Abstract
Evoked potentials (EPs) are observed in motor cortical local field potentials (LFPs) during movement execution (movement-related potentials [MRPs]) and in response to relevant visual cues (visual evoked potentials [VEPs]). Motor cortical EPs may be directionally selective, but little is known concerning their relation to other aspects of motor behavior, such as task timing and performance. We recorded LFPs in motor cortex of two monkeys during performance of a precued arm-reaching task. A time cue at the start of each trial signaled delay duration and thereby the pace of the task and the available time for movement preparation. VEPs and MRPs were strongly modulated by the delay duration, VEPs being systematically larger in short-delay trials and MRPs larger in long-delay trials. Despite these systematic modulations related to the task timing, directional selectivity was similar in short and long trials. The behavioral reaction time was positively correlated with MRP size and negatively correlated with VEP size, within sessions. In addition, the behavioral performance improved across sessions, in parallel with a slow decrease in the size of VEPs and MRPs. Our results clearly show the strong influence of the behavioral context and performance on motor cortical population activity during movement preparation and execution.
INTRODUCTION
The local field potential (LFP) is thought to mainly reflect the synaptic input activity in large populations of neurons around the electrode tip, with additional contributions from spike afterpotentials and intrinsic transmembrane current changes (Logothetis et al. 2007; Mitzdorf 1985). Accordingly, the LFP in motor cortex should be a sensitive indicator of ongoing motor (preparatory) processes. A prominent feature of the LFP in motor cortex is the movement-related potential (MRP). Typically it starts with a positivity (P1) prior to movement onset followed by a negativity (N1) around onset and slower positive and negative (P2, N2) components during and immediately after the movement (Donchin et al. 2001). Visual evoked potentials (VEPs) were also described in motor cortex, induced by visual cues that are related to the upcoming movement (Asher et al. 2010; Ledberg et al. 2007; O'Leary and Hatsopoulos 2006; see also Gemba et al. 1981, 1990).
Motor cortical MRPs are directionally selective in amplitude (Cardoso de Oliveira et al. 2001; Mehring et al. 2003, 2004; Rickert et al. 2005). In addition, Roux et al. (2006) recently found that the MRP premovement (P1) component also modulates in size with the level of expectancy and preprocessing, suggesting that at least certain components of motor cortical evoked potentials (EPs) are influenced by the behavioral context.
If motor cortical EPs reflect ongoing processing during movement preparation and execution (Asher et al. 2010; Cardoso de Oliveira et al. 2001; Donchin et al. 2001; Ledberg et al. 2007; Mehring et al. 2003, 2004; O'Leary and Hatsopoulos 2006; Rickert et al. 2005; Roux et al. 2006), one would indeed expect their modulations to be correlated with changes in behavioral performance, as well as by changes in the context in which the movement is performed. To study motor cortical EPs under different behavioral contexts, we recorded LFPs from two macaque monkeys during the execution of a precued arm-reaching task in six directions. The task included two successive delays, which varied in duration from trial to trial in a precued fashion, allowing the monkey to anticipate the time of occurrence of the visual spatial cue at the end of the first delay and the GO signal at the end of the second delay (i.e., temporal expectation; Coull and Nobre 2008). The available time for movement preparation and the overall pace of the task thereby varied from trial to trial, whereas the visual spatial cues and required movements were the same in short-delay and long-delay trials.
We found significant modulations in VEP and MRP sizes in relation to both the precued delay duration and the animal's behavioral performance (reaction times [RTs]). This clearly shows that apart from reflecting the specific parameters of the movement being prepared/executed (e.g., movement direction), these population activity measures are strongly influenced by the behavioral context in which the movement is made. The results suggest a complementarity of early preparation (VEP) and late preparation/execution activity (MRP) in motor cortex.
Some preliminary results were previously presented (Kilavik and Riehle 2010; Kilavik et al. 2007).
METHODS
Experimental procedures
Two adult male rhesus monkeys (T and M, both 9 kg) participated in this study. Care and treatment of the animals during all stages of the experiments conformed to the European and French Government Regulations.
After learning the behavioral task the monkeys were prepared for multielectrode recordings in the right hemisphere of motor cortex, contralateral to the trained arm. A cylindrical stainless steel recording chamber inner diameter: [ID] 15 mm in monkey T and 19 mm in monkey M) was implanted under aseptic conditions and general isoflurane anesthesia (<2.5% in air). A T-bar was cemented to the skull to fixate the animal's head during recording sessions. Before and after surgery, antibiotics and analgesics were administered.
The chamber locations above motor cortex were verified with T1-weighted magnetic resonance imaging (MRI) scans in both monkeys (see Fig. 1A for monkey T) and additionally with intracortical microstimulation in monkey M. Microstimulation allowed further subdivision into primary motor cortex (M1) and dorsal premotor cortex (PMd) in monkey M (Fig. 1B), based on observed microstimulation thresholds for inducing muscle twitches (microstimulation protocol as in Asanuma and Rosén 1972). Locations in which microstimulation <20 μA current induced muscle twitches were defined as M1. Other locations, where higher current was needed to induce twitches, were denoted as PMd. Twitches could also be evoked at the most anterior recording locations, indicating we remained within the caudal part of PMd (F2), where there are direct projections to the spinal cord (Boudrias et al. 2010; Dum and Strick 1991). Only 37 of 490 (3 of 37 in M1, the rest in PMd) of the analyzed LFPs in monkey M were recorded from “nonarm regions” (i.e., the evoked muscle twitches at threshold were not in hand/arm/shoulder muscles; note that in several of these so-called nonarm regions, arm movements were also evoked when applying currents slightly above threshold). These LFPs were included in the analysis because their evoked potentials contained similar effects (e.g., modulations in evoked potential size in relation to delay duration) as LFPs recorded from arm-related regions. It is also clear that even if the monkeys were seated in a primate chair with the head fixated and the nonactive hand restrained, and performing an arm-movement task, we cannot exclude systematic task-related small shifts in body posture, for example, possibly involving other muscle groups.
Fig. 1.
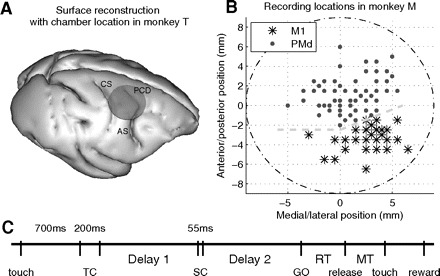
A: reconstruction of recording location in monkey T, based on T1-weighted magnetic resonance (MR) scans. The circle indicates the position of the recording chamber (ID: 15 mm). CS, central sulcus; AS, arcuate sulcus; PCD, precentral dimple. Anterior is toward the right. B: recording positions within the chamber (ID: 19 mm) in monkey M. Locations are assigned to primary motor cortex (M1) or dorsal premotor cortex (PMd) based on microstimulation controls. The gray dashed lines indicate the estimated location of the border between M1 and PMd. Zero is chamber center. Anterior is up, medial is left (chamber in right hemisphere). C: behavioral task; each vertical line indicates the onset or offset of a task or behavioral event (see methods for full description). TC, time cue; SC, spatial cue; RT, reaction time; MT, movement time.
A multielectrode, computer-controlled microdrive (MT-EPS; AlphaOmega, Nazareth Illit, Israel) was used to transdurally insert up to four or eight (in monkeys T and M, respectively) microelectrodes (0.5–1.2 MΩ at 1,000 Hz; glass or epoxy insulated tungsten electrodes; FHC, Bowdoinham, ME). In monkey T the electrodes were organized in a bundle in one common guide tube holding the individual electrode guides, with an interelectrode distance <400 μm (MT; AlphaOmega). However, since the electrodes were driven independently, their position in depth varied for each electrode. In monkey M, on some days electrodes were organized in a bundle as for monkey T and on others the electrodes were positioned independently within the chamber (ID: 19 mm) with separate guide tubes (Flex-MT; AlphaOmega). From each electrode, signals were amplified with a gain of 5,000 to 10,000 (MCP+, AlphaOmega) and separated into two channels with different band-pass filtering by using in-house hardware (active filtering): from 0.3 to 10 kHz for spiking activity and from 1 to 250 Hz for LFPs. The LFPs were stored with a temporal resolution of 1 kHz (AlphaMap; AlphaOmega), along with the behavior that was transmitted on-line to AlphaMap from the CORTEX software (NIMH, http://www.cortex.salk.edu), which was used to control the task. In the following, the abbreviation “LFP” refers to the LFP signal recorded on one electrode during one experimental session. Timed x and y hand position information was recorded with CORTEX and aligned with the neuronal signals off-line (see Fig. 2E).
Fig. 2.
