein: Data from the Multiethnic Study of Atherosclerosis (MESA) cohort. American Heart Journal. 2006;152:593–598. doi: 10.1016/j.ahj.2006.02.015. doi:10.1016/j.ahj.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Landi F, Liperoti R, Russo A, Capoluongo E, Barillaro C, Pahor M, Onder G. Disability, more than multimorbidity, was predictive of mortality among older persons aged 80 years and older. Journal of Clinical Epidemiology. 2010;63:752–759. doi: 10.1016/j.jclinepi.2009.09.007. doi:10.1016/j.jclinepi.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Love GD, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS National study: Protocol, measures, sample, and comparative context. Journal of Aging and Health. 2010;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analyses. Annual Review of Psychology. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, Fratiglioni L. Aging with multimorbidity: A systematic review of the literature. Ageing Research Reviews. 2011;10:430–439. doi: 10.1016/j.arr.2011.03.003. doi:10.1016/j.arr.2011.03. 003. [DOI] [PubMed] [Google Scholar]
- Marengoni A, von Strauss E, Rizzuto D, Winblad B, Fratiglioni L. The impact of chronic multimorbidity and disability on functional decline and survival in elderly persons. A community-based, longitudinal study. Journal of Internal Medicine. 2009;265:288–295. doi: 10.1111/j.1365-2796.2008.02017.x. doi:10.1111/j.1365-2796.2008.02017.x. [DOI] [PubMed] [Google Scholar]
- Marsland AL, McCaffery JM, Muldoon MF, Manuck SB. Systemic inflammation and the metabolic syndrome among middle-aged community volunteers. Metabolism. 2010;59:1801–1808. doi: 10.1016/j.metabol.2010.05.015. doi:10.1016/j.metabol.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffery JM, Marsland AL, Strohacker K, Muldoon MF, Manuck SB. Factor structure underlying components of allostatic load. PLoS ONE. 2012;7(10):e47246. doi: 10.1371/journal.pone.0047246. doi:10.1371/journal.pone.0047246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. Journal of the American Medical Directors Association. 2013;14:877–882. doi: 10.1016/j.jamda.2013.05.009. doi:10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Murtagh KN, Hubert HB. Gender differences in physical disability among an elderly cohort. American Journal of Public Health. 2004;94:1406–1411. doi: 10.2105/ajph.94.8.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugler WE, Karin M. The wolf in sheep’s clothing: The role of inter-leukin-6 in immunity, inflammation and cancer. Trends in Molecular Medicine. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. doi:10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Oehlert GW. A note on the delta method. American Statistician. 1992;46:27–29. [Google Scholar]
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Wilmore JH. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. Journal of the American Medical Association. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- Peake J, Della Gatta P, Cameron-Smith D. Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2010;298:R1485–R1495. doi: 10.1152/ajpregu.00467.2009. doi:10.1152/ajpregu.00467.2009. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, Vinicor F. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for health-care professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Rigdon EE. Demonstrating the effects of unmodeled random measurement error. Structural Equation Modeling. 1994;1:375–380. [Google Scholar]
- Rothrock NE, Hays RD, Spritzer K, Yount SE, Riley W, Cella D. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS). Journal of Clinical Epidemiology. 2010;63:1195–1204. doi: 10.1016/j.jclinepi.2010.04.012. doi:10.1016/j.jclinepi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider KM, O'Donnell BE, Dean D. Prevalence of multiple chronic conditions in the United States’ Medicare population. Health and Quality of Life Outcomes. 2009;7 doi: 10.1186/1477-7525-7-82. Article 82. doi:10.1186/1477-7525-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeni RF, Martin LG, Andreski PM, Freedman VA. Persistent and growing socioeconomic disparities in disability among the elderly: 1982-2002. American Journal of Public Health. 2005;95:2065–2070. doi: 10.2105/AJPH.2004.048744. doi:10.2105/ajph. 2004.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John PD, Tyas SL, Menec V, Tate R. Multimorbidity, disability, and mortality in community-dwelling older adults. Canadian Family Physician/ Medecin de famille canadien. 2014;60(5):e272–e280. [PMC free article] [PubMed] [Google Scholar]
- Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences. 2000;55(12):M709–M715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- Thacker SB, Stroup DF, Carande-Kulis V, Marks JS, Roy K, Gerberding JL. Measuring the public’s health. Public Health Reports. 2006;121:14–22. doi: 10.1177/003335490612100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinetti ME, McAvay GJ, Chang SS, Newman AB, Fitzpatrick AL, Fried TR, Peduzzi PN. Contribution of multiple chronic conditions to universal health outcomes. Journal of the American Geriatrics Society. 2011;59:1686–1691. doi: 10.1111/j.1532-5415.2011.03573.x. doi:10.1111/j.1532-5415.2011.03573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugge LM, Jette AM. The disablement process. Social Science & Medicine. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Ward BW, Schiller JS. Prevalence of multiple chronic conditions among US adults: Estimates from the National Health Interview Survey, 2010. Preventing Chronic Disease. 2013;10 doi: 10.5888/pcd10.120203. Article E65. doi:10.5888/pcd10.120203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Archives of Internal Medicine. 2002;162:2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Bentler SE, Hockenberry J, Jones MP, Obrizan M, Weigel PA, Wallace RB. Long-term declines in ADLs, IADLs, and mobility among older Medicare beneficiaries. BMC Geriatrics. 2011;11 doi: 10.1186/1471-2318-11-43. Article 43. doi:10.1186/1471-2318-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]