Abstract
Objective
Patients suffering from corneal neuropathy may present with photoallodynia; i.e., increased light sensitivity, frequently with a normal slit-lamp examination. This study aimed to evaluate the efficacy of autologous serum tears (AST) for treatment of severe photoallodynia in corneal neuropathy and correlate with corneal subbasal nerve alterations by in vivo confocal microscopy (IVCM).
Methods
Retrospective case control study with 16 patients with neuropathy-induced severe photoallodynia compared to 16 normal controls. Symptom severity, clinical examination and bilateral corneal IVCM scans were recorded.
Results
All patients suffered from extreme photoallodynia (8.8±1.1) with no concurrent ocular surface disease. Subbasal nerves were significantly decreased at baseline in patients compared to controls; total nerve length (9208 ±1264 vs 24714 ±1056 μm/mm2; p<.0001) and total nerve number (9.6±1.4 vs 28.6±2.0; p<.0001), respectively. Morphologically, significantly increased reflectivity (2.9±0.2 vs 1.8±0.1; p<.0001), beading (in 93.7%), and neuromas (in 62.5%) were seen. AST (3.6±2.1 months) resulted in significantly decreased symptom severity (1.6±1.7; p=.02). IVCM demonstrated significantly improved nerve parameters (p< .005), total nerve length (15451±1595 μm/mm2), number (13.9±2.1), and reflectivity (1.9±0.1). Beading and neuromas were seen in only 56.2% and 7.6% of patients.
Conclusion
Patients with corneal neuropathy-induced photoallodynia show profound alterations in corneal nerves. AST restores nerve topography through nerve regeneration, and this correlated with improvement in patient-reported photoallodynia. The data support the notion that corneal nerve damage results in alterations in afferent trigeminal pathways to produce photoallodynia.
Keywords: autologous serum tears, corneal neuropathy, laser in vivo confocal microscopy, light sensitivity, nerve growth factor, photoallodynia, regeneration
I. INTRODUCTION
Corneal neuropathy, a recently described entity, is characterized by dysfunctional nerves following direct damage to the trigeminal nerve endings.1 Chronic and persistent ectopic activity of injured corneal nerves causing pain in response to both innocuous stimulation (allodynia) and noxious stimuli (hyperalgesia) of the cornea is the hallmark feature of neuropathy, with patients typically reporting severe spontaneous corneal pain.1–3 In addition, a large number of these patients may experience increased light sensitivity, which may serve as a proxy symptom to the pain. The severe ocular discomfort in response to light in these neuropathic patients is referred to as photoallodynia, which has a serious debilitating impact on the quality of life of the patients.2,3 Patients may be unable to carry out activities of daily living, even when wearing dark glasses, resulting in loss of work hours, impaired social functioning, frustration, and increased anxiety and depression.4
Despite the severe impact on the quality of life, corneal neuropathy-induced photoallodynia is often overlooked by ophthalmologists and remains severely undertreated.4 Because evidence of nerve injury is required for the diagnosis of neuropathy, routine slit-lamp biomicroscopy does not allow for diagnosis of this condition. The recent advent of laser in vivo confocal microscopy (IVCM), which is a noninvasive, high-resolution, real-time imaging device, has allowed visualization of the cornea at 800-times magnification with a lateral digital resolution of 1μm/pixel. Layer-by-layer analysis of the corneal ultrastructure in both ocular health and disease, including detailed visualization of corneal nerves and immune cells, is now possible. Both quantitative and qualitative assessments of the cellular and nerve properties can be performed and provide insight into the underlying disease pathogenesis, diagnosis, and efficacy of treatments.5–14 Very recently, laser IVCM has demonstrated corneal nerve abnormalities in patients with severe symptoms of pain or photoallodynia, despite the lack of clinical signs on slit-lamp examination, thus prompting a diagnosis of corneal neuropathy.2,3,15–17
Given that the basis of the extreme corneal neuropathy-induced photoallodynia is associated with nerve injury, therapeutic strategies resulting in regeneration of damaged nerves may help alleviate patient symptoms. This rationale is based on reports on the use of neurotrophic factors, particularly nerve growth factor (NGF) to reduce allodynia in animal models with neuropathic pain.18,19 By reducing reactive astrocytosis and reversing the glial morphomolecular modulation, NGF reduces both allodynia and hyperalgesia. Administration of neurotrophic factors results in post-injury repair of peripheral nerves and their functional recovery.20 Successful corneal nerve regeneration following the use of autologous plasma in neurotrophic keratopathy,21 as well as with autologous serum tears (AST) in dry eye patients, prompted us to consider the possible application of AST in patients suffering from corneal neuropathy-induced photoallodynia.22
We hypothesized that AST would result in corneal nerve regeneration and subsequent functional recovery of the damaged peripheral corneal nerves in patients suffering from corneal neuropathy, leading to symptomatic improvement in photoallodynia. The purpose of this pilot study is to assess corneal nerve changes in patients with corneal neuropathy associated with photoallodynia and to evaluate the clinical efficacy of AST therapy as correlated with IVCM.
II. METHODS
A. Study Design and Patients
All subjects were recruited from the Cornea Service of the Massachusetts Eye & Ear Infirmary, Department of Ophthalmology, Harvard Medical School, Boston, MA, between 2009 to 2012. The protocol was approved by the Institutional Review Board/ Ethics Committee, complied with the Health Insurance Portability and Accountability Act (HIPAA), and adhered to the tenets of the Declaration of Helsinki.
This was a retrospective, single-center study. Sixteen patients (10 females and 6 males, mean age 61.8 ± 4.4, range 27–88 years) with the diagnosis of corneal neuropathy were included in the study and were compared to 16 controls (10 females and 6 males, mean age 56.4 ± 3.1, range 25–74 years; p=0.6). Inclusion criteria included symptoms of severe photoallodynia, a presence of a normal slit-lamp examination, and the absence of clinical signs of ocular surface disease by slit-lamp examination at the time of baseline examination. Specific exclusion criteria included any anterior or posterior segment pathology that could independently cause the symptoms of light sensitivity, such as corneal abrasion, ulcer, ocular infections, uveitis, and iridocyclitis. All the patients had tried frequent lubrication for at least 3 months without any relief in symptoms of photoallodynia, and were currently being treated with 20% AST 8 times/day for the purpose of inducing corneal nerve regeneration, in order to address symptoms of photoallodynia. Both eyes of all patients were studied; however, only one eye was randomly selected for analysis.
B. Clinical Chart Review
The charts of all the patients were reviewed for symptoms, activities of daily life that were affected, and previous ocular history. The severity of photoallodynia symptoms was recorded based on the patient’s assessment on a scale of 0–10, 10 being the maximum. Results of ophthalmic examination, including slit-lamp biomicroscopy findings, corneal fluorescein staining, conjunctival lissamine green staining, Schirmer’s test with anesthesia, and tear film breakup time (TFBUT) were recorded in addition to the treatment regimen and duration of therapy. Two time points, before (baseline) and after treatment with 20% autologous serum drops were assessed.