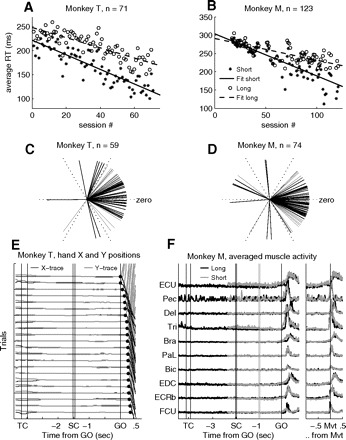
A and B: average RTs in short (stars) and long (open circles) delay trials in each recording session, plotted chronologically for monkey T (A) and monkey M (B). The lines are least-square fits to the data. C and D: difference between short and long trials (circular distance) of preferred directions (direction with the fastest RTs in average) for monkey T (C) and monkey M (D) for all recording sessions where all 6 movement directions were included; 57/59 and 59/74 of the sessions were significantly directionally selective in RTs in either short or long trials for monkeys T and M, respectively. The more the lines point toward zero, the smaller the circular difference in behavioral preference in short and long trials. E: example of hand X and Y positions for monkey T, from multiple correct repetitions of a long trial type. The x-axis indicates the timing of events. Each pair of black (x-direction of movement) and gray (y-direction of movement) lines corresponds to one trial (trials are displaced in steps of 0.5 cm on the y-axis for clarity). Filled circles after GO indicate movement onset. Trials are sorted off-line for increasing RT. F: averaged muscle activity from 10 arm and upper body muscles on the active side, recorded in representative and dedicated sessions in monkey M. Activity is aligned to the signals, at the left, and to movement onset (Mvt), at the right. Short trials are shown in gray, long trials in black, both for movements to the lower right (T2). The time of spatial cue (SC) onset and offset in long trials is indicated by 2 vertical black lines. The 2 vertical gray lines indicate SC in short trials. The selected muscles were (from top to bottom): extensor carpi ulnaris (ECU), pectoralis (Pec), deltoideus (Del), triceps brachii (Tri), brachioradialis (Bra), palmaris longus (PaL), biceps brachii (Bic), extensor digitalis communis (EDC), extsensor carpi radialis brevis (ECRb), and flexor carpi ulnaris (FCU). Artifacts attributed to the heartbeat can be observed on the electromyogram (EMG) taken from the pectoralis muscle.
Behavioral task
We trained the monkeys in a delayed center-out task to make arm movements in six directions in the horizontal plane, from a common center position. The movement was executed by holding a handle, freely movable in the two-dimensional plane, which contained a pen transmitting a signal to a digital tablet (WACOM Intuos2). The monkey had continuous monitor feedback about pen and target positions, the center target in yellow and the six peripheral target locations as red outlines.
The monkey started each trial by moving the handle to the center and holding it there for 700 ms before a temporal cue (TC) was presented (Fig. 1C). TC consisted of an auditory signal of 200-ms duration, its pitch indicating the delay duration to be estimated (low pitch for short-delay and high pitch for long-delay duration). In monkey M, a visual cue was additionally presented centrally on the monitor, simultaneously with the sound cue, a blue square for short and pink triangle for long-delay duration, in an attempt to observe visual evoked neuronal activity to TC. Two delays of equal duration were presented successively in each trial, the first (delay 1), starting with TC offset, demanded attention to perceive the briefly (55 ms) presented visual spatial cue (SC) at the end of it. During the second delay (delay 2) the movement indicated by SC had to be prepared. Apart from the initial TC, short and long trials were identical in terms of visual input and movements required; only the task timing was different. The short- and long-delay durations were fixed to 700 and 1,500 ms for monkey T and 1,000 and 2,000 ms for monkey M. Delay duration was changed from trial to trial, in a pseudorandom fashion.
For an exact temporal precision of SC we used light-emitting diodes (LEDs) as cues, placed over the target positions on the screen. After illumination of the target LED as SC, the target was masked by the additional illumination of the five remaining LEDs, marking the start of the preparatory delay (delay 2). At its end all the LEDs were turned off, providing the GO signal to swiftly execute the movement to the cued SC location. The distance between the central and peripheral targets (center to center) was 6.75 cm in the movement plane. Their diameters (required touch precision) were 0.91 and 2.24 cm, respectively. In some sessions we reduced the number of conditions by presenting only two opposite targets (4 conditions instead of 12). These sessions were included in the analysis, apart from those in the studies of directional selectivity of behavioral performance and EP size. Trials were considered to be correct when the movement was initiated within 500 ms of GO signal occurrence and completed to the correct target within another 500 ms, plus a final period of holding the handle at the target position of 300 ms. Only correct trials were rewarded by a drop of water (∼0.3 ml) and included in the analysis. When quantifying the percentage of errors we considered only late errors, occurring after the GO signal.
Data analysis
All analyses were conducted off-line by using Matlab (The MathWorks, Natick, MA).
REACTION TIMES AND MOVEMENT TRAJECTORIES.
To control behavior, RTs were defined on-line by CORTEX as the time between extinguishing the target LEDs (GO) and leaving the center target. For analysis, however, correct RTs were determined off-line from the movement trajectories. Single trial movement trajectories were measured (in mm) in x and y coordinates by acquiring two vectors in time. The final time resolution was 1 ms. Missing values (because of the temporal imprecision of MS Win XP) were extrapolated from the surrounding values. The mean of each x and y trajectory in each correct trial was calculated during the 500 ms before GO, to determine the movement's starting position. The moment when reaching a 2-mm deviation from the starting position, minus a fixed latency of 35 ms (average movement duration from the starting position to the threshold), was then determined as movement onset. From each of the two vectors (x and y), the shortest time was defined as RT. These values were controlled by visual inspection of single trial trajectories (example in Fig. 2E).
EMG ACTIVITY.
Electromyographic (EMG) activity was recorded from 10 muscles of the active (left) arm, shoulder, and chest of monkey M in multiple representative and dedicated sessions by using differential surface electrodes on the skin. Raw EMG signals were recorded at 1-kHz resolution and subsequently full-wave rectified and integrated off-line (see Fig. 2F).
LOCAL FIELD POTENTIALS.
Each LFP was normalized prior to further analysis by using the z-score. Since there is no DC offset in the LFP signal, the 0 V baseline is the overall mean of the LFP over time. This normalization allowed comparison of EP sizes across LFPs. To measure single-trial EP sizes (see following text), the LFPs were first band-pass filtered between 1 and 15 Hz (4th-order Butterworth filter; example of single-trial filtered LFPs are shown in Fig. 3A). This frequency range corresponds to the main frequency band of these signals, as shown in the spectrogram in Fig. 3B. Furthermore, it excludes the prominent beta oscillations (20–25 Hz), which were phase-locked neither to the SC nor to movement onset, but partly overlapping in time (not in frequency) with the EPs. Exclusion of the beta-band activity in the present study does not imply that we consider it to be noninformative regarding, for instance, the task timing (e.g., Kilavik and Riehle 2010), but here we focus on the evoked activity. The spectrogram in Fig. 3B was calculated using Welch's method, including all short trials in a single representative LFP recorded in monkey T. The data were analyzed in 300-ms sliding windows, with 50-ms offsets.
Fig. 3.
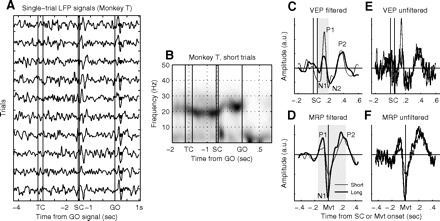
A: example of local field potential (LFP) recorded from one electrode during one recording session in monkey T. Each trace corresponds to a single trial in the same condition, filtered between 1 and 15 Hz and sorted off-line for increasing RT (filled circles after GO). Data are aligned to the signals. B: spectrogram of a representative LFP recorded in monkey T. All short trials were included in the analysis. Darker gray indicates increased power. Beta oscillations (around 20–25 Hz) were strong during long periods of the task. The evoked potentials after SC and around movement onset (after GO) are also visible as short-lasting increases in power <10 Hz. C and D: average visual evoked potential (VEP) and movement-related potential (MRP) of a single LFP for short and long trials in the same movement direction (same LFP as in A). The LFP was prefiltered off-line between 1 and 15 Hz before trial averaging. Components are tagged. Note, that the P2 component of VEPs was rarely observed in monkey T. The gray zones indicate the trial epochs in which the root-mean-square (rms) was calculated. E and F: the same data as shown in C and D, but without off-line prefiltering (only on-line filter during recording between 1 and 250 Hz). The main trial-averaged evoked potential (EP) components that are tagged in C and D can also be found in the raw data. The y-axis scales are the same in E and F as those in C and D, respectively.
Evoked potentials.
EPs were analyzed in epochs that were selected based on visual inspection of trial-averaged LFPs (prior to filtering). The main MRP epoch was −150 to +250 ms around movement onset for both monkeys, selected to capture the three main components: P1, N1, and P2 (see Figs. 3D and 6D). The main VEP epoch was +60 to +200 ms (monkey T) and +60 to +190 ms (monkey M) after SC onset, selected to capture the two main components N1 and P1, which were the most frequent VEP components (see Figs. 3C and 6C). The size of EPs was determined in single trials by calculating the area in the selected epochs using the root-mean-square (rms). The areas were subsequently averaged across trials for each condition. To test the significance of the EPs, the sizes of VEPs and MRPs were compared with a control epoch (the rms area during the 200 ms prior to TC), by using a one-sided t-test (P < 0.05) including all single-trial values, separately for short- and long-delay trials. If a particular EP was significant, all single-trial values were included in further analysis.
Fig. 6.
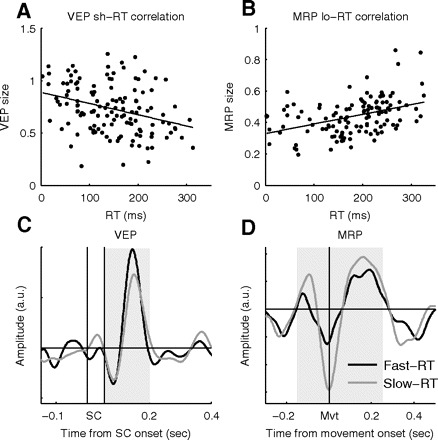
A: example of a significant negative trial-by-trial correlation between VEP size and RT for one LFP (short trials; r = −0.33, P < 0.001). B: example of another LFP with significant positive trial-by-trial correlation between MRP size and RT for one LFP (long trials; r = +0.41, P ≪ 0.001). C and D: comparison of the average VEP-short (C) and MRP-long (D) waveforms in the 50% of trials with fast (black) and slow (gray) RTs, for the same LFPs as shown in A and B. The gray zones indicate the trial epochs in which the rms was calculated.
Single EP components.
