Abstract
Background
Depression is characterized by poor executive function, but—counterintuitively—it is associated with highly accurate performance on certain cognitively demanding tasks. The psychological mechanisms responsible for this paradoxical finding are unclear. To address this issue, we applied a drift diffusion model (DDM) to flanker task data from depressed and healthy adults participating in the multi-site Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care for Depression (EMBARC) study.
Methods
One hundred unmedicated, depressed adults and forty healthy controls completed a flanker task. We investigated the effect of flanker interference on accuracy and response time, and used the DDM to examine group differences in three cognitive processes: prepotent response bias (tendency to respond to the distracting flankers), response inhibition (necessary to resist prepotency), and executive control (required for execution of correct response on incongruent trials).
Results
Consistent with prior reports, depressed participants responded more slowly and accurately than controls on incongruent trials. The DDM indicated that although executive control was sluggish in depressed participants, this was more than offset by decreased prepotent response bias. Among the depressed participants, anhedonia was negatively correlated with a parameter indexing the speed of executive control (r = -0.28, p = 0.007).
Conclusions
Executive control was delayed in depression but this was counterbalanced by reduced prepotent response bias, demonstrating how participants with executive function deficits can nevertheless perform accurately in a cognitive control task. Drawing on data from neural network simulations, we speculate that these results may reflect tonically reduced striatal dopamine in depression.
How does depression affect higher-order cognition? Given its association with maladaptive rumination (Nolen-Hoeksema, 1991) and abnormal frontal lobe function (Wagner et al., 2006), one might expect depression to weaken executive function, which encompasses the exertion of cognitive control to achieve goals despite obstacles. Indeed, a meta-analysis of 113 studies found broadly negative effects of Major Depressive Disorder (MDD) on executive function (Snyder, 2013), linking MDD to impaired performance on tasks tapping inhibition, set-shifting, and working memory updating. Thus, the negative relationship between depression and executive function is well-established.
However, a close reading of the literature reveals a puzzle: several studies report positive effects of depression and sad mood on tasks that would seem to depend on executive function. For instance, Snyder et al. (2014) reported that, although anxiety impaired selection from amongst competing response options in three language tasks, increased depression facilitated selection (after accounting for variance associated with anxiety). Along similar lines, Au et al. (2013) assessed the effects of sad, positive, and neutral moods on decision-making during financial trading. Across two experiments, sad mood was associated with accurate decisions and conservative allocation strategies, leading to financial gains. By contrast, positive mood was linked to inaccurate decisions coupled with aggressive allocations, leading to poor outcomes: while participants in sad moods profited, those in positive moods incurred net losses. Although sad mood and depression are not equivalent, the fact that excessive sadness is a cardinal symptom of depression (American Psychiatric Association, 2013) makes these results surprising: one might have expected a negative effect of sad mood on complex financial decisions, which surely involve executive function.
Research with the Eriksen flanker task (Eriksen & Eriksen, 1974) has also yielded counterintuitive findings. Several versions of the flanker task exist, but they share a common structure: participants must report the identity of a centrally presented stimulus that is surrounded by flankers, which call for either the same response as the central stimulus (congruent condition) or the opposite response (incongruent condition). In the arrow flanker task, participants report the direction (left or right) of a central arrow that is flanked by arrows pointing in the same direction (congruent: ≪≪< or ≫≫) or the opposite direction (incongruent: ≪>≪ or ≫<≫). Typically, response time (RT) is slower and accuracy is lower in the incongruent condition due to interference introduced by the misleading flankers. Resisting this interference suggests intact executive function.
Against this backdrop, results from two flanker studies are striking (Dubal et al., 2000; Dubal & Jouvent, 2004). In these studies, undergraduates with severe anhedonia responded more slowly but also more accurately on incongruent trials than did healthy participants, suggesting that executive function was delayed but intact. Because anhedonia is the second cardinal symptom of MDD (American Psychiatric Association, 2013), these data accentuate the paradox: MDD is associated with executive dysfunction, but its defining symptoms—anhedonia and sadness—are sometimes associated with high accuracy on cognitive control tasks. Finally, there is evidence that this result extends to clinical samples, as several studies that have administered the flanker and Stroop tasks to adults with MDD and healthy controls have found slower but more accurate responses in depressed participants, although the accuracy effect is typically non-significant in small samples (e.g., Chiu & Deldin, 2007; Holmes & Pizzagalli, 2010; Siegle et al., 2004).
If depression involves diminished executive function, what explains this pattern? To date, answers to this question have appealed to cognitive styles. Depressed individuals adopt a deliberative, analytical stance towards information processing (Andrews et al., 2007; Andrews & Thomson, 2009). When a task calls for rapid, intuitive decisions, this is counterproductive and accuracy suffers (e.g., Ambady & Gray, 2002). But when fast responses are likely to produce errors, the careful approach associated with depression can support high accuracy.
This naturally raises a second question: why is depression associated with a systematic information processing style? One possibility is that depressed individuals are especially motivated to avoid the negative emotions triggered by errors (e.g., Robinson et al., 2007). Alternatively, depression rumination may be an evolved response that serves to limit distraction and focus cognitive resources in order to identify the causes of low mood (Andrews & Thomson, 2009). These explanations are intriguing, but it may prove useful to study depression in the context of computational models of response inhibition, which provide quantitative estimates of specific cognitive processes that may be sensitive to MDD. We take this approach by applying a modified drift diffusion model (DDM; Noorani & Carpenter, 2013; Ratcliff & McKoon, 2008) to flanker data from healthy controls and a large depressed sample.
