Abstract
Objectives
Efforts to improve the clinical outcome for patients with localized high-risk prostate cancer have led to the development of neoadjuvant systemic therapies. We review the different modalities of neoadjuvant therapies for localized prostate cancer and highlight emerging treatment approaches including immunotherapy and targeted therapy.
Methods
We performed a PubMed search of clinical trials evaluating preoperative systemic therapies for treating high-risk prostate cancer published after 2000, and those studies with the highest clinical relevance to current treatment approaches were selected for review. The database at clinicaltrials.gov was queried for neoadjuvant studies in high-risk prostate cancer, and those evaluating novel targeted therapies and immunotherapies are spotlighted here.
Results
Neoadjuvant chemotherapy has become standard of care for treating some malignancies, including breast and bladder cancers. In prostate cancer, preoperative hormonal therapy or chemotherapy has failed to demonstrate improvements in overall survival. Nevertheless, the emergence of novel treatment modalities such as targeted small molecules and immunotherapy has spawned neoadjuvant clinical trials that provide a unique vantage from which to study mechanism of action and biological potency. Tissue-based biomarkers are being developed to elucidate the biological efficacy of these treatments. With targeted therapy, these can include phospho-proteomic signatures of target pathway activation and deactivation. With immunotherapies, including sipuleucel-T and ipilimumab, recruitment of immune cells to the tumor microenvironment can also be used as robust markers of a biological effect. Such studies can provide insight not only into mechanism of action for these therapies but can also provide paths forward to improving clinical efficacy like with rationally designed combinations and dose selection.
Conclusions
The use of neoadjuvant androgen-deprivation therapy and chemotherapy either singly or in combination before radical prostatectomy is generally safe and feasible while reducing prostate volume and tumor burden. However, pathologic complete response rates are low and no long-term survival benefit has been observed with the addition of neoadjuvant therapies over surgery alone at present, and therefore preoperative therapy is not the current standard of care in prostate cancer treatment.
Keywords: Prostate cancer, Neoadjuvant, Preoperative, Targeted therapy, Immunotherapy
Introduction
Neoadjuvant therapy, the administration of systemic treatments before surgical resection or definitive radiation therapy, has demonstrable clinical benefit in several malignancies, including bladder cancer [1]. Conceptually, neoadjuvant therapy may reduce the primary tumor burden as well as treat undetected micrometastases. This would facilitate a more complete surgical resection in the case of radical prostatectomy (RP) and could reduce the treatment area in the case of radiation therapy. Over the past 2 decades, significant effort has gone into determining whether neoadjuvant treatment improves clinical outcomes in prostate cancer.
For radiation therapy, numerous studies have shown improved outcomes with the addition of neoadjuvant, concurrent, and adjuvant androgen-deprivation therapy (ADT) in treating intermediate- and high-risk diseases. RTOG 8610 was the first randomized phase III trial to evaluate neoadjuvant ADT started 2 months before, and then 2 months concurrently with, external beam radio-therapy (EBRT) in men with locally advanced prostate cancer [2]. The ADT plus EBRT arm had statistically significant improvements in 10-year prostate cancer–specific mortality (23% vs. 36%, P = 0.01), occurrence of distant metastasis (35% vs. 47%, P = 0.006), disease-free survival (11% vs. 3%, P < 0.001), biochemical failure (65% vs. 80%, P < 0.0001), and a trend toward improved 10-year overall survival (OS) [43% vs. 34%, P = 0.12] compared with the radiation alone arm. D'Amico et al. [3] compared radiation alone versus radiation plus 6 months of ADT (2 mo each of neoadjuvant, concurrent, and adjuvant ADT) in men with high-grade clinically localized prostate cancer (intermediate and high risk by NCCN risk stratification) and showed statistically significant higher OS (88% vs.78%, P = 0.04), lower prostate cancer–specific mortality (0 events vs. 6 events, P = 0.02), and higher survival free of salvage ADT (82% vs. 57%, P = 0.002) at 5 years, favoring the combination arm. The 10-year results of the European Organization for Research and Treatment of Cancer 22,863 by Bolla et al. [4] supported the addition of long-term ADT to EBRT in treating high-risk prostate cancer. In this randomized phase III trial, EBRT alone was compared with EBRT plus ADT for 3 consecutive years beginning concurrently with radiation. The improvements in 10-year disease-free survival (22.7% vs. 47.7%, P < 0.0001), OS (39.8% vs. 58.1%, P = 0.0004), and prostate cancer–specific mortality (30.4% vs. 10.3%, P < 0.0001) all favored long-term ADT plus EBRT; importantly, no significant difference in cardiovascular mortality was observed between the treatment arms.
In contrast, neoadjuvant therapies (both ADT and chemotherapy) administered before RP have yet to show a definitive clinical benefit. This stems from the numerous trials that have demonstrated a lack of statistically significant improvement in both progression-free survival and OS [5]. Nevertheless, neoadjuvant trials provide a unique opportunity to study treatment effects within the actual tumor microenvironment. In this review, we discuss the more recent experience with neoadjuvant therapies administered before definitive RP in men with localized prostate cancer. We also highlight how neoadjuvant trials can be used to study the mechanism of action for different targeted agents as well as immunotherapies.
Neoadjuvant androgen-deprivation therapy
ADT represents one of the original targeted therapies for cancer (Fig. 1). Two decades of trials testing neoadjuvant ADT alone or combined with chemotherapy have demonstrated that this treatment can induce measureable changes in the local disease burden at the time of surgery (recent, prospective, randomized controlled trials are summarized in Table 1). Though end points differed between studies, they generally included higher rates of organ-confined disease, reduced rates of extracapsular extension, and reduced rates of positive surgical margins. Affected systemic end points included reduced incidence of lymph node involvement, reduced testosterone levels, and PSA responses. Unfortunately, improved local control rates obtained in these trials did not translate into OS benefits; granted, the majority of these studies were underpowered to detect statistically significant differences in biochemical relapse-free survival.
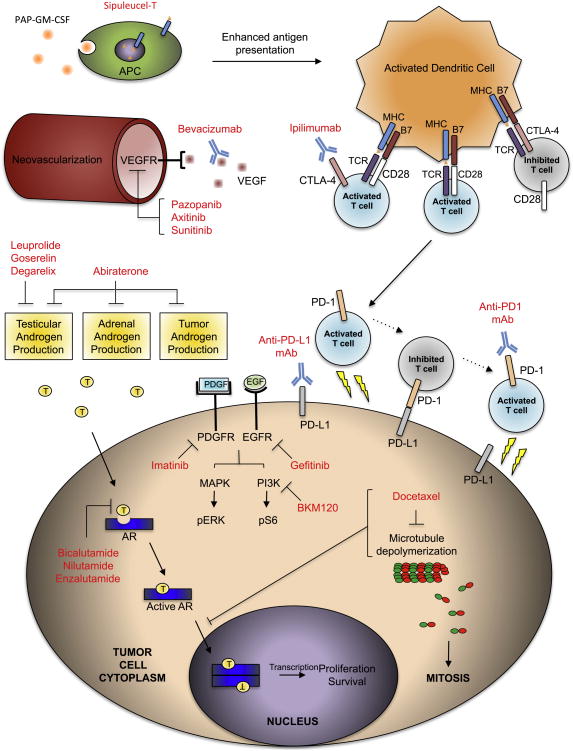
Fig. 1.
