Abstract
Background
A developmental improvement of symptoms in Attention-Deficit/Hyperactivity Disorder (ADHD) is frequently reported, but the underlying neurobiological substrate has not been identified. The aim of this study was to determine whether white matter microstructure is related to developmental improvement of ADHD symptoms.
Methods
A cross-sectional Magnetic Resonance Imaging (MRI) analysis was embedded in a prospective follow-up of an adolescent cohort of ADHD and control subjects (NeuroIMAGE). Mean age at baseline was 11.9 years, mean interval of follow-up was 5.9 years. 75.3% of the original cohort was retained successfully. Data of 101 participants with ADHD combined type at baseline and 40 healthy controls was analysed. ADHD symptoms were measured with semi-structured, investigator-based interviews and Conners' questionnaires, on the basis of DSM-IV criteria. Fractional anisotropy (FA) and mean diffusivity (MD) indices of white matter microstructure were measured using whole brain diffusion tensor imaging at follow-up only. In a dimensional analysis FA and MD were related to change in ADHD symptoms. To link this analysis to DSM-IV diagnoses, a post-hoc categorical group analysis was conducted comparing participants with persistent (n=59) versus remittent (n=42) ADHD and controls.
Results
Over time, participants with ADHD showed improvement mainly in hyperactive/impulsive symptoms. This improvement was associated with lower FA and higher MD values in the left corticospinal tract at follow-up. Findings of the dimensional and the categorical analysis strongly converged. Changes in inattentive symptoms over time were minimal and not related to white matter microstructure.
Conclusions
The corticospinal tract is important in the control of voluntary movements, suggesting the importance of the motor system in the persistence of hyperactive/impulsive symptoms.
Keywords: ADHD, hyperactivity/impulsivity, white matter, DTI, recovery, development
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental disorder characterised by excessive levels of inattention and/or hyperactivity and impulsivity (American Psychiatric Association., 2000). Childhood onset ADHD persists into adulthood in 15%–65% of cases, depending on the definition of persistence (Barkley, Fischer, Smallish, & Fletcher, 2002; Biederman, Petty, Evans, Small, & Faraone, 2010; Faraone, Biederman, & Mick, 2006; Mannuzza, Klein, & Moulton, 2003). Hyperactive/impulsive symptoms are found to decline at a higher rate than inattentive symptoms (Biederman, Mick, & Faraone, 2000). Although various psychiatric factors have been associated with persistence of ADHD into adulthood (Biederman et al., 1996; Biederman et al., 2000; Mick et al., 2011), relatively little is known about associated biological mechanisms. A neurodevelopmental theory associates improvement of ADHD symptoms with the development of the prefrontal cortex and related top-down executive control (Halperin & Schulz, 2006). Within this theory, a non-cortical dysfunction is hypothesised to be static over time in all patients with a childhood ADHD diagnosis. Behavioural studies examining the relation between executive control functioning and remission of ADHD reported inconsistent results (Biederman et al., 2000; Fischer, Barkley, Smallish, & Fletcher, 2005; Halperin, Trampush, Miller, Marks, & Newcorn, 2008; Mick et al., 2011).
Few studies have examined the neurobiological underpinnings of remission of ADHD. A functional MRI study reported lower thalamo-cortical activation during response preparation for a cued reaction time task in adults with childhood ADHD (Clerkin et al., 2013). These findings suggested dysfunction of the thalamus in both remitters and persisters. Stronger functional integration of the thalamo-cortical network did parallel symptom recovery, supporting the neurodevelopmental theory. A structural brain imaging study reported that remission of symptoms was associated with normalisation of the developmental trajectory of cortical thickness, particularly in the right parietal cortex (Shaw et al., 2006). Persistent ADHD was characterised by a fixed thinning of the medial prefrontal cortex (Proal et al., 2011; Shaw et al., 2006), indicating a role of the prefrontal cortex in the improvement of ADHD symptoms.
In recent years, neural models of ADHD are shifting focus from the study of regional brain abnormalities to disturbed connectivity in networks (K. Konrad & Eickhoff, 2010). Structural connectivity and white matter (WM) abnormalities are key elements in these models. An established modality for investigating WM microstructure is diffusion tensor imaging (DTI (Basser, Mattiello, & LeBihan, 1994)). Two commonly used diffusion tensor derivative measures are mean diffusivity (MD), which measures the magnitude of diffusion and fractional anisotropy (FA), which quantifies the directionality of diffusion. Cross-sectional DTI studies in children and adults with ADHD have shown inconsistent results. A recent meta-analysis of DTI studies in ADHD which dealt mainly with children, reported WM abnormalities in the anterior corona radiata, cerebellum, internal capsule and forceps minor (van Ewijk, Heslenfeld, Zwiers, Buitelaar, & Oosterlaan, 2012). DTI studies of adults with persistent ADHD revealed abnormal WM microstructure in the temporal, orbitomedial prefrontal lobe and right anterior cingulate bundle (Casey et al., 2007; A. Konrad et al., 2010; Makris et al., 2008). The only study to date that has investigated the role of WM structure in remission of ADHD reported lower FA values in several WM tracts in ADHD subjects compared to controls, but no differences in DTI measures between persisters and remitters (Cortese et al., 2013).
In the current study, we aimed to investigate whether developmental improvement of ADHD symptoms is linked to the microstructure of white matter (WM) tracts. To that end, a large childhood ADHD cohort was followed longitudinally over 6 years. The association between developmental improvement of ADHD symptoms over adolescence and WM microstructure at follow-up was examined in a dimensional analysis. This analysis was corroborated with a categorical analysis that compared remitters and persisters head-to-head while also using a healthy control group. In accordance with the neurodevelopmental model, we hypothesised that developmental improvement of ADHD symptoms would be related to the WM microstructure of the fronto-striatal circuitry involved in executive control. Furthermore, we expected WM microstructure 1) of participants with remittent ADHD to be similar to healthy controls, and 2) of participants with persistent ADHD to differ from WM of both remittent ADHD and healthy controls.