C. In Vivo Confocal Microscopy
Laser IVCM (Heidelberg Retina Tomograph 3 with the Rostock Cornea Module [HRT/RCM], Heidelberg Engineering GmbH, Heidelberg, Germany) of the central cornea was performed in all patients bilaterally. Laser IVCM is performed routinely for all patients who present to our clinic with ocular surface disease, pain, or light sensitivity at baseline and follow-up visits, as an adjunct device for the assessment of corneal nerves and immune cell alterations in the cornea, given minimal slit-lamp findings in many patients. Only one randomly selected eye was chosen for quantitative analysis and was compared to the central corneas of normal age- and gender-matched reference controls as previously described.7
The microscope uses a 670-nm red wavelength diode laser source and is equipped with a 63× objective immersion lens with a numerical aperture of 0.9 (Olympus, Tokyo, Japan). A coronal section of the cornea of 400×400 μm (horizontal × vertical) is represented by each image. Adjacent images are separated by 1 μm, with a lateral resolution of 1 μm/pixel. Digital images are recorded at a speed of 30 frames/second. During confocal microscopy scanning, a disposable, sterile polymethylmethacrylate cap (Tomo-Cap; Heidelberg Engineering GmbH, Heidelberg, Germany), filled with a layer of hydroxypropyl methylcellulose 2.5% (GenTeal gel; Novartis Ophthalmics, East Hanover, NJ) in the bottom, is mounted on the cornea module. Both eyes are anesthetized using one drop of topical anesthesia, 0.5% proparacaine hydrochloride (Alcaine; Alcon, Fort Worth, TX), followed by a drop of hydroxypropyl methylcellulose 2.5% (GenTeal gel, Novartis Ophthalmics). One drop of hydroxypropyl methylcellulose 2.5% is also placed on the outside of the cap to improve optical coupling. The equipment is manually advanced until the gel on the cap comes in contact with the surface of the central cornea.
Six to eight scans were performed on the full thickness of the central cornea using the sequence mode. With this approach, the subbasal plexus is observed in the subepithelial area, immediately at or posterior to the basal epithelial layer and anterior to the Bowman’s layer, typically at a depth of 50–80 μm. Three images most representative of the subbasal nerve plexus were chosen for analysis by a masked observer. The criteria for selecting the images were the best focused images, in a single layer, without folds, and good contrast. Two masked observers then evaluated the confocal images for morphology and density of subbasal nerves.
The nerve analysis was performed using the semiautomated tracing program NeuronJ, (http://www.imagescience.org/meijering/software/neuronj/)23 a plug-in for ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/http://rsb.info.nih.gov/ij/) as previously described.5 Metrics were assessed as described below..
Total nerve density was assessed by measuring the total length of the nerve fibers in micrometers per frame (160,000 μm2 [Figure 1]).
Main nerve trunks were defined as the total number of main nerve trunks in one image after analyzing the images anterior and posterior to the analyzed image to confirm that these did not branch from other nerves.
Nerve branching was defined as the total number of nerve branches in one image.
The number of total nerves measured was defined as the number of all nerves, including main nerve trunks and branches, in one image. The data were expressed as density (μm/mm2) ± SEM.
The grade of nerve tortuosity and reflectivity was classified in four grades according to a relative grading scale reported by Oliveira-Soto and Efron.24
Neuromas represent stumps of severed nerves and were identified as abrupt endings of a nerve fiber on confocal images.
Beading was defined as discrete small beads along the length of the nerves.24 The presence of beading and neuromas was reported qualitatively as present or absent.
The images were also analyzed for the density of the dendritiform cells (DCs) present in the subbasal layer using the cell counter function in Image J, as previously described.7
Figure 1.
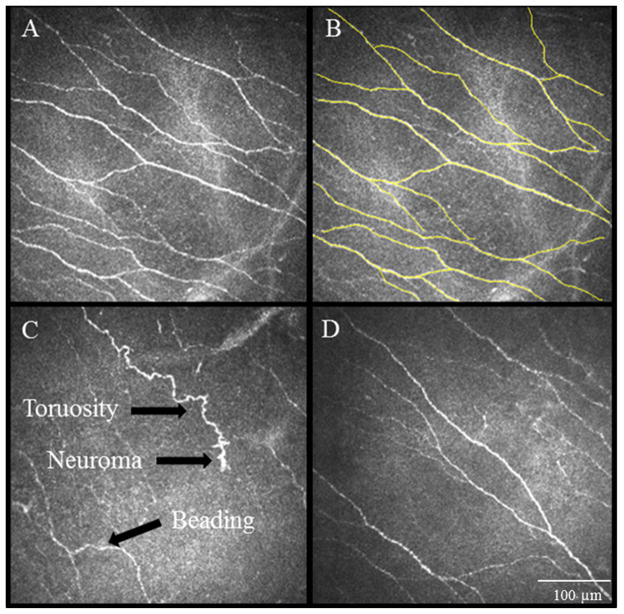
IVCM images obtained at the level of the corneal subbasal nerve plexus demonstrate nerve alterations in patients of corneal neuropathy. A. Normal corneal subbasal nerve plexus. B. Nerve tracings using Neuron J to quantify the density of nerves. C. IVCM images showing significantly decreased corneal subbasal nerve plexus at baseline, prior to autologous serum tears therapy. Note the decrease in length and number of nerves, increased tortuosity, reflectivity, beading and formation of neuromas. D. IVCM image showing post-treatment findings of the patient shown at panel C. Note improvement in the plexus, still abnormal as compared to normal controls. Size bar=100μm.
D. Preparation and Administration of Autologous Serum Tears
Patients were tested for blood-borne infections, including hepatitis B and C, syphilis, and HIV serology. A positive serology excluded the patients from use of AST. 100 ml of whole blood was drawn by venipuncture at the antecubital fossa under aseptic conditions and left standing for 2 hours to ensure complete clotting. The blood was then centrifuged for 15 minutes at 3000 rpm. The serum was separated in a sterile manner and filtered using MILLLEX-HP PES 5 micron low protein binding filter and diluted to 20% using sterile saline solution. The final preparation was allocated into 5 ml bottles. The bottles were kept frozen at −20°C until ready for use. After thawing, expiration was 2 weeks (unopened) and 1 week (after opening). To protect from ultraviolet light, an amber colored sleeve was provided with the bottles. The patients were instructed to keep the bottles under refrigeration. AST was applied 8 times daily bilaterally.
E. Statistical Analysis
Statistical analysis was performed by analysis of variance (ANOVA) to compare the pre-treatment, post-treatment, and control groups. Paired Student’s t-test was used to compare pre- and post-treatment groups. Differences were considered statistically significant for p values less than 0.05. Analyses were performed with statistical analysis software in STATA ™ (StataCorp, version 11).
F. Literature Review
A literature review was performed in PubMed to confirm the novelty of use of autologous serum drops for treatment of corneal neuropathic symptoms-- specifically, increased light sensitivity or photoallodynia-- and the correlation with in vivo confocal microscopy findings. The following search terms were used: corneal neuropathy, light sensitivity, photophobia, photoallodynia, autologous serum, corneal nerve regeneration with autologous serum in neuropathy, in vivo confocal microscopy and neuropathy, in vivo confocal microscopy and nerves.
III. RESULTS
A. Demographics
The cornea of one of the eyes in each of the 16 patients suffering from severe photoallodynia were studied and the data collected was compared with 16 age-matched control eyes. Demographic data of patients and controls are presented in Table 1.
Table 1.