Briefly, the model decomposes performance in the flanker task into separate parameters that reflect prepotent response bias, response inhibition, and executive function, providing an opportunity to determine which (if any) of these parameters is affected by depression (Pe et al., 2013; Hübner et al., 2010; White et al. 2011). Furthermore, because the DDM has been fit to data from neural network simulations of cortico-striatal-thalamic circuits (Ratcliff & Frank, 2012; see also Wiecki & Frank, 2013), results from the DDM may suggest hypotheses about brain function in depression. Response inhibition depends on fronto-striatal circuits that receive dopaminergic projections from the midbrain (Wiecki & Frank, 2013), and that are dysfunctional in anhedonic depression (Dillon et al., 2014; Epstein et al., 2006; Pizzagalli et al., 2009; Treadway & Zald, 2011). Consequently, slow but accurate performance in tasks that probe response inhibition—such as the flanker task—may reflect dysfunction in fronto-striatal circuitry, and results from the DDM could suggest which aspects of this circuitry are most strongly affected by depression (Montague et al., 2012; Wiecki et al., in press).
Method
The data described here were collected in a multi-site study entitled “Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care for Depression” (EMBARC) (http://clinicaltrials.gov/show/NCT01407094). Recruiting sites are Columbia University Medical Center in New York City, Massachusetts General Hospital in Boston, the University of Texas Southwestern Medical Center in Dallas, and the University of Michigan in Ann Arbor. Participants with unipolar depression completed several behavioral, self-report, and physiological assessments prior to enrolling in a double-blind, placebo-controlled clinical trial designed to identify biomarkers of response to sertraline and bupropion. Data collection is ongoing and the blind is unbroken, thus we are unable to consider treatment outcomes. We present an analysis of flanker task data from the first 100 depressed participants enrolled in the study and 40 healthy controls. McLean Hospital was responsible for analysis of flanker data.
Participant recruitment, eligibility criteria, and reimbursement
Participants were recruited using flyers and posters, and by research coordinators who visited local clinics. Participants provided informed consent following procedures approved by site IRBs. Adults aged 18–65 of all races and ethnicities were invited to participate. Eligible depressed participants met DSM-IV criteria for nonpsychotic MDD, as assessed via the SCID-I/P (First et al., 2002), and scored 14 or above on the self-report version of the 16-item Quick Inventory of Depression Symptomatology (QIDS-SR16; Rush et al., 2003); this cut-off corresponds to moderate depression. Exclusion criteria included: lifetime psychotic depressive, schizophrenic, bipolar, schizoaffective, or other Axis I psychotic disorder; current primary diagnosis of obsessive compulsive disorder; substance dependence in the past six months (excluding nicotine) or substance abuse in the past two months; active suicidality; or unstable medical conditions that would likely require hospitalization during the study. Critically, no depressed participant was being treated with antidepressant or other psychotropic medication for at least three weeks when the data described here were collected.
Data from two depressed individuals were excluded due to difficulty following instructions and technical problems, leaving a sample of 98 depressed participants and 40 healthy controls. Controls did not meet criteria for any current or lifetime history of mood, anxiety, eating, dementing, or psychotic disorder, did not meet any of the other exclusion criteria in place for the depressed group, and had a QIDS-SR score lower than 8. Participants were paid $50 for the session, which included additional testing not described here.
Questionnaires
Participants in the EMBARC study complete an extensive clinical evaluation battery, including clinician- and participant-rated instruments probing various domains, including lifetime diagnosis, personality traits, and social functioning. Because flanker performance is sensitive to anhedonia (Dubal et al., 2000; Dubal & Jouvent, 2004) and may be influenced by depressive severity (Chiu & Deldin, 2007), we concentrate on data from the QIDS-SR16 and the Snaith Hamilton Pleasure Scale (SHAPS; Snaith et al., 1995). The QIDS-SR16 is a self-report instrument that assesses core DSM-IV diagnostic criteria for MDD. It has acceptable psychometric properties and demonstrates convergent validity with other measures of depression (Rush et al., 2003). The SHAPS was used to assess hedonic capacity and was scored dimensionally, with higher scores indicating greater anhedonia (Franken et al., 2007). Finally, we also tested the hypothesis that flanker performance would be negatively associated with the number of depressive episodes reported.1 The distribution of number of depressive episodes was positively skewed, thus we binned these data into six categories (number of episodes: 1, n = 10; 2–3, n = 17; 4–5, n = 13; 6–10, n = 27; 11–30, n = 16; over 30, n = 7; unrecorded, n = 2).
Flanker task
Participants completed a 30-trial practice session that included 15 congruent and 15 incongruent trials. The flanking arrows were presented alone (duration: 100 ms) and were then joined by the central arrow (50 ms); the total stimulus duration was thus 150 ms. Participants were asked to indicate whether the center arrow pointed left or right by pressing a button, and accuracy and RT were recorded.
Participants then completed five blocks of 70 trials (46 congruent, 24 incongruent), for a total of 350 trials (230 congruent, 120 incongruent). To ensure adequate difficulty, a response deadline corresponding to the 85th percentile of the RT distribution on incongruent trials in the preceding block was established; for the first block, the practice RT distribution was used (Holmes et al., 2010). Stimulus presentation was followed by a fixation cross (1400 ms). If the participant did not respond by the response deadline, a screen reading “TOO SLOW!” was presented (300 ms). Participants were told that if they saw this screen, they should speed up. If a response was made before the deadline, the “TOO SLOW!” screen was omitted and the fixation cross remained onscreen for the 300 ms interval. Finally, each trial ended with presentation of the fixation cross for an additional 200–400 ms. Thus, trial duration varied between 2050–2250 ms. The sequence of congruent and incongruent trials was created using optseq2 (http://surfer.nmr.mgh.harvard.edu/optseq/) and was identical across participants.