Methods
Participants
Subjects with ADHD combined type (ADHD-C) and healthy controls (HC) participated in the Dutch part of the International Multicenter ADHD Genetics study (Muller et al., 2011a, 2011b). In short, all children were Caucasian, between 6–18 years (M=11.9, SD=2.6) and with an IQ≥70. Exclusion criteria were a diagnosis of autism, epilepsy, brain, or genetic disorders. IQ was estimated based on the WISC or WAIS–III vocabulary and block design subtests at baseline and at follow-up. Extensive diagnostic, neurocognitive and genetic data were collected at the VU University Amsterdam and Radboud UMC in Nijmegen. All participants were invited for follow-up measurement with a mean follow-up period of 5.9 years (SD=0.6). At the second assessment a similar phenotypic protocol was followed and complemented with the acquisition of MRI brain scans.
In the current analyses we included all subjects diagnosed with ADHD-C at baseline and a DTI-scan of high quality at follow-up (n=101; Figure S1 available online). There were no differences between the subjects included in current analysis and the complete sample on measures of ADHD severity (p=0.935), age (p=0.206) and gender (p=0.134). 40 age-matched HC subjects were additionally included, they were recruited from schools and did not meet criteria for ADHD, neither did their first-degree relatives. The study was approved by the regional ethics committee and written informed consent was given by the children and their parents.
Diagnostic assessment
Diagnosis was based on a semi-structured, investigator-based interview and Conners' questionnaires. The interview was the Dutch version of the Parental Account of Children’s Symptoms (PACS (Taylor, 1991; Taylor, Schachar, Thorley, & Wieselberg, 1986)) at baseline and the Schedule for Affective Disorders and Schizophrenia for Children (K-SADS(Kaufman et al., 1997)) at follow-up. Both interviews are compatible with the DSM-IV-TR (American Psychiatric Association., 2000). Initially, all participants were administered the screening interview. Participants with elevated scores on any of the screen items were administered the full ADHD supplement. The Conners' questionnaires were the teacher report (Conners’ Teacher Rating Scale: Long version (CTRS-R:L)) applied for children <18 years or a self-report (Conners’ Adult ADHD Rating Scales–Self-Report: Long version (CAARS-S:L)) applied for children ≥18 years (Conners, Sitarenios, Parker, & Epstein, 1998; Conners et al., 1997). Using a diagnostic algorithm, a combined symptom count was calculated by adding symptom counts on the interview and CTRS-R:L (for participants <18) or CAARS-S:L (for participants ≥18), both providing operational definitions of each of the 18 behavioral symptoms defined by the DSM-IV (American Psychiatric Association., 2000). Symptoms of the Conners’ questionnaires were only added to the combined symptom count if at least two symptoms were reported, to avoid the Conners’ score to put too much weight on the diagnosis. Of the Conners’ ADHD questionnaires, the following scales were used: DSM Inattentive behaviour (Scale L of the CPRS-R:L/CTRS-R:L; Scale E of the CAARS-S:L), DSM Hyperactive/Impulsive behaviour (Scale M of the CPRS-R:L/CTRS-R:L; Scale F of the CAARS-S:L) and DSM Total (Scale N of the CPRS-R:L/CTRS-R:L; Scale G of the CAARS-S:L). For participants using medication, ratings were done of children’s functioning off medication.
For the dimensional analyses, we calculated a measure of symptom change over time by subtracting the symptom count at baseline from the count at follow-up. For the categorical analyses, participants with a combined symptom count of ≥6 symptoms of hyperactive/impulsive and/or inattentive behaviour were diagnosed with persistent ADHD, provided they a) met the DSM-IV criteria for pervasiveness and impact of the disorder, b) had an age of onset before 7, and c) received a standardised T-score ≥63 on at least one DSM ADHD scale of the ADHD questionnaire. ADHD participants not meeting criteria for persistence were categorised as remitted. Completely remitted participants and controls had to score T<63 on all scales of the ADHD questionnaire and had ≤3 symptoms derived from the combined symptom counts of the K-SADS and CTRS-R:L/CAARS-S:L.
Diffusion tensor imaging and (pre-) processing
MRI data were acquired at follow-up only with 1.5T scanners from Siemens (MAGNETOM-Sonata and AVANTO) at two scan locations. Both scanners were equipped with the same 8-channel phased-array head coil. For each participant, whole brain diffusion-weighted images were collected (twice refocused PGSE EPI; 60 diffusion-weighted directions; b-factor 1000s/mm2; 5 non-diffusion-weighted images; interleaved slice acquisition; TE/TR=97/8500ms; GRAPPA-acceleration 2; phase full Fourier; voxelsize 2.0 × 2.0 × 2.2mm). DTI images were realigned and corrected for residual eddy-current (SPM8; http://www.fil.ion.ucl.ac.uk/spm, London, UK) and for artefacts from head and/or cardiac motion using robust tensor modelling (PATCH (Zwiers, 2010)). All DTI-scans were visually inspected to assess quality of the data. When the quality was insufficient the data of the subject was excluded (n=21, see Figure S1). Diffusion tensors and derived FA and MD values were then calculated for each voxel (FSL 4.1.7; (Behrens et al., 2003).
The group analysis of diffusion parameters was performed using Tract-Based Spatial Statistics (FSL-TBSS (Smith et al., 2006)). FA and MD images of each participant were nonlinearly registered to the FMRIB-58_FA template (MNI152-space). Subsequently, a group mean FA-image was created to produce a mean skeleton map of WM-tracts. Finally, diffusion parameters of each subject were projected onto the group skeleton, which was thresholded to FA≥0.3 to exclude peripheral tracts (Smith et al., 2006). Non-parametric permutation tests (5000 random permutations; FSL-randomise) were conducted voxel-wise on the whole brain WM-skeleton, while resulting p-values were corrected for multiple comparisons (p<0.05). The JHU DTI-based WM-atlas (available in FSL) was used to label WM-tracts.
