Abstract
Background
The objective of this study was to investigate the change of platelet function and platelet mitochondrial membrane potential in contentious-flow left ventricular assist device (CF-LVAD) implanted heart failure (HF) patients with or without systemic inflammatory response syndrome (SIRS).
Methods and Results
We recruited 31 CF-LVAD patients (16 SIRS and 15 Non-SIRS) and 11 healthy volunteers as the control. Pre and post implant blood samples were collected. We used PFA-100 to test the platelet functionality. Mitochondrial potential sensitive dye was used to detect platelet dysfunction (ΔΨm) via flow cytometry. The percentage of depolarized ΔΨm platelets was found to be pre-existing conditions in all HF patients prior to CF-LVAD implantation compared to controls (10.3±6.3vs.2.8±2.2%,p<0.001). As evident from PFA-100 test, The HF patients who developed SIRS after CF-LVAD implantation had significantly higher qualitative platelet defects and thrombocytopathies compared to baseline level. After implantation, the depolarized platelets in the SIRS patients increased by 2-fold compared to the baseline (18.2±8.4vs.9.0±6.6%,p<0.01); while no change was noticed in the Non-SIRS patients (10.9±6.2vs.11.7±5.8%,p=0.75).
Conclusions
We identified that the platelet function and mitochondrial damage were enhanced in CFLVAD patients with SIRS. Our findings suggest that depolarization of mitochondrial membrane potential is associated with SIRS after CF-LVAD implant surgery.
Keywords: Heart failure, left ventricular assist device, systemic inflammatory response syndrome, platelets mitochondrial dysfunction
Introduction
Continuous-flow Left ventricular assist device (CF-LVAD) therapy has evolved into a standard therapy for patients with advanced heart failure (HF), either as a destination therapy or a bridge to cardiac transplantation or a bridge to myocardial recovery.1 Despite demonstrating significant improvements in survival with CF-LVAD when compared to the older, pulsatile devices; systemic inflammatory response syndrome (SIRS), major infections, renal dysfunction, non-surgical bleeding, transient ischemic attack or stroke, respiratory failure and right ventricular failure continues to be the major obstacles in advancing the LVAD therapy.2–5
SIRS is a whole-body inflammation and still remains a major clinical problem, despite improved diagnostics and therapy. Clinically, SIRS is identified by two or more symptoms including fever or hypothermia, tachycardia, tachypnoea and change in blood leucocyte count.6 Thus peripheral vasodilatation, loss of volume due to capillary leakage, myocardial dysfunction and dysfunction of major organs, are also summarized as SIRS.7–9 Cardiac surgery for the placement of CF-LVAD may be associated with the development of a systemic inflammatory response that can often lead to dysfunction of major organs. The systemic inflammation can be assessed by measuring concentrations of inflammatory mediators in plasma and tissues but the concentrations, however, do not always correlate with the degree of observed organ dysfunction.9
Although the primary function of platelets is to prevent excessive bleeding after an injury and to survey and maintain the integrity of the endothelium, there is growing evidence that platelets play an active role in fighting infections and in innate immunity.10-13 It has been reported that the depolarization of platelet mitochondrial membrane potential (ΔΨm) is associated with the progression of SIRS in critically ill patients.14 Mitochondrial membrane potential (ΔΨm) is the parameter that reflects mitochondrial function and is an indicator of mitochondrial energy status.15 Mitochondrial membrane alterations have been observed in chronic disorders such as heart failure,16 sepsis-induced multi-organ failure,17 right ventricular failure,18 end-stage renal disease19 and diabetes mellitus.20
SIRS frequently occurs in HF patients after CF-LVAD implantation. The knowledge of platelet functionality in this sub-group of CF-LVAD patients is limited. Thus, the purpose of the present study was to investigate the change of platelet function and platelet mitochondrial membrane potential in circulating blood of CF-LVAD patients with SIRS and to clarify whether depolarized ΔΨm platelets can reflect systemic mitochondrial dysfunction in clinical settings after CF-LVAD surgery and might be a useful marker for post implant SIRS development in CF-LVAD patients.