Demographic Data of Reference Controls and Patients with increased light sensitivity
| Controls | Patients | P value | |
|---|---|---|---|
| No. of Patients | 16 | 16 | |
| Age (years) | 56.4 ± 3.1 (range 25–74) | 61.8 ± 4.4 (range 27–88) | P = 0.6 |
| Gender (female/male) | 10/6 | 10/6 | P = 0.4 |
Values are mean ±standard error of mean unless otherwise specified.
B. Clinical Features
All patients complained of increased and debilitating photoallodynia. Severity of symptoms ranged from 7 to 10, with a mean of 8.8±1.1. The impact on quality of life of patients was significant. All patients reported difficulty in reading, watching TV, and driving. Eleven patients (64.7%) were dependent on sunglasses, even indoors. Four of 16 patients (25%) lost their jobs and one had to drop out of graduate school due to inability to tolerate any form of light. Patients also reported significant social impairment, as they tended to spend days in dark rooms, refusing to tolerate any form of artificial or natural light. Severity of symptoms, activities affected, impact on quality of life, and the possible underlying mechanism leading to corneal neuropathy are shown in Table 2.
Table 2.
Clinical Features
| Pt no. | Symptom duration | Activities affected | Impact | Underlying mechanism | Severity of photoallodynia (0/10) | Initial patient- reported symptom improvement (months) | |
|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | ||||||
| 1 | 1 yr | TV, Reading | Eyes closed at all times | DES | 7 | 0 | 8 |
| 2 | 3 yrs | Reading, driving | SDEI; Social impairment | DES | 8 | 3 | 3 |
| 3 | 3 yrs | TV, Comp., driving | SDEI | DES | 10 | 4 | 3 |
| 4 | 6 mths | TV | Social impairment | Radiation keratopathy | 8 | 0 | 3 |
| 5 | 6 mths | Reading | SDEI, dropped out of school | DES | 10 | 4 | 7 |
| 6 | 3 yrs | Reading, driving | Social impairment | DES | 8 | 0 | 3 |
| 7 | 5 yrs | TV, Reading | SDEI, lost job | MGD | 9 | 0 | 1 |
| 8 | 1 yr | Reading, Driving | SDEI, lost job | UV exposure | 8 | 0 | 3 |
| 9 | 2 yrs | TV, Reading | SDEI | DES | 10 | 1 | 1 |
| 10 | 1 yr | TV, Comp, Driving | SDEI | DES | 10 | 5 | 6 |
| 11 | 4 yrs | Reading, Driving | Social impairment | DES | 9 | 0 | 4 |
| 12 | 1 yr | Reading | SDEI | LASIK | 8 | 4 | 2 |
| 13 | 5 mths | Walking around | SDEI | Radiation keratopathy | 9 | 2 | 6 |
| 14 | 1 yr | TV, Comp | SDEI, lost job | CE, ABMD | 10 | 2 | 3 |
| 15 | 9 mths | Reading, Driving | SDEI; Social impairment | LASIK | 7 | 0 | 4 |
| 16 | 8 mths | Walking around | SDEI, lost job | DES | 10 | 1 | 1 |
CE = Cataract Extraction; Comp. = Computer Use; DES = Dry Eye Syndrome; MGD = Meibomian Gland Dysfunction; UV = Ultraviolet; LASIK = Laser-Assisted in situ Keratomileusis; ABMD = Anterior basement membrane dystrophy; mths = months; OS = left eye; OU = Both eyes; SDEI = Sunglasses dependent even indoors; TV = Watching television; yrs = years
None of the patients suffered from a concurrent active ocular surface disease, as evidenced by absence of clinical signs, including fluorescein and lissamine green staining, decreased Schirmer’s test results, or TFBUT. Slit-lamp biomicroscopy of the eyelid margins and conjunctiva did not reveal any active blepharitis, meibomian gland dysfunction, or conjunctivitis. Twelve eyes had a best-corrected visual acuity of 20/20, two had 20/25, while the other two eyes were 20/30 and 20/50 due to cataracts.
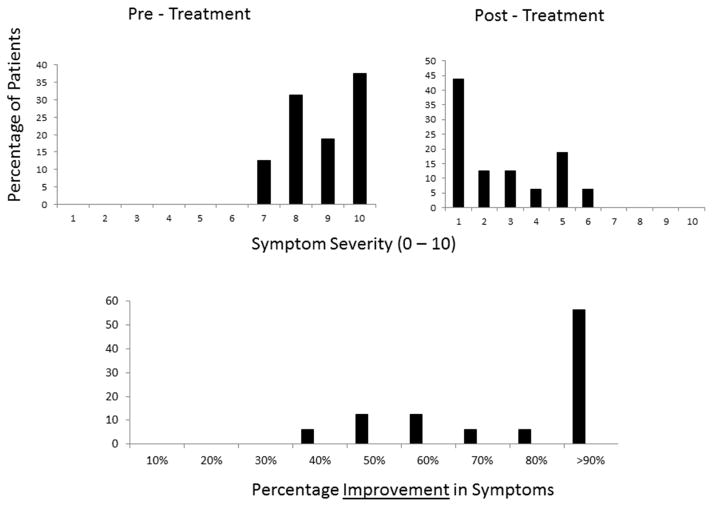
Following treatment with AST, the patients reported a significant reduction in symptoms, with 9/16 patients (56.25%) demonstrating more than 90 % improvement and the remaining 7/16 reporting 40–60% improvement in the symptom of photoallodynia. The average symptom severity was reduced significantly to 1.6±1.7 (range 0–5; p =.02). Figure 2 demonstrates the improvement in symptom severity post-treatment. The mean interval for improvement was 3.6 ± 2.1 months, ranging from 1 to 8 months. The severity of symptoms post-treatment with AST, percentage improvement, and duration for improvement are presented in Figure 2 and Table 2.
Figure 2.
Severity of symptoms pre- and post-treatment and improvement in symptom severity post-treatment.
C. In Vivo Confocal Microscopy Findings
A summary of the nerve parameters for the patients, pre- and post-treatment with AST, and normal control groups is presented in Table 3. Figure 3 shows the improvement in corneal subbasal nerve plexus for three representative patients following treatment.
Table 3.
Corneal Subbasal nerve parameters in controls and eyes with photoallodynia before and after treatment with autologous serum tears
| IVCM Parameter | Controls | Patients | |
|---|---|---|---|
| Pre-Treatment | Post-Treatment | ||
| No. of total nerves (n/frame) | 28.6 ± 2 | 9.6 ± 1.4* | 13.9 ± 2*† |
| Total nerve length (μm/mm2) | 24,714 ± 1056 | 9,208 ± 1264* | 15,451 ± 1595*† |
| No. of main nerve trunks (n/frame) | 4.3 ± 0.2 | 1.6 ±0.2* | 2.6 ± 0.2*† |
| Length of main nerve trunks (μm/mm2) | 11,214 ± 602 | 3,958 ±460* | 6,525 ± 601*† |
| No. of nerve branches (n/frame) | 24.3 ± 2 | 7.9 ±1.2* | 11.3 ± 1.9*† |
| Length of nerve branches (μm/mm2) | 13,519 ± 807 | 5,483 ± 838* | 8,925 ± 1228*† |
| Tortuosity (0–4) | 1.7 ±0.1 | 2.2 ± 0.2 | 1.7 ± 0.2† |
| Reflectivity (0–4) | 1.7 ± 0.1 | 2.9 ± 0.2* | 1.9 ± 0.1*† |
All values are reported as mean ± Standard error of mean unless otherwise specified.
Statistically significant as compared to controls, p < 0.0001
Statistically significant as compared to pre-treatment.
Figure 3.
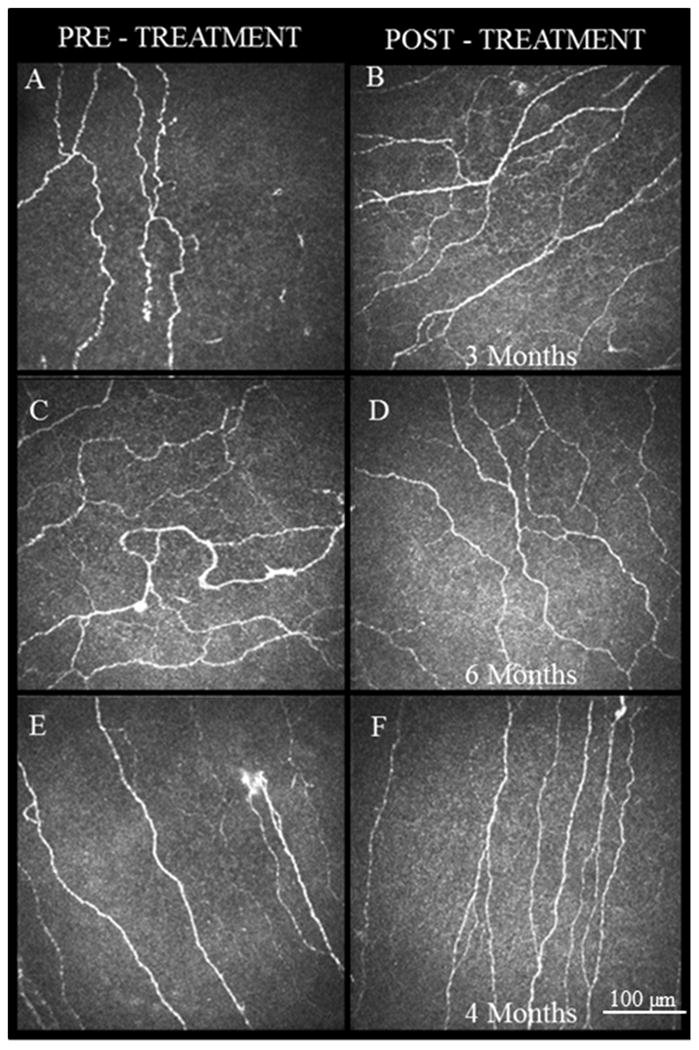
IVCM images obtained at the level of the corneal subbasal nerve plexus demonstrate the improvement in the nerve density and morphology following treatment with autologous serum tears. A, C, E. Images at baseline showing increased reflectivity and tortuosity of nerves with presence of beading and neuromas. B, D, F. IVCM images post-treatment corresponding to same patients as A, C, E. Images after 3, 6 and 4 months, respectively, showing increased nerve density and decreased reflectivity, tortuosity, beading, and neuromas.
1. Pre–Treatment Baseline IVCM Findings
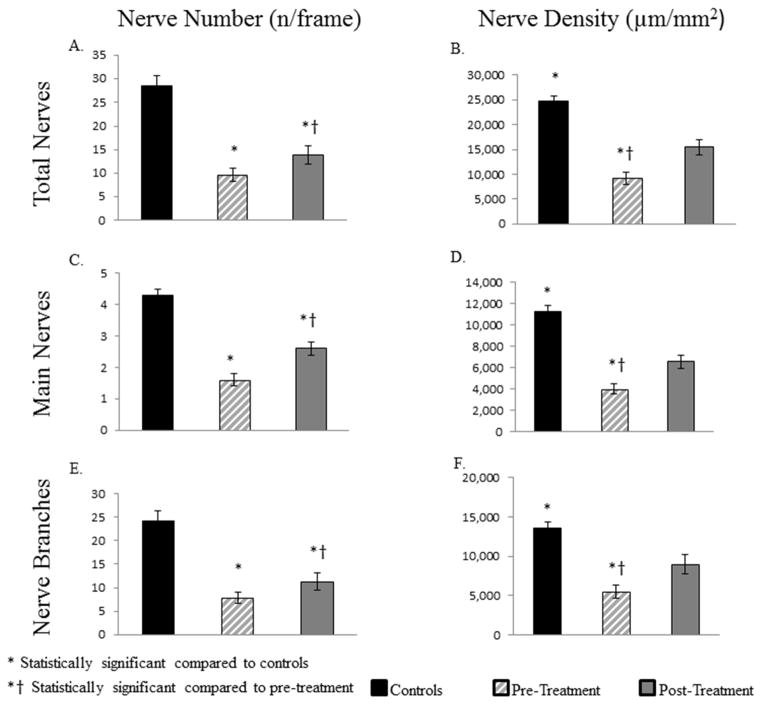
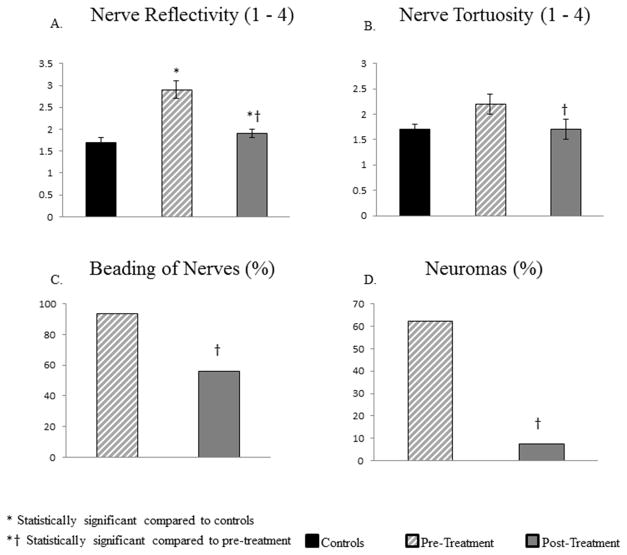
The subbasal nerve plexus density in patients pre-treatment was profoundly decreased compared to controls (Figure 4), including total nerve length (9,208±1,264 μm/mm2 vs 24,714 ±1,056 μm/mm2; p<.0001), total number of nerves (9.6±1.4 vs 28.6±2.0; p<.0001), main nerve length (3,958±460 μm/mm2 vs 11,214±602 μm/mm2; p<.0001), number of main nerves (1.6±0.2 vs. 4.3±0.2; p<.0001), nerve branch length (5,483±838 μm/mm2 vs 13,519 ±807 μm/mm2; p<.0001), and number of nerve branches (7.9±1.2 vs 24.3±2.0; p<.0001), respectively. Morphologically, the nerves showed significant increase in reflectivity (2.9±0.2 vs 1.8±0.1; p<.0001), and demonstrated the presence of beading and neuromas in 93.7% and 62.5% of patients, respectively. The nerves also showed increased tortuosity (2.2±0.1), although this was not significant compared to controls (1.8±0.2; p=.06). There was no significant difference in the density of DCs compared to controls (54.2±21.3 vs. 17.3±4.4 cells/mm2; p=.1).
Figure 4.