Probabilistic tractography
To visualise the most likely pathways extending from significant FA clusters, we estimated voxel-wise fibre orientation distributions and probabilistic streamline tractography (FSL-Probtrackx (Behrens et al., 2003)). Significant voxels of the TBSS analysis were mapped into each subject's native space and used as seed-masks for tractography. To remove spurious connections, the resulting connectivity maps of individual subjects were thresholded to include only voxels which had at least 50 samples passing through them (out of 5000 generated streamlines). Finally, the individual thresholded images were projected into standard space, binarised, summed over individuals and thresholded to include tracts that were present in at least 75% of the subjects (Boorman, O'Shea, Sebastian, Rushworth, & Johansen-Berg, 2007).
Statistical analysis
Demographic between-group differences were tested using F-tests for continuous variables and X2-tests for categorical variables. For both the dimensional as the categorical MRI analysis, general linear models (GLMs) were built with FA or MD values as dependent variable and gender, age, duration of medication use and scanner site as covariates of no interest. We corrected for multiple comparisons by implementing threshold-free cluster enhancement (FSL-TFCE (Smith & Nichols, 2009)).
Dimensional analysis: Symptom change over time
In this within-group analysis among subjects with ADHD (n=101) (Table 1) we tested voxelwise whether WM microstructure differed as a function of hyperactive/impulsive and inattention symptom change. Therefore, univariate linear regressions were run with FA or MD values as dependent variable, symptom change as predictor variable and symptom count at follow-up as additional nuisance regressor to correct for differences in WM related to symptom count at scan time.
Table 1.
Demographic and clinical characteristics for the ADHD group at T1 and T2
| n = 101 | T1 | T2 | Test statistic | ||
|---|---|---|---|---|---|
| Demographic | |||||
| Age | 11.9 | (2.7) | 17.8 | (2.7) | t(100)=77.494** |
| Estimated IQ | 95.4 | 13.4 | 95.0 | (14.2) | t(76)=−0.605 |
| Clinical | |||||
| Interview hyperactivea | 8.1 | (1.0) | 5.8 | (2.4) | t(100)=9.763** |
| Interview inattentivea | 8.2 | (1.0) | 7.1 | (1.6) | t(100)=6.251** |
Note: M(SD); Estimated IQ based on Wechsler Intelligence Scale for Children or Wechsler Adult Intelligence Scale–III Vocabulary and block design; estimated IQ missing for 24 subjects at baseline.
Symptom count. Maximal 9 symptoms per dimension (≥6 is clinical threshold).
p<.05,
p < .001.
Categorical ROI analysis: Persistent and remittent groups in relation to healthy controls
To link our analysis to traditional DSM-IV diagnoses and relate our findings to previous reports, we divided the same subjects as in the dimensional analysis into persisters (n=59) and remitters (n=42) based on the hyperactive/impulsive symptom count at follow-up and added an age-matched HC group (n=40) (Table 2). Significant clusters of the dimensional analysis were used as regions of interest (ROIs) to conduct a voxel wise comparison between persisters, remitters and HC. FA and MD of the three groups was compared using an ANCOVA with gender, age, duration of medication use and scanner site as covariates of no interest.
Table 2.
Demographic and clinical characteristics of the hyperactive/impulsive remittent, persistent and healthy control group
| T1: IMAGE | Remit (n=42) | Persist (n=59) | Control (n=40) | Test Statistic | Post-hoc | |||
|---|---|---|---|---|---|---|---|---|
| Demographic | ||||||||
| Age | 11.9 | (2.4) | 11.9 | (2.8) | 12.9 | (2.3) | F(2, 138)=2.092 | - |
| Gender, % male | 85.7 | n=36 | 76.3 | n=45 | 25.0 | n=10 | χ2(2)=39.101** | R=P>C |
| Estimated IQ | 94.5 | (12.8) | 95.9 | ((13.9) | 106.4 | (13.4) | F(2, 114)=8.954** | R=P<C |
| Gestational ageb, weeks | 39.5 | (1.7) | 39.6 | (2.3) | 39.1 | (1.8) | F(2, 93)=0.578 | - |
| Birth weightc, kg | 3.3 | (0.5) | 3.5 | (0.5) | 3.4 | (0.6) | F(2, 96)=0.723 | - |
| Clinical | ||||||||
| PACS hyperactivea | 7.93 | (1.1) | 8.27 | (0.9) | - | - | t(99)=−1.623 | - |
| PACS inattentivea | 8.26 | (0.9) | 8.07 | (1.1) | - | - | t(99)=0.968 | - |
| T2: NeuroIMAGE | Remit | Persist | Control | |||||
| Demographic | ||||||||
| Age | 17.9 | (2.5) | 17.8 | (2.7) | 17.8 | (2.1) | F(2, 138)=0.034 | - |
| Estimated IQ | 96.3 | (13.2) | 94.1 | (15.1) | 106.7 | (13.5) | F(2, 136)=10.010** | R=P<C |
| Duration medication use, months | 82.7 | (51.8) | 77.1 | (60.5) | - | - | t(99)=0.845 | - |
| Scansite, % in Nijmegen | 59.5 | n=25 | 61.0 | n=36 | 60.0 | n=24 | χ2(2)=0.025 | - |
| Motion during scanningd | 28.0 | (10.2) | 31.0 | (14.8) | 26.5 | (8.1) | F(2, 138)=1.886 | - |
| Handness, % right handed | 88.1 | n=37 | 89.7 | n=52 | 90.0 | n=36 | χ2(2)=2.863 | - |
| ODD, % | 10.9 | n=11 | 23.8 | n=24 | 0 | n=0 | χ2=2.274 | - |
| CD, % | 2.0 | n=2 | 5.9 | n=6 | 0 | n=0 | χ2=1.032 | - |
| Follow-up interval, years | 6.0 | (0.7) | 5.9 | (0.6) | 4.9 | (0.7) | F(2, 138)=36.098** | R=P>C |
| Clinical | ||||||||
| K-SADS hyperactivea | 3.4 | (1.4) | 7.6 | (1.1) | - | - | t(99)=15.284** | R<P |
| K-SADS inattentivea | 7.1 | (1.3) | 7.2 | (1.8) | - | - | t(99)=−0.387 | - |
Note: M(SD); Estimated IQ based on Wechsler Intelligence Scale for Children or Wechsler Adult Intelligence Scale–III Vocabulary and block design.