We also quantified individual VEP and MRP components separately. The MRP N1 quantification was done as in Roux et al. (2006), similar to the method used by Donchin et al. (2001). For each LFP and each condition, N1 was determined by its negative peak (see Fig. 3D) and the preceding and subsequent zero-line crossings (using trial-averaged LFP traces). The epoch between these two zero crossings was then defined as N1 (typically of ∼100-ms duration). In the preceding 100 ms and subsequent 200 ms we also quantified the MRP P1 and P2 components, respectively.
The individual VEP components were analyzed in a similar way, but because of the more variable amplitude of the components in different conditions and LFPs (making the method described earlier less reliable), we used fixed epochs, selected for each animal based on visual inspection. For monkey T, three components could be distinguished: N1 (60–120 ms after SC onset), P1 (120–200 ms), and N2 (200–310 ms; less frequent and thus not included in the main VEP analysis epoch). In monkey M, four VEP components could be distinguished: N1 (60–110 ms), P1 (110–190 ms), N2 (190–290 ms), and P2 (290–460 ms), the latter two not being part of the main VEP analysis epoch because of their less frequent occurrence across LFPs. We mainly report findings obtained from analyses of the complete VEPs and MRPs because results were very similar for the individual components (mentioned in results).
Peak amplitudes of single components.
We also calculated the peak amplitudes on single trials for the largest and most frequent VEP and MRP components: VEP P1 and MRP N1. Peak amplitude was defined as the positive/negative deviation from the zero baseline. The VEP P1 peak amplitude was measured in the epoch 120–200 or 110–190 ms after SC onset, in monkey T and M, respectively. Similarly, peak amplitude for the MRP N1 component was selected in the epochs used to measure the N1 rms areas (see preceding text). In results we mainly report findings obtained from rms measurements. However, very similar results were obtained when observing the peak amplitudes, as mentioned on several occasions in results.
Directional selectivity.
We determined directional selectivity of RTs and EP sizes. For the EPs we used the P1 component of VEPs and the N1 component of MRPs because they were the largest and most frequent of the EP components. The EP “preferred direction” was defined as the direction of the vector sum of the average single-trial rms values in the six directions. The “preferred direction” of the average RTs was defined as the opposite direction of the vector sum, to indicate the direction with the fastest (smallest) RTs.
We also estimated the significance of the directional selectivity using a resampling procedure (similar to that used by Asher et al. 2007). The length of the vector sum was taken as the strength of directional selectivity. For each EP (or session for RTs), single-trial rms (or RT) values were then randomly reassigned to one of the six movement directions and a new vector sum was calculated. This procedure was repeated 1,000 times and the length of the vector sum of the original data was then compared with the vector sums of the resampled data. If the original vector sum was >950 of the 1,000 resampled vector sums (P < 0.05), the EP (or the RTs in this session) was deemed directionally selective.
Because we were interested in comparing preferred directions in short- and long-delay trials, we subsequently calculated the circular distance (angle) between preferred directions in short and long trials of RTs for all recorded sessions (Fig. 2, C and D) and for all EPs that were significantly directionally selective both in short and in long trials (Fig. 5).
Fig. 5.
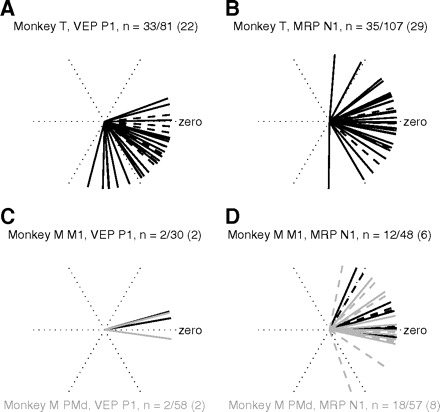
The circular distance (angle) between preferred directions in short and long trials for all statistically significant EPs (denominator in heading) and selective (numerator in heading) in short and long trials. Each line represents the circular difference between short and long trials of one EP. Zero indicates no difference in preferred directions in short and long trials. Dashed lines indicate all EPs that are selective in short and long trials, whereas overlying solid lines indicate the subset (numbers in parentheses in heading) that are additionally significantly modulated by delay duration. M1 in black and PMd in gray (monkey M).
Circular statistics were done using the circular statistics toolbox for Matlab (http://www.mathworks.com/matlabcentral/fileexchange/10676).
RESULTS
We recorded 287 LFPs in motor cortex from monkey T in 90 sessions across 37 days and 759 LFPs in monkey M in 151 sessions across 73 days. Of those, 183 LFPs (71 sessions; 59 of those recorded with all 12 behavioral conditions) in monkey T and 490 LFPs (132 from M1 and 358 from PMd; in a total of 123 sessions; 74 of the sessions recorded with all 12 conditions) in monkey M were selected for further analysis. The remaining LFPs were excluded because of too many artifacts or due to lack of sufficient correct trials (if <10 correct trials per condition; typically 20 correct trials per condition were obtained). In the selected LFPs, some trials with obvious artifacts detected by visual inspection were further excluded from analysis.
Behavioral performance in relation to delay duration and long-term task practice
Recordings started when the monkeys were proficient in the task, performing with a success rate of 75 and 69% on short and long trials, respectively, for monkey T and 84 and 75%, respectively, for monkey M, averaged across all included recording sessions. Both monkeys performed the task with significantly fewer errors in short trials (paired t-test across sessions, P ≪ 0.001 for both). Additionally, the percentage of errors decreased across sessions in short and long trials in both monkeys (P < 0.01 for all). Although the majority of the behavioral errors was directional (>67% of errors in both monkeys), it was mainly the percentage of other types of errors that decreased across sessions (e.g., responding or completing the movement too late; P < 0.001 in both monkeys in short and long trials). The percentage of directional errors decreased across sessions only in long trials for monkey M (P < 0.01). The directional information was presented only during a very brief epoch at the end of a timed interval (55 ms, following a 700- to 2,000-ms delay) and subsequently masked. Directional information thus had to be kept in working memory during the subsequent movement preparation delay, possibly explaining the higher proportion of directional errors in long-delay trials.
Averaged RTs across sessions in short and long trials, respectively, were 167 and 210 ms in monkey T and 236 and 257 ms in monkey M. RTs were significantly different (P ≪ 0.001) in short and long trials and decreased significantly (P ≪ 0.001) across sessions, for both monkeys (see Fig. 2, A and B). The mean interquartile ranges (the difference between the upper limit for the lower quartile and the lower limit for the upper quartile of single-trial values) of RTs in short and long trials, respectively, were 77 and 54 ms in monkey T (significantly different, paired t-test, P ≪ 0.001), 67 and 60 ms in monkey M (P < 0.001). Note that for simplicity we use the term “reaction time” here, although we are entirely aware of its questionable use in instructed delay tasks. It is evident that subjects do not necessarily react to the GO signal, but most often anticipate its occurrence. Monkey T, in particular, had relatively fast RTs in short trials, combined with a higher trial-by-trial RT variability, indicating a stronger tendency to anticipate the occurrence of the GO signal. However, the distributions of RTs across all trials were not bimodal in short or long trials (not shown) for either of the two animals. In the following analysis we therefore did not attempt to select only those trials with sufficiently late RTs to qualify as “reactions” to the GO signal.
In summary, although both animals performed the task sufficiently well in short and long trials, their performance was significantly different depending on the delay duration. In short trials, RTs were faster but more variable and fewer errors were made. In addition, there was a significant improvement in performance across sessions, due to the extensive daily practice.
RTs were not the same for the six movement directions, but the preferred directions for each animal (with the fastest average RTs) tended to be similar in different sessions (Rayleigh's test, P < 0.05). Preferred direction was calculated using the vector sum (see methods). Figure 2, C and D shows circular distance (angle) between the behaviorally preferred movement directions in short and long trials for both monkeys. Each line in the plots corresponds to one session. In both monkeys the preferred directions were similar in short and long trials (circular mean +15 and −5° in monkeys T and M, respectively), with the mean circular distance significantly different from zero only for monkey T (P < 0.01). Thus even if the overall RTs were strongly modulated by delay duration (see preceding text), the directional selectivity of RTs was similar in short and long trials.
Figure 2E shows representative single-trial movement trajectories during the delays [separated in simultaneous left/right (X) and toward/away from the body (Y) pen positions on the digital tablet] in one movement direction, recorded in monkey T in one session. Clearly no systematic movements were made after the hand position stabilized on the center before TC and until after the GO signal. Filled circles after GO indicate movement onset (see methods).
In monkey M, we recorded EMG activity in several sessions. As a result, we find that the main arm and upper body muscles that were activated during movement execution (see Fig. 2F, right; data aligned to movement onset) showed no systematic activity modulation during the delay periods (Fig. 2F, left). These behavioral controls confirm that neither of the two animals made systematic overt movements during periods when they should not move, i.e., during delays and following SC.
Motor cortical visual evoked potentials to the spatial cue
Figure 3A shows filtered (1–15 Hz) single-trial LFPs from multiple repetitions of a long trial type during one session. Trials are aligned to the GO signal. MRPs are clearly visible in single trials around movement onset (marked with filled circles; trials sorted off-line for increasing RT) and VEPs are visible after SC. In the example LFP shown in Fig. 3A, magnitudes of the VEPs and MRPs were indeed similar (trials aligned to signals). However, this was not always the case because it was more typical to observe MRPs than VEPs in single-trial traces.
In this study we analyzed VEPs in an epoch following SC onset, with single-trial LFPs aligned to the signals, whereas MRPs were analyzed in an epoch around movement onset, with single-trial LFPs aligned in each trial to the movement onset (see methods). Figure 3B shows the spectrogram for all short trials in a representative LFP from monkey T. Apart from the clear increases in power <10 Hz after SC and GO, reflecting the EPs, there was also increased power in the beta frequency range (20–25 Hz). The prefiltering between 1 and 15 Hz minimally compromised the size of EPs on single trials while excluding beta oscillations that were partly overlapping in time with the EPs, but not phase-locked with SC or movement onset. Figure 3, C–F shows trial-averaged VEP and MRP waveforms from filtered and unfiltered LFPs recorded in short- and long-delay trials (same LFP as in Fig. 3A).
Figure 4A shows the percentage of LFPs that contained significantly identified VEPs and MRPs in short and long trials in both monkeys. For monkey M, LFPs from M1 and PMd are quantified separately. VEPs and MRPs were frequently observed in both monkeys, in both M1 and PMd. In monkey T, MRPs occurred more often than VEPs, a difference that was significant in long trials (chi-square, P < 0.01). In monkey M, VEPs occurred significantly more often than MRPs in short trials (chi-square, P < 0.05; data pooled across M1 and PMd not shown). There was a decrease in the percentage of significant EPs across sessions. In a comparison of the first third and last third of recorded LFPs, there was a decrease from 98 to 72% in monkey T and from 75 to 40% in monkey M (summing across VEPs and MRPs in short and long trials). This suggests that there was a decrease in average EP size compared with the baseline epoch for later sessions.
Fig. 4.
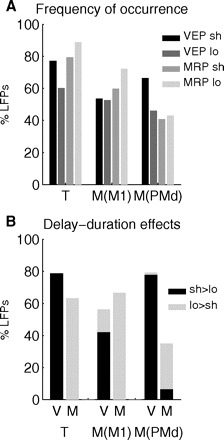
A: overall percentage of LFPs containing statistically significant VEPs and MRPs, in short and long trials, in the 2 animals (T and M), shown separately for M1 and PMd in monkey M. B: percentage of VEPs (V) and MRPs (M), with a significant effect of delay duration in the 2 animals, separated for M1 and PMd in monkey M. Percentages are calculated with respect to the population containing significant EPs in either short or long trials (see A). The bars indicate the proportion of EPs whose size was significantly larger (black) or smaller (gray) in short than in long trials.
Although VEPs and MRPs were frequent in M1 and PMd, MRPs occurred significantly more often in M1 than in PMd (60 vs. 41% in short and 72 vs. 43% in long trials, chi-square, P < 0.01 for both comparisons). VEPs were more often present in PMd than in M1, in short trials (66 vs. 53%, although not significant, P = 0.09). In summary, the most frequently occurring EP in M1 was the MRP in long trials, whereas in PMd it was the VEP in short trials.
Delay duration modulates the VEP and MRP sizes
The difference in the number of significant EPs in short and long trials suggests a difference in their size in short and long trials. Figure 3, C–F shows an example of an LFP where the VEP was significantly larger in short trials, whereas the MRP was larger in long trials. We find that this was indeed a general effect in the majority of LFPs in both animals. Figure 4B shows the percentage of EPs (of all LFPs with significant VEPs/MRPs in either short or long trials, as shown in Fig. 4A), with a significant difference in size in short and long trials (determined with a two-way ANOVA, with delay duration and movement direction as the two factors; P < 0.05). Altogether, 35–79% of the EPs were significantly modulated by delay duration. In both monkeys, and in both M1 and PMd, the large majority of VEPs with a significant effect of delay duration was larger in short trials. The opposite was true for MRPs, for which almost all were larger in long trials (Fig. 4B). A significant delay duration effect was more frequently observed for the VEP in PMd and for the MRP in M1 (chi-square, P < 0.01 for both comparisons). The results were very similar for the individual VEP and MRP components. In fact, only VEP N2 in monkey T, which was the least frequent VEP component in this monkey, was more often significantly larger in long trials. All other VEP components in both monkeys were more often significantly larger in short than in long trials. All MRP components in both monkeys were more often significantly larger in long trials.
As a control, we also measured peak amplitudes of the VEP P1 and the MRP N1 components (see methods for details). The same conclusions as described earlier were reached when comparing peak amplitudes in short and long trials. In particular, all VEP P1 components with significantly different peak amplitudes in short and long trials had larger peak amplitudes in short trials in both monkeys. Correspondingly, a large majority of the MRP N1 components with a significant delay-duration effect had larger peak amplitudes in long trials in both monkeys.
In summary, VEPs were more often present and were larger in short-delay trials, whereas MRPs were more often present and were larger in long-delay trials. The effect was strong for both monkeys and in both M1 and PMd.
Do delay-duration–related modulations of EP size affect directional selectivity?
Motor cortical MRPs were shown to be directionally selective (e.g., Cardoso de Oliveira et al. 2001; Mehring et al. 2003; Rickert et al. 2005), as confirmed by our data. Also directional selectivity of stimulus-locked oscillations in different frequency ranges was recently reported in motor cortex (O'Leary and Hatsopoulos 2006). They may partly reflect the directional-selective VEPs we are reporting on. We estimated directional selectivity separately in VEPs and MRPs in short and long trials. We calculated the vector sum of the average VEP P1 and MRP N1 components in the six movement directions (for all LFPs that were recorded in sessions with 12 conditions and contained statistically significant EPs). Only the VEP P1 and MRP N1 components were taken into account because they were the most frequently occurring components. Preferred direction was defined as the direction of the vector sum. The significance of directional selectivity was estimated with a resampling procedure (see methods). Many VEPs and MRPs were significantly directionally selective (in monkey T between 42 and 58% of VEPs and MRPs in short and long trials, similar for VEPs and MRPs; in monkey M, 25% of VEPs in short trials and 13% in long trials and ∼40% of MRPs). The preferred directions were significantly nonhomogeneously distributed around the circle (Rayleigh's test, P < 0.05).
Quantification of directional selectivity of EPs in our study and other studies was based on different measures of size or “amplitude” of the signal (e.g., rms area as used here and in Asher et al. 2007; rms of band-pass filtered data in different frequency bands in O'Leary and Hatsopoulos 2006; waveform amplitude in Rickert et al. 2005; peak-to-peak size in Cardoso de Oliveira et al. 2001). In the previous section we described how delay duration strongly modulated the EP size in a majority of LFPs. The behavioral results show that even if RTs were strongly modulated by delay duration, the animal's preferred direction remained the same because the direction with the shortest RTs was similar in both short and long trials (see Fig. 2, C and D). We were therefore interested in whether the EP modulation induced by delay duration interacted with the modulation related to movement direction. We calculated the circular distance (angle) between the preferred directions in short and long trials, for all significant EPs that were directionally selective in both short and long trials. In Fig. 5 we show the circular distance for VEPs (Fig. 5, A and C) and MRPs (Fig. 5, B and D) for the two monkeys. Solid lines mark EPs that were directionally selective in short and long trials and additionally significantly modulated by delay duration, whereas the dashed lines indicate EPs that were only directionally selective. Note that in monkey M only a few VEPs were directionally selective in both short and long trials (cf. Fig. 5C). In general, the circular distances of all EP signals were clustered within less than ±1 target (neighboring target distances were 60°). The mean angles of circular difference between short and long trials in monkey T were −35° (significantly different from zero, P < 0.0001) and 2° (not significant), for VEPs and MRPs, respectively. In monkey M the mean circular difference of VEPs was +8° (not significantly different from zero, but only four data points) and that of MRPs +10° (not significant).
There was no evident difference between the subsets that were modulated by delay duration (solid lines) and the nondelay-sensitive populations of selective EPs (underlying dashed lines). Similarly, there was no systematic difference between M1 and PMd in monkey M (black vs. gray lines). Another interesting point is that the proportions of the directionally selective EPs with delay-duration effects were similar to the proportions found in all LFPs (Fig. 4B). Overall, these results indicate that the preferred direction remained stable both in short and in long trials, even when the size of the EP was significantly modulated by delay duration.
Trial-by-trial relationship between evoked potentials and RTs
Earlier we have shown that the size of motor cortical EPs is strongly modulated by the temporal context. If these modulations reflect the amount of ongoing motor processing, then EP size should also correlate with RT, which varied considerably from trial to trial (see earlier text in results). We therefore correlated EP size (measured in single trials) with RT, for all movement directions together, for each LFP. Figure 6A shows an example LFP with a significant negative correlation between VEP and RT, in short trials. Figure 6B shows another LFP with a significant positive correlation between MRP size and RT, in long trials. In Fig. 6, C and D we show the average waveforms of the same EPs as in Fig. 6, A and B, separated for the 50% of trials with faster versus slower RTs in each direction, plotted together for all directions. The amplitude is different in fast and slow RT trials (mainly P1 for the VEP), whereas the latency of the component peaks remains similar. This indicates that the significant trial-by-trial correlations between EP size and RT were mainly due to modulations in amplitude of the EP component, and not to a systematic shift in latency, which could have led to a misalignment of the rms analysis epochs with respect to the EP components. The same conclusion was reached when inspecting component latency and amplitude of other LFPs with significant EP–RT correlations (also see results of more detailed analysis of peak amplitudes in the following text).
Figure 7, A and B shows all correlation coefficients from correlation analyses between the EP size and RT, for all EPs in both monkeys. In the plots, data from short and long trials are grouped together, as well as data from M1 and PMd in monkey M, for clarity. All LFPs are included, irrespective of whether the correlations were significant. In both monkeys, most of the MRP–RT correlation coefficients were positive (monkey T: 94 and 94% and monkey M: 90 and 79%, in short and long trials, respectively; significantly more positive than negative, chi-square, P ≪ 0.001 for all). Correlations between VEP and RT were weaker, as could be expected because of the long separation in time between SC and movement onset (700 ms to 2 s delay duration). Still, there was a weak majority of negative correlation coefficients (monkey T: 80 and 50% and monkey M: 57 and 55%, in short and long trials, respectively; significantly more negative than positive for short trials in monkey T, chi-square, P ≪ 0.001), reflecting the slight shift of the VEP population to the left of zero in Fig. 7A, for monkey T. For the entire population of LFPs we additionally calculated the average EP size in each session, separately for the two halves of trials with the fastest and slowest RTs (selected separately for each movement direction). A comparison between the two distributions revealed that VEPs were larger in fast RT trials (significant for short trials in monkey T and for short and long trials in monkey M, paired t-test, P ≪ 0.001), whereas MRPs were larger in slow RT trials (P ≪ 0.001 for all comparisons). When looking separately at EPs recorded in M1 and in PMd in monkey M, only VEPs in short trials in M1 were not significantly different in fast and slow RT trials (P < 0.05 VEP in long trials in M1; P ≪ 0.001 for the other comparisons).
Fig. 7.
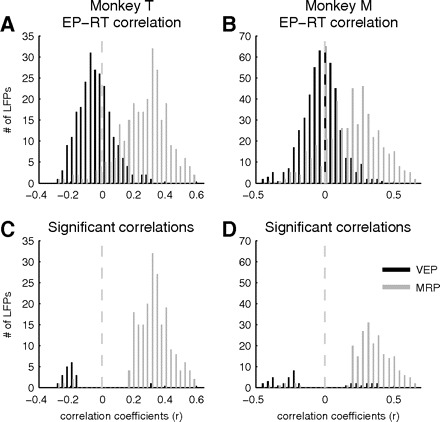
A and B: distributions of correlation coefficients (r) from trial-by-trial EP–RT correlations for monkey T (A) and monkey M (B). C and D: same as A and B, showing only the r values of significant correlations.
In Fig. 7, C and D, only the significant (P < 0.05) correlation coefficients are shown. Almost all significant MRP–RT correlations were positive (41–77% significant, 95% or more of these were positive correlations in short and long trials for both monkeys; short and long trials grouped in the plots). Only few individual VEPs were significantly correlated with RT, but most of the significant correlations were negative [monkey T: 12% (all 16 cases negative) in short, but only 3% (2/3 negative) in long trials; monkey M: only 4% (6/12 negative) in short, but 13% (22/28 negative) in long trials]. LFPs in which RT was significantly correlated with both VEP and MRP size were rare (11/118 in short-delay and 3/101 in long-delay trials in monkey T; 4/170 in short-delay and 8/144 in long-delay trials in monkey M).
Significant MRP–RT correlations were much more frequent in M1 (83 and 66%, in short and long trials, respectively) than in PMd (33 and 26%, respectively; significant difference between M1 and PMd in both short and long trials, chi-square, P ≪ 0.001).
We also assessed RT correlations for the individual components. There were only a few significant VEP–RT correlations, with a weak majority of negative significant correlations. For the MRP components, the results were very similar to those described earlier, with a large majority of positive correlations. In addition, we analyzed the peak amplitudes of the components (see methods) in fast and slow RT trials. In particular, we compared peak amplitudes in the 50% of trials with fast and slow RTs, for individual LFPs (e.g., Fig. 6, C and D). For VEP P1, a majority of significant cases had larger peak amplitudes in trials with fast RTs, compared with trials with slow RTs (monkey T: 83% larger on fast RT trials of the 34% with significant difference in short trials and 48% of 23% in long trials; monkey M: 70% of 21% in short trials and 90% of 29% in long trials). This is consistent with the negative VEP–RT correlations described earlier. The MRP N1 component, whenever significantly different in fast and slow RT trials, was larger in trials with slower RTs, consistent with the positive MRP–RT correlations described earlier.
To assess whether MRP correlations with RT could be due to underlying visual evoked activity to the GO signal (all six LEDs turned off), we measured the trial-by-trial variance of the peak latency of the MRP P1 component with respect to GO signal occurrence and movement onset (see Roux et al. 2006). This component is more likely to reflect GO signal-related activity than any other MRP component. For each LFP with a significant MRP P1 component, we defined an index Q = (var X − var Y)/(var X + var Y), in which var X is the variance of the P1 peak latency with respect to GO signal occurrence and var Y is the variance with respect to movement onset. A positive Q indicates that the component is more related to movement onset than to GO signal occurrence. The means of Q for all LFPs were 0.69 ± 0.10 and 0.54 ± 0.18 in short and long trials for monkey T and 0.54 ± 0.17 and 0.64 ± 0.16 for monkey M. Only two individual Q values were negative across all four data sets (both individual Q values were less negative than −0.05; total n = 256). Thus MRP P1 peak latency varied much less with respect to movement onset than to GO signal onset, indicating that it reflects movement-related activity to a much stronger degree than any GO signal-related activity. This confirms the conclusion of Roux et al. (2006) who found P1 to be movement-related in the context of complete information concerning time and direction of movement.
Finally, because RTs varied with movement direction (see Fig. 2, C and D), we also analyzed the correlations between EP rms size and RT in each movement direction separately. Again, a majority of the relatively few significant correlations between VEP size and RT were negative. A large majority of the significant MRP–RT correlations were positive, confirming the results from the analysis done across all directions together, described earlier.
In summary, we find that RT was weakly negatively correlated with VEP size, but strongly positively correlated with MRP size.
Trial-by-trial correlations between VEP and MRP size
Earlier we described that several individual VEPs and many MRPs were correlated in size with RTs, on a trial-by-trial basis, but only a minority of LFPs had significant correlations with RT for both VEPs and MRPs. However, if VEPs and MRPs in the same LFPs were correlated with RTs, we could also expect a direct correlation between VEP and MRP size in some LFPs. In particular, because most VEP–RT correlations were negative and most MRP–RT correlations were positive, we could expect most of the significant VEP–MRP correlations to be negative. We therefore quantified VEP–MRP trial-by-trial size correlations in all LFPs with significant VEPs and MRPs, separately for short and long trials. It turned out that a small subset of LFPs in monkey T indeed had significant VEP–MRP correlations [17/118 (14%), in short-delay trials; 10/102 (10%), in long-delay trials] and a majority of the significant correlations were negative (13/17 and 8/10, in short- and long-delay trials, respectively). However, only very few correlations were significant in monkey M, with a small majority of positive correlations [9% (15/170, 4 negative) in short-delay trials; 6% (9/144, 3 negative) in long-delay trials]. It is noteworthy that we found larger proportions of significant correlations in conditions under which the delay durations were shorter; i.e., in both monkeys more often in short than in long trials and more often in monkey T, who had shorter delay durations than those of monkey M (indeed in monkey M so few correlations are significant that it possibly reflects the significance level set at 5%). Monkey T, who had a larger proportion of significant correlations, had a majority of negative VEP–MRP correlations, in agreement with the inverse VEP–RT and MRP–RT correlations described earlier.
Long-term modulations of motor cortical EP size and improvement in performance
So far we have exclusively studied context- and performance-related modulations in motor cortical EPs on a trial-by-trial basis, within single LFPs and sessions. As mentioned initially, however, there was a significant improvement in performance across sessions, with a reduction in RTs and the percentage of errors. We therefore compared EP sizes across sessions (Fig. 8). All EPs in monkey T decreased significantly in size across sessions. Also in monkey M, VEPs significantly decreased in area M1 in short trials and in area PMd in short and long trials, whereas MRPs significantly decreased only in short trials, in area M1.
Fig. 8.
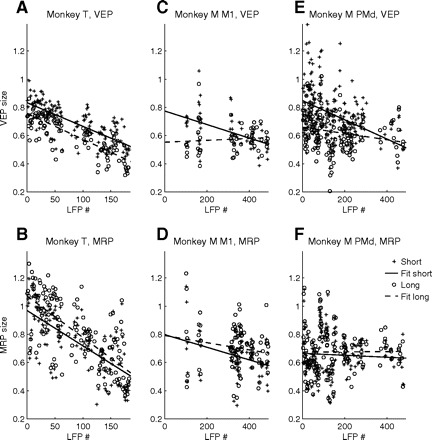
EP sizes of LFPs recorded during chronologically ordered sessions. Top plots show VEP sizes in short and long trials in monkey T (A) and in monkey M in M1 (C) and PMd (E). Bottom plots show MRP sizes. Lines are least-square fits to the data. A correlation analysis of EP size with session number resulted in significant negative correlations for all EPs in monkey T (r < −0.69, P ≪ 0.001 for all measures). In monkey M, VEPs decreased significantly in size across sessions in area M1 in short trials (r = −0.48, P < 0.001) and in area PMd in short (r = −0.43, P ≪ 0.001) and long (r = −0.21, P < 0.01) trials. MRPs decreased significantly across sessions only in short trials, in area M1 (r = −0.36, P < 0.01).
Given the highly significant reduction in RT across sessions for both monkeys, it is not surprising that we also find significantly positive across-session correlations between average EP size and RTs. Indeed, all EPs were significantly positively correlated with RT in monkey T (r > 0.6, P ≪ 0.001 for all). In monkey M, again, VEPs were more strongly correlated with RTs across sessions than MRPs. In M1, VEPs and MRPs were correlated with RT in short trials (r = 0.53 and P ≪ 0.001, r = 0.34 and P < 0.001, respectively). In PMd, VEPs in short trials were correlated with RT (r = 0.25, P < 0.001). Note that monkey M had a much stronger RT reduction in short-delay than that in long-delay trials (Fig. 2B). Finally, it is also not surprising to find VEP and MRP sizes to be positively correlated across sessions (r > 0.8, P ≪ 0.001 in monkey T; r > 0.3, P ≪ 0.001 in monkey M; analyzed separately in short and long trials; see also Asher et al. 2010).
The across-session effects observed in the global EPs were similar for the individual VEP and MRP components. In particular, the size of all individual VEP and MRP components decreased significantly across sessions in monkey T. In monkey M, several of the individual VEP components decreased across sessions (significant for N1, P1, and P2 in short trials in area M1; for all components apart from N2 and P2 in long trials in PMd). Only a few of the MRP components in monkey M decreased across sessions (significant for P1 in long and N1 in short trials in M1; only for P1 in short trials in PMd). Note that none of the components increased in size across sessions.
Because we use the z-score to normalize each LFP, a decrease in EP size could in principle imply a systematic increase in SD across sessions (and a decrease in signal-to-noise ratio). Monkey T, who had the strongest decrease in EP sizes across sessions, had a weak decrease also in SD across sessions (r = −0.19, P = 0.01). In monkey M, there was a weak increase in SD (r = 0.38, P < 0.001).
DISCUSSION
The amplitude of motor cortical EPs is related to several aspects of motor performance, observed for both MRPs around movement onset (Cardoso de Oliveira et al. 2001; Donchin et al. 2001; Roux et al. 2006) and VEPs as response to cues (Asher et al. 2010; Ledberg et al. 2007; O'Leary and Hatsopoulos 2006). Here we show for the first time that both types of EPs are strongly influenced by the precued delay duration and are correlated with the behavioral performance on a trial-by-trial basis, as well as on a slower timescale, as performance improves across several months of daily practice.
Motor cortical VEPs: visual processing or movement preparation?
Interestingly, whereas a majority of motor cortical LFPs recorded in our study contained VEPs in response to SC, only a handful of them showed an evoked response to TC, even in monkey M, where TC consisted of simultaneously presented auditory and visual stimuli. The lack of evoked responses to TC suggests that stimulus-induced evoked activity in motor cortex is tightly linked to movement preparation, not merely reflecting sensory inputs. This is not to say that single neurons in motor cortex cannot be selective for visual stimuli (Riehle 1991; Wannier et al. 1989; Zach et al. 2008), modulating their activity even when a movement is not to be executed (Miller et al. 1992).
In our data set from monkey M, VEPs (in response to SC) were observed in both M1 and PMd, even if more frequently in PMd than in M1. This suggests that there is no clear-cut difference of visually related early movement preparatory processing between PMd and M1 (Asher et al. 2010; Ledberg et al. 2007; O'Leary and Hatsopoulos 2006), as already proposed for preprocessing activity in single neurons (Riehle 2005; Riehle and Requin 1989).
Motor cortical MRPs
MRPs have been studied extensively, but their origin is still controversial and might depend on the behavioral context. The phasic component preceding movement onset (epidural negativity; probably corresponding to our intracortical MRP P1) was described to originate in motor cortex, whereas the component following movement onset (epidural positivity; probably corresponding to our MRP N1) was found to be generated in both pre- and postcentral cortices (Arezzo and Vaughan Jr 1975, 1980; Pieper et al. 1980). The premovement component (MRP P1) was mainly interpreted as reflecting input to motor cortex to launch the movement or, more directly, the descending motor volley itself (Donchin et al. 2001; Gemba et al. 1981; Vaughan Jr et al. 1970), but it was found to be modulated with expectancy and prior information, indicating that it also partly reflects preparatory processes (Roux et al. 2006). In motor cortex the postmovement component MRP N1 was found to be unaffected by deafferentation (Rothwell et al. 1982; Vaughan Jr et al. 1970), but it was absent in somatosensory cortex of a deafferented patient (Kristeva et al. 2006). Our motor cortical MRP N1 might therefore reflect central feedback to motor cortex, whereas the MRP N1 in somatosensory cortex probably reflects feedback from the periphery (Kristeva et al. 2006). After cerebellar hemispherectomy, the phasic motor cortical MRP components preceding and following movement onset disappeared in a simple visually triggered RT task (Sasaki et al. 1981), but they remained largely unchanged if a warning signal preceded the visual GO signal with a fixed delay (Sasaki et al. 1990). Thus motor cortical MRPs might reflect cerebellar input only to a minor degree in delay tasks such as ours. Finally, the late component, probably corresponding to our MRP P2, seems to originate in motor cortex and is interpreted as being evoked by sensory feedback from the periphery and from lower motor centers (Cui and Deecke 1999; their MEP1 component). Donchin et al. (2001) suggested that MRP P2 (and the preceding N1) reflect recurrent synaptic activation within motor cortex.
It is clear that much work still remains to permit comparing electroencephalographic (EEG) and LFP signals and results in monkey and human subjects. However, it seems likely that the phasic premovement MRP component reflects preparatory and movement-launching processes, whereas postmovement components reflect a variety of subcortical and peripheral feedback and recurrent activity in motor cortex, to optimize the ongoing movement. In our task the monkey uses visual feedback during movement execution (i.e., feedback of hand trajectory and the target position presented on the monitor) more than during the simple wrist or finger extension movements that were performed in most of the studies mentioned earlier. The modulations in size of the different MRP components in relation to delay duration and RT might therefore reflect the degree to which the animal relies on visual feedback during execution.
Task timing modulates evoked potentials
Recently it has been shown that the firing rate of single neurons is modulated by delay duration (e.g., Roesch and Olsen 2005; Roux et al. 2003; reviewed in Kilavik and Riehle 2010); higher firing rates are mainly associated with shorter delays.
A few studies on human participants have explored EEG activity using temporal orienting paradigms (e.g., Correa et al. 2006; Miniussi et al. 1999; Praamstra et al. 2006). These studies mainly reported on EPs over occipital areas to validly (presented at the precued time) and invalidly (presented earlier than indicated by the precue) cued targets. VEPs were larger to validly cued targets (Correa et al. 2006; Praamstra et al. 2006). However, these studies did not directly compare (Correa et al. 2006; Miniussi et al. 1999) or failed to observe differences between (Praamstra et al. 2006) EPs to targets after short and long intervals (validly cued). It is therefore questionable whether modulations in the neuronal responses to the targets after the delay in relation to precue validity can be directly compared with effects of delay duration.
Correa et al. (2006) found in the EEG larger P1 responses to cues predicting short delays than predicting long delays before target onset. When comparing their cue to our SC and their target to our GO signal, even if the paradigms are not completely analogous, their results correspond to ours, with larger VEPs in trials with less time between the cue and the upcoming movement.
Why are VEPs and MRPs modulated by the delay duration? One can speculate that the effect of delay duration on VEPs is partly related to time estimation, which increases in variability with increasing delay duration (scalar property of time estimation processes; Gibbon 1977). The smaller VEP and the higher fraction of (directional) errors in long trials could partly reflect less optimal estimation of the time of SC occurrence (Rolke and Hoffmann 2007). However, as mentioned earlier, an effect of delay duration prior to the onset of a visual target was not observed in human EPs (Praamstra et al. 2006), indicating VEP size modulations might rather reflect the following than the preceding delay duration.
The fact that MRPs are also modulated suggests that delay-duration effects reflect a behavioral strategy related to the pace of the trial. In long trials, the animal has more time available to prepare the movement than in short trials. The long interval between SC and GO might indeed make it less beneficial to prepare detailed aspects of the movement early after SC (1,500/2,000 ms before movement onset) because this means the specifics of the movement must be kept “on alert” for up to 2 s before actually being executed. Additionally, it might be more difficult to estimate the exact time of GO occurrence in long-delay trials (Niemi and Näätänen 1981). It might therefore be beneficial, in long-delay trials, to wait for the GO signal before finalizing movement preparation, whereas in short-delay trials much of the preparation might be done shortly after SC, resulting in anticipating the GO signal. This is supported by the fact that average RTs were longer in long-delay trials and only a few trials with very short RTs were observed, suggesting little anticipation. Thus the pattern of RTs, RT variability, and errors suggest an anticipatory response mode in short-delay trials and a reactive response mode in long-delay trials. This implies a complementarity between early preparatory processes (partly reflected in the VEP) and late movement preparation/execution-related processes (partly reflected in the MRP), adapted to the temporal constraints of the task. It is interesting to note that the effects of delay duration were similar in the individual components of the EPs, indicating to a large degree a task-related scaling of the global EP.
Trial-by-trial correlations between EP size and RT
The size of many MRPs was significantly positively correlated with RT on a trial-by-trial basis, whereas VEPs were mainly negatively correlated with RT, albeit very seldom significantly for single LFPs. The weaker negative correlation between VEP and RT, mainly visible when pooling across all LFPs, could be due to the long time interval between SC and movement onset. Trial-by-trial modulations in RT and neuronal responses might be related to fluctuations in attention, which could easily vary during the time course of a single trial. Likewise, this might partly be the reason that we found only few significant correlations between VEP and MRP sizes in individual LFPs, with more significant correlations in the conditions with shorter delays. Monkey T, who had a larger proportion of significant correlations, had mainly negative VEP–MRP correlations, corroborating the inverse pattern of VEP–RT and MRP–RT correlations. However, VEPs and MRPs (and task-related modulations thereof) were not necessarily observed in LFPs recorded on the same electrodes. One reason for this might be that MRP effects were more often observed in M1, whereas VEP effects were more often observed in PMd (discussed in the following text). It is therefore not evident that one should expect to observe significant VEP–MRP correlations in LFPs recorded on the same electrode.
The firing rates of up to 40% of single neurons in motor cortex show a significant negative correlation with RT, during the last few hundreds of milliseconds before GO (Kubota and Hamada 1979; Lecas et al. 1986; Requin et al. 1990; Riehle and Requin 1993; reviewed in Riehle 2005); in other words, the higher the firing rate during the preparatory delay, the shorter the RT. However, when spike data are aligned to movement onset and tallied during the RT interval, there is either no correlation or the correlation with RT becomes positive (Riehle 2005), consistent with the positive MRP–RT correlations we observe. The much higher percentage of LFPs with significant MRP–RT correlations in M1 than in PMd suggests a stronger correlation between M1 activity and motor performance during movement execution.