Comparison of subbasal nerve plexus density in patients suffering from corneal neuropathy, controls, and following treatment with ASTs. Bar graphs show corneal nerve alterations in pre-treatment and post-treatment eyes in patients and in the control group. A. Total number of nerves. B. Length of total nerves. C. Number of main nerve trunks. D. Length of main nerve trunks. E. Number of nerve branches. F. Length of nerve branches. *P<.001, compared to the control group by analysis of variance, †P<.001, compared to the pre-treatment group by paired t-test.
2. Post-Treatment IVCM Findings
IVCM showed a significant improvement in nerve density (total nerve length 15,451±1595 μm/mm2 and number 13.9±2.1, main nerve length 6,525±601 μm/mm2 and number 2.6±0.2, branch nerve length 8,925±1,228 μm/mm2 and number 11.3±1.9; p<0.005) after therapy with AST (Figure 3). The density of total nerves, main nerve trunks, and nerve branches increased by 68%, 65%, and 63%, respectively. In addition to the changes in density (Figures 3 and 4), IVCM demonstrated significant improvements in nerve morphology after therapy with AST (Figures 3 and 5). A significant decrease in reflectivity (1.9±0.1; p=.001) and tortuosity (1.7±0.2; p=.001) of nerves was noted. Beading and neuromas were now seen in only 56.2% and 7.6% of patients, respectively, a decrease of 37.5% and 56.25%, respectively (p=.01) compared to pre-treatment. These changes are reported at the time point when patients reported the first symptomatic improvement in photoallodynia, average being 3.8±0.5 months, with a range of 1–8 months.
Figure 5.
Comparison of the subbasal nerve plexus morphology in patients suffering from corneal neuropathy, controls, and following treatment with ASTs. Bar graphs showing corneal nerve alterations in pre-treatment and post-treatment eyes in patients and in the control group. A. Reflectivity of nerves. B. Tortuosity of nerves. C. Beading of nerves. D. Neuromas. *P<.001, compared to the control group by analysis of variance, †P<.001, compared to the pre-treatment group by paired t-test.
There was no significant difference in the DC density after treatment 29.2±8.4 cells/mm2; p=.1). When clinical symptom severity was correlated to IVCM parameters, a significant negative correlation was found between photoallodynia symptom severity and total nerve density (r=−0.61, p=.002). Similarly, significant positive correlation was seen between symptom severity and reflectivity of nerves (r=0.41, p=.02). However, no significant correlation was seen between symptoms and tortuosity of nerves (r=0.17, p=.35).
IV. DISCUSSION
In the current study, all patients suffered from extreme photoallodynia. Despite their severe symptoms, the clinical signs on slit-lamp examination were unremarkable, but decreased nerve density and abnormal nerve morphology was observed as measured by IVMC. Such changes are consistent with corneal neuropathy.2,3,16 In addition, these patients demonstrated decreased or diminished photoallodynia with application of proparacaine, suggesting that neural blockade of afferent input from the cornea is the contributing factor to the photoallodynia.
Corneal neuropathy-induced photoallodynia should be differentiated from other etiologies, including traumatic and inflammatory ocular conditions. Both anterior segment pathology, such as corneal diseases, iritis, iridocyclitis, and blepharitis, as well as posterior segment pathology, such as uveitis, vitritis, retinitis pigmentosa, and retinal and cone dystrophies (reviewed by Digre and Brennan4) can result in photophobia. Various neurological conditions can result in photophobia, including headaches (most commonly migraine headaches), benign essential blepharospasm, and trigeminal neuralgia, as well as intracranial conditions, including meningitis, subarachnoid hemorrhage, and pituitary tumors.4 A clear history and clinical examination can help to rule out the above conditions. Further, corneal neuropathy-induced photoallodynia does not improve with cycloplegic drops or ultraviolet filters, and use of these may aid in clinical differentiation of the underlying etiology.3
IVCM can reveal corneal subbasal nerve damage and aberrant nerve regeneration and has shown excellent repeatability in recent studies.25,26 Both decreased density of nerves and morphological changes have been described in various ocular surface diseases.5–14 Formation of neuromas, seen as abrupt swelling of injured nerve endings5 and neurite sprouting27,28 are seen after injury and abnormal regeneration of nerves. In addition, increased beading patterns have been hypothesized to represent the mitochondria and are increased in response to metabolic stress.24 Further, increased reflectivity and tortuosity of corneal subbasal nerves are seen following chronic damage in various conditions, including dry eye disease,29 diabetes, and systemic neuropathies.30,31 Moreover, discontinuous nerves and decreased nerve density have been shown in post-refractive surgery patients.32 Finally, several studies have demonstrated profoundly decreased density and altered morphology of nerves in patients suffering from corneal neuropathy.1,15,16
In the current study, IVCM demonstrated profound damage to the corneal nerves in patients with photoallodynia. The density of total nerves, main nerves, and nerve branches were all decreased in patients compared to controls. Further, morphologically, the nerves showed increased reflectivity. In addition, corneal nerves were more tortuous, albeit the difference was not significant compared to controls; this could be due to the relatively small sample size. Finally, beading and neuromas were present in most patients. However, after application of AST for a mean duration of 3.6 months, patients demonstrated significant improvement in the severity of symptoms of photoallodynia, which resulted in improved quality of life within months. Concurrently, IVCM demonstrated significant increase in nerve density and reversal of morphological alterations noted at baseline, including decreased presence of beading and neuromas.
We demonstrated that the clinical symptom severity of photoallodynia was significantly correlated to decreased total nerve density and increased reflectivity of nerves, suggesting that providing neurotrophic support and subsequent nerve regeneration achieves alleviation of symptoms. Several experiments in rat models have shown that administration of growth factors, including NGF and NGF-mimetic factors, help to reduce symptoms of neuropathic pain.18,19 NGF plays a role in survival, differentiation, and growth of sensory and sympathetic neurons.33,34 NGF has, further, a direct effect on regeneration of neurons and restoring function in injured neuronal cells.34 Matsumoto et al reported that NGF concentration in autologous serum is several times higher than that in tears and reported an improvement in corneal sensation following topical serum use after a mean duration of 15 months.36
In addition to NGF, AST supplies the eye surface with several epithelial and neurotrophic growth factors, such as vitamin A, epidermal growth factor, transforming growth factor β, fibronectin, substance P, and insulin-like growth factor-1. More recently, Rao et al demonstrated improvement in corneal nerve density following autologous plasma use.21 However, although therapy with serum drops is safe and no substantial side effects have been reported, it is currently unclear whether a single factor or a combination of several factors are responsible for the therapeutic effects of AST.
There may be skepticism regarding the success of AST in the treatment of corneal neuropathy,36 as it is possible that AST leads to the epithelial healing as well. A healthy epithelium could potentially also result in nerve regeneration, as shown by in vitro studies on rabbit corneas.37 However, we would like to point out that all our patients had no active ocular surface disease at the time of initiation of AST therapy. While approximately half of the patients had been treated for ocular surface disease in the past, including the use of topical steroids, they continued to experience severe symptoms of photoallodynia, despite resolution of ocular surface disease; this suggests that their symptoms were likely related to chronic nerve changes as observed by IVCM. Further, the fact that the patients with no history of ocular surface disease had similar IVCM findings of nerve degeneration and demonstrated a similar response and improvement in symptoms suggests that AST may produce patient improvement through nerve regeneration.