An overview of mechanisms of action. Overall, 5 categories of treatments are illustrated here including androgen-deprivation therapy (ADT), chemotherapy, immunotherapy, antiangiogenic approaches, and small molecule inhibitors of signaling pathways. ADT treatments such as luteinizing hormone–releasing hormone agonists (leuprolide and goserelin) and gonadotropin-releasing hormone (GnRH) receptor antagonists (degarelix) reduce testicular androgen production. Androgen receptor (AR) antagonists (bicalutamide, nilutamide, and enzalutamide) inhibit the binding of testosterone (T) to AR. Abiraterone, a P450c17 inhibitor, blocks testicular, adrenal, and tumor androgen production. Docetaxel stabilizes microtubules to interfere with cell division and also antagonizes AR nuclear translocation. Sipuleucel-T is composed of antigen-presenting cells (APC) primed with the prostatic acid phosphatase granulocyte-macrophage colony-stimulating factor (PAP-GM-CSF) fusion protein that results in enhanced antigen presentation to activated dendritic cells. The activated dendritic cells then activate T cells through paired interactions of the following costimulatory molecules: antigen-major histocompatibility complex (MHC) to T-cell receptor (TCR), and B7 to CD28 in an IL-2 dependent fashion. T cells are inactivated by B7 binding to cytotoxic T-lymphocyte–associated receptor 4 (CTLA-4) on dendritic cells, or to programmed cell death ligand (PD-L1) on tumor cells. Antibodies targeting CTLA-4 (ipilimumab) activate inhibited T cells. Similarly, antibodies targeting PD-L1 and PD-1 (programmed death receptor) activate T cells inactivated by tumor. Bevacizumab neutralizes vascular endothelial growth factor (VEGF), whereas pazopanib, axitinib, and sunitinib inhibit several isoforms of VEGF receptor to reduce neovascularization. Small molecule tyrosine kinase inhibitors including imatinib and gefitinib have been ineffective in trials to date. The PI3K (phosphoinositide 3-kinase) inhibitor, BKM120, is currently being evaluated in clinical trials.
Table 1. Randomized clinical trials of neoadjuvant androgen-deprivation therapy.
| References | n | Clinical T stage | Neoadjuvant treatment | Positive margin rate (%) | Biochemical PFS (%) | OS (%) | Median follow-up (mo) |
|---|---|---|---|---|---|---|---|
| Schulman et al. [6] | 402 | T2–T3 | C vs. G + F | 47.5 vs. 26.2, P < 0.05 | 67 vs. 74, P = 0.18 | 95 vs. 93, P = 0.64 | 48 |
| Aus et al. [49] | 126 | T1b–T3a | C vs. T + Cy | 45.5 vs. 23.6, P = 0.016 | 51.5 vs. 49.8, P = 0.588 | No difference, P = 0.513 | 82 |
| Klotz et al. [16] | 213 | T1b–T2c | C vs. Cy | 65 vs. 28, P = 0.001 | 68.2 vs. 60.2, P = 0.73 | 93.9 vs. 88.4, P = 0.38 | 72 |
| Soloway et al. [17] | 282 | T2b | C vs. L + F | 48 vs. 18, P < 0.001 | 67.6 vs. 64.8, P = 0.663 | 60 | |
| Yee et al. [50] | 148 | T1b–T3 | C vs. G + F | 38 vs. 19, P = 0.022 | 78 vs. 80, P = 0.07 | 96 | |
| Prezioso et al. [51] | 167 | T1a–T2b | C vs. L + Cy | 60 vs. 39, P = 0.01 | |||
| Selli et al. [52] | 393 | T2a–b | C vs. G + B 12 wk and 24 wk | 46.5 vs. 25.9 and 18.7, P = 0.003 and <0.001 | |||
| T3a–b | C vs G + B 12 wk and 24 wk | 75.9 vs. 34.3 and 35.5, P = 0.002 and 0.004 | |||||
| Gravina et al. [53] | 119 | T2–T3a | C vs. B | 34.5 vs. 13.1, P = 0.011 | |||
| Gleave et al. [8] | 547 | T1b–T2 | L + F 3 mo vs. 8 mo | 23 vs. 12, P = 0.0106 |
N = number of patients, PFS = progression-free survival, OS = overall survival, C = control, G = goserelin, F = flutamide, T = triptorelin, Cy = cyproterone, L = leuprolide, B = bicalutamide.
Schulman and colleagues were among the first to report on both progression-free survival (PFS) and OS in a large, randomized trial of neoadjuvant ADT [6]. This study randomized 402 men with clinical T2 or T3 localized prostate cancer to receive either 3 months of total androgen deprivation with neoadjuvant goserelin plus flutamide before RP or RP alone. The rates of pathologic downstaging in the neoadjuvant ADT group compared with the prostatectomy alone group were 15% and 7% (P < 0.01), and the positive surgical margin rates were 47.5% and 26.3% (P < 0.05), respectively. After 4 years of follow-up, there were no significant differences in biochemical progression-free survival (67% vs. 76%, P = 0.18) or OS (95% vs. 94%, P = 0.64) between the 2 treatment groups.
Kumar et al. [7] performed a systematic review and meta-analysis of neoadjuvant hormone therapy in localized or locally advanced prostate cancer. Overall, 10 randomized clinical trials testing the role of hormonal therapy given before prostatectomy between 1966 and 2006 were included. Only 3 of the trials provided OS data, and 5 provided biochemical progression-free survival data. The authors showed that neoadjuvant hormonal therapy before prostatectomy did not improve OS (OR = 1.11; 95% CI: 0.67–1.85; P = 0.69) despite significant reductions in the positive surgical margin rates (OR = 0.34; 95% CI: 0.27–0.42; P < 0.00001) and significant improvements in other clinical outcome measures including lymph node involvement, pathologic staging, and organ-confined rates. Neoadjuvant treatment resulted in a borderline significant reduction in disease recurrence rates (OR = 0.74; 95% CI: 0.55–1.0; P = 0.05). The authors concluded that neoadjuvant hormone therapy given before prostatectomy is associated with significant clinical benefits in the form of improved local control but does not result in improved OS. As a result, neoadjuvant ADT before prostatectomy is not considered the standard of care.
One possible reason for the lack of survival benefit was the short 3-month duration of treatment used in most of the studies. We now know from the neoadjuvant-EBRT experience in high-risk prostate cancer that longer duration (3 y) of androgen suppression is associated with improved outcomes [4]. In the study by Gleave, 8 months of preoperative ADT was compared with 3 months (Table 1) and showed a significant reduction in the rate of positive surgical margins from 23% to 12% (P = 0.0106) with longer duration of ADT; mean serum PSA decreased by 98% after 3 months, with a further 57% decrease from 3 to 8 months. Although this suggested that 3 months of ADT was insufficient to achieve optimal local control, survival data were not reported, so conclusions regarding the ideal length of preoperative ADT could not be determined from this study [8].