Materials and Methods
Subjects
From 2010 to 2014, we were only able to consent 46 HF patients who were implanted with LVADs, either as a bridge to transplant or destination therapy, at the University of Maryland Medical Center. Not all the patients were consented for the study. Out of 46, we were able to find only 16 patients who meet the criteria for the SIRS within first week after CF-LVAD implantation (SIRS-group). SIRS in those patients was defined according to the guidelines of American College of Chest Physicians and the Society of Critical Care Medicine.21 On the other hand we had selectively chosen 15 patients form rest of 30 consented patients who did not fall into SIRS criteria. These 15 patients (Non-SIRS) were selected in such a way so that they can match with the SIRS group in respect to their demographic and baseline clinical characteristics. So this study did not represent the whole patient’s population during that time frame rather a subgroup of 31 patients from 46 patients. The CF-LVADs implanted included the HeartMate II (Thoratec Corp, Pleasanton, CA) in 12 patients (6 Non-SIRS and 6 SIRS), the Jarvik 2000 (Jarvik Heart, New York, NY) in 10 patients (6 Non-SIRS and 4 SIRS), and the HeartWare HVAD (HeartWare Inc, Framingham, MA) in 9 patients (4 Non-SIRS and 5 SIRS). All the procedures involving collection of human blood were approved by the Institutional Review Board (IRB). All the patients and volunteers gave their written informed consent and were informed about the aim of the study.
Anticoagulation/Antiplatelet Treatment
After CF-LVAD implantation, anticoagulation was initiated with a titrated heparin dose with the goal for partial thromboplastin time of 40-45s once chest drainage was less than 30 mL/h for at least 4 hours. Thereafter the goal was aimed to have an anti-Xa activity level of 0.1-0.15 U/mL. The anticoagulation medication was subsequently converted to warfarin with a targeted international normalized ratio (INR) from 1.8 to 2.3 for the HeartMate II, 2 to 3 for the Jarvik and the HeartWare. Antiplatelet agents were added to the anticoagulation regimen and the dosage was titrated based on measurements of platelet function using a platelet function analyzer (PFA-100® (Dade Behring, Inc, Deerfield, IL) and thrombelastogram (TEG) (TEG® 5000 Thrombelastograph® Hemostasis Analyzer System, Haemonetics Corporation, Braintree, MA). All the patients received pentoxifylline to improve RBC deformability in the hope of mitigating shear-induced hemolysis.
Collection and Preparation of Blood Sample
EDTA/Citrate-anticoagulated blood samples from the HF patients were collected at baseline (pre-operative: Pre-OP) and one week after the CF-LVAD implant surgery. Based on the expected post-transfusion recovery and life span of transfused platelets, we had collected blood samples after 24 to 48 hours of platelet transfusion (if any). Blood samples from the healthy donors were collected once. All the blood samples from the HF patients and the healthy volunteers were aliquoted and processed immediately according to the standardized study protocols.
Platelet Function Measurements
Measurements of platelet function with the PFA-100® were performed according to the manufacturer’s instruction using the collagen/adenosine-5′-diphosphate (CAPD) cartridge and the collagen/epinephrine (CEPI) cartridge. The Platelet Function Analyzer (PFA)-100 measures platelet function in a high shear environment and is well suited to the detection of platelet dysfunction in the clinical setting. The instrument records the time for platelets to occlude an aperture with a membrane coated with collagen and either epinephrine (CEPI) or ADP (CADP). CEPI cartridges detect qualitative platelet defects while CADP cartridges detect only thrombocytopathies which is characterized by dysfunctional platelets resulting in prolonged bleeding time, defective clot formation, and a tendency to hemorrhage. We also used the TEG parameters to assess the platelet function. The TEG parameters: TEG-maximum amplitude (TEG-MA), kinetic time (TEG-KT) and angle (TEG-Angle) were analyzed.
Measurement of Platelet Mitochondrial Damage
Platelet mitochondrial damage was measured by flow cytometry using mitochondrial membrane potential (ΔΨm)-sensitive dye, tetramethylrhodamine ethyl ester (TMRE), a commercially available MitoPT® TMRE Assay Kit (ImmunoChemistry Technologies, LLC, Bloomington, MN). TMRE is a cell permeant, positively-charged, red-orange dye that readily accumulates in active mitochondria due to their relative negative charge. Depolarized or inactive mitochondria have decreased membrane potential and fail to sequester TMRE. Thus TMRE measured the mitochondrial integrity of platelets by monitoring the loss of fluorescence intensity. The level of platelet mitochondrial damage was displayed as % of depolarized ΔΨm platelets.