Inflammation can produce corneal pain and symptoms of photoallodynia. At the TFOS (Tear Film and Ocular Surface) International Workshop on Contact Lens Discomfort, the subcommittee on neurobiology reviewed the role of ocular inflammatory mediators in ocular pain and discomfort.38 However, in our current study, we found no significant increase in DCs in the subbasal layer in patients compared to controls. Further, the subset of patients who had received anti-inflammatory treatment, such as steroids, in the past had continued to experience symptoms of light sensitivity despite anti-inflammatory therapy. Thus, while inflammation may be involved in the pathogenesis of photoallodynia, such as in the initial induction of nerve damage, the current data suggest that it is primarily the neurotrophic support through AST that resulted in the improvement in symptoms. Interestingly, several studies have shown that corneal epithelial DCs may contain melanin.39 Thus, one could speculate that these cells may act by sensing light in the cornea and provide an initial stimulus for firing of the trigeminal nociceptors in response to light, as corneal DCs are frequently seen in close contact to injured nerve terminals.7
Physiologic responses of corneal nerves to injury have been described in detail by Belmonte et al.40–42 Nerve injury results in release of pro-inflammatory neuropetides, including substance P and calcitonin gene-related peptide (CGRP), from both injured nerves and healthy nerves due to antidromic propagation of nerve impulses from the site of injury. These neuropeptides then produce plasma extravasation and vasodilation and stimulate the release of pro-inflammatory cytokines that induce inflammatory cells around the injured nerve terminals. One important effect of the inflammatory cytokines is the neuromodulation of the ion channels concentrated in the trigeminal nerve endings43 through mechanisms that include altered protein expression and ion channel trafficking.44,45 Thus, chronic inflammation produces permanent changes in nociceptive terminals and diminishes neurite outgrowth in dorsal root ganglion cells by augmenting sodium load and reversing the operation of the sodium-calcium exchanger, ultimately resulting in increased calcium influx across cell membranes and thus axonal degeneration.46 Further, this results in the increased firing rate of the nociceptors to all forms of stimuli, in respect to both frequency and amplitude. Similar to peripheral neuropathies, sensitization is a prominent feature of corneal nociceptors, i.e., repeated noxious stimuli lead to a decrease in the threshold.47 In addition, removal of stimuli only transiently abolishes the response, with the discharge of impulses becoming irregularly sustained with a lower frequency, irrespective of the presence of stimulus.40 This is the physiologic basis for the development of neuropathy, called “phantom cornea.”48
Currently, the pathophysiology of corneal neuropathy and the associated photoallodynia are not well understood, given the very recent description of this entity and the very limited available literature. Whatever the mechanism, the process needs to explain how light can produce discomfort or pain. A number of putative mechanisms have been suggested: 1) trigeminal systems and 2) retinal systems, or 3) a combination of both.
Damage to trigeminal corneal nerves can occur in association with surgical procedures (e.g., keratorefractive surgery),54 dry eye disease,55 systemic neuropathic conditions such as diabetes,56,57 infections such as herpes zoster ophthalmicus,58 toxic damage during chemotherapy, recurrent corneal erosions, and exposure to noxious fumes or radiation and infections.2,3 Both spontaneous pain and evoked pain exacerbated by pressure or cold may relate to damaged nerves, as has been well described in peripheral neuropathies, and the altered morphology of nerve fibers observed is thus fully consistent with a primary neuropathic condition.49 Spontaneous pain arises from damaged small fibers,50 and the exacerbation of pain may be due to altered/lowered membrane potential as a result of such mechanisms as increased sodium channel expression.51–53 Using functional magnetic resonance imaging, Moulton et al showed that exposure to light in a photophobic subject activates the trigeminal system.59 Furthermore, through a series of experiments in rats, Okamoto et al have proposed that intense light activates a trigeminal nociceptive pathway via increased parasympathetic outflow.60 They identified a population of nociceptive nuclei in the spinal trigeminal nucleus, which are activated by mechanical stimulation of the ocular surface.60 Electrophysiological measures evaluating neuronal activity show an increased firing rate in response to light, suggesting a reflex circuit that results in the stimulation of trigeminal nociceptors via exposure to light.61,62 Another potential pathway could be present via the autonomic fibers that accompany the trigeminal nerve. These fibers have been shown to mediate symptoms of light sensitivity in various neurological conditions through the trigeminovascular and trigeminoautonomic reflexes.63
The second possible pathway involves the retinal systems. Photosensitive retinal cells may contribute in part to light aversion.64 Presence of photophobia in blind patients65 and after optic nerve resection in rats66 demonstrates that an intact optic nerve is not essential for sensitivity to light. Such photosensitive non-image forming cells have been directly implicated in photophobia in migraine, during which light increases the pain.66,67 The potential pathway could involve melanopsin-containing photosensitive cells in the retina and the iris, the intrinsically photosensitive retinal ganglion cells that detect light and bypass conventional pathways through the retina and optic nerve.67
Combination of trigeminal and retinal systems is also a plausible mechanism, as inputs from the retinal photoreceptors and trigeminal afferents converge at the level of the thalamus.68 A crosstalk between these converging fibers may cause the perception of ocular discomfort and pain with light. From the thalamus, these afferents then project into various areas of the somatosensory, visual, and association cortices, explaining the broadly distributed response to light stimuli. Dolgonos et al have suggested the presence of an intraretinal mechanism, acting through associational ganglion cells, that modulates the trigeminal system to produce the ocular discomfort associated with exposure to bright light.66
Our study has several limitations, including the relatively small sample size. However, no epidemiological data are currently available on corneal neuropathy, and many cases likely remain undiagnosed. Nevertheless, our results achieved clear statistical significance. Another limitation is the retrospective nature of this study. Given the promising results of this pilot study for a disease without any current treatment options, future larger randomized trials are now warranted to confirm and expand our findings. Performing long-term randomized studies in this patient population will be challenging, given the nature of the disease and severe impact on the quality of life.
We have used patient-reported severity and impact on quality of life as the parameters for severity of the condition. As photoallodynia is a very recently described entity, there are no established and validated objective tests for it. For future prospective studies, objective tests such as glare or contrast sensitivity testing, light scatter, corneal topography, and Fourier’s analysis could be used as potential measures. Another limitation of this retrospective study is the lack of availability of corneal esthesiometry and correlation of corneal sensation with nerve density, which should be included in future prospective studies. However, as Belmonte et al have demonstrated before, abnormal nerve function following nerve injury may be associated with decreased sensation.48 Hence, in patients with corneal neuropathy, sensitivity measurement may not correlate with nerve morphology as in normal subjects. Finally, our study has a relatively short follow-up time, and it is unclear if the outcomes and corneal nerve changes are retained long-term or are potentially reversible when the treatment with AST is discontinued. Future prospective studies on the use of AST for corneal neuropathy with a larger sample size and longer follow-up will likely provide more definitive answers on the long-term effects of AST on corneal nerves.
In conclusion, treatment with AST produces significant improvement in both patient-reported severity of symptoms of photoallodynia and recovery of corneal subbasal nerves as shown by IVCM. AST is easy to prepare, has limited side effects, and is a highly effective treatment for symptoms of corneal neuropathy, which have previously been severely undertreated and underdiagnosed.
Acknowledgments
Financial Support: NIH K08-EY020575 (PH), NIH R01-EY022695 (PH), Research to Prevent Blindness Career Development Award (PH), Falk Medical Research Trust (PH), and the New England Corneal Transplant Research Fund (PH). The funding organizations had no role in the design or conduct of this research.
Footnotes
This work was presented in part at the Association of Research in Vision and Ophthalmology/International Society for Imaging in the Eye (ARVO/ISIE) conference, Seattle, WA, May 2013; the Tear Film and Ocular Surface Society Meeting, Sicily, Italy, September 2013; and the American Academy of Ophthalmology Meeting, New Orleans, LA, November 2013.
The authors have no commercial or proprietary interest in any concept of product discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borsook D, Rosenthal P. Chronic (neuropathic) corneal pain and blepharospasm: five case reports. Pain. 2011;152:2427–31. doi: 10.1016/j.pain.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain: is it real? Ocul Surf. 2009;7:28–40. doi: 10.1016/s1542-0124(12)70290-2. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal P, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf. 2012;10:2–14. doi: 10.1016/j.jtos.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Digre KB, Brennan KC. Shedding light on photophobia. J Neuroophthalmol. 2012;32:68–81. doi: 10.1097/WNO.0b013e3182474548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 2010;25:171–7. doi: 10.3109/08820538.2010.518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamrah P, Cruzat A, Dastjerdi MH, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117:1930–6. doi: 10.1016/j.ophtha.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruzat A, Witkin D, Baniasadi N, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011;52:5136–43. doi: 10.1167/iovs.10-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamrah P, Cruzat A, Dastjerdi MH, et al. Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study. Ophthalmology. 2013;120:40–7. doi: 10.1016/j.ophtha.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guthoff RF, Zhivov A, Stachs O. In vivo confocal microscopy, an inner vision of the cornea - a major review. Clin Experiment Ophthalmol. 2009;37:100–17. doi: 10.1111/j.1442-9071.2009.02016.x. [DOI] [PubMed] [Google Scholar]
- 10.Patel DV, McGhee CN. Quantitative analysis of in vivo confocal microscopy images: a review. Surv Ophthalmol. 2013;58:466–75. doi: 10.1016/j.survophthal.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Zhivov A, Winter K, Peschel S, et al. Quantitative analysis of corneal subbasal nerve plexus with in vivo confocal laser scanning microscopy. Klin Monbl Augenheilkd. 2011;228:1067–72. doi: 10.1055/s-0031-1281663. [DOI] [PubMed] [Google Scholar]
- 12.Tavakoli M, Quattrini C, Abbott C, et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010;33:1792–7. doi: 10.2337/dc10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labbe A, Alalwani H, Van Went C, et al. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012;53:4926–31. doi: 10.1167/iovs.11-8708. [DOI] [PubMed] [Google Scholar]
- 14.Hamrah P, Sahin A, Dastjerdi MH, et al. Cellular changes of the corneal epithelium and stroma in herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2012;119:1791–7. doi: 10.1016/j.ophtha.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Xu J, Hong J, Le Q. Dry eye like symptoms without dessicated signs implies corneal neuropathy: an in vivo confocal microscopy study. Invest Ophthalmol Vis Sci. 2014;55:ARVO E-Abstract 3646. [Google Scholar]
- 16.Qazi Y, Aggarwal S, Cavalcanti B, et al. In vivo confocal microscopy demonstrates a profound increase in immune dendritic cells and decrease in corneal nerves in patients with post-refractive surgery keratoneuralgia. Invest Ophthalmol Vis Sci. 2013;54:ARVO E-Abstract 3711. [Google Scholar]
- 17.Shahatit B, Hamrah P, Dastjerdi MH, et al. Corneal nerve changes in patients with corneal allodynia: an in vivo confocal microscopy study. Invest Ophthalmol Vis Sci. 2009;50:ARVO E-Abstract 3707. [Google Scholar]
- 18.Cirillo G, Cavaliere C, Bianco MR, et al. Intrathecal NGF administration reduces reactive astrocytosis and changes neurotrophin receptors expression pattern in a rat model of neuropathic pain. Cell Mol Neurobiol. 2010;30:51–62. doi: 10.1007/s10571-009-9430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colangelo AM, Bianco MR, Vitagliano L, et al. A new nerve growth factor-mimetic peptide active on neuropathic pain in rats. J Neurosci. 2008;28:2698–709. doi: 10.1523/JNEUROSCI.5201-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takemura Y, Imai S, Kojima H, et al. Brain-derived neurotrophic factor from bone marrow-derived cells promotes post-injury repair of peripheral nerve. PLoS One. 2012;7:e44592. doi: 10.1371/journal.pone.0044592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao K, Leveque C, Pflugfelder SC. Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma therapy. Br J Ophthalmol. 2010;94:584–91. doi: 10.1136/bjo.2009.164780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruzat A, Cavalcanti B, Trinidad M, et al. Corneal nerve regeneration with autologous serum eye drops in treatment of severe dry eye syndrome. International Society for Eye Research Biennial Meeting. 2012;109:O320. http://www.iser.org/files/2012_abstracts.pdf. [Google Scholar]
- 23.Meijering E, Jacob M, Sarria JC, et al. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A. 2004;58:167–76. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20:374–84. doi: 10.1097/00003226-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Dehghani C, Pritchard N, Edwards K, et al. Morphometric stability of the corneal subbasal nerve plexus in healthy individuals: a 3-year longitudinal study using corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2014;55:3195–9. doi: 10.1167/iovs.14-13959. [DOI] [PubMed] [Google Scholar]
- 26.Petropoulos IN, Manzoor T, Morgan P, et al. Repeatability of in vivo corneal confocal microscopy to quantify corneal nerve morphology. Cornea. 2013;32:e83–9. doi: 10.1097/ICO.0b013e3182749419. [DOI] [PubMed] [Google Scholar]
- 27.Tuisku IS, Lindbohm N, Wilson SE, Tervo TM. Dry eye and corneal sensitivity after high myopic LASIK. J Refract Surg. 2007;23:338–42. doi: 10.3928/1081-597X-20070401-05. [DOI] [PubMed] [Google Scholar]
- 28.Tuominen IS, Konttinen YT, Vesaluoma MH, et al. Corneal innervation and morphology in primary Sjogren’s syndrome. Invest Ophthalmol Vis Sci. 2003;44:2545–9. doi: 10.1167/iovs.02-1260. [DOI] [PubMed] [Google Scholar]
- 29.Alhatem A, Cavalcanti B, Hamrah P. In vivo confocal microscopy in dry eye disease and related conditions. Semin Ophthalmol. 2012;27:138–48. doi: 10.3109/08820538.2012.711416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kallinikos P, Berhanu M, O’Donnell C, et al. Corneal nerve tortuosity in diabetic patients with neuropathy. Invest Ophthalmol Vis Sci. 2004;45:418–22. doi: 10.1167/iovs.03-0637. [DOI] [PubMed] [Google Scholar]
- 31.Mocan MC, Durukan I, Irkec M, Orhan M. Morphologic alterations of both the stromal and subbasal nerves in the corneas of patients with diabetes. Cornea. 2006;25:769–73. doi: 10.1097/01.ico.0000224640.58848.54. [DOI] [PubMed] [Google Scholar]
- 32.Linna TU, Vesaluoma MH, Perez-Santonja JJ, et al. Effect of myopic LASIK on corneal sensitivity and morphology of subbasal nerves. Invest Ophthalmol Vis Sci. 2000;41:393–7. [PubMed] [Google Scholar]