The opposite correlations of VEPs and MRPs with respect to RT and also with respect to delay duration (see preceding text) suggest that motor cortical EPs are associated with preparatory processes. Prior information provided by SC that is already processed during the delay does not need to be processed again after the GO signal. Early preparation (preprocessing) leads to shortening of RTs (e.g., Riehle 2005) and might partly be reflected in the VEP, whereas late movement preparation after the GO signal and on-line adjustment during the movement might partly be reflected in the MRP. Whenever there is a reduced preprocessing after SC, possibly also related to a task-solving strategy (see preceding text), this will result in slower RTs and might also be reflected in smaller VEPs and larger MRPs. Thus the movement production might rely more on feedforward, or automatic, processes in short-delay and/or fast-RT trials, and on sensory feedback in long-delay and/or slow-RT trials, reflected in larger MRPs.
Directional selectivity and task timing
We found many EPs to be directionally selective with preferred directions being similar in short and long trials. This was the same for the behavioral preferences, observed in RTs. This stability might not be surprising by itself because the visual spatial cues and the required movements were the same in short and long trials. However, it becomes interesting by the fact that the delay duration strongly modified both the RTs and the EP sizes. The effect of task timing (trial pace) and directional selectivity thus seem to be largely independent processes. This suggests that a modulation induced by directional information may “ride” on top of a more general modulation induced by delay duration because duration-related modulations were indeed more frequent and consistent than those related to movement direction. The result is relevant when considering the potential use of EPs in brain–machine interfaces (Andersen et al. 2004; Mehring et al. 2003) because it shows that similar directional information is available even if the overall size of evoked potentials changes considerably with the behavioral context of the movement. As suggested earlier, the observed delay-related modulations can partly reflect a task-solving strategy related to the relative urgency of preparation; however, the correct movement must be prepared between the spatial cue and movement onset, in both short and long trials. The VEPs and MRPs (and their directional selectivity) presumably partly reflect synaptic input to different neuronal populations because many single neurons that are active after SC are silent after GO and vice versa (for examples, see Kilavik and Riehle 2010; Roux et al. 2003). The complementarity of VEPs and MRPs therefore implies flexibility in the neuronal participation in movement preparation, highly adaptable to the pace of the task.
Correlations between EP size and RT across sessions reflecting long-term practice
VEPs and MRPs decreased in size across sessions as performance improved. This indicates that multiple processes are reflected in EP modulations, related to, on the one hand, trial-by-trial changes in network activity (see preceding text), possibly reflecting fluctuations in the level of attention and readiness and, on the other hand, long-term changes in network activity, reflecting repeated task practice (for studies of motor cortical spiking activity in relation to long-term task practice also see Kilavik et al. 2009; Matsuzaka et al. 2007; Schieber 2002). Most studies of motor cortical plasticity during motor learning were related to relatively short periods, concentrating on single sessions or a few days (for a review see Sanes and Donoghue 2000). Sasaki and Gemba (1982) studied EPs during earlier phases of learning visually initiated hand movements and found EPs to increase in size (stabilize) as the monkey learned to perform the task. Here we observe a slow decrease in EP size with long-term practice, well after the task was acquired, possibly reflecting a more gradual optimization of task timing.
The decrease in EP size across sessions is probably not related to neural tissue damage due to the prolonged recordings because performance continued to improve across sessions. Importantly, we recorded for a longer period (1 yr) in monkey M, with up to eight electrodes simultaneously and only for 5 mo in monkey T, with up to four electrodes simultaneously, and the across-session effects were weaker in monkey M. A deterioration of signal quality (which might be seen as an increase in SD) across sessions also cannot adequately explain the changes in EP size. The SD decreased across sessions in monkey T and increased in monkey M only. Rather we note that in monkey T both RTs and EP sizes decreased in short and in long trials across sessions and in monkey M there were larger across-session improvements of RTs in short trials (Fig. 2) and also larger decreases in EP sizes in short trials across sessions (Fig. 8). This suggests that the selective decrease in EP sizes is indeed related to animals' improvement in performance. Note also that the z-score normalization was, per definition, the same for short and long trials (and for VEPs and MRPs) in each LFP. If the increase in SD would have been a major cause of the apparent decrease in the normalized EP sizes in monkey M, we would expect similar changes in short and in long trials (and in VEPs and MRPs), which was not the case.
Asher et al. (2010) reported a negative correlation between VEP and RT and a positive correlation between VEP and MRP. A correlation between RT and MRP size was not mentioned. Unfortunately, the authors did not specify whether there were any systematic modulations across sessions either in EP size or in RTs. This prohibits a direct comparison with our finding of a decrease in RT and in EP size across sessions, reflecting long-term task practice.
Differences between M1 and PMd
There were several quantitative differences between M1 and PMd. In particular, MRPs were observed more often, were more often modulated by delay duration, and were more often correlated with RT in M1 than in PMd. On the other hand, VEPs were more frequently observed, were more often modulated by delay duration, and were more strongly correlated with RT in PMd than in M1. The fact that all results described earlier were indeed observed in both areas, however, indicates only a gradual difference in neuronal representations of visual information and movement preparation in the two areas (e.g., O'Leary and Hatsopoulos 2006), as also suggested for single-neuron activity (reviewed in Riehle 2005).
Conclusions
We have shown that motor cortical EPs are strongly modulated in relation to the temporal context and the behavioral performance. Our results suggest a complementarity of early and late motor cortical evoked activity during preparation and execution of trained arm movements, dynamically adapting to the behavioral context on a trial-by-trial basis. The LFP signal provides a sensitive measure of changes in the population activity in relation to modulations in behavioral performance.
GRANTS
This work was supported by Agence National de la Recherche Grant ANR-NEUR-05-045-1; AXA Research Fund grant to B. E. Kilavik; Consejo Nacional de Ciencia y Tecnología de México (Mexican government) grant to A. Ponce-Alvarez; Ministère Recherche et Technologie (French government) grant to J. Confais; European Union Grant 15879 (Fast Analog Computing with Emergent Transient States [FACETS]) to M. Diesmann; and Ministry of Education, Culture, Sports, Science and Technology (Japan) Next-Generation Supercomputer project grant to M. Diesmann.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank J. Ito, A. Ledberg, and W. A. MacKay for stimulating comments regarding the manuscript; E. Procyk and M. Meunier (Institut National de la Santé et de la Recherche Médicale, Lyon, France) for making available to us the MRI scanner facility; S. Takerkart for help with MRI image processing; T. Brochier for many interesting discussions and for generously sharing needed equipment; I. Balansard, M. Martin, and C. Mazenq for animal care; and J. Baurberg, A. De Moya, and X. Degiovanni for technical assistance.
REFERENCES
- Andersen et al., 2004. Andersen RA, Musallam S, Pesaran B. Selecting the signals for a brain-machine interface. Curr Opin Neurobiol 14: 720–726, 2004. [DOI] [PubMed] [Google Scholar]
- Arezzo and Vaughan, 1975. Arezzo J, Vaughan HG., Jr Cortical potentials associated with voluntary movements in the monkey. Brain Res 88: 99–104, 1975. [DOI] [PubMed] [Google Scholar]
- Arezzo and Vaughan, 1980. Arezzo J, Vaughan HG., Jr Intracortical sources and surface topography of the motor potential and somatosensory evoked potential in the monkey. Prog Brain Res 54: 77–83, 1980. [DOI] [PubMed] [Google Scholar]
- Asanuma and Rosén, 1972. Asanuma H, Rosén I. Topographical organization of cortical efferent zones projecting to distal forelimb muscles in the monkey. Exp Brain Res 14: 243–256, 1972. [DOI] [PubMed] [Google Scholar]
- Asher et al., 2007. Asher I, Stark E, Abeles M, Prut Y. Comparison of direction and object selectivity of local field potentials and single units in macaque posterior parietal cortex during prehension. J Neurophysiol 97: 3684–3695, 2007. [DOI] [PubMed] [Google Scholar]
- Asher et al., 2010. Asher I, Zinger N, Yanai Y, Israel Z, Prut Y. Population-based corticospinal interactions in macaques are correlated with visuomotor processing. Cereb Cortex 20: 241–252, 2010. [DOI] [PubMed] [Google Scholar]
- Boudrias et al., 2010. Boudrias MH, PcPherson RL, Frost SB, Cheney PD. Output properties and organization of the forelimb representation of motor areas on the lateral aspect of the hemisphere in rhesus macaques. Cereb Cortex 20: 169–186, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso de Oliveira et al., 2001. Cardoso de Oliveira S, Gribova A, Donchin O, Bergman H, Vaadia E. Neural interactions between motor cortical hemispheres during bimanual and unimanual arm movements. Eur J Neurosci 14: 1881–1896, 2001. [DOI] [PubMed] [Google Scholar]