Symptom count. Maximal 9 symptoms per dimension (≥6 is clinical threshold).
Gestational age missing for 18 remitters, 22 persisters, and 5 controls
Birth weight missing for 18 remitters, 22 persisters, and 2 controls
Motion during scanning quantified as the mean average displacement score of the realignment parameters
p<.05,
p < .001.
Results
Clinical outcome
Tables 1 and 2 present the demographics of the sample. Both the hyperactive/impulsive and the inattentive symptom count were significantly lower at follow-up compared to baseline (Table 1). Remittance was strongly skewed towards the hyperactive/impulsive domain of ADHD, with a mean symptom change of −2.30 symptoms (SD=2.37), and less towards the inattentive symptoms (mean change: −1.02 symptoms, SD=1.65). In fact, only n=3 (3%) of the childhood ADHD sample were completely remitted. We therefore focused on changes in the hyperactive/impulsive symptom count in the analyses. Persistent and remitted ADHD groups did not differ significantly in hyperactive/impulsive symptom counts at baseline. As expected, there were differences between the groups in terms of hyperactive/impulsive symptom score at follow-up and change of symptoms over time (Table 2). Between baseline and follow-up 86.1% of the subjects with ADHD used methylphenidate and 2.0% dexamphetamine. Pearson correlations between the change in symptoms and, age at follow-up (r(99)=−0.07, p=0.466), duration of medication use (r(99) =0.03, p=0.751), or baseline symptom count (r(99)=0.2, p=0.796) were low in the ADHD cases. No differences in follow-up interval were found between the persistent and remittent ADHD groups.
MRI results
Dimensional analysis: Symptom change over time
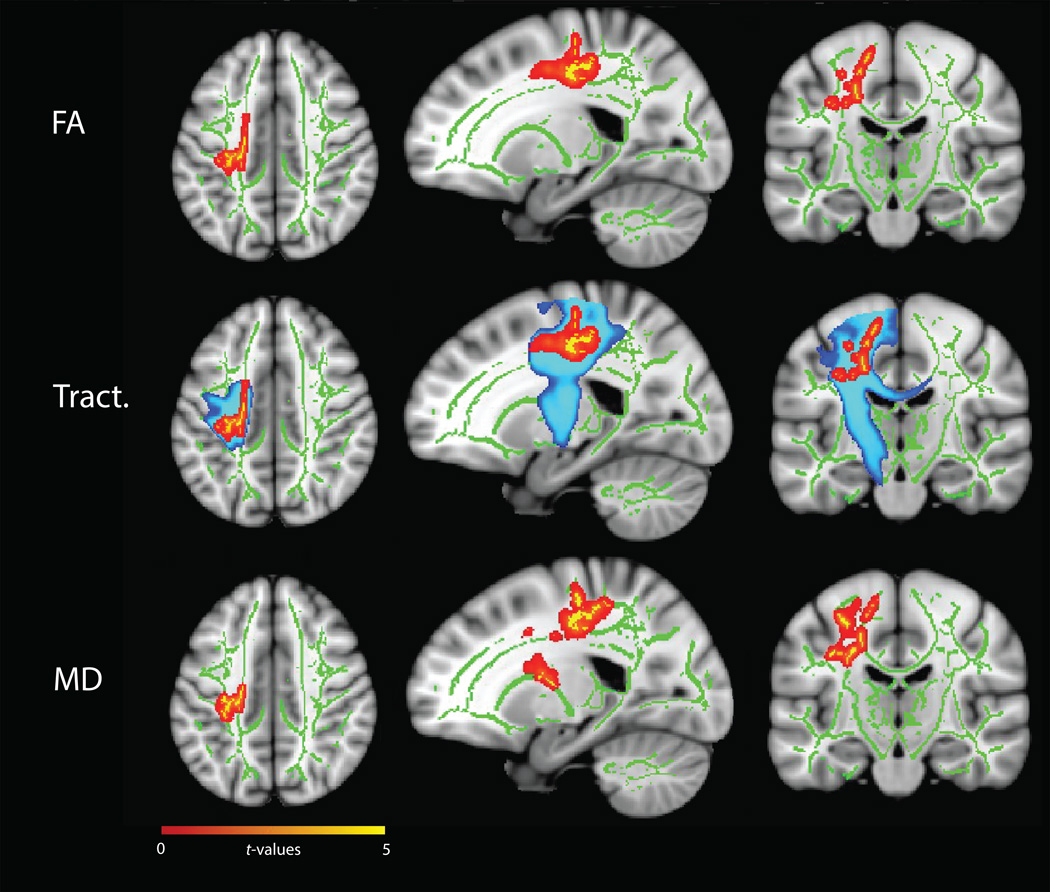
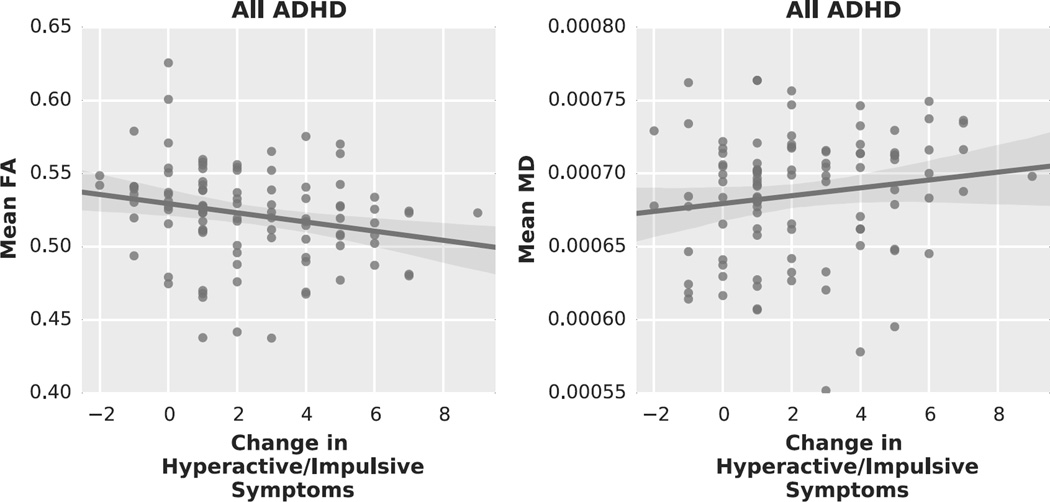
FA values varied as a function of hyperactive/impulsive symptom change over age in the left superior longitudinal fasciculus and corona radiata (peak voxel: t=4.84, p=0.03) (Figure 1 upper panel, Table 3). Lower FA values were associated with a stronger decrease (i.e. remission) in hyperactive/impulsive symptoms (Figure 2, left). Probabilistic tractography using significant FA clusters as seed regions indicated that these regions were most likely part of the corticospinal tract, running from the motor cortex through the corona radiata into the internal capsula (Figure 1, middle panel). MD values also varied as a function of hyperactive/impulsive symptom change (Figure 1, lower panel). Higher MD values were associated with stronger remission of hyperactive/impulsive symptoms (Figure 2, right), and significant clusters were located in similar regions as the FA results. We identified these regions as the bilateral corona radiata (peak voxel: t=4.78, p=0.04) and superior longitudinal fasciculus (peak voxel: t=4.77, p=0.02), extending to the internal and external capsula, corpus callosum (genu, body and splenium) and cingulum.
Figure 1.
Dimensional TBSS analysis showing significant associations (red-yellow) between MD and FA values and change in hyperactive/impulsive symptoms over time. Mean FA skeleton across all subjects (green) was overlaid on an MNI template image for presentation (x=−20, y=−17, z=41). The results were thickened for visualisation (FSL TBSS-fill). Top: Lower FA values are associated with a larger decrease in hyperactive/impulsive symptoms. Middle: The FA results were used as seeds for probabilistic tractography (blue) revealing the left corticospinal tract (JHU White-Matter Tractography Atlas). Bottom: Higher MD values are significantly associated (red-yellow) with a larger decrease in hyperactive/impulsive symptoms.
Table 3.
Peak voxels and localization of significant clusters of categorical and dimensional analysis
| MNI (Peak voxel) | |||||||
|---|---|---|---|---|---|---|---|
| Analysis | WM | n Voxels | X | Y | Z | tmax | p-value |
| Dimensional: hyperactive/impulsive symptom change | |||||||
| FA | SLF L | 431 | 119 | 102 | 111 | 4.84 | 0.03 |
| MD | SLF L | 785 | 119 | 99 | 111 | 4.77 | 0.02 |
| SCR L | 152 | 117 | 123 | 101 | 4.78 | 0.04 | |
| ROI Categorical: remit - persist - control | |||||||
| FA Control < persist | SCR L | 86 | 113 | 104 | 110 | 3.79 | 0.01 |
| Remit < persist | SCR L | 29 | 113 | 101 | 113 | 4.57 | 0.03 |
| MD Control > persist | SLF L | 918 | 117 | 107 | 102 | 4.99 | 0.001 |
| Remit > persist | SCR L | 556 | 112 | 102 | 109 | 4.08 | 0.002 |
min. 10 voxels per cluster; WM, white matter; FA, fractional anisotropy; MD, mean diffusion; L, left; R, right; MNI, Montreal Neurological Institute; SLF, Superior longitudinal fasciculus; ACR, Anterior corona radiata; AIC, Anterior internal capsula; SCR, Superior corona radiata.
Figure 2.
Graphs illustrating the relationship between (Left) mean FA values extracted from the significant results of the dimensional analysis and change in hyperactive/impulsive symptom count; (Right) mean MD values and change in hyperactive/impulsive symptom count.
Change in inattentive symptom count was not found to be significantly associated with altered FA or MD values. A series of control and sensitivity analyses showed that: 1) Neither FA nor MD values were found to be associated with IQ or medication use; 2) Adding comorbid ODD/CD diagnosis to or removing symptom counts at follow-up from the model did not significantly change the results; 3) Results were replicated (although not reaching significance due to reduced statistical power) in each scan site separately and in two age-groups based on a median split (at the age of 17.9); and 4) No interaction effects were found between the change in symptoms and medication use, gender, or age in predicting FA and MD values (see also Figure S2).
Categorical ROI analysis: Persistent and remittent groups in relation to healthy controls
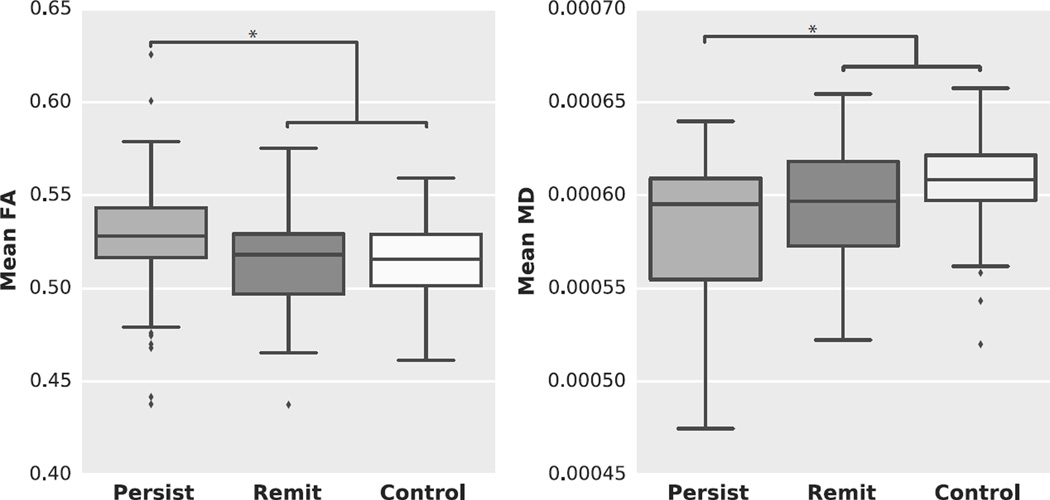
In the ROIs created based on the results of the dimensional analyses, both the HC and remittent group exhibited lower FA and higher MD values compared to the persistent hyperactive/impulsive group (Table 3 and Figure 3). The remittent hyperactive/impulsive group did not differ in FA, nor in MD values from HC.
Figure 3.
Tukey box plots illustrating the results of the categorical group comparison between persistent, remittent and healthy control groups. (Left) mean FA values for the three groups; (Right) mean MD values for the three groups.
Discussion
We investigated the relationship between WM microstructure and developmental improvement of ADHD symptoms in a longitudinal study of a large childhood ADHD cohort. Consistent with previous reports we observed that hyperactive/impulsive symptoms improved to a greater extent than inattentive symptoms (Biederman et al., 2000; Hart, Lahey, Loeber, Applegate, & Frick, 1995). Complete remission was rare. Hence, we focused our analyses on the WM correlates of improvement in hyperactive/impulsive symptoms. Persistence of diagnostic status was associated with higher FA and lower MD values in the left superior longitudinal fasciculus and corona radiata, which are part of the corticospinal tract. These motor?associated changes might reflect the findings of a stronger remittance of hyperactive symptoms compared to enduring attention deficits (Biederman et al., 2000; Lahey et al., 1994; Lahey et al., 2004; Molina et al., 2009). Also, in these regions, FA and MD values of subjects with remittent hyperactive/impulsive symptoms did not differ from those of HC, while subjects with persistent symptoms did show higher FA and lower MD values in this region compared to both remitters and HC. This is in line with and an extension of our previous findings showing that higher FA and lower MD are widely associated with more behavioural ADHD symptoms (van Ewijk et al., 2014).
In contrast to our hypothesis, no associations were found between the developmental improvement of ADHD symptoms and microstructure of fronto-striatal WM tracts. Importantly, this does not imply the absence of associations between fronto-striatal grey matter and ADHD remission. Although not hypothesised, the change in hyperactive/impulsive symptoms was related to WM microstructure of the corticospinal tract. This tract is of interest because of the motor hyperactivity subjects with ADHD exhibit and the essential role this tract plays for the motor system. It contains fibres running from the primary motor, premotor, supplementary motor, somatosensory, parietal and cingulate cortex to the spine and, thus, is involved in the control of posture, but also the control of more complex voluntary movements (Rizzolatti & Luppino, 2001). Our findings were unilateral, which may stem from the fact that 89% of our subjects were right-handed (note that motor axons cross to the contralateral side before reaching the lower motor neurons). Our data does not allow us to make inferences about causality; however, we could speculate that the findings in the corticospinal tract might be down-stream results of improved prefrontal executive control in remitting subjects with ADHD, which in turn may result in less stimulation of these motor tracts.
Although it is generally difficult to interpret FA or MD in terms of the individual elements that constitute the tissue microstructure (Beaulieu, 2002), our higher FA in conjunction with lower MD findings associated with persistent hyperactive/impulsive symptoms may indicate higher efficiency in motor signal transmission, either because the associated axonal fibres are more densely myelinated or because they are more numerous. However, the higher FA values can also be attributed to decreased neuronal branching (Suzuki, Matsuzawa, Kwee, & Nakada, 2003) in brain areas where healthy subjects have many crossing fibres (Mori, Wakana, & Van Zijl, 2005), though this is less likely to be accompanied with lower MD values. The finding of higher FA values in subjects with ADHD compared to controls has been frequently reported for various regions, including the superior longitudinal fasciculus, anterior corona radiata, uncinate fasciculus and thalamic radiation (Davenport, Karatekin, White, & Lim, 2010; A. Konrad et al., 2010; Silk, Vance, Rinehart, Bradshaw, & Cunnington, 2009; Tamm, Barnea-Goraly, & Reiss, 2012). However, there is a large degree of heterogeneity in DTI results, with studies also reporting lower FA in various regions including the internal capsula, corona radiata and corpus callosum in ADHD compared to control subjects (van Ewijk et al., 2012). The current results differ from those of a recent study using a longitudinal design in adults where the investigators found no difference in WM properties between remitters and persisters (Cortese et al., 2013). This may be explained by the relatively small statistical power given the sample size of their groups (persisters: n=15, remitters: n=25) and the variation in the ADHD subtypes of the persisters (6 inattentive, 6 hyperactive/impulsive and 3 combined).
Although this study was based on a prospective longitudinal design, brain structure was assessed at follow-up only, hence we can just speculate as to the WM status at baseline. It is possible that differences between subjects with remittent and persistent hyperactive/impulsive symptoms were already present at baseline; in this case, the corticospinal WM may be an early marker of remission of hyperactive/impulsive symptoms over time. Another possibility is that no WM differences between subjects with remittent and persistent symptoms were present at baseline. This implies normalisation of the corticospinal WM of subjects with remittent hyperactive/impulsive symptoms.
In this study we followed a large sample of children with ADHD-C over a period of 6 years. This period covers a developmental window that is ideally suited to the investigation of neural correlates of hyperactive/impulsive symptom change over time. However, there are a number of limitations to the study. Diagnostic interviews differed between baseline and follow-up, however Conner's questionnaires were used both at baseline and follow-up, and for consistency symptoms were always counted according to DSM-IV criteria. Secondly, this was an observational study in which the vast majority of ADHD participants were taking stimulant medications. More in depth medication studies are warranted to determine the precise effects of medication use on WM microstructure in relation to persistence and remission. Lastly, follow-up assessments at later age may allow for larger developmental change of inattentive symptoms and investigation of the neural underpinnings of this change.
In conclusion, we found in the current ADHD cohort foremost developmental improvement of hyperactive/impulsive symptoms of ADHD, confirming previous studies. The developmental improvement in hyperactive/impulsive symptoms was related to the white matter structure in the left corticospinal tract, which has an important role in the motor system. Participants with remittent symptoms did not show differences in this tract compared to healthy controls, while both those groups differed significantly from participants with persistent symptoms.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant R01MH62873 (to Stephen V. Faraone), NWO Large Investment Grant (1750102007010 to Jan Buitelaar), NWO Brain & Cognition grant (056-13-015 to Jan Buitelaar) and grants from Radboud University Nijmegen Medical Center, University Medical Center Groningen and Accare and VU University Amsterdam.
Footnotes
The authors have declared that they have no competing or potential conflicts of interest.
Supporting information
Additional supporting information is provided along with the online version of this article.
Figure S1. Flowchart of subject withdrawal throughout the longitudinal study
Figure S2. Graphs illustrating the relationship between mean FA values extracted from the significant results
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol. 2002;111(2):279–289. [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103(3):247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone S, Milberger S, Curtis S, Chen L, Marrs A, Spencer T. Predictors of persistence and remission of ADHD into adolescence: results from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1996;35(3):343–351. doi: 10.1097/00004583-199603000-00016. doi: S0890-8567(09)63465-1 [pii] 10.1097/00004583-199603000-00016. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157(5):816–818. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Evans M, Small J, Faraone SV. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299–304. doi: 10.1016/j.psychres.2009.12.010. doi:10.1016/j.psychres.2009.12.010 S0165-1781(09)00519-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman ED, O'Shea J, Sebastian C, Rushworth MF, Johansen-Berg H. Individual differences in white-matter microstructure reflect variation in functional connectivity during choice. Curr Biol. 2007;17(16):1426–1431. doi: 10.1016/j.cub.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, Glover G. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. Am J Psychiatry. 2007;164(11):1729–1736. doi: 10.1176/appi.ajp.2007.06101754. doi:164/11/1729 [pii] 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- Clerkin SM, Schulz KP, Berwid OG, Fan J, Newcorn JH, Tang CY, Halperin JM. Thalamo-cortical activation and connectivity during response preparation in adults with persistent and remitted ADHD. Am J Psychiatry. 2013;170(9):1011–1019. doi: 10.1176/appi.ajp.2013.12070880. doi: 10.1176/appi.ajp.2013.12070880 1734467 [pii] [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26(4):279–291. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- Conners CK, Wells KC, Parker JD, Sitarenios G, Diamond JM, Powell JW. A new self-report scale for assessment of adolescent psychopathology: factor structure, reliability, validity, and diagnostic sensitivity. J Abnorm Child Psychol. 1997;25(6):487–497. doi: 10.1023/a:1022637815797. [DOI] [PubMed] [Google Scholar]
- Cortese S, Imperati D, Zhou J, Proal E, Klein RG, Mannuzza S, Castellanos FX. White Matter Alterations at 33-Year Follow-Up in Adults with Childhood Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.02.025. doi: S0006-3223(13)00221-7 [pii] 10.1016/j.biopsych.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport ND, Karatekin C, White T, Lim KO. Differential fractional anisotropy abnormalities in adolescents with ADHD or schizophrenia. Psychiatry Res. 2010;181(3):193–198. doi: 10.1016/j.pscychresns.2009.10.012. doi: 10.1016/j.pscychresns.2009.10.012 S0925-4927(09)00245-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159–165. doi: 10.1017/S003329170500471X. doi: S003329170500471X [pii] 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- Fischer M, Barkley RA, Smallish L, Fletcher K. Executive functioning in hyperactive children as young adults: attention, inhibition, response perseveration, and the impact of comorbidity. Dev Neuropsychol. 2005;27(1):107–133. doi: 10.1207/s15326942dn2701_5. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychol Bull. 2006;132(4):560–581. doi: 10.1037/0033-2909.132.4.560. doi:2006-08436-006 [pii] 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Trampush JW, Miller CJ, Marks DJ, Newcorn JH. Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. J Child Psychol Psychiatry. 2008;49(9):958–966. doi: 10.1111/j.1469-7610.2008.01926.x. doi:10.1111/j.1469-7610.2008.01926.x JCPP1926 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EL, Lahey BB, Loeber R, Applegate B, Frick PJ. Developmental change in attention-deficit hyperactivity disorder in boys: a four-year longitudinal study. J Abnorm Child Psychol. 1995;23(6):729–749. doi: 10.1007/BF01447474. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. doi: S0890-8567(09)62555-7 [pii] 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Konrad A, Dielentheis TF, El Masri D, Bayerl M, Fehr C, Gesierich T, Winterer G. Disturbed structural connectivity is related to inattention and impulsivity in adult attention deficit hyperactivity disorder. Eur J Neurosci. 2010;31(5):912–919. doi: 10.1111/j.1460-9568.2010.07110.x. doi: 10.1111/j.1460-9568.2010.07110.xEJN7110 [pii] [DOI] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp. 2010;31(6):904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, McBurnett K, Biederman J, Greenhill L, Hynd GW, et al. DSM-IV field trials for attention deficit hyperactivity disorder in children and adolescents. Am J Psychiatry. 1994;151(11):1673–1685. doi: 10.1176/ajp.151.11.1673. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Kipp H, Ehrhardt A, Lee SS, Massetti G. Three-year predictive validity of DSM-IV attention deficit hyperactivity disorder in children diagnosed at 4–6 years of age. Am J Psychiatry. 2004;161(11):2014–2020. doi: 10.1176/appi.ajp.161.11.2014. [DOI] [PubMed] [Google Scholar]
- Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, Seidman LJ. Attention and executive systems abnormalities in adults with childhood ADHD: A DT-MRI study of connections. Cereb Cortex. 2008;18(5):1210–1220. doi: 10.1093/cercor/bhm156. doi: bhm156 [pii] 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Moulton JL., 3rd Persistence of Attention-Deficit/Hyperactivity Disorder into adulthood: what have we learned from the prospective follow-up studies? J Atten Disord. 2003;7(2):93–100. doi: 10.1177/108705470300700203. [DOI] [PubMed] [Google Scholar]
- Mick E, Byrne D, Fried R, Monuteaux M, Faraone SV, Biederman J. Predictors of ADHD persistence in girls at 5-year follow-up. J Atten Disord. 2011;15(3):183–192. doi: 10.1177/1087054710362217. doi:10.1177/1087054710362217 1087054710362217 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS Group, M. T. A. Cooperative. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, Van Zijl PCM. MRI atlas of human white matter. 1st ed. Amsterdam, The Netherlands: Boston: Elsevier; 2005. [Google Scholar]
- Muller UC, Asherson P, Banaschewski T, Buitelaar JK, Ebstein RP, Eisenberg J, Steinhausen HC. The impact of study design and diagnostic approach in a large multi-centre ADHD study. Part 1: ADHD symptom patterns. BMC Psychiatry. 2011a;11:54. doi: 10.1186/1471-244X-11-54. doi: 10.1186/1471-244X-11-54 1471-244X-11-54 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller UC, Asherson P, Banaschewski T, Buitelaar JK, Ebstein RP, Eisenberg J, Steinhausen HC. The impact of study design and diagnostic approach in a large multi-centre ADHD study: Part 2: Dimensional measures of psychopathology and intelligence. BMC Psychiatry. 2011b;11:55. doi: 10.1186/1471-244X-11-55. doi: 10.1186/1471-244X-11-55 1471-244X-11-55 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proal E, Reiss PT, Klein RG, Mannuzza S, Gotimer K, Ramos-Olazagasti MA, Castellanos FX. Brain gray matter deficits at 33-year follow-up in adults with attention-deficit/hyperactivity disorder established in childhood. Arch Gen Psychiatry. 2011;68(11):1122–1134. doi: 10.1001/archgenpsychiatry.2011.117. doi: 10.1001/archgenpsychiatry.2011.117 68/11/1122 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31(6):889–901. doi: 10.1016/s0896-6273(01)00423-8. doi: S0896-6273(01)00423-8 [pii] [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Rapoport J. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63(5):540–549. doi: 10.1001/archpsyc.63.5.540. doi: 63/5/540 [pii] 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R. White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum Brain Mapp. 2009;30(9):2757–2765. doi: 10.1002/hbm.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. doi: S1053-8119(06)00138-8 [pii] 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. doi: 10.1016/j.neuroimage.2008.03.061 S1053-8119(08)00297-8 [pii] [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Matsuzawa H, Kwee IL, Nakada T. Absolute eigenvalue diffusion tensor analysis for human brain maturation. NMR Biomed. 2003;16(5):257–260. doi: 10.1002/nbm.848. [DOI] [PubMed] [Google Scholar]
- Tamm L, Barnea-Goraly N, Reiss AL. Diffusion tensor imaging reveals white matter abnormalities in Attention-Deficit/Hyperactivity Disorder. Psychiatry Res. 2012;202(2):150–154. doi: 10.1016/j.pscychresns.2012.04.001. doi: 10.1016/j.pscychresns.2012.04.001 S0925-4927(12)00062-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E. Developmental neuropsychiatry. J Child Psychol Psychiatry. 1991;32(1):3–47. doi: 10.1111/j.1469-7610.1991.tb00002.x. [DOI] [PubMed] [Google Scholar]
- Taylor E, Schachar R, Thorley G, Wieselberg M. Conduct disorder and hyperactivity: I. Separation of hyperactivity and antisocial conduct in British child psychiatric patients. Br J Psychiatry. 1986;149:760–767. doi: 10.1192/bjp.149.6.760. [DOI] [PubMed] [Google Scholar]
- van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2012;36(4):1093–1106. doi: 10.1016/j.neubiorev.2012.01.003. doi: 10.1016/j.neubiorev.2012.01.003 S0149-7634(12)00010-3 [pii] [DOI] [PubMed] [Google Scholar]
- van Ewijk H, Heslenfeld DJ, Zwiers MP, Faraone SV, Luman M, Hartman CA, Oosterlaan J. Different mechanisms of white matter abnormalities in attention-deficit/hyperactivity disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2014;53(7):790–799. e793. doi: 10.1016/j.jaac.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Zwiers MP. Patching cardiac and head motion artefacts in diffusion-weighted images. Neuroimage. 2010;53(2):565–575. doi: 10.1016/j.neuroimage.2010.06.014. doi: 10.1016/j.neuroimage.2010.06.014 S1053-8119(10)00858-X [pii] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.