Statistical Analyses
The data are presented as mean±SD (standard deviation) or median with interquartile range (IQR) and statistically analyzed using SPSS statistical software (Statistical Package for Social Sciences for windows, release 18.0; SPSS Inc., Chicago, IL, USA). Statistical differences were determined by using Chi-square test, Student’s t-test and Mann-Whitney U test, as applicable. Univariate analysis was carried out using Spearman’s rank correlation test to find out the relation between two measurable parameters as continuous variables, and the result was expressed as ρ (rho) value. Statistical significance was assigned at p<0.05.
Results
SIRS and demography
In our study, we were only able to include 16 patients who developed SIRS within first week after CF-LVAD implantation. Comparative analyses of demographic and clinical characteristics of the patients in the SIRS group and those who did not experience SIRS (Non-SIRS group) before CF-LVAD implantation were summarized in Table 1.
Table 1.
Demographic and baseline clinical characteristics of HF patients prior to CF-LVAD implantation
| Characteristics | Pre-implant HF patients (n = 31) |
|
|---|---|---|
| Non-SIRS (n = 15) |
SIRS (n = 16) |
|
| Demography | ||
| Age in years, median (IQR) | 63 (25 – 76) | 63 (48 – 71) |
| Sex, n (% male) | 13 (86.7 %) | 14 (87.5 %) |
| Race | ||
| Caucasian white, n (%) | 7 (46.7%) | 8 (50.0%) |
| Black, n (%) | 8 (53.3 %) | 8 (50.0 %) |
| Height in meter, median (IQR) | 1.8 (1.7 – 1.9) | 1.8 (1.6 – 1.9) |
| Weight in kilograms, median (IQR) | 84.0 (53.0 – 119.4) | 88.0 (68.9 – 127.9) |
| Body mass index (kg/m2), median (IQR) | 26.7 (19.5 – 39.0) | 31.2 (23.0 – 38.2) |
| Body surface area (m2), median (IQR) | 2.1 (1.6 – 2.4) | 2.2 (1.8 – 2.5) |
| History of smoking, n (%) | 3 (20 %) | 4 (25.0 %) |
| History of substance abuse | ||
| Ethyl alcohol abuse, n (%) | 2 (13.3 %) | 3 (18.7 %) |
| Drug abuse, n (%) | 2 (13.3 %) | 4 (25.0 %) |
| Vital signs | ||
| Systolic blood pressure (mmHg), mean±SD | 103.4 ± 18.5 | 100.3 ± 7.1 |
| Diastolic blood pressure (mmHg), mean±SD | 67.0 ± 18.2 | 64.5 ± 6.8 |
| Etiology of heart disease | ||
| Ischemic cardiomyopathy, n (%) | 7 (46.7%) | 5 (31.3 %) |
| Non-ischemic cardiomyopathy, n (%) | 6 (40.0%) | 6 (37.5 %) |
| Idiopathic Cardiomyopathy, n (%) | 2 (13.3 %) | 5 (31.3 %) |
| Echocardiographic parameters | ||
| Left ventricular end diastolic diameter (mm), mean±SD |
72.0 ± 11.0 | 67.6 ±11.4 |
| Left ventricular ejection fraction, (%), mean±SD |
12.5 ± 3.8 | 13.3 ± 4.1 |
Adverse Events after Implantation
Table 2 lists adverse events and clinical complications of the HF patients in both the Non-SIRS and SIRS groups after CF-LVAD implantation. Adverse clinical complications after CF-LVAD implantation were found to be more prominent in the SIRS group (Table 2).
Table 2.
Adverse events of HF patients with or without SIRS after CF-LVAD implantation
| Adverse events | Pre-implant HF patients (n=31) |
|
|---|---|---|
| Non-SIRS (n =15) |
SIRS (n =16) |
|
| Major infections | 0 | 5 |
| Renal dysfunction | 4 | 4 |
| Bleeding | 5 | 7 |
| Respiratory failure | 2 | 4 |
| Stroke | 2 | 4 |
| Right ventricular failure | 3 | 6 |
| Device malfunction | 1 | 1 |
Laboratory Hematology and Blood Chemistry
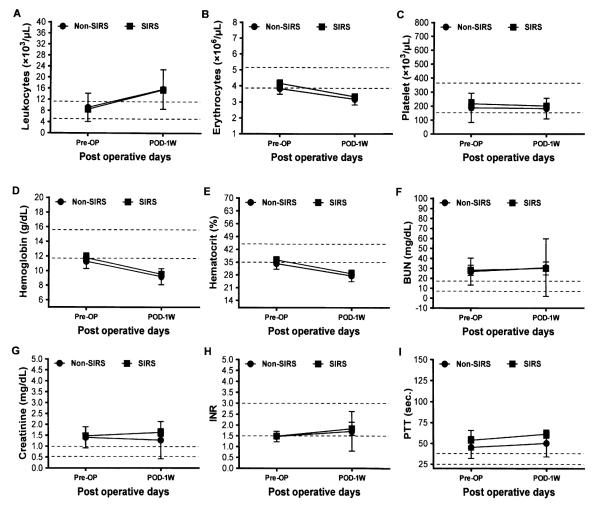
The routine laboratory hematology and the blood chemistry tests of the patients in each group before and after implantation are summarized in Figure 1. There were no significant differences in the hematology and blood chemistry parameters between the two groups before and after implantation although we noticed higher leukocyte counts in the two groups compared to their baseline values. The decreasing trends of erythrocytes, hemoglobin and hematocrit counts were also similar in the two groups (Figure 1).