While data collection was ongoing, block-by-block feedback was added to the paradigm to maintain performance at desired levels. If participants made fewer than three incongruent errors in a block, the following instructions were presented after the block: “Remember to respond as QUICKLY as possible while still being accurate”. If six or more incongruent errors were made, the screen read, “Remember to respond as ACCURATELY as possible while still being fast”. Otherwise, the screen read, “Please respond as quickly and accurately as possible”. Block-by-block feedback was presented to 7 of 40 controls and 42 of 98 depressed participants.
Quality control
Quality control checks were used to exclude datasets characterized by unusually poor performance. First, for each participant outlier trials were defined as those in which the raw RT was less than 150 ms or the log-transformed RT exceeded the participant’s mean±3SD, computed separately for congruent and incongruent stimuli. Second, we excluded datasets with: 35 or more RT outliers (greater than 10% of trials), fewer than 200 outlier-free congruent trials, fewer then 90 outlier-free incongruent trials, or lower than 50% correct for congruent or incongruent trials. Data from 92 depressed and 37 healthy participants passed these checks and constitute the final sample. Trials characterized by RT outliers were excluded from all analyses.
Analysis of flanker interference effects on accuracy and RT
We computed linear mixed models on trial-level RT and accuracy data using the lme4 package (version 1.1.7) in the R software environment (R Core team, 2013). In the first model, RT was the dependent variable. We expected depressed adults to respond more slowly than controls, particularly in response to incongruent stimuli (Snyder, 2013), thus we included Group and Stimulus as independent variables. Furthermore, errors are typically faster than correct responses on incongruent but not congruent trials in the flanker task (Wiecki & Frank, 2013), and there is evidence of hypersensitivity to errors in MDD (Chiu & Deldin, 2007; Holmes & Pizzagalli, 2008), thus Accuracy was added as another independent variable. Site was included as covariate. In the second model, accuracy was the dependent variable and the independent variables were Group and Stimulus, with Site as a covariate. Because accuracy was scored as 0 or 1, logistic regression was used for this model. Participant was entered as a random effect in both models. Follow-up tests for significant interactions were conducted using the lsmeans (version 2.11) package in R. Additional analyses focused on congruency sequence effects (Gratton, Coles, & Donchin, 1992) and post-error behavioral adjustments (Laming, 1979; Rabbitt, 1966) did not reveal any group differences; they are presented in the Appendix.
Computational modeling
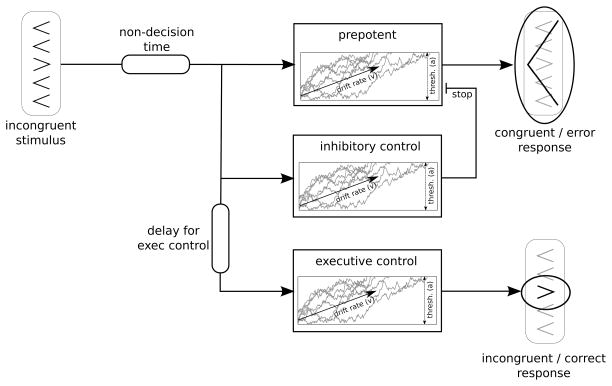
Our computational model uses a modified version of the Linear Approach to Threshold with Ergodic Rate (LATER) (Noorani & Carpenter, 2013); the key modification is that noise in the accumulation process is explicitly modeled. This should not yield qualitatively different interpretations as these models are closely related (Bogacz et al., 2006; Donkin et al., 2011). Behavior reflects the output of three mechanisms: (i) a prepotent, reflexive mechanism biased to respond according to the distracting flankers; (ii) inhibitory control, which can suppress the prepotent response tendency; and (iii) executive control, needed to initiate the correct response on incongruent trials. As shown in Figure 1, each mechanism is modeled as a drift process that progresses towards a shared threshold (i.e., one model parameter—denoted “a”—controls the threshold setting for all three mechanisms). The drift processes reflect the accumulation of evidence from the stimulus presented on each trial, and they are noisy to simulate noise in the environment and sensory systems. The response executed by the model depends on which drift process crosses its threshold first. See the Appendix for details regarding the algorithm used to simulate trial-level data.
Figure 1.
Computational model, adapted from LATER model (Noorani & Carpenter, 2013) for application to the flanker task.
On congruent trials, responses are committed when the prepotent accumulator reaches threshold; because all arrows point in one direction on congruent trials, the inhibitory and executive control mechanisms are inactive. By contrast, the less frequent incongruent trials involve a race between the prepotent accumulator, which responds in agreement with the flanking arrows (Figure 1, top), and the executive control unit, which responds according to the central arrow (Figure 1, bottom). Onset of the executive control accumulator is delayed by a constant (Figure 1, bottom left) that simulates time needed to retrieve and apply rules on incongruent trials (i.e., respond to the central arrow, not the flankers; Wiecki & Frank, 2013). If the prepotent accumulator crosses its threshold first, the model commits an error (Figure 1, top right). By contrast, if the executive control accumulator wins the race, the model makes the correct response (Figure 1, bottom right). Finally, the inhibitory control accumulator acts as a brake, stopping the prepotent accumulator when its threshold is reached (Figure 1, middle). Thus, the model has the following parameters: a shared threshold setting for all accumulators; drift-rates for the prepotent, inhibitory, and executive control accumulators; a delay to onset for the executive control accumulator; and a constant, non-decision time capturing motor execution (Figure 1, upper left). RT corresponds to the passage time of the winning accumulator.
We fit the DDM to each participant’s full distribution of RT data from congruent and incongruent trials simultaneously, and used Powell-optimization (Powell, 1964) with basin hopping (Wales & Doye, 1997) to find the best-fitting model parameters while avoiding local maxima. Threshold settings and prepotent drift-rate were shared across congruent and incongruent trials, while the inhibitory and executive control parameters were only fit to data from incongruent trials. Model fit was evaluated by probability density approximation (PDA; Turner & Sederberg, 2014), which uses kernel density estimation of samples generated by the model (see Appendix for details) and does not require a closed-form solution of the likelihood function. Optimal model fits were found by maximizing the summed log-likelihood (evaluated using PDA) of the RT and choice data from each subject. Weakly informative priors were placed on model parameters to constrain extreme model fits. Finally, we compared best-fitting DDM parameters across the groups.
Results
Demographics and clinical measures
There were no group differences (ts < 1.1, ps > 0.27) in age (controls: 36.22±14.32; depressed: 39.16±12.99) or years of education (controls: 15.77±4.52; depressed: 15.06±2.43). QIDS-SR16 scores were higher in depressed participants (18.48±2.87) versus controls (1.46±1.30), reflecting eligibility criteria. The mean QIDS-SR16 score in the depressed group indicates moderate depression (Rush et al., 2003). SHAPS scores were higher in depressed participants (33.83±5.99) versus controls (21.05±5.37), t(127) = 11.27, p < 0.001.
Flanker interference effects
RT
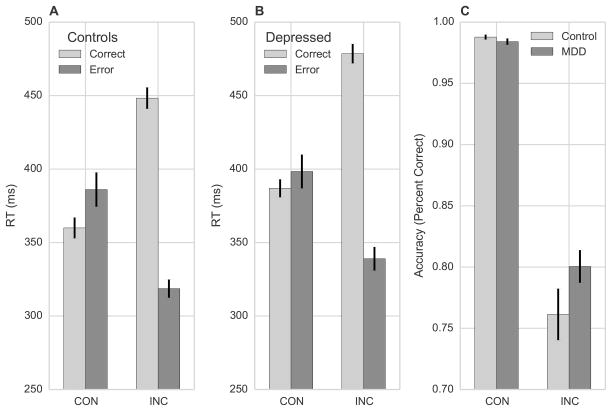
Controls (Figure 2a) and depressed participants (Figure 2b) responded more quickly on correct congruent trials versus correct incongruent trials, consistent with flanker interference. Both groups showed the opposite pattern when making errors, generating faster RTs on incorrect incongruent trials versus incorrect congruent trials. This pattern led to a Stimulus x Accuracy interaction, Z = 22.82, p < 0.001.
Figure 2.
Flanker interference effects on (A) RT in controls, (B) RT in depressed participants, and (C) accuracy in both groups. Error bars indicate standard error of the mean.
The model also returned a Group x Accuracy interaction, Z = 3.28, p = 0.001, and a Group x Stimulus interaction, Z = 2.05, p = 0.040. Follow-up contrasts linked the Group x Accuracy interaction to a difference on correct trials: depressed participants were slower than controls, Z = -2.49, p = 0.013. There was no difference on error trials (p = 0.424). The Group x Stimulus interaction reflected a difference on incongruent trials, with depressed participants responding more slowly than controls, Z = -2.09, p = 0.037. Depressed participants were also slower on congruent trials, but this difference was not significant (p = 0.242). Thus, depressed participants responded more slowly than controls, with significant differences for correct responses and responses to incongruent stimuli. Slow responses on incongruent trials are common in depressed samples, consistent with executive function deficits (Snyder, 2013).
Accuracy
As shown in Figure 2c, both groups were more accurate when responding to congruent versus incongruent stimuli, consistent with flanker interference. However, depressed participants were more accurate than controls on incongruent trials, leading to a Group x Stimulus interaction, Z = 3.90, p < 0.001. Follow-up linear contrasts confirmed a (marginal) Group effect on incongruent trials, Z = -1.94, p = 0.053 that was absent on congruent trials, Z = 0.68, p = 0.495. This result echoes reports of better accuracy on incongruent trials in sad and anhedonic samples (Au et al., 2013; Dubal et al., 2004).
Block-by-block feedback
Two analyses investigated whether including block-by-block feedback for some participants influenced the RT and accuracy results. First, the linear models were re-computed with Version (feedback, no-feedback) as an additional covariate. Version was not significant in either model, and all interactions reported above remained significant. Second, we re-computed the original models including only participants who did not receive feedback (33 controls, 42 MDD). Again, all reported interactions remained significant. Thus, including block-by-block feedback for some participants did not strongly influence the findings.
Computational modeling
Table 1 shows best-fitting parameter values from the model. The executive control drift-rate on incongruent trials was lower in depressed versus healthy participants, t(127) = -2.05, p = 0.043, consistent with slower executive function. However, the prepotent drift-rate was also lower in depressed participants, t(127) = -2.40, p = 0.018. This is intriguing because weak prepotent bias might offset the executive control deficit (see Appendix for distributions of both parameters).
Table 1.
Mean (±SD) best fitting parameter values from the Drift Diffusion Model
| Model parameter | Healthy Controls | Depressed Participants |
|---|---|---|
| Non-decision time (ms) | 212 ±34 | 207±59 |
| Prepotent drift-rate* | 7.00±1.45 | 6.37±1.28 |
| Inhibitory drift-rate | 9.76±1.95 | 9.67±2.18 |
| Executive control: drift-rate* | 10.38±2.61 | 9.28±2.80 |
| Executive control: delay to onset (ms) | 131.23±25.27 | 138.97±34.99 |
| Threshold | 1.05±0.33 | 1.14±0.44 |
Depressed < Controls, p < 0.05.