- 33.Wiesmann C, de Vos AM. Nerve growth factor: structure and function. Cell Mol Life Sci. 2001;58:748–59. doi: 10.1007/PL00000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Fausto V, Fiore M, Tirassa P, et al. Eye drop NGF administration promotes the recovery of chemically injured cholinergic neurons of adult mouse forebrain. Eur J Neurosci. 2007;26:2473–80. doi: 10.1111/j.1460-9568.2007.05883.x. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto Y, Dogru M, Goto E, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004;111:1115–20. doi: 10.1016/j.ophtha.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 36.Benitez-Del-Castillo JM, Acosta MC, Wassfi MA, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007;48:173–81. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- 37.Chan KY, Haschke RH. Action of a trophic factor(s) from rabbit corneal epithelial culture on dissociated trigeminal neurons. J Neurosci. 1981;1:1155–62. doi: 10.1523/JNEUROSCI.01-10-01155.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stapleton F, Marfurt C, Golebiowski B, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the subcommittee on neurobiology. Invest Ophthalmol Vis Sci. 2013;54:TFOS71–97. doi: 10.1167/iovs.13-13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segawa K. Electron microscopy of dendritic cells in the human corneal epithelium: e modified Masson’s ammoniated silver nitrate stain. Invest Ophthalmol. 1965;4:264–9. [PubMed] [Google Scholar]
- 40.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78:513–25. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 41.Belmonte C, Aracil A, Acosta MC, et al. Nerves and sensations from the eye surface. Ocul Surf. 2004;2:248–53. doi: 10.1016/s1542-0124(12)70112-x. [DOI] [PubMed] [Google Scholar]
- 42.Rivera L, Gallar J, Pozo MA, Belmonte C. Responses of nerve fibres of the rat saphenous nerve neuroma to mechanical and chemical stimulation: an in vitro study. J Physiol. 2000;527(Pt 2):305–13. doi: 10.1111/j.1469-7793.2000.t01-1-00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMahon S, Koltzenburg M. The changing role of primary afferent neurones in pain. Pain. 1990;43:269–72. doi: 10.1016/0304-3959(90)90024-8. [DOI] [PubMed] [Google Scholar]
- 44.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt M, Dubin AE, Petrus MJ, et al. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron. 2009;64:498–509. doi: 10.1016/j.neuron.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Persson AK, Liu S, Faber CG, et al. Neuropathy-associated Nav1. 7 variant I228M impairs integrity of dorsal root ganglion neuron axons. Ann Neurol. 2013;73:140–5. doi: 10.1002/ana.23725. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez GG, Garcia de la Rubia P, Gallar J, Belmonte C. Reduction of capsaicin-induced ocular pain and neurogenic inflammation by calcium antagonists. Invest Ophthalmol Vis Sci. 1993;34:3329–35. [PubMed] [Google Scholar]
- 48.Belmonte C. Eye dryness sensations after refractive surgery: impaired tear secretion or “phantom” cornea? J Refract Surg. 2007;23:598–602. doi: 10.3928/1081-597X-20070601-11. [DOI] [PubMed] [Google Scholar]
- 49.Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008;131:1912–25. doi: 10.1093/brain/awn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serra J, Sola R, Quiles C, et al. C-nociceptors sensitized to cold in a patient with small-fiber neuropathy and cold allodynia. Pain. 2009;147:46–53. doi: 10.1016/j.pain.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 51.Kerstman E, Ahn S, Battu S, et al. Neuropathic pain. Handb Clin Neurol. 2013;110:175–87. doi: 10.1016/B978-0-444-52901-5.00015-0. [DOI] [PubMed] [Google Scholar]
- 52.Bennett DL, Woods CG. Painful and painless channelopathies. Lancet Neurol. 2014;13:587–99. doi: 10.1016/S1474-4422(14)70024-9. [DOI] [PubMed] [Google Scholar]
- 53.Black JA, Nikolajsen L, Kroner K, et al. Multiple sodium channel isoforms and mitogen-activated protein kinases are present in painful human neuromas. Ann Neurol. 2008;64:644–53. doi: 10.1002/ana.21527. [DOI] [PubMed] [Google Scholar]
- 54.Chao C, Golebiowski B, Stapleton F. The role of corneal innervation in LASIK-induced neuropathic dry eye. Ocul Surf. 2014;12:32–45. doi: 10.1016/j.jtos.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 55.Benitez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci. 2004;45:3030–5. doi: 10.1167/iovs.04-0251. [DOI] [PubMed] [Google Scholar]
- 56.Nitoda E, Kallinikos P, Pallikaris A, et al. Correlation of diabetic retinopathy and corneal neuropathy using confocal microscopy. Curr Eye Res. 2012;37:898–906. doi: 10.3109/02713683.2012.683507. [DOI] [PubMed] [Google Scholar]
- 57.Patel DV, McGhee CN. In vivo confocal microscopy of corneal stromal nerves in patients with peripheral neuropathy. Arch Neurol. 2009;66:1179–80. doi: 10.1001/archneurol.2009.191. author reply 80. [DOI] [PubMed] [Google Scholar]
- 58.Rowbotham MC, Petersen KL. Zoster-associated pain and neural dysfunction. Pain. 2001;93:1–5. doi: 10.1016/S0304-3959(01)00328-1. [DOI] [PubMed] [Google Scholar]
- 59.Moulton EA, Becerra L, Borsook D. An fMRI case report of photophobia: activation of the trigeminal nociceptive pathway. Pain. 2009;145:358–63. doi: 10.1016/j.pain.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okamoto K, Tashiro A, Thompson R, et al. Trigeminal interpolaris/caudalis transition neurons mediate reflex lacrimation evoked by bright light in the rat. Eur J Neurosci. 2012;36:3492–9. doi: 10.1111/j.1460-9568.2012.08272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okamoto K, Tashiro A, Chang Z, Bereiter DA. Bright light activates a trigeminal nociceptive pathway. Pain. 2010;149:235–42. doi: 10.1016/j.pain.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okamoto K, Thompson R, Tashiro A, et al. Bright light produces Fos-positive neurons in caudal trigeminal brainstem. Neuroscience. 2009;160:858–64. doi: 10.1016/j.neuroscience.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Noseda R, Burstein R. Advances in understanding the mechanisms of migraine-type photophobia. Curr Opin Neurol. 2011;24:197–202. doi: 10.1097/WCO.0b013e3283466c8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matynia A, Parikh S, Chen B, et al. Intrinsically photosensitive retinal ganglion cells are the primary but not exclusive circuit for light aversion. Exp Eye Res. 2012;105:60–9. doi: 10.1016/j.exer.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 65.Amini A, Digre K, Couldwell WT. Photophobia in a blind patient: An alternate visual pathway. Case report. J Neurosurg. 2006;105:765–8. doi: 10.3171/jns.2006.105.5.765. [DOI] [PubMed] [Google Scholar]
- 66.Dolgonos S, Ayyala H, Evinger C. Light-induced trigeminal sensitization without central visual pathways: another mechanism for photophobia. Invest Ophthalmol Vis Sci. 2011;52:7852–8. doi: 10.1167/iovs.11-7604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–3. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 68.Noseda R, Constandil L, Bourgeais L, et al. Changes of meningeal excitability mediated by corticotrigeminal networks: a link for the endogenous modulation of migraine pain. J Neurosci. 2010;30:14420–9. doi: 10.1523/JNEUROSCI.3025-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]