Figure 1.
Laboratory hematology and blood chemistry tests of the Non-SIRS and SIRS groups of the HF patients before (Pre-OP) and after one week (POD-1W) of CF-LVAD implantation. Data are expressed as mean±SD. The dotted lines in each line diagram indicating the normal high and normal low values of each parameter measured.
Whole blood hemostasis tests
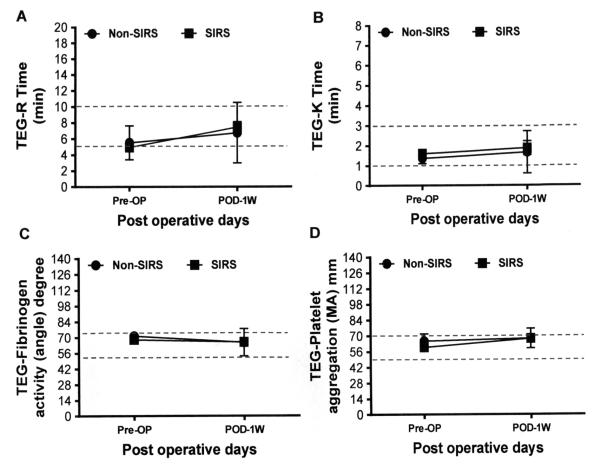
The differences in the thromboelastogram data between the Non-SIRS and the SIRS groups are shown in Figure 2. There were no significant differences in the severity of the primary hemostatic defect indicated by these tests between the Non-SIRS and SIRS groups.
Figure 2.
Parameters of whole blood hemostasis test of the Non-SIRS and SIRS groups of HF patients before (Pre-OP) and after one week (POD-1W) of CF-LVAD implantation. Data are expressed as mean±SD. The dotted lines in each line diagram indicating the normal high and normal low values of each parameter measured.
Platelet Function Tests
We noticed that the mean closure times (CTs) for the CADP (186.8±30.8 vs. 118.0±11.7 sec., p=0.028, Student’s t-test) and CEPI (208.5±21.8 vs. 168.7±18.2 sec., p=0.1781) cartridges before CFLVAD implantation were higher in the Non-SIRS group when compared to the SIRS group. In the Non-SIRS group, the mean CTs for the CADP and the CEPI cartridges were 27% (186.8±30.8 vs. 237.4±15.2 sec., p>0.05) and 16% (208.5± vs. 241.3±14.0 sec., p>0.05) higher after CF-LVAD implantation in comparison to the baseline values, respectively. But in the SIRS group, the post-implant mean CTs for the CADP and the CEPI cartridges significantly increased by 2-fold (214.4±13.5 vs. 118.0±11.7 sec., p<0.0001) and 1.6-fold (266.7±8.2 vs. 168.7±18.2 sec., p<0.0001), respectively, when compared to their corresponding baseline values.
Change in Platelet Mitochondrial Membrane Potential
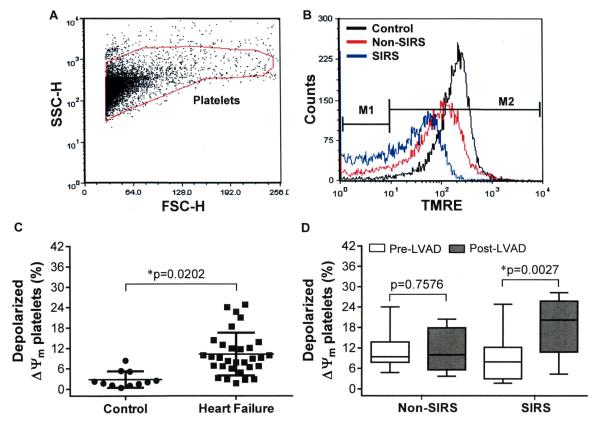
The flow cytometric analysis of the platelet mitochondrial dysfunction is depicted in the figure 3A and B. Compared to the healthy volunteers, the HF patients had 3.7-fold higher % of depolarized ΔΨm platelets prior to CF-LVAD implantation, indicating a pre-existing condition of platelet mitochondrial damage in these patients (Figure 3C). The difference in the baseline levels of depolarized platelets between the Non-SIRS and SIRS groups were not statistically significant (11.7±5.8 vs 9.0±6.6%, p=0.2463, Students t-test). We noticed the depolarized platelets became doubled in the SIRS group after CF-LVAD implantation in comparison to the baseline level while no significant change was noticed in the Non-SIRS group (Figure 3D). Thus the platelet mitochondrial dysfunction was more prominent in the SIRS group after CF-LVAD implantation.
Figure 3.
The change in % depolarized ΔΨm platelets among the Non-SIRS and SIRS groups before and after CF-LVAD implantation. (A) FSC versus SSC dot plot. The gate indicates position of platelets. (B) The distribution histograms show the change in TMRE fluorescence in the control, Non-SIRS and SIRS groups. (C) Scatter plot representing the differences in % depolarized ΔΨm platelets between the healthy controls and the HF patients at the baseline. *p<0.05 is considered significant in Student’s t-test. (D) Box-whisker plot shows the differences in % depolarized ΔΨm platelets before and after CF-LVAD implantation in the Non-SIRS and SIRS groups. The lines across each box plot represent the median value. The lines that extend from the top and the bottom of each box represent the lowest and highest observations still inside the lower and upper limit of confidence. *p<0.05 is considered significant in Mann–Whitney U-test.
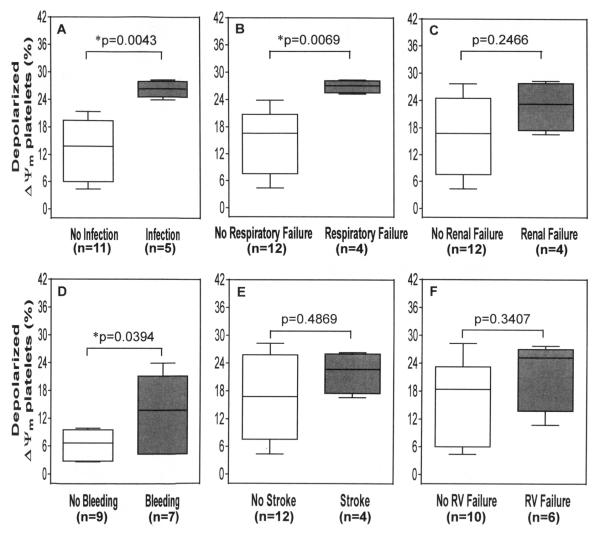
Figure 4 shows the differences in depolarized platelets in the SIRS group with respect to different clinical complications after CF-LVAD implantation. The SIRS patients with major infection, respiratory failure and major bleeding had significantly higher depolarized platelets than the patients with SIRS alone. The depolarized platelets were also higher in the SIRS patients with renal failure, stroke and right ventricular failure but these differences were not found to be statistically significant (Figure 4).
Figure 4.
The change in % depolarized ΔΨm platelets associated with different adverse events after CF LVAD implantation among the SIRS group. The lines across each box plot represent the median value. The lines that extend from the top and the bottom of each box represent the lowest and highest observations still inside the lower and upper limit of confidence. *p<0.05 is considered significant in Mann–Whitney U-test.