Most of these studies, however, were performed before formal guidelines for risk stratification, so these patient populations were very heterogeneous and may have diluted the effects of treatment. For example, the data presented by Gleave, discussed previously, trended toward more dramatic responses (PSA decline and negative margin rate) in men with intermediate-risk disease by current standards and highlights the importance of stratifying by risk. It is possible that the most commonly reported end points of positive surgical margins, extracapsular extension, lymph node involvement, and seminal vesicle invasion were good markers of local control but poor indicators for predicting long-term clinical outcomes. Given the neoadjuvant chemo-therapy experience in breast and bladder cancers, the pCR rate will probably be a better indicator of OS. Lastly, the identification of tumor within androgen-ablated prostates can be difficult, resulting in erroneously low rates of surgical margin involvement when in fact the specimen is margin positive.
The reasons for the failure of neoadjuvant ADT to improve survival are unclear and complex. However, the biology of castration-resistant prostate cancer (CRPC), defined as progressive disease despite castrate levels of testosterone (less than 50 ng/dl), has taught us that despite maximum suppression of androgen synthesis and activity using LHRH agonists/antagonists and antiandrogens, residual levels of testosterone are sufficient to drive continued growth of prostate cancer left behind with surgery. It is possible that the degree of androgen suppression achieved with these medications was not profound enough to ultimately affect survival rates. Abiraterone is a novel first-in-class inhibitor of 17α-hydroxylase/C17, 20-lyase (CYP17), a critical enzyme in testicular, adrenal, and tumor androgen biosynthesis (Fig. 1). By suppressing androgen synthesis beyond what is achievable using LHRH agonists and antagonists, abiraterone was shown to increase PFS and OS in men with metastatic CRPC in both the pre– and post– docetaxel administration patient populations [9,10]. A neoadjuvant trial combining abiraterone with leuprolide has since been performed [11]. Overall, 58 men with high-risk prostate cancer (cT3–T4, Gleason score greater than or equal to 7, PSA greater than or equal to 20 ng/ml, or PSA velocity greater than 2 ng/ml/y) were randomized to receive treatment with abiraterone plus prednisone in combination with leuprolide or leuprolide alone for the first 12 weeks. All patients then subsequently received an additional 12 weeks of combined abiraterone plus prednisone with leuprolide before prostatectomy for 24 weeks of treatment. The primary end point of the study was a comparison of intraprostatic testosterone (and DHT) levels in interim prostate biopsies obtained at 12 weeks. The secondary end points of PSA, pathologic complete response (pCR), and near pCR (less than or equal to 5 mm of residual tumor) were assessed on the surgical specimen. Patients receiving the 24 weeks of abiraterone treatment had a trend toward improved combined pCR/near pCR rates (34% vs. 15%, P = 0.0894) compared with those treated with abiraterone for only the final 12 weeks. These results suggest that abiraterone may enhance the potency of traditional androgen deprivation in early prostate cancer and provide a rationale to further study this combination in that disease setting.
Neoadjuvant chemotherapy
Neoadjuvant chemotherapy represents the standard of care for a number of malignancies including bladder and breast cancers, where neoadjuvant treatment can sometimes achieve pCR at surgery as well as significant gains in PFS and OS [1,12]. Docetaxel in combination with prednisone has been the standard of care in the treatment of metastatic CRPC (mCRPC) for the past 10 years (Fig. 1) [13]. Given the efficacy of docetaxel in the metastatic setting, several studies assessed the utility of docetaxel with and without hormonal therapy in the neoadjuvant setting (summarized in Table 2). The reasoning behind this approach stemmed from the experience in neoadjuvant ADT in prostate cancer where pCRs were rarely observed, suggesting that castrate-resistant clones were present at early stages of disease, warranting cytotoxic approaches up front.
Table 2. Clinical trials of neoadjuvant chemotherapy alone and in combination with androgen-deprivation therapy.
| References | n | Clinical T stage | Neoadjuvant treatment | Positive margin rate (%) | pCR rate (%) | Biochemical PFS (%) | Median follow-up (mo) |
|---|---|---|---|---|---|---|---|
| Dreicer et al. [14] | 29 | T2b–T3 | D | 4 | 0 | 71 | 23 |
| Febbo et al. [54] | 19 | T3 | D | 0 | |||
| Friedman et al. [55] | 15 | T2–T4 | D+C | 54.5 | 0 | ||
| Garzotto et al. [15] | 57 | T2c– T3a | D+M | 33 | 0 | 65.5 at 2 y 49.8 at 5 y |
63 |
| Hussain et al. [56] | 21 | T2b–T3 | D+E | 30 | 0 | 71 | 15.1 |
| Prayer-Galetti et al. [57] | 22 | T3- | T then D + E | 26 | 5 | 42 | 53 |
| Chi et al. [18] | 72 | T1c–T3 | D + Bu + N | 27 | 3 | 70 | 42.7 |
| Mellado et al. [58] | 57 | T1c–T2 | D+G+F | 35.3 | 6 | 64.7 | 35 |
| Womble et al. [59] | 22 | T3- | D+K | 42 | 0 | 36 | 18 |
| Narita et al. [60] | 18 | T3- | L + B then D + E | 0 | 11 | 77.8 | 18 |
N = number of patients, pCR = pathologic complete response, PFS = progression-free survival, D = docetaxel, C=capecitabine, M = mitoxantrone, E = estramustine, T = triptorelin, Bu = buserelin, N = nilutamide, G = goserelin, F = flutamide, K = ketoconazole, B = bicalutamide, L = leuprolide.
A phase II trial (Dreicer et al.) evaluated 29 men with locally advanced prostate cancer (cT2b, PSA greater than 15 ng/ml, and Gleason score 8–10) treated with weekly docetaxel for 6 weeks before RP [14]. The primary end point was feasibility of RP after 6 weeks of neoadjuvant chemotherapy, and secondary end points included changes in serum PSA levels, histologic effects (local response and pathologic outcomes), and time to biochemical failure. No unexpected toxicities, surgical delays, or intraoperative complications occurred. Of the 28 men who had an RP, only 3 (11%) had organ-confined disease whereas 25 men (89%) had extracapsular extension. There were no cases of pCR although 26 men (93%) had an undetectable serum PSA postoperatively (and none preoperatively). There was a statistically significant difference in prechemotherapy vs. postchemotherapy mean PSA levels (12 vs. 8.42 ng/ml, P < 0.03) favoring treatment; serum PSA level declines greater than 50% were observed in 24% of docetaxel-treated men. At a mean follow-up of 36 months, 20 patients (71%) were biochemically disease free.