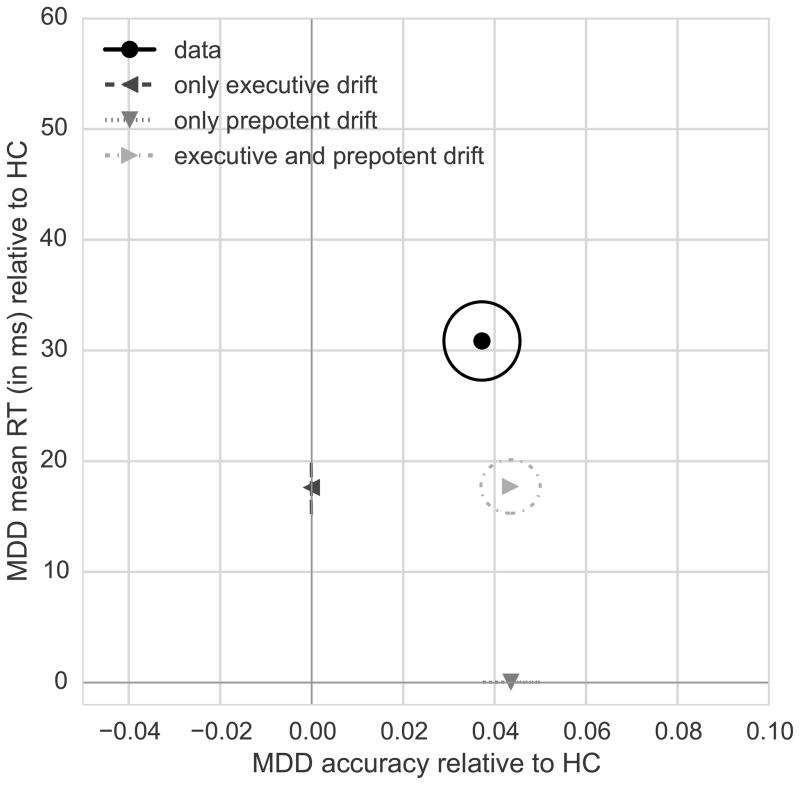
To test this hypothesis, we conducted simulations in which the model was used to generate artificial RT and accuracy data. In the first simulation, all model parameters were set to the best-fitting values for controls with the exception of the executive control drift-rate, which was matched to the best-fitting value for the depressed participants. As shown in Figure 3, this resulted in prolonged incongruent RT but no group difference in accuracy. In the second simulation, we returned the executive control drift-rate to the controls’ value but set the prepotent drift-rate to the best-fitting value for depressed participants. As can be seen in Figure 3, this modulation accounted for the increase in accuracy but failed to capture the slow RTs seen on correct incongruent trials in depressed participants. The fact that there is no variability in accuracy when allowing executive control drift-rate to change nor variability in RT when allowing prepotent drift-rate to change is expected because these two parameters affect incongruent RT and accuracy independently in this parameter setting. In the third simulation, we set both the executive control and prepotent drift-rates to best-fitting values for the depressed group, leaving all other parameters set to optimal values for controls. This yielded the pattern most similar to data from depressed participants (Figure 3): responding on correct incongruent trials was slower, and the incongruent error rate was reduced. We corroborated this finding by performing model comparison using the Bayesian Information Criterion (BIC): lower scores indicate better model fit. The best-fitting model was the one that allowed for group differences in executive control and prepotent drift-rate (BIC=115.13); manipulating only executive drift-rate (BIC=124.33) or prepotent drift-rate (BIC=179.03) produced worse fits. Thus, accurate performance can emerge if executive control is sluggish, provided prepotent response bias is also decreased. The combination is critical: neither factor alone could account for the data.
Figure 3.
RT simulations isolate the effects of particular cognitive processes on behavior. Points indicate accuracy on incongruent trials (x-axis) and mean RT on correct incongruent trials (y-axis) for MDD subjects relative to healthy controls (HC). Ellipses indicate standard error of the mean. The collected data revealed that depressed participants were slower and more accurate than controls on incongruent trials (‘data’). Simulated data show that manipulation of only the executive control drift-rate (‘only executive drift’) captured slowing in the MDD group but not the group difference in accuracy (difference relative to model with all parameters set to healthy control values). Conversely, manipulation of only the prepotent drift-rate (‘only prepotent drift’) captured increased accuracy in the MDD group but not the increased RT (difference relative to model with all parameters set to healthy control values). Simultaneous adjustment of the executive control and prepotent drift-rates (‘executive and prepotent drift’) yielded results that most closely match the combination of increased accuracy and prolonged incongruent RT in depressed participants. Note that the overall model fit displayed here is sub-optimal because, in order to isolate the effect of manipulating the prepotent and executive drift-rates, all other parameters were set to best-fitting values from controls; overall model fit is improved when all parameters are set to optimal values for the MDD group.
Correlations
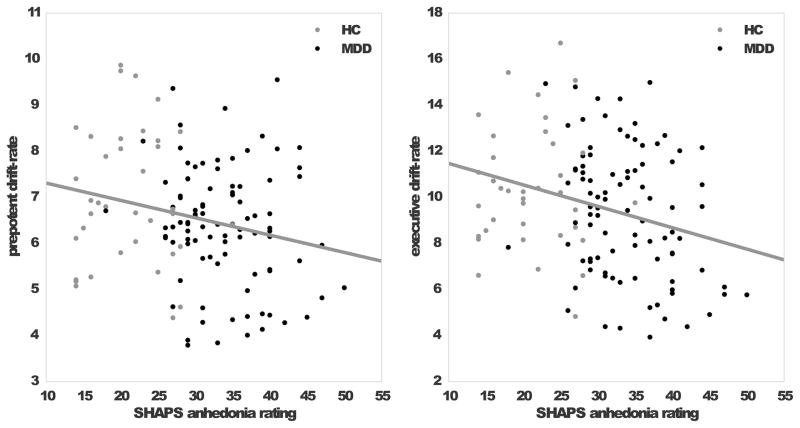
As shown in Figure 4, we found a significant Pearson correlation between anhedonia, as assessed by the SHAPS total score, and executive control in the MDD group, r = -0.28, p = 0.007. The correlation with prepotent drift-rate was not significant, r = -0.09, p = 0.40, and neither drift-rate was correlated with QIDS-SR16 scores in the MDD group. Number of depressive episodes was positively correlated with congruent accuracy (r = 0.23, p = 0.03) and median correct RT on incongruent trials (r = 0.22, p = 0.04).
Figure 4.
Self-reported anhedonia was negatively correlated with the prepotent drift-rate (left) and executive control drift-rate (right panel) across the two groups.
Discussion
This study yielded three main results. First, responding on incongruent trials was slower but (marginally) more accurate in depressed versus healthy participants. Second, the DDM uncovered slow executive control and reduced prepotent response bias in the MDD group, with simulations indicating that the combination of these two factors best accounted for the observed group differences in accuracy and RT. Third, executive control was negatively correlated with anhedonia in depressed participants. These findings extend recent DDM research in depression and point to a candidate neural mechanism: tonically reduced striatal dopamine.
Using the DDM to probe depression
This study extends recent work by Vallesi et al. (2015) that used the standard DDM to analyze data from a color perception task. Relative to controls, depressed participants in that study showed a lower drift rate that was negatively correlated with Hamilton Depression Rating Scale (HDRS) scores (Hamilton, 1960). The current findings extend this study in two ways. First, the modified DDM afforded increased precision: we also found a negative effect of depression on drift rate, but it was specific to executive control and prepotency. Second, because the HDRS is sensitive to several facets of depression (Bagby et al., 2004), it is difficult to know which ones are related to reduced drift rate. We found a negative relationship between executive control drift rate and anhedonia, linking this particular aspect of depression to deficits in executive function. Assessing the reliability and specificity of this relationship is a key goal for future work.
Additional points of convergence with the report by Vallesi et al. (2015) merit consideration. First, neither study found a group difference in non-decision time, which captures processes such as response execution. This suggests that the observed group differences do not reflect psychomotor slowing. Second, neither study found an effect of depression on decision threshold. Consequently, the results do not simply reflect a speed-accuracy trade-off in depressed participants, because such a trade-off should yield a threshold difference (Dutilh et al., 2012).
Reduced striatal dopamine in depression
We speculate that reduced executive and prepotent drift-rates in depression reflects dysfunction in circuits that connect the basal ganglia to the frontal cortex, and that receive dopaminergic innervation from the midbrain. Selective activation of basal ganglia neurons in the Go and NoGo pathways acts to facilitate or suppress action plans stored in frontal cortex, making their execution more or less likely (Chevalier & Deniau, 1990; Mink, 1996). The balance between facilitation and suppression is modulated by dopamine, which excites Go neurons and inhibits NoGo neurons (Frank, 2005). Low concentrations of striatal dopamine disinhibit NoGo neurons and weakly activate Go neurons, leading to response slowing (Wiecki & Frank 2010; Wiecki et al., 2009). This is true for habitual actions (parameterized by the prepotent drift-rate) and volitional actions (parameterized by the executive control drift-rate) (Wiecki & Frank 2013). Thus, low striatal dopamine could account for reduced prepotent and executive control drift-rates in depressed participants, consistent with independent evidence of abnormal striatal dopamine concentration and function (Dillon et al., 2014; Treadway & Zald, 2011). To resolve the paradox introduced earlier, tonically reduced striatal dopamine may help explain the coexistence of executive function deficits and accurate performance in depressed samples. Moreover, the positive correlation between number of depressive episodes and correct RT on incongruent trials indicates that mechanisms supporting action selection may be sensitive to cumulative effects of depression. Neuroimaging techniques sensitive to dopamine are needed to test these conjunctures.
Our data are intriguing in light of work on predictors of antidepressant treatment response. Specifically, psychomotor slowing predicts a poor response to selective serotonin reuptake inhibitors (SSRIs; Bruder et al., 2014; Taylor et al., 2006) but a good response to bupropion (Bruder et al., 2014; Herrera-Guzmán et al., 2008), which is a dopamine/noradrenaline reuptake inhibitor. The current results raise the possibility that slow but accurate performance on the flanker task, although not directly attributable to psychomotor slowing, may predict a better response to bupropion versus SSRIs. When treatment outcome data from the EMBARC study are available, we will be able to test this hypothesis.
Limitations
Two important limitations deserve mention. First, negative effects of depression are typically strongest in unconstrained tasks (Hertel, 1997). The flanker task features clear instructions and few response options, thus it may be less sensitive to depression and depressive rumination than more open-ended tasks. Second, while the computational model used here has been validated on the related antisaccade task (Noorani & Carpenter, 2013), other models have been successfully applied to the flanker task (Hübner et al. 2010; White et al., 2011). The relationship between these models is not well-established, and they might suggest negative effects of depression on different parameters.
Conclusions
Depressed participants responded more slowly and accurately than controls in the flanker task, consistent with prior findings. Because depression impairs executive function, highly accurate performance has been difficult to explain. We used computational modeling to show that reduced prepotent response bias offset slow executive control in MDD, and we speculate that these abnormalities may reflect reduced striatal dopamine.
Supplementary Material
Acknowledgments
Financial Support
The EMBARC study was supported by the National Institute of Mental Health of the National Institutes of Health under award numbers U01MH092221 (Trivedi, M.H.) and U01MH092250 (McGrath, P.J., Parsey, R.V., Weissman, M.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Valeant Pharmaceuticals donated the Wellbutrin XL used in the study. This work was supported by the EMBARC National Coordinating Center at UT Southwestern Medical Center, Madhukar H. Trivedi, M.D., Coordinating PI, and the Data Center at Columbia and Stony Brook Universities. Dr. Dillon was supported by NIMH grant R00MH094438.
Footnotes
Conflict of Interest
D.G.D., T.W., P.P, C.W., F.G., L.M., P.J.M., M.W., R.P., B.K., P.A., T.C., S.W., K.S.-W., M.T., M.A.O., C.C., P.D., and G.B. declare no financial, professional, or personal relationships with the potential to bias this work. Madhukar H. Trivedi is or has been an advisor/consultant to: Abbott Laboratories, Inc., Abdi Ibrahim, Akzo (Organon Pharmaceuticals Inc.), Alkermes, AstraZeneca, Axon Advisors, Bristol-Myers Squibb Company, Cephalon, Inc., Cerecor, Concert Pharmaceuticals, Inc., Eli Lilly & Company, Evotec, Fabre Kramer Pharmaceuticals, Inc., Forest Pharmaceuticals, GlaxoSmithKline, Janssen Global Services, LLC, Janssen Pharmaceutica Products, LP, Johnson & Johnson PRD, Libby, Lundbeck, Meade Johnson, MedAvante, Medtronic, Merck, Mitsubishi Tanabe Pharma Development America, Inc., Naurex, Neuronetics, Otsuka Pharmaceuticals, Pamlab, Parke-Davis Pharmaceuticals, Inc., Pfizer Inc., PgxHealth, Phoenix Marketing Solutions, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Products Ltd., Sepracor, SHIRE Development, Sierra, SK Life and Science, Sunovion, Takeda, Tal Medical/Puretech Venture, Targacept, Transcept, VantagePoint, Vivus, and Wyeth-Ayerst Laboratories. In addition, he has received research support from: Agency for Healthcare Research and Quality (AHRQ), Corcept Therapeutics, Inc., Cyberonics, Inc., National Alliance for Research in Schizophrenia and Depression, National Institute of Mental Health, National Institute on Drug Abuse, Novartis, Pharmacia & Upjohn, Predix Pharmaceuticals (Epix), and Solvay Pharmaceuticals, Inc. Benji Kurian has received grant support from the following additional sources: Targacept, Inc.; Pfizer, Inc.; Johnson & Johnson; Evotec; Rexahn; Naurex; Forest Pharmaceuticals. Melvin McInnis has consulted for and been on speakers bureau with Janssen, Merck, and Lily Pharmaceuticals in the past five years. For a comprehensive list of lifetime disclosures of Dr. Fava, see http://mghcme.org/faculty/faculty-detail/maurizio_fava. Over the past three years, Dr. Pizzagalli has received honoraria/consulting fees from Advanced Neuro Technology North America, AstraZeneca, Otsuka America Pharmaceutical, Pfizer, and Servier.
References
- Ambady N, Gray HM. On being sad and mistaken: mood effects on the accuracy of thin-slice judgments. Journal of Personality and Social Psychology. 2002;83:947–961. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Andrews PW, Aggen SH, Miller GF, Radi C, Dencoff JE, Neale MC. The functional design of depression’s influence on attention: A preliminary test of alternative control-process mechanisms. Evolutionary Psychology. 2007;5:584–604. [Google Scholar]
- Andrews PW, Thomson JA., Jr The bright side of being blue: Depression as an adaptation for analyzing complex problems. Psychological Review. 2009;116:620–654. doi: 10.1037/a0016242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au K, Chan F, Wang D, Vertinsky I. Mood in foreign exchange trading: Cognitive processes and performance. Organizational Behavior and Human Decision Processes. 2003;91:322–328. [Google Scholar]
- Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? American Journal of Psychiatry. 2004;161:2163–2177. doi: 10.1176/appi.ajp.161.12.2163. [DOI] [PubMed] [Google Scholar]
- Bogacz R, Brown E, Moehlis J, Holmes P, Cohen JD. The physics of optimal decision making: a formal analysis of models of performance in two-alternative forced-choice tasks. Psychological Review. 2006;113:700–765. doi: 10.1037/0033-295X.113.4.700. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Alvarenga JE, Alshculer D, Abraham K, Keilp JG, Hellerstein DJ, Stewart JW, McGrath PJ. Neurocognitive predictors of antidepressant clinical response. Journal of Affective Disorders. 2014;166:108–114. doi: 10.1016/j.jad.2014.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends in Neuroscience. 1990;13:277–280. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- Chiu PH, Deldin PJ. Neural evidence for enhanced error detection in major depressive disorder. American Journal of Psychiatry. 2007;164:608–616. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Rosso IM, Pechtel P, Killgore WDS, Rauch SL, Pizzagalli DA. Peril and pleasure: an RDoC-inspired examination of threat responses and reward processing in anxiety and depression. Depression and Anxiety. 2014;31:233–249. doi: 10.1002/da.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkin C, Brown S, Heathcote A, Wagenmakers E-J. Diffusion versus linear ballistic accumulation: different models but the same conclusions about psychological processes? Psychonomic Bulletin & Review. 2011;18:61–69. doi: 10.3758/s13423-010-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal S, Jouvent R. Time-on-task effect in trait anhedonia. European Psychiatry. 2004;19:285–291. doi: 10.1016/j.eurpsy.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Dubal S, Pierson A, Jouvent R. Focused attention in anhedonia: a P3 study. Psychophysiology. 2000;37:711–714. [PubMed] [Google Scholar]
- Dutilh G, Vandekerckhove J, Forstmann BU, Keuleers E, Brysbaert M, Wagenmakers E-J. Testing theories of post-error slowing. Attention, Perception & Psychophysics. 2012;74:454–465. doi: 10.3758/s13414-011-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. American Journal of Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Franken IHA, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS) Journal of Affective Disorders. 2007;99:83–89. doi: 10.1016/j.jad.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. Optimizing the use of information: Strategic control of activation of responses. Journal of Experimental Psychology: General. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Dynamic dopamine modulation in the basal ganglia: A neurocomputational account of cognitive deficits in medicated and non-medicated parkinsonism. Journal of Cognitive Neuroscience. 2005;17:51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel PT. On the contributions of deficient cognitive control to memory impairments in depression. Cognition and Emotion. 1997;11:569–583. [Google Scholar]
- Holmes AJ, Bogden R, Pizzagalli DA. Serotonin transporter genotype and action monitoring dysfunction: a possible substrate underlying increased vulnerability to depression. Neuropsychopharmacology. 2010;35:1186–1197. doi: 10.1038/npp.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Archives of General Psychiatry. 2008;65:179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Effects of task-relevant incentives on the electrophysiological correlates of error processing in major depressive disorder. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:119–128. doi: 10.3758/CABN.10.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner R, Steinhauser M, Lehle C. A dual-stage two-phase model of selective attention. Psychological Review. 2010;117:759–784. doi: 10.1037/a0019471. [DOI] [PubMed] [Google Scholar]
- Laming D. Autocorrelation of choice-reaction times. Acta Psychologica. 1979;43:381–412. doi: 10.1016/0001-6918(79)90032-5. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Progress in Neurobiology. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Montague PR, Dolan RJ, Friston KJ, Dayan P. Computational psychiatry. Trends in Cognitive Sciences. 2012;16:72–80. doi: 10.1016/j.tics.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Noorani I, Carpenter RHS. Antisaccades as decisions: LATER model predicts latency distributions and error responses. European Journal of Neuroscience. 2013;37:330–338. doi: 10.1111/ejn.12025. [DOI] [PubMed] [Google Scholar]
- Pe ML, Vandekerckhove J, Kuppens P. A diffusion model account of the relationship between the emotional flanker task and rumination and depression. Emotion. 2013;13:739–747. doi: 10.1037/a0031628. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal of Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell MJD. An efficient method for finding the minimum of a function of several variables without calculating derivatives. Computer Journal. 1964;7:155–162. [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. URL http://www.R-project.org/ [Google Scholar]
- Rabbitt PM. Errors and error correction in choice-response tasks. Journal of Experimental Psychology. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Frank MJ. Reinforcement-based decision making in corticostriatal circuits: Mutual constraints by neurocomputational and diffusion models. Neural Computation. 2012;24:1186–1229. doi: 10.1162/NECO_a_00270. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, McKoon G. The diffusion decision model: theory and data for two-choice decision tasks. Neural Computation. 2008;20:873–922. doi: 10.1162/neco.2008.12-06-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, Meier BP, Wilkowski BM, Ode S. Introversion, inhibition, and displayed anxiety: The role of error reactivity processes. Journal of Research in Personality. 2007;41:558–578. [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-item Quick Inventory of Depressive Symptomatology (QIDS) Clinician Rating (QIDS-C) and Self-Report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME. Pupillary assessment and computational modeling of the Stroop task in depression. International Journal of Psychophysiology. 2004;52:63–76. doi: 10.1016/j.ijpsycho.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone: the Snaith-Hamilton Pleasure Scale. British Journal of Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychological Bulletin. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Kaiser RH, Whisman MA, Turner AEJ, Guild RM, Munakata Y. Opposite effects of anxiety and depressive symptoms on executive function: the case of selecting among competing options. Cognition and Emotion. 2014;28:893–902. doi: 10.1080/02699931.2013.859568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BP, Bruder GE, Stewart JW, McGrath PJ, Halperin J, Ehrlichman H, Quitkin FM. Psychomotor slowing as a predictor of fluoxetine nonresponse in depressed outpatients. American Journal of Psychiatry. 2006;163:73–78. doi: 10.1176/appi.ajp.163.1.73. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neuroscience and Biobehavioral Reviews. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BM, Sederberg PB. A generalized, likelihood-free method for posterior estimation. Psychonomic Bulletin & Review. 2014 doi: 10.3758/s13423-013-0530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallesi A, Canalaz F, Balestrieri M, Brambilla P. Modulating speed-accuracy strategies in major depression. Journal of Psychiatric Research. 2015;60:103–108. doi: 10.1016/j.jpsychires.2014.09.017. [DOI] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Köhler S, Marinou V, Mentzel HJ, Sauer H, Schlösser RG. Cortical inefficiency in patients with unipolar depression: an event-related fMRI study with the Stroop task. Biological Psychiatry. 2006;59:958–965. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Wales DJ, Doye JPK. Global optimization by basin-hopping and the lowest energy structures of Lennard-Jones clusters containing up to 110 atoms. Journal of Physical Chemistry A. 1997;101:5111–5116. [Google Scholar]
- White CN, Ratcliff R, Starns JJ. Diffusion models of the flanker task: discrete versus gradual attentional selection. Cognitive Psychology. 2011;63:210–238. doi: 10.1016/j.cogpsych.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiecki TV, Frank MJ. Neurocomputational models of motor and cognitive deficits in Parkinson’s disease. Progress in Brain Research. 2010;183:275–297. doi: 10.1016/S0079-6123(10)83014-6. [DOI] [PubMed] [Google Scholar]
- Wiecki TV, Frank MJ. A computational model of inhibitory control in frontal cortex and basal ganglia. Psychological Review. 2013;120:329–355. doi: 10.1037/a0031542. [DOI] [PubMed] [Google Scholar]
- Wiecki TV, Poland JS, Frank MJ. Model-based cognitive neuroscience approaches to computational psychiatry: clustering and classification. Clinical Psychological Science (in press) [Google Scholar]
- Wiecki TV, Riedinger K, Meyerhofer A, Schmidt W, Frank MJ. A neurocomputational account of catalepsy sensitization induced by D2-receptor-blockade in rats: Context-dependency, extinction, and renewal. Psychopharmacology. 2009;204:265–277. doi: 10.1007/s00213-008-1457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.