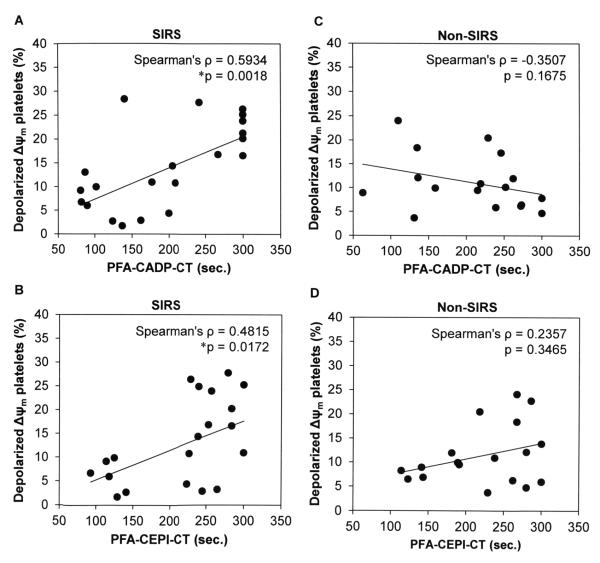
Relationship between mitochondrial dysfunction and platelet function in SIRS
To examine any relationship between mitochondrial dysfunction and platelet function tests, we conducted Spearman’s rank correlation test between the depolarized ΔΨm platelets and PFA-100 tests among both the Non-SIRS and SIRS groups of the CF-LVAD patients. Considering the SIRS group, when the percentages of depolarized ΔΨm platelets were plotted against the PFA-CADP-CT and PFA-CEPI-CT values, we noticed a statistically significant positive association especially with PFA-CADP-CT (Figure 5A-B). Contrary to this, in the Non-SIRS group, we did not notice any significant association between the mitochondrial dysfunction and platelet function tests; rather a non-signifant negative correlation was established between depolarized platelets and PFA-CADP-CT (Figure 5C). In the Non-SIRS group, the mitochondrial dysfunction was not correlated with the PFA-CEPI-CT (Figure 5D).
Figure 5.
Relationship between mitochondrial dysfunction and platelet function tests in both the SIRS (A,B) and Non-SIRS groups (C,D).
Discussion
This is the first report that evaluated platelet functionality and the change of platelet mitochondrial membrane potential in CF-LVAD patients with SIRS. In our study, The HF patients who developed SIRS after CF-LVAD implantation had significantly higher qualitative platelet defects (PFA-CEPI-CT) and thrombocytopathies (PFA-CADP-CT) when compared to the baseline levels. The elevated CT for the CEPI cartridge in the Non-SIRS group might be related to drug therapy or others before implantation when compared to the SIRS group. The finding that both the CADP and CEPI are significantly increased by 2-fold and 1.6 fold, respectively, in the SIRS group suggests that platelet function is abnormal in those HF patients who developed SIRS after CF-LVAD implantation while no significant changes were observed in the CF-LVAD patients without SIRS. Like platelet function, the depolarization of platelet mitochondrial membrane potential also significantly rose in the CF-LVAD patients with SIRS compared with that in the CF-LVAD patients without SIRS and the healthy volunteers. Circulating normal blood platelets contain fully functional mitochondria. Accumulating evidence indicates that these mitochondria regulate the pro-thrombotic function of platelets through energy generation.22 The change of platelet mitochondrial membrane potential is an indicator of mitochondrial energy status.15,23,24 Thus mitochondrial membrane potential is the parameter that reflects mitochondrial function and can be used to evaluate abnormality of platelet function.
Besides SIRS, the HF patients also experienced other adverse events and clinical complications after CF-LVAD implantation. In the SIRS group, 5 patients had major infections either at the driveline of the CF-LVAD or in bloodstream, GI tract and mediastinum due to bacterial attack. One patient in the SIRS group had pulmonary infection with unknown cause. The SIRS patients with infection are considered as sepsis. A recent study suggests that mitochondrial dysfunction of platelets correlates with clinical disease activity in sepsis.25 In our study, the depolarization of mitochondrial membrane potential became doubled in sepsis condition (SIRS with major infection) when compared to SIRS only. Non-surgical bleeding is another important adverse event occurred in HF patients with CF-LVAD support irrespective of the SIRS. Forty four percent of the SIRS patients had bleeding complication. The depolarized platelets were also significantly higher among these patients. In a recent study we showed that platelet dysfunction is related to non-surgical bleeding after CF-LVAD implantation .26,27 Thus, apart from contributing towards the cell’s energy metabolism, the role of mitochondria as pivotal mediators of intrinsic pathway of apoptosis was also established in our previous study. Moreover, the occurrence of respiratory failure and the right ventricular failure were also common in the SIRS patients with CF-LVAD when compared to the Non-SIRS patients and the platelet mitochondrial membrane depolarization elevated in these conditions. Our finding is consistent with other reports in which the patients with SIRS and multiorgan dysfunction had elevated depolarized platelets. Thus the change in platelet mitochondrial membrane potential may be associated with the progression of SIRS.14
In the present study, the % of depolarization ΔΨm platelet significantly correlated with CTs of CADP and CEPI in the CF-LVAD patients with SIRS, suggesting that the mitochondrial energy status is associated with qualitative platelet defects and thrombocytopathies. While in Non-SIRS CF-LVAD patients, we did not notice any significant correlation. Thus, the present study revealed that the platelet functional abnormalities were related to the change of mitochondrial energy status only in the patients who developed SIRS after CF-LVAD implantation. Despite having a relatively common physiologic pathway, SIRS has numerous triggers, and patients may response in various manners. The exact mechanisms of how the change in mitochondrial energy status modulates platelet function in the CFLVAD patients with SIRS are still not clear and further studies are warranted in this exciting field.