- Correa et al., 2006. Correa Á, LupiÁñez J, Madrid E, Tudela P. Temporal attention enhances early visual processing: a review and new evidence from event-related potentials. Brain Res 1076: 116–128, 2006. [DOI] [PubMed] [Google Scholar]
- Coull and Nobre, 2008. Coull JT, Nobre AC. Dissociating explicit timing from temporal expectation with fMRI. Curr Opin Neurobiol 18: 137–144, 2008. [DOI] [PubMed] [Google Scholar]
- Cui and Deecke, 1999. Cui RQ, Deecke L. High resolution DC-EEG analysis of the Bereitschaftspotential and post movement onset potentials accompanying uni- or bilateral voluntary finger movements. Brain Topogr 11: 233–249, 1999. [DOI] [PubMed] [Google Scholar]
- Donchin et al., 2001. Donchin O, Gribova A, Steinberg O, Bergman H, Cardoso de Oliveira S, Vaadia E. Local field potentials related to bimanual movements in the primary and supplementary motor cortices. Exp Brain Res 140: 46–55, 2001. [DOI] [PubMed] [Google Scholar]
- Dum and Strick, 1991. Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 11: 667–689, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemba et al., 1981. Gemba H, Hashimoto S, Sasaki K. Cortical field potentials preceding visually initiated hand movements in the monkey. Exp Brain Res 42: 435–441, 1981. [DOI] [PubMed] [Google Scholar]
- Gemba et al., 1990. Gemba H, Sasaki K, Tsujimoto T. Cortical field potentials associated with hand movements triggered by warning and imperative stimuli in the monkey. Neurosci Lett 113: 275–280, 1990. [DOI] [PubMed] [Google Scholar]
- Gibbon, 1977. Gibbon J. Scalar expectancy theory and Weber's law in animal timing. Psych Rev 84: 279–325, 1977. [Google Scholar]
- Kilavik et al., 2007. Kilavik BE, Ponce-Alvarez A, Riehle A. Beta oscillations in monkey motor cortical LFPs are stronger during temporal attention than motor preparation. Soc Neurosci Abstr 6642, 2007. [Google Scholar]
- Kilavik and Riehle, 2010. Kilavik BE, Riehle A. Timing structures neuronal activity during preparation for action. In: Attention and Time, edited by Coull J, Nobre A. Oxford, UK: Oxford Univ. Press, 2010, p. 257–271. [Google Scholar]
- Kilavik et al., 2009. Kilavik BE, Roux S, Ponce-Alvarez A, Confais J, Grün S, Riehle A. Long-term modifications in motor cortical dynamics induced by intensive practice. J Neurosci 29: 12653–12663, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristeva et al., 2006. Kristeva R, Chakarov V, Wagner M, Schulte-Mönting J, Hepp-Reymond MC. Is the movement-evoked potential mandatory for movement execution? A high-resolution EEG study in a deafferented patient. NeuroImage 31: 677–685, 2006. [DOI] [PubMed] [Google Scholar]
- Kubota and Hamada, 1979. Kubota K, Hamada I. Preparatory activity of monkey pyramidal tract neurons related to quick movement onset during visual tracking performance. Brain Res 168: 435–439, 1979. [DOI] [PubMed] [Google Scholar]
- Lecas et al., 1986. Lecas JC, Requin J, Anger C, Vitton N. Changes in neuronal activity of the monkey precentral cortex during preparation for movement. J Neurophysiol 56: 1680–1702, 1986. [DOI] [PubMed] [Google Scholar]
- Ledberg et al., 2007. Ledberg A, Bressler SL, Ding M, Coppola R, Nakamura R. Large-scale visuomotor integration in the cerebral cortex. Cereb Cortex 17: 44–62, 2007. [DOI] [PubMed] [Google Scholar]
- Logothetis et al., 2007. Logothetis NK, Kayser C, Oeltermann A. In vivo measurement of cortical impedance spectrum in monkeys: implications for signal propagation. Neuron 55: 809–823, 2007. [DOI] [PubMed] [Google Scholar]
- Matsuzaka et al., 2007. Matsuzaka Y, Picard N, Strick PL. Skill representation in the primary motor cortex after long-term practice. J Neurophysiol 97: 1819–1832, 2007. [DOI] [PubMed] [Google Scholar]
- Mehring et al., 2004. Mehring C, Nawrot MP, Cardoso de Oliveira S, Vaadia E, Schulze-Bonhage A, Aertsen A, Ball T. Comparing information about arm movement direction in single channels of local and epicortical field potentials from monkey and human motor cortex. J Physiol (Paris) 98: 498–506, 2004. [DOI] [PubMed] [Google Scholar]
- Mehring et al., 2003. Mehring C, Rickert J, Vaadia E, Cardoso de Oliveira S, Aertsen A, Rotter S. Inference of movement direction from local field potentials in monkey motor cortex. Nat Neurosci 6: 1253–1254, 2003. [DOI] [PubMed] [Google Scholar]
- Miller et al., 1992. Miller J, Riehle A, Requin J. Effects of preliminary perceptual output on neuronal activity of the primary motor cortex. J Exp Psychol Hum Percept Perform 18: 1121–1138, 1992. [DOI] [PubMed] [Google Scholar]
- Miniussi et al., 1999. Miniussi C, Wilding EL, Coull JT, Nobre AC. Orienting attention in time. Modulation of brain potentials. Brain 122: 1507–1518, 1999. [DOI] [PubMed] [Google Scholar]
- Mitzdorf, 1985. Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev 65: 37–100, 1985. [DOI] [PubMed] [Google Scholar]
- Niemi and Näätänen, 1981. Niemi P, Näätänen R. Foreperiod and simple reaction time. Psychol Bull 89: 133–162, 1981. [Google Scholar]
- O'Leary and Hatsopoulos, 2006. O'Leary JG, Hatsopoulos NG. Early visuomotor representations revealed from evoked local field potentials in motor and premotor cortical areas. J Neurophysiol 96: 1492–1506, 2006. [DOI] [PubMed] [Google Scholar]
- Pieper et al., 1980. Pieper CF, Goldring S, Jenny AB, McMahon JP. Comparative study of cerebral cortical potentials associated with voluntary movements in monkey and man. Electroencephalogr Clin Neurophysiol 48: 266–292, 1980. [DOI] [PubMed] [Google Scholar]
- Praamstra et al., 2006. Praamstra P, Kourtis D, Kwok HF, Oostenveld R. Neurophysiology of implicit timing in serial choice reaction-time performance. J Neurosci 26: 5448–5455, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requin et al., 1990. Requin J, Lecas JC, Vitton N. A comparison of preparation-related neuronal activity changes in the prefrontal, premotor, primary motor and posterior parietal areas of the monkey cortex: preliminary results. Neurosci Lett 111: 151–156, 1990. [DOI] [PubMed] [Google Scholar]
- Rickert et al., 2005. Rickert J, Cardoso De Oliveira S, Vaadia E, Aertsen A, Rotter S, Mehring C. Encoding of movement direction in different frequency ranges of motor cortical local field potentials. J Neurosci 25: 8815–8824, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle, 1991. Riehle A. Visually induced signal-locked neuronal activity changes in precentral motor areas of the monkey: hierarchical progression of signal processing. Brain Res 540: 131–137, 1991. [DOI] [PubMed] [Google Scholar]
- Riehle, 2005. Riehle A. Preparation for action: one of the key functions of the motor cortex. In: Motor Cortex in Voluntary Movements: A Distributed System for Distributed Functions, edited by Riehle A, Vaadia E. Boca Raton, FL: CRC Press, 2005, p. 213–240. [Google Scholar]
- Riehle and Requin, 1993. Riehle A, Requin J. The predictive value for performance speed of preparatory changes in neuronal activity of the monkey motor and premotor cortex. Behav Brain Res 53: 35–49, 1993. [DOI] [PubMed] [Google Scholar]
- Roesch and Olson, 2005. Roesch MR, Olson CR. Neuronal activity in primate orbitofrontal cortex reflects the value of time. J Neurophysiol 94: 2457–2471, 2005. [DOI] [PubMed] [Google Scholar]
- Rolke and Hofmann, 2007. Rolke B, Hofmann P. Temporal uncertainty degrades perceptual processing. Psychon Bull Rev 14: 522–526, 2007. [DOI] [PubMed] [Google Scholar]
- Rothwell et al., 1982. Rothwell JC, Traub MM, Day BL, Obeso JA, Thomas PK, Marsden CD. Manual motor performance in a deafferented man. Brain 105: 515–542, 1982. [DOI] [PubMed] [Google Scholar]
- Roux et al., 2003. Roux S, Coulmance M, Riehle A. Context-related representation of timing processes in monkey motor cortex. Eur J Neurosci 18: 1011–1016, 2003. [DOI] [PubMed] [Google Scholar]
- Roux et al., 2006. Roux S, MacKay WA, Riehle A. The pre-movement component of motor cortical local field potentials reflects the level of expectancy. Behav Brain Res 169: 335–351, 2006. [DOI] [PubMed] [Google Scholar]
- Sanes and Donoghue, 2000. Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci 23: 393–415, 2000. [DOI] [PubMed] [Google Scholar]
- Sasaki and Gemba, 1982. Sasaki K, Gemba H. Development and change of cortical field potentials during learning processes of visually initiated hand movements in the monkey. Exp Brain Res 48: 429–437, 1982. [DOI] [PubMed] [Google Scholar]
- Sasaki et al., 1981. Sasaki K, Gemba H, Hashimoto S. Influences of cerebellar hemispherectomy upon cortical potentials preceding visually initiated hand movements in the monkey. Brain Res 205: 425–430, 1981. [DOI] [PubMed] [Google Scholar]
- Sasaki et al., 1990. Sasaki K, Gemba H, Tsujimoto T. Cortical field potential associated with hand movement on warning-imperative visual stimulus and cerebellum in the monkey. Brain Res 519: 343–346, 1990. [DOI] [PubMed] [Google Scholar]
- Schieber, 2002. Schieber MH. Training and synchrony in the motor system. J Neurosci 22: 5277–5281, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan et al., 1970. Vaughan HG, Jr, Gross EG, Bossom J. Cortical motor potential in monkeys before and after upper limb deafferentation. Exp Neurol 26: 253–262, 1970. [DOI] [PubMed] [Google Scholar]
- Wannier et al., 1989. Wannier TMJ, Maier MA, Hepp-Reymond MC. Responses of motor cortex neurons to visual stimulation in the alert monkey. Neurosci Lett 98: 63–68, 1989. [DOI] [PubMed] [Google Scholar]
- Zach et al., 2008. Zach N, Inbar D, Grinvald Y, Bergman H, Vaadia E. Emergence of novel representations in primary motor cortex and premotor neurons during associative learning. J Neurosci 28: 9545–9556, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


