Abstract
Like humans, songbirds learn vocal sounds from “tutors” during a sensitive period of development. Vocal learning in songbirds therefore provides a powerful model system for investigating neural mechanisms by which memories of learned vocal sounds are stored. This study examined whether NCM (caudo-medial nidopallium), a region of higher level auditory cortex in songbirds, serves as a locus where a neural memory of tutor sounds is acquired during early stages of vocal learning. NCM neurons respond well to complex auditory stimuli, and evoked activity in many NCM neurons habituates such that the response to a stimulus that is heard repeatedly decreases to approximately one-half its original level (stimulus-specific adaptation). The rate of neural habituation serves as an index of familiarity, being low for familiar sounds, but high for novel sounds. We found that response strength across different song stimuli was higher in NCM neurons of adult zebra finches than in juveniles, and that only adult NCM responded selectively to tutor song. The rate of habituation across both tutor song and novel conspecific songs was lower in adult than in juvenile NCM, indicating higher familiarity and a more persistent response to song stimuli in adults. In juvenile birds that have memorized tutor vocal sounds, neural habituation was higher for tutor song than for a familiar conspecific song. This unexpected result suggests that the response to tutor song in NCM at this age may be subject to top-down influences that maintain the tutor song as a salient stimulus, despite its high level of familiarity.
Keywords: auditory cortex, vocal learning, songbird, neural habituation, stimulus-specific adaptation
songbirds, like humans, acquire a memory of the sounds used for vocal communication during a sensitive period of development based on hearing adult “tutors” (Bottjer and Arnold 1997; Doupe and Kuhl 1999; Marler 1970). Thus the first essential step in vocal learning is to acquire a neural representation of tutor sounds based on auditory experience, a template memory. Several lines of recent work suggest that a neural memory of tutor sounds in the songbird brain may be present in higher level regions of auditory cortex, including NCM (caudo-medial nidopallium) (Bolhuis and Gahr 2006). Because NCM receives strong projections from primary auditory cortex and provides indirect input to vocal-control nuclei, it is well-positioned to serve as one primary locus where the memory of tutor songs may be encoded (Mello et al. 1998; Vates et al. 1996). Playback of various song stimuli (but not tones or noise) induces robust expression of the immediate early gene product ZENK (early growth response protein 1) in NCM, but not in primary auditory cortex or in sensorimotor vocal-control nuclei (Jarvis and Nottebohm 1997; Mello et al. 1992; Mello and Clayton 1994). In addition, the level of ZENK expression in adult NCM induced by playback of tutor song correlates with the strength of vocal imitation of that song (Terpstra et al. 2004). ZENK is regulated by ERK (extracellular signal-regulated kinase), and local blockade of ERK signaling in NCM during periods of exposure to tutor song in juvenile birds prevents them from subsequently developing an accurate imitation of that song as adults (London and Clayton 2008). This disruption of imitative learning was not due to basic impairments in auditory processing, since blockade of ERK signaling did not impair the ability to discriminate between novel songs. Likewise, lesions of NCM in adult birds do not disrupt song production or the ability to discriminate between male and female vocal calls, but do impair the ability to recognize tutor song (Gobes and Bolhuis 2007).
Electrophysiological studies have shown that NCM neurons in both juvenile and adult birds respond strongly to diverse complex sounds, including songs and human speech (Chew et al. 1995, 1996; Stripling et al. 1997, 2001). Evidence for strong differences in response selectivity to different song stimuli is sparse: multiunit studies have typically found greater response strength to conspecific than to heterospecific songs, but no studies have found a stronger response to each bird's own song (BOS) than to conspecific song (CON). However, despite the fact that NCM neurons as a population are not highly selective, Stripling et al. (2001) reported that ∼10% of single units in NCM of juvenile birds were selective for CON compared with the same song played as a mirror-image reverse. Surprisingly, no electrophysiological studies have described response strength or selectivity of NCM neurons to playback of tutor song. The major focus of this study was to compare the responsivity of NCM neurons to tutor vs. other songs at different stages of song development.
A subregion of NCM contains a high density of neurons that show stimulus-specific habituation in adult birds: multiunit responses evoked by complex sounds decrease with repeated presentations (Chew et al. 1995, 1996; Stripling et al. 1997, 2001) (cf. Ayala and Malmierca 2012; Netser et al. 2011; Ulanovsky et al. 2003). The degree to which a song stimulus is novel vs. familiar can be assessed by the rate of habituation of NCM neural activity: an increased rate of habituation is observed for novel sounds compared with those that have been heard repeatedly (Chew et al. 1995). Phan et al. (2006) reported that the rate of habituation in adult NCM was lower for tutor songs compared with novel conspecific songs in tutored birds, but not in untutored birds, suggesting that the lower habituation rate in tutored birds reflected a recognition memory of the learned tutor song. Furthermore, the degree to which adult birds achieved an accurate imitation of tutor song correlated with a lower rate of neural habituation to the tutor song relative to novel conspecific song (Phan et al. 2006). This pattern is consistent with the idea that NCM in adult birds is part of a circuit encoding an auditory memory of tutor sounds that serves as a template for vocal learning.