Platelet function was tightly associated with the process of systemic inflammation. The study of platelet dysfunction may be an important marker for CF-LVAD associated systemic inflammation. Platelets are also a valuable tool to study human mitochondria. We identified that the platelet function and mitochondrial damage were enhanced in CF-LVAD patients with SIRS. Our findings may suggest that depolarization of mitochondrial membrane potential is associated with SIRS after CF-LVAD implant surgery. The rapid advancement of our understanding of platelet mitochondrial biology not only provides information about the mechanisms governing haemostasis, but also contributes to our ability to translate mitochondrial research to clinical complications associated with CF-LVAD implantation in heart failure patients.
We acknowledge that there are some limitations in this prospective observational study. This is a single-center study and the sample size was relatively small. Not all CF-LVAD patients were enrolled for this study. The effects of antiplatelet drugs on platelet function and change in mitochondrial membrane potential may need to be explored. The anticoagulation treatments for the two groups were similar and had no impact on platelet function in our current study. Other medication side effects or pharmacologic properties may either induce or mask SIRS. Recent changes in medications should be addressed in further studies to rule out drug-drug interactions or a new side effect. A larger cohort of CF-LVAD patients with SIRS and other associated clinical complications is needed to confirm our initial findings.
Acknowledgments
Source of Funding: The described research was partially sponsored by the National Institutes of Health (Grant R01 HL 088100).
Footnotes
Disclosure
All authors report no disclosures relevant to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, Burke M, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355:1873–84. doi: 10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]
- 2.Lietz K. Destination therapy: patient selection and current outcomes. J Card Surg. 2010;25:462–71. doi: 10.1111/j.1540-8191.2010.01050.x. [DOI] [PubMed] [Google Scholar]
- 3.Lahpor J, Khaghani A, Hetzer R, Pavie A, Friedrich I, Sander K, et al. European results with a continuous-flow ventricular assist device for advanced heart failure patients. Eur J Cardiothorac Surg. 2010;37:357–61. doi: 10.1016/j.ejcts.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 4.Crow S, John R, Boyle A, Shumway S, Liao K, Colvin-Adams M, et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;137:208–15. doi: 10.1016/j.jtcvs.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Stern DR, Kazam J, Edwards P, Maybaum S, Bello RA, D’Alessandro DA, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg. 2010;25:352–6. doi: 10.1111/j.1540-8191.2010.01025.x. [DOI] [PubMed] [Google Scholar]
- 6.Comstedt P, Storgaard M, Lassen AT. The Systemic Inflammatory Response Syndrome (SIRS) in acutely hospitalised medical patients: a cohort study. Scand J Trauma Resusc Emerg Med. 2009;17:67. doi: 10.1186/1757-7241-17-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieman G, Searles B, Carney D, McCann U, Schiller H, Lutz C, et al. Systemic inflammation induced by cardiopulmonary bypass: a review of pathogenesis and treatment. J Extra Corpor Technol. 1999;31:202–10. [PubMed] [Google Scholar]
- 8.Prondzinsky R, Müller-Werdan U, Pilz G, Witthaut R, Stabenow I, Werdan K, et al. Systemic inflammatory reactions to extracorporeal therapy measures (II): Cardiopulmonary bypass. Wien Klin Wochenschr. 1997;109:346–53. [PubMed] [Google Scholar]
- 9.Asimakopoulos G. Systemic inflammation and cardiac surgery: an update. Perfusion. 2001;16:353–60. doi: 10.1177/026765910101600505. [DOI] [PubMed] [Google Scholar]
- 10.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 11.Kraemer BF, Campbell RA, Schwertz H, Cody MJ, Franks Z, Tolley ND, et al. Novel anti-bacterial activities of β-defensin 1 in human platelets: suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 2011;7:e1002355. doi: 10.1371/journal.ppat.1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semple JW1, Freedman J. Platelets and innate immunity. Cell Mol Life Sci. 2010;67:499–511. doi: 10.1007/s00018-009-0205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speth C, Rambach G1, Lass-Flörl C. Platelet immunology in fungal infections. Thromb Haemost. 2014;112(3) doi: 10.1160/TH14-01-0074. [DOI] [PubMed] [Google Scholar]
- 14.Yamakawa K, Ogura H, Koh T, Ogawa Y, Matsumoto N, Kuwagata Y, et al. Platelet mitochondrial membrane potential correlates with severity in patients with systemic inflammatory response syndrome. Trauma Acute Care Surg. 2013;74:411–7. doi: 10.1097/TA.0b013e31827a34cf. [DOI] [PubMed] [Google Scholar]
- 15.Cossarizza A, Baccarani-Contri M, Kalashnikova G, Franceschi C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1) Biochem Biophys Res Commun. 1993;197:40–5. doi: 10.1006/bbrc.1993.2438. [DOI] [PubMed] [Google Scholar]
- 16.Maloyan A, Sanbe A, Osinska H, Westfall M, Robinson D, Imahashi K, et al. Mitochondrial dysfunction and apoptosis underlie the pathogenic process in alpha-B-crystallin desmin-relatedcardiomyopathy. Circulation. 2005;112:3451–61. doi: 10.1161/CIRCULATIONAHA.105.572552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014;5:66–72. doi: 10.4161/viru.26907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagendran J, Gurtu V, Fu DZ, Dyck JR, Haromy A, Ross DB, et al. A dynamic and chamber specific mitochondrial remodeling in right ventricular hypertrophy can be therapeutically targeted. J Thorac Cardiovasc Surg. 2008;136:168–78. doi: 10.1016/j.jtcvs.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 19.Raj DS, Boivin MA, Dominic EA, Boyd A, Roy PK, Rihani T, et al. Haemodialysis induces mitochondrial dysfunction and apoptosis. Eur J Clin Invest. 2007;37:971–7. doi: 10.1111/j.1365-2362.2007.01886.x. [DOI] [PubMed] [Google Scholar]
- 20.Guo X, Wu J, Du J, Ran J, Xu J. Platelets of type 2 diabetic patients are characterized by high ATP content and low mitochondrial membrane potential. Platelets. 2009;20:588–93. doi: 10.3109/09537100903288422. [DOI] [PubMed] [Google Scholar]
- 21.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 22.Zharikov S, Shiva S. Platelet mitochondrial function: from regulation of thrombosis to biomarker of disease. Biochem Soc Trans. 2013;41:118–23. doi: 10.1042/BST20120327. [DOI] [PubMed] [Google Scholar]
- 23.Rottenberg H, Wu S. Quantitative assay by flow cytometry of the mitochondrial membrane potential in intact cells. Biochim Biophys Acta. 1998;1404:393–404. doi: 10.1016/s0167-4889(98)00088-3. [DOI] [PubMed] [Google Scholar]
- 24.Vayssier-Taussat M, Kreps SE, Adrie C, Dall’Ava J, Christiani D, Polla BS. Mitochondrial membrane potential: a novel biomarker of oxidative environmental stress. Environ Health Perspect. 2002;110:301–5. doi: 10.1289/ehp.02110301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gründler K, Angstwurm M, Hilge R, Baumann P, Annecke T, Crispin A, et al. Platelet mitochondrial membrane depolarization reflects disease severity in patients with sepsis and correlates with clinical outcome. Crit Care. 2014;18:R31. doi: 10.1186/cc13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu J, Mondal NK, Sorensen EN, Cai L, Fang HB, Griffith BP, et al. Platelet glycoprotein Ibα ectodomain shedding and non-surgical bleeding in heart failure patients supported by continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2014;33:71–9. doi: 10.1016/j.healun.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mondal NK, Sorensen EN, Hiivala NJ, Feller ED, Pham SM, Griffith BP, et al. Intraplatelet reactive oxygen species, mitochondrial damage and platelet apoptosis augment non-surgical bleeding in heart failure patients supported by continuous-flow left ventricular assist device. Platelets. 2014 doi: 10.3109/09537104.2014.948840. (in press) DOI: 10.3109/09537104.2014.948840. [DOI] [PubMed] [Google Scholar]