Febbo and colleagues evaluated a longer period of preoperative docetaxel and enrolled 19 patients with high-risk prostate cancer (cT1c–T3, PSA greater than 20 ng/ml, and Gleason score 8–10) to a 6-month course of weekly docetaxel before RP. In addition to measuring pathologic response, frozen sections of the surgical prostate specimens were harvested for microarray expression analysis to measure gene expression as a window to discover possible mechanisms of chemotherapy resistance. A total of 16 men completed chemotherapy and went on to RP; there were no pathologic complete responses reported. Serum PSA declines of greater than 50% were observed in 11 of 19 patients (58%), and reductions in prostate volume of at least 25% were observed in 13 of 19 patients (68%). Applying gene set enrichment analysis to the microarray data, the authors determined that a set of genes involved in androgen and estrogen metabolism was up-regulated in docetaxel-treated specimens; specifically, enzymes that decreased levels of active androgen were enriched whereas enzymes that increased levels of active androgen were diminished. The authors postulated that chemotherapy alters the underlying biology of prostate cancer cells such that androgen utilization is reduced, cells divide less frequently and assume a more stem cell like phenotype, and become less sensitive to the antimitotic effects of docetaxel. The pathologic outcomes in this study agreed with findings from the previous study of Dreicer et al. [14] and foreshadowed the impending failure of future studies of neoadjuvant chemotherapy to demonstrate improvement in pCR rates or survival benefit (summarized in Table 2), although no completed phase III studies have been reported.
Garzotto et al. [15] evaluated neoadjuvant combination chemotherapy in a phase I/II study in which 57 men with high-risk prostate cancer (cT2c–T3, Gleason Score greater than 7, and PSA greater than or equal to 15 ng/ml) were treated with weekly docetaxel plus escalating mitoxantrone on a 3 of 4 week cycle for 4 cycles before RP. The authors reasoned that mitoxantrone and docetaxel have different mechanisms of antitumor activity and cited evidence for incomplete cross-resistance between mitoxantrone and docetaxel as a potential source of synergy when administered in combination. The primary end point was to determine the 5-year recurrence-free survival, where recurrence was defined as a confirmed PSA of greater than 0.4 ng/ml. Negative surgical margins were attained in 67% of cases, but no pCRs were reported. Postoperative PSA was less than 0.2 ng/ml in 80% (95% CI: 66%–89%) of patients. The recurrence-free survival at 2 years was 65.5% (95% CI: 53%–78%) and at 5 years was 49.8% (95% CI: 35.5%–64.1%); historical controls from the neoadjuvant ADT experience for 5-year recurrence-free survival following RP alone were approximately 68% (Soloway, Table 1), although these patients generally had lower-risk disease [16,17]. A tissue microarray was constructed from each prostatectomy specimen to facilitate biomarker studies by immunohistochemistry (IHC). The tissue expressions of Ki-67, CD10, and p16 were not predictive of clinical outcomes, whereas tissue VEGF expression (but not plasma VEGF concentrations) was an independent predictor of early recurrence (HR = 3.5, 95% CI: 1.2–10.4).
Chi et al. [18] postulated that earlier neoadjuvant ADT trials failed to prolong survival owing to several factors, including inefficient eradication of micrometastatic disease and the emergence of androgen-independent subpopulations of cancer cells following ADT. Therefore, they rationalized that the addition of docetaxel to ADT in the neoadjuvant setting would address these 2 issues and improve on earlier trials that used neoadjuvant ADT alone. In a phase II multicenter study, 72 men with high-risk prostate cancer (primary criteria of greater than 2 cores showing cT3, Gleason score greater than 7, and PSA greater than 20 ng/ml) were treated with buserelin acetate subcutaneously every 8 weeks for 3 cycles (with nilutamide daily for the first 4 wk) in combination with docetaxel weekly for 6 of 8 weeks for 3 cycles before RP. Two (3%) of 64 patients completing the trial had pCRs, and 16 patients (25%) had less than 5% tumor in their surgical specimen. A total of 17 (27%) patients had positive surgical margins. At a median follow-up of 42.7 months, 19 men (30%) had PSA recurrence; the median PSA recurrence-free survival has not been reached but was estimated at 65.1 months.
A randomized phase III clinical trial sponsored by the Alliance (CALGB 90203) is currently underway evaluating the effect of neoadjuvant docetaxel and ADT before RP in men with localized, high-risk prostate cancer. This study plans to enroll 750 men (cT1–T3, Gleason score 8–10, and PSA less than 100 ng/ml) for randomization into 2 treatment arms: (1) ADT with goserelin subcutaneously every 4 weeks, or leuprolide intramuscularly every 12 weeks for 18 to 24 weeks combined with docetaxel once every 3 weeks for up to 6 cycles (18 wk) before RP plus staging pelvic lymphadenectomy or (2) immediate RP plus staging pelvic lymphadenectomy within 60 days of randomization. The primary end point will be a comparison of the 3-year biochemical progression-free survival (bPFS) between the 2 arms.
Firm conclusions regarding the long-term clinical outcomes of neoadjuvant chemotherapeutic approaches cannot be made from these studies as they are all nonrandomized phase I and II trials without control arms. Instead, they focused on demonstrating feasibility and safety, while measuring immediate-term intratumoral changes and serum markers. Notably, the rates of pCR were uniformly low, suggesting that there likely existed chemotherapy-resistant clones early in the course of disease; alternatively, it is unclear how extensive the pathology reviews were in determining pCR status, or if they were certified laboratories, so underreporting of pCR rates is possible. The key to successful neoadjuvant therapy likely lies in improved treatments able to eradicate these resistant cell populations and tested in randomized phase III trials powered to detect survival differences.
Other targeted therapies
Beyond the AR, other targeted therapies are being developed to enhance tumor-specific cell killing while minimizing off-target systemic toxicities. Inhibitors of several potential targets (Fig. 1), including platelet-derived growth factor receptor (PDGFR), epidermal growth factor receptor (EGFR), and vascular endothelial growth factor receptor, showed encouraging antitumor activity in preclinical and animal studies but did not translate into improved pathologic or clinical outcomes in early-phase clinical trials. These studies are summarized in Table 3. Although most of these neoadjuvant studies are exploratory and not designed to evaluate clinical outcomes such as PFS and OS, they hold great potential to inform us about treatment activity and also to detect early biological activity of novel agents in a disease with an otherwise lengthy natural history.
Table 3. Published clinical trials evaluating neoadjuvant targeted agents.
| References | n | Clinical T stage | Neoadjuvant treatment | Target | Positive margin rate (%) | pCR rate (%) | PR rate (%) | bPFS rate (%) | Median follow-up (mo) |
|---|---|---|---|---|---|---|---|---|---|
| Ross et al. [36] | 41 | T2–T3 | D+B | VEGF | 32.0 | 0 | |||
| Vuky et al. [30] | 31 | T2b–T3 | D+G | EGFR | 33 | 0 | 94 | ||
| Febbo et al. [61] | 11 | T1c–T2c | I | PDGFR | 0 | ||||
| Mathew et al. [24] | 36 | T2 | L+D+I | PDGFR | 18 | 0 | 53 | 39 | |
| Vuky et al. [62] | 6 | D + GVAX | Immune | 0 | 40 | 36 |
N = number of patients, pCR = pathologic complete response, PR = partial response, bPFS = biochemical progression-free survival, D = docetaxel, B = bevacizumab, G = gefitinib, I = imatinib, L = leuprolide.
One of the earliest receptor tyrosine kinases to be targeted in prostate cancer was PDGFR after its expression was detected in both primary tumor and metastatic bone deposits [19–21]. One sequence of studies by Mathew et al. [22,23] evaluating the combination of docetaxel with imatinib in metastatic prostate cancer with bone metastases was initially promising in the phase I study but subsequently poorly tolerated and ineffective in the phase II study. Nevertheless, the authors then conducted a neoadjuvant trial in 39 men combining imatinib with docetaxel and ADT, postulating that earlier exposure to imatinib may prevent bone metastases and result in increased PFS [24]. There were no pCR, and 47% of the treated patients experienced treatment failure; the remaining patients had a 2-year progression-free survival rate of 57% (95% CI: 43%–77%) after a median follow-up period of 39 months; the authors concluded that the results were not better than historical controls. Notably, the authors performed a quantitative assessment of the association between PDGFR inhibition and PFS by comparing the expression levels of phosphorylated PDGFR (p-PDGFR) in peripheral blood leukocytes (at baseline and immediately before surgery). The probability of p-PDGFR decrease was 0.49, but this was not significantly associated with PFS. Although the small sample size was a limitation, it would have been interesting to assess the p-PDGFR status in the resected prostate tissue as a potential predictor of clinical outcome as this would be a direct measure of drug activity on the target tissue of interest.
EGFR has also been identified as a potential therapeutic target in prostate cancer; the association between the onset of androgen independence and up-regulation of EGFR expression has been well described [25–27]. Preclinical studies combining docetaxel with the EGFR inhibitor gefitinib were also encouraging [28,29]. Vuky et al. [30] performed a phase II study to evaluate the combination of docetaxel with gefitinib, a small molecule inhibitor of EGFR, in men with high-risk prostate cancer (cT2b–T3, PSA greater than 20 ng/ml, and Gleason score 8–10). Of the 30 men who completed treatment and RP, none achieved a pCR. The positive surgical margin rate was 33%, similar to the rates reported in previous studies evaluating neoadjuvant ADT or docetaxel or both. In correlative studies, the authors used a semiquantitative IHC-based method to score pretreatment biopsy and posttreatment RP specimens for degree of HER2/neu and EGFR expression. Most prostate tumor samples (both before and after treatment) showed negative staining for HER2/neu, whereas 62% of pretreatment samples showed positive staining for EGFR compared with 70% of posttreatment samples. There was no correlation between expression levels of EGFR, HER2/neu, and response to gefitinib treatment. Gefitinib is now known to be more active against tumors harboring (non-T790M) activating mutations in EGFR. Therefore, it may have been informative to correlate the frequency of EGFR mutations (albeit a rare event in prostate cancer), instead of EGFR expression level, to PFS, although the link between EGFR mutations and response to EGFR inhibitors was likely not known at the time this study was designed [31–33]. The addition of gefitinib to conventional chemotherapy did not improve pathologic outcomes in the neoadjuvant setting.
Vascular endothelial growth factor is a key mediator of both physiologic and pathologic angiogeneses [34,35]. Ross et al. [36] evaluated neoadjuvant docetaxel plus bevacizumab in patients with high-risk localized prostate cancer, hypothesizing that interfering with angiogenesis in the micrometastatic setting (tumor deposits 1–2 mm3) would limit future outgrowth of these deposits. Of the 41 men who were treated, 37 underwent RP, but none of them had a pCR, and the positive margin rate was 32%. Twelve of 41 patients (29%; 95% CI: 16%–41%) achieved a greater than 50% reduction in tumor volume assessed by endorectal magnetic resonance imaging (eMRI). PSA decline was seen in 76% of the patients, and PSA decline greater than 50% was seen in 22% (95% CI: 11%–38%) of treated men; these results were no better than prior studies of neoadjuvant docetaxel alone. Notably, tumor volume reduction detected by eMRI did not directly correlate with PSA decline, calling into question the utility of PSA decline in the neoadjuvant setting. Conversely, detection of tumor downstaging by eMRI has not yet been validated as an intermediate end point that correlates with PFS or OS, so determining clinically relevant end points will be a major challenge facing investigators performing these neoadjuvant studies. Correlative studies gauging the biological effect of bevacizumab were not performed. Despite these findings, interest in vascular endothelial growth factor receptor inhibition remains high as evidenced by several ongoing clinical trials evaluating sunitinib, pazopanib, and axitinib in the neoadjuvant setting (Table 4). Some of these studies incorporate correlative outcome measures to determine the intraprostatic biochemical changes that occur because of the experimental treatments. For example, one study (NCT01385059) evaluating neoadjuvant axitinib will determine if there is an association between biochemical recurrence and the expression of the following markers in tumor tissue: lysyl oxidase, and phosphorylated signal transducer and activator of transcription 3. Additionally, recruitment of myeloid-derived suppressor cell to tumor tissues will also be correlated with biochemical recurrence.
Table 4. Current clinical trials evaluating neoadjuvant targeted agents.
| Identifier | P.I. sponsor | Target | Neoadjuvant treatment | outcome measures |
|---|---|---|---|---|
| NCT01695473 | Febbo UCSF/Novartis | PI3K | BKM-120 | Quantification of phospho-S6, phospho-AKT, phospho-4EBP1, and PSA response |
| NCT00526591 | Garcia case Comprehensive Cancer Center | mToR | Everolimus | Rates of pCR, extracapsular extension; margin status, toxicity, PSADT, and IHC for intraprostatic biomarkers |
| NCT00138918 | Chi University of British Columbia/Department of Defense | Clusterin | oGX-011 | Rate of pCR, quantify intraprostatic expression of clusterin, oGX-011; associate oGX-011 with PBMNC and serum clusterin |
| NCT00589472 | Slovin NCI | HDAC | Vorinostat | Rate of pCR, before and after levels of testosterone, DHT, DHEA, and DHEA-S in prostate tissue; gene and expression analysis of AR genes, PSA, and TMPRSS2 |
| NCT01832259 | Agarwal University of Utah | VEGFR | Pazopanib | Decrease premetastatic niche (LN disease), PFS, and toxicity |
| NCT01409200 | Zurita MD Anderson/Pfizer | VEGFR | Axitinib | PFS |
| NCT01385059 | Pal City of Hope | VEGFR | Axitinib | Premetastatic niche density: quantitate VEGFR1 clusters, pSTAT3, MDSC, LoX, angiogenic factors, and bPFS |
| NCT00329043 | Zurita MD Anderson/Pfizer | VEGFR | Sunitinib | Rate of pCR |
| NCT00321646 | Taplin lBIDMC, Duke, Genentech, Sanofi | VEGF | Bevacicumab + docetaxel | Efficacy, safety |
| NCT00715104 | Fong Dendreon/UCSF | Dendritic cells | Sip-T | Intraprostatic T-cell infiltration |
| NCT00305669 | Fong UCSF/NCI | Immune | GM-CSF | Intraprostatic T-cell and dendritic cell infiltration |
| NCT01194271 | Sharma MD Anderson | Immune | Ipilimumab + ADT | Tumor and blood effector to regulatory T-cell ratio, CD4+ICoS+ T cells, CD8+ICoS+ T cells, NY-ESo-1 antibodies (only in blood), and absolute lymphocyte count (only in blood) |
| NCT00400517 | Dreicer, Klein Cleveland Clinic | Immune | GM-CSF + Thalidomide | Rates of pCR, negative surgical margins, PSA response, and PFS |
| NCT01696877 | Antonarakis Sidney Kimmel Comprehensive Cancer Center | Immune | GVAX | Intraprostatic CD8+ T-cell infiltration, intraprostatic CD4+ T-cell and Treg infiltration, quantification of tissue androgens and markers of apoptosis, serum antibodies to prostatic antigens, rate of pCR and PSA response, and PFS |
| NCT01804712 | Howell Genentech | CD-20 | Rituximab | Histologic response rate, change in: PSA, peripheral blood B cell count, and serum CXCL13 level |
The phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamyin pathway is a key signaling pathway that has been linked to tumorigenesis, aggressive clinical behavior, and drug resistance in many tumor types including prostate cancer [37]. Febbo et al. at the University of California, San Francisco, are currently evaluating the PI3K inhibitor, BKM120, in a phase II neoadjuvant setting for high-risk prostate cancer (NCT01695473). The primary end point is to determine the proportion of men with downstream target inhibition of PI3K in prostate tumor tissue as measured by phospho-S6 levels using IHC; secondary outcome measures include IHC quantitation of additional key downstream effectors of PI3K signaling including phospho-4EBP1 and phospho-AKT [38–40]. Markers of cell proliferation, apoptosis, and autophagy including Ki67, p27, cleaved caspase-3, and lipidated LC3 will also be assessed by IHC.
Immunotherapies
The FDA approval of sipuleucel-T as a treatment for prostate cancer established immunotherapy as another treatment modality for prostate cancer. Although sipuleucel-T treatment leads to improvements in OS, PFS is not affected by this treatment. The actual mechanism of action is not well understood [41]. Nevertheless, this immunotherapy presumably induces T-cell immune responses to the prostate by coculturing patient-derived cells with an antigen comprised of prostate acid phosphatase fused to granulocytemonocyte colony-stimulating factor. This treatment can induce circulating antibody and T-cell responses to this antigen (Fig. 1) [42]. We have recently completed a neoadjuvant trial administering sipuleucel-T to patients with localized prostate cancer to assess for immune effects in the prostate microenvironment (Neo-ACT, NCT00715104). We found that sipuleucel-T treatment induced the recruitment of activated effector CD3+ T cells into the tumor rim [43]. These results support the proposed mechanism of action for sipuleucel-T where T-cell responses induced by the treatment can manifest as enhanced immune infiltration at the tumor.
Another immunotherapy under development in prostate cancer is ipilimumab, an anti-CTLA-4 antibody [44]. By blocking CTLA-4, an immunologic checkpoint on T-cell responses, this treatment may release a crucial brake on the immune system leading to an amplified antitumor immune response (Fig. 1) [45]. This antibody is FDA approved for the treatment of metastatic melanoma [46]. Sharma et al. performed a neoadjuvant ipilimumab study in bladder cancer where immune responses were assessed within the resected prostate [47,48]. These studies identified ICOS as a potential marker for tumor-specific T cells. Neoadjuvant trials with ipilimumab in prostate cancer are ongoing (NCT01194271).
Conclusions
To date, administration of what are thought to be highly active conventional therapies such as ADT and chemotherapy before surgery has not favorably affected OS and progression-free survival in men with high-risk prostate cancer. This appears discordant with encouraging results demonstrating improvements in intermediate end points including reduced prostate volume, reduced positive surgical margin rates, and reduced serum PSA with neoadjuvant treatment. However, given the low rate of pCRs with neoadjuvant therapy, resistant cancer cell populations most likely exist early on, making the unchanged long-term outcomes not entirely surprising as existing treatments are unable to eradicate these resistant cancer clones. This underscores the fact that in spite of advances in our fundamental knowledge of prostate cancer, we do not fully understand the biology of prostate cancer regarding disease progression, dissemination, and acquired or innate resistance to medical therapies.
Nevertheless, neoadjuvant trials provide an important opportunity to further our understanding of the biology of prostate cancer. Because tumor tissue can be analyzed before and after treatment, direct comparisons can be made to elucidate the mechanisms of drug action and resistance. Understanding how treatment affects the primary tumor may give us insights to how treatments work, or fail, at metastatic sites. Both targeted therapies and immunotherapies are designed to work through very specific mechanisms, such that conventional anatomic measures of treatment response may not be applicable. For example, noncytotoxic immunotherapies that can take several months to work may not result in pCR at prostatectomy given the relatively short timeframe of neoadjuvant trials. Instead, biologically selected readout activities should be developed such as immune cell infiltration and the presence or absence of an inflammatory response. Likewise for therapies targeting key effectors of signal transduction pathways, the development of phospho-proteomic signatures may directly reflect drug response on a molecular level to differentiate responders from nonresponders early in the course of treatment. Because these readouts can be quantitative, these neoadjuvant trials could also be used to assess for relative potency at hitting a biological target between different treatments, combinations, or even dose levels of the same treatment. Indeed, many drugs under investigation do not have dose-limiting toxicities. Studying these drugs in the neoadjuvant setting could allow for more rationale drug dose selection in a smaller number of patients.
This novelty of studying the effects of neoadjuvant treatments on prostate tumors also underscores the limitations and challenges to this approach. For most of these targeted small molecule and immunotherapies, the intermediate biological end points of treatment response are still being defined and hence are not clinically validated or standardized for application to larger study populations. Potential differences in the quality and quantity of pretreatment (prostate core biopsies) and posttreatment (surgical specimen) tissues may complicate interpretation of treatment effect as it does in androgen-ablated prostate tissue. The biology of androgen-dependent and CRPC is fundamentally different, perhaps accounting for why therapies that show survival benefit in the metastatic setting show no survival benefit in early-stage disease; conversely, regimens developed from neoadjuvant applications in early-stage disease may not work in advanced disease unless they can exploit common, yet undiscovered, vulnerabilities. In conclusion, although neoadjuvant ADT and chemotherapy are not currently recommended in high-risk prostate cancer, innovative targeted and immunotherapies, by virtue of changing the underlying tumor biology and tumor environment, may hold enormous potential to positively affect long-term outcomes.
References
- 1.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 2.Roach M, 3rd, Bae K, Speight J, Wolkov HB, Rubin P, Lee RJ, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol. 2008;26:585–91. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 3.D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. J Assoc Med Assoc. 2004;292:821–7. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 4.Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–73. doi: 10.1016/S1470-2045(10)70223-0. [DOI] [PubMed] [Google Scholar]
- 5.Pendleton J, Pisters LL, Nakamura K, Anai S, Rosser CJ. Neoadjuvant therapy before radical prostatectomy: where have we been? Where are we going? Urol Oncol. 2007;25:11–8. doi: 10.1016/j.urolonc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Schulman CC, Debruyne FM, Forster G, Selvaggi FP, Zlotta AR, Witjes WP. 4-year follow-up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2-3N0M0 prostate cancer. European Study Group on neoadjuvant treatment of prostate cancer. Eur Urol. 2000;38:706–13. doi: 10.1159/000020366. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Shelley M, Harrison C, Coles B, Wilt TJ, Mason MD. Neoadjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD006019.pub2. CD006019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gleave ME, Goldenberg SL, Chin JL, Warner J, Saad F, Klotz LH, et al. Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: biochemical and pathological effects. J Urol. 2001;166:500–6. discussion 506-507. [PubMed] [Google Scholar]
- 9.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taplin ME, Montgomery RB, Logothetis C, Bubley GJ, Richie JP, Dalkin BL, et al. Effect of neoadjuvant abiraterone acetate (AA) plus leuprolide acetate (LHRHa) on PSA, pathological complete response (pCR), and near pCR in localized high-risk prostate cancer (LHRPC): results of a randomized phase II study. J Clin Oncol. 2012;30(suppl) doi: 10.1200/JCO.2013.53.4578. abstr 4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 13.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 14.Dreicer R, Magi-Galluzzi C, Zhou M, Rothaermel J, Reuther A, Ulchaker J, et al. Phase II trial of neoadjuvant docetaxel before radical prostatectomy for locally advanced prostate cancer. Urology. 2004;63:1138–42. doi: 10.1016/j.urology.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Garzotto M, Higano CS, O'Brien C, Rademacher BL, Janeba N, Fazli L, et al. Phase 1/2 study of preoperative docetaxel and mitoxantrone for high-risk prostate cancer. Cancer. 2010;116:1699–708. doi: 10.1002/cncr.24960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klotz LH, Goldenberg SL, Jewett MA, Fradet Y, Nam R, Barkin J, et al. Long-term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J Urol. 2003;170:791–4. doi: 10.1097/01.ju.0000081404.98273.fd. [DOI] [PubMed] [Google Scholar]
- 17.Soloway MS, Pareek K, Sharifi R, Wajsman Z, McLeod D, Wood DP, Jr, et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol. 2002;167:112–6. [PubMed] [Google Scholar]
- 18.Chi KN, Chin JL, Winquist E, Klotz L, Saad F, Gleave ME. Multicenter phase II study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer. J Urol. 2008;180(2):565–70. doi: 10.1016/j.juro.2008.04.012. discussion 570. [DOI] [PubMed] [Google Scholar]
- 19.Chott A, Sun Z, Morganstern D, Pan J, Li T, Susani M, et al. Tyrosine kinases expressed in vivo by human prostate cancer bone marrow metastases and loss of the type 1 insulin-like growth factor receptor. Am J Pathol. 1999;155:1271–9. doi: 10.1016/S0002-9440(10)65229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fudge K, Wang CY, Stearns ME. Immunohistochemistry analysis of platelet-derived growth factor A and B chains and platelet-derived growth factor alpha and beta receptor expression in benign prostatic hyperplasias and Gleason-graded human prostate adenocarcinomas. Mod Pathol. 1994;7:549–54. [PubMed] [Google Scholar]
- 21.Ko YJ, Small EJ, Kabbinavar F, Chachoua A, Taneja S, Reese D, et al. A multi-institutional phase ii study of SU101, a platelet-derived growth factor receptor inhibitor, for patients with hormone-refractory prostate cancer. Clin Cancer Res. 2001;7:800–5. [PubMed] [Google Scholar]
- 22.Mathew P, Thall PF, Bucana CD, Oh WK, Morris MJ, Jones DM, et al. Platelet-derived growth factor receptor inhibition and chemo-therapy for castration-resistant prostate cancer with bone metastases. Clin Cancer Res. 2007;13:5816–24. doi: 10.1158/1078-0432.CCR-07-1269. [DOI] [PubMed] [Google Scholar]
- 23.Mathew P, Thall PF, Jones D, Perez C, Bucana C, Troncoso P, et al. Platelet-derived growth factor receptor inhibitor imatinib mesylate and docetaxel: a modular phase I trial in androgen-independent prostate cancer. J Clin Oncol. 2004;22:3323–9. doi: 10.1200/JCO.2004.10.116. [DOI] [PubMed] [Google Scholar]
- 24.Mathew P, Pisters LL, Wood CG, Papadopoulos JN, Williams DL, Thall PF, et al. Neoadjuvant platelet derived growth factor receptor inhibitor therapy combined with docetaxel and androgen ablation for high risk localized prostate cancer. J Urol. 2009;181:81–7. doi: 10.1016/j.juro.2008.09.006. discussion 87. [DOI] [PubMed] [Google Scholar]
- 25.Traish AM, Morgentaler A. Epidermal growth factor receptor expression escapes androgen regulation in prostate cancer: a potential molecular switch for tumour growth. Br J Cancer. 2009;101:1949–56. doi: 10.1038/sj.bjc.6605376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah RB, Ghosh D, Elder JT. Epidermal growth factor receptor (ErbB1) expression in prostate cancer progression: correlation with androgen independence. Prostate. 2006;66:1437–44. doi: 10.1002/pros.20460. [DOI] [PubMed] [Google Scholar]
- 27.Barton J, Blackledge G, Wakeling A. Growth factors and their receptors: new targets for prostate cancer therapy. Urology. 2001;58:114–22. doi: 10.1016/s0090-4295(01)01253-5. [DOI] [PubMed] [Google Scholar]
- 28.Pienta KJ. Preclinical mechanisms of action of docetaxel and docetaxel combinations in prostate cancer. Semin Oncol. 2001;28:3–7. doi: 10.1016/s0093-7754(01)90148-4. [DOI] [PubMed] [Google Scholar]
- 29.Sirotnak FM, Zakowski MF, Miller VA, Scher HI, Kris MG. Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res. 2000;6:4885–92. [PubMed] [Google Scholar]
- 30.Vuky J, Porter C, Isacson C, Vaughan M, Kozlowski P, Picozzi V, et al. Phase II trial of neoadjuvant docetaxel and gefitinib followed by radical prostatectomy in patients with high-risk, locally advanced prostate cancer. Cancer. 2009;115(4):784–91. doi: 10.1002/cncr.24092. [DOI] [PubMed] [Google Scholar]
- 31.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 32.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 33.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 34.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 35.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 36.Ross RW, Galsky MD, Febbo P, Barry M, Richie JP, Xie W, et al. Phase 2 study of neoadjuvant docetaxel plus bevacizumab in patients with high-risk localized prostate cancer: a prostate cancer clinical trials consortium trial. Cancer. 2012;118:4777–84. doi: 10.1002/cncr.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bitting RL, Armstrong AJ. Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer. Endocr Relat Cancer. 2013;20:R83–99. doi: 10.1530/ERC-12-0394. [DOI] [PubMed] [Google Scholar]
- 38.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 39.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 40.von Manteuffel SR, Dennis PB, Pullen N, Gingras AC, Sonenberg N, Thomas G. The insulin-induced signalling pathway leading to S6 and initiation factor 4E binding protein 1 phosphorylation bifurcates at a rapamycin-sensitive point immediately upstream of p70s6k. Mol Cell Biol. 1997;17:5426–36. doi: 10.1128/mcb.17.9.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cha E, Fong L. Immunotherapy for prostate cancer: biology and therapeutic approaches. J Clin Oncol. 2011;29:3677–85. doi: 10.1200/JCO.2010.34.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wesley JD, Whitmore J, Trager J, Sheikh N. An overview of sipuleucel-T: autologous cellular immunotherapy for prostate cancer. Hum Vaccin Immunother. 2012;8:520–7. doi: 10.4161/hv.18769. [DOI] [PubMed] [Google Scholar]
- 43.Fong L, Weinberg VK, Chan SE, Corman JM, Amling CL, Stephenson RA, et al. Neoadjuvant sipuleucel-T in localized prostate cancer: effects on immune cells within the prostate tumor micro-environment. J Clin Oncol. 2012;30(suppl) abstr 2564. [Google Scholar]
- 44.Kwek SS, Cha E, Fong L. Unmasking the immune recognition of prostate cancer with CTLA4 blockade. Nat Rev Cancer. 2012;12:289–97. doi: 10.1038/nrc3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwon ED, Foster BA, Hurwitz AA, Madias C, Allison JP, Greenberg NM, et al. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc Natl Acad Sci U S A. 1999;96(26):15074–9. doi: 10.1073/pnas.96.26.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen H, Liakou CI, Kamat A, Pettaway C, Ward JF, Tang DN, et al. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc Natl Acad Sci U S A. 2009;106:2729–34. doi: 10.1073/pnas.0813175106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carthon BC, Wolchok JD, Yuan J, Kamat A, Ng Tang DS, Sun J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16:2861–71. doi: 10.1158/1078-0432.CCR-10-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aus G, Abrahamsson PA, Ahlgren G, Hugosson J, Lundberg S, Schain M, et al. Three-month neoadjuvant hormonal therapy before radical prostatectomy: a 7-year follow-up of a randomized controlled trial. BJU Int. 2002;90:561–6. doi: 10.1046/j.1464-410x.2002.02982.x. [DOI] [PubMed] [Google Scholar]
- 50.Yee DS, Lowrance WT, Eastham JA, Maschino AC, Cronin AM, Rabbani F. Long-term follow-up of 3-month neoadjuvant hormone therapy before radical prostatectomy in a randomized trial. BJU Int. 2010;105:185–90. doi: 10.1111/j.1464-410X.2009.08698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prezioso D, Lotti T, Polito M, Montironi R. Neoadjuvant hormone treatment with leuprolide acetate depot 3.75 mg and cyproterone acetate, before radical prostatectomy: a randomized study. Urol Int. 2004;72:189–95. doi: 10.1159/000077113. [DOI] [PubMed] [Google Scholar]
- 52.Selli C, Montironi R, Bono A, Pagano F, Zattoni F, Manganelli A, et al. Effects of complete androgen blockade for 12 and 24 weeks on the pathological stage and resection margin status of prostate cancer. J Clin Pathol. 2002;55:508–13. doi: 10.1136/jcp.55.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gravina GL, Festuccia C, Galatioto GP, Muzi P, Angelucci A, Ronchi P, et al. Surgical and biologic outcomes after neoadjuvant bicalutamide treatment in prostate cancer. Urology. 2007;70:728–33. doi: 10.1016/j.urology.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 54.Febbo PG, Richie JP, George DJ, Loda M, Manola J, Shankar S, et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2005;11:5233–40. doi: 10.1158/1078-0432.CCR-05-0299. [DOI] [PubMed] [Google Scholar]
- 55.Friedman J, Dunn RL, Wood D, Vaishampayan U, Wu A, Bradley D, et al. Neoadjuvant docetaxel and capecitabine in patients with high risk prostate cancer. J Urol. 2008;179:911–5. doi: 10.1016/j.juro.2007.10.064. discussion 915-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hussain M, Smith DC, El-Rayes BF, Du W, Vaishampayan U, Fontana J, et al. Neoadjuvant docetaxel and estramustine chemotherapy in high-risk/locallyadvanced prostate cancer. Urology. 2003;61:774–80. doi: 10.1016/s0090-4295(02)02519-0. [DOI] [PubMed] [Google Scholar]
- 57.Prayer-Galetti T, Sacco E, Pagano F, Gardiman M, Cisternino A, Betto G, et al. Long-term follow-up of a neoadjuvant chemohormonal taxane-based phase II trial before radical prostatectomy in patients with nonmetastatic high-risk prostate cancer. BJU Int. 2007;100:274–80. doi: 10.1111/j.1464-410X.2007.06760.x. [DOI] [PubMed] [Google Scholar]
- 58.Mellado B, Font A, Alcaraz A, Aparicio LA, Veiga FJ, Areal J, et al. Phase II trial of short-term neoadjuvant docetaxel and complete androgen blockade in high-risk prostate cancer. Br J Cancer. 2009;101(8):1248–52. doi: 10.1038/sj.bjc.6605320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Womble PR, VanVeldhuizen PJ, Nisbet AA, Reed GA, Thrasher JB, Holzbeierlein JM. A phase II clinical trial of neoadjuvant ketoconazole and docetaxel chemotherapy before radical prostatectomy in high risk patients. J Urol. 2011;186:882–7. doi: 10.1016/j.juro.2011.04.087. [DOI] [PubMed] [Google Scholar]
- 60.Narita S, Tsuchiya N, Kumazawa T, Maita S, Numakura K, Obara T, et al. Short-term clinicopathological outcome of neoadjuvant chemohormonal therapy comprising complete androgen blockade, followed by treatment with docetaxel and estramustine phosphate before radical prostatectomy in Japanese patients with high-risk localized prostate cancer. World J Surg Oncol. 2012;10:1. doi: 10.1186/1477-7819-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Febbo PG, Thorner A, Rubin MA, Loda M, Kantoff PW, Oh WK, et al. Application of oligonucleotide microarrays to assess the biological effects of neoadjuvant imatinib mesylate treatment for localized prostate cancer. Clin Cancer Res. 2006;12:152–8. doi: 10.1158/1078-0432.CCR-05-1652. [DOI] [PubMed] [Google Scholar]
- 62.Vuky J, Corman JM, Porter C, Olgac S, Auerbach E, Dahl K. Phase II trial of neoadjuvant docetaxel and CG1940/CG8711 followed by radical prostatectomy in patients with high-risk clinically localized prostate cancer. Oncologist. 2013;18:687–8. doi: 10.1634/theoncologist.2011-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]