This study sought to extend these results by investigating the pattern of habituation in NCM to tutor vs. other songs during vocal development. We reasoned that if a lower rate of habituation to tutor song reflects a higher level of familiarity due to vocal learning, then NCM of juvenile birds that have memorized a tutor song should show a lower rate of habituation compared with fledgling birds that are at the onset of vocal learning. However, this prediction was not born out. Older juvenile birds that had accrued sufficient auditory experience to learn the tutor song had relatively higher rates of neural habituation to the tutor song than to familiar conspecific songs, suggesting that tutor song is treated as a salient or “novel” stimulus by NCM neurons at this age, despite its high level of familiarity.
METHODS
Subjects
All birds were bred and raised in group breeding aviaries at the University of Southern California by their parents such that they received normal tutor song exposure and social experience. Each breeding aviary measured 3 × 6 × 5 m and contained 6 breeding pairs of adult zebra finches (Taeniopygia guttata); a single large room contained 14 such aviaries. Birds were tested at the following ages: 20 days (range = 19–22 days, n = 11), 35 days (range = 34–36 days, n = 13), and adult (>100 days, n = 20). Each bird was tested at only one age. Because some birds were not tested with all song stimuli, the actual sample sizes for statistical comparisons ranged from 9 to 11 for 20-day-old birds, and from 19 to 20 for adult birds. At 20 days of age birds have just fledged from the nest and are beginning to learn vocal sounds from their father (their tutor). By 35 days birds have largely finished memorizing the tutor song and are on the cusp of beginning to produce babbling vocalizations (Böhner 1990; Roper and Zann 2006). Adult birds have finished learning to imitate the tutor song and produce highly stable vocal patterns, including songs and calls. All procedures were specifically approved by the University of Southern California Institutional Animal Care and Use Committee and followed the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Juvenile birds remained in the aviaries until they were removed for testing, and thus were exposed to a rich repertoire of vocal auditory stimuli, including the songs of their father and other adult males. Adult birds were removed from their breeding aviary at ∼45–60 days of age and housed in a group aviary with other male birds in the same room as the breeding aviaries until the time of testing. Birds raised under these naturalistic conditions (i.e., by their biological parents within a colony) memorize the song of their father (Böhner 1983, 1990; Catchpole and Slater 1995; Clayton 1987; Eales 1985; Immelmann 1969; Mann and Slater 1995; Roper and Zann 2006), and previous work in our laboratory has shown that the rearing conditions of our group aviaries provide sufficient tutor exposure to develop an accurate copy of the father's song (Bottjer and Altenau 2010; Foster and Bottjer 2001). Because the songs of individual adult zebra finches vary considerably, each juvenile bird learns a specific and unique tutor song. Thus we considered that the tutor for each bird was its biological father.
However, because juveniles could interact directly with other adult male breeders in their group aviary, they may have memorized some aspects of other males' songs (Williams 1990). To address this question directly, we compared the songs of six of the experimental adult birds in this study to the songs of all six breeder males from the aviary in which they had hatched: the song of their father as well as the songs of the other five breeding males in that aviary. Briefly, each syllable in the adult “pupil” song was compared with those in each song from the six adult breeders from the aviary in which the bird was raised to search for corresponding syllables with high similarity (Basham et al. 1996; Bottjer and Altenau 2010). For each comparison, each syllable in the pupil song was given a similarity score as follows: 0 = no similarity, 1 = slightly similar, 2 = highly similar, 3 = matched. Two experimenters with no knowledge of the fathers' identity independently analyzed sonograms for each pupil bird, and their scores were averaged for each pupil-breeder comparison. Syllables with a score >2 were operationally defined as having been learned from the tutor, whereas syllables with scores <2 were judged as not having been learned from the tutor.
Vocal Recording and Song Stimuli for Playback
Songs of all adult male breeders in all aviaries were recorded prior to this experiment and used as playback stimuli during electrophysiological recordings. Song recordings were made by placing each individual bird in a cage within an acoustic isolation box (no females were present; 44 kHz sampling rate, Sound Analysis Pro) (Tchernichovski et al. 2000). Representative songs consisting of two motifs sung in a row were selected for each bird, high-pass filtered at 400 Hz, and matched for amplitude (Goldwave). Song stimuli were presented at an amplitude of 60–65 dB, measured with a sound level meter (Extech Instruments). Each bird's own song was not used for playback in this experiment because 20- and 35-day-old birds do not yet produce song vocalizations (in our breeding population, birds begin to produce song-related vocalizations at 36 days, on average).
Tutors for every bird tested in this study were identified prior to the time that birds fledged by observing which breeding males entered each nest box in each aviary; parents enter only the nestbox that contains their own clutch, so this constitutes a highly reliable method for identifying the biological father. Based on this information, tutor songs (TUT) were selected for each bird from the database of songs previously recorded from all adult male breeders. Reversed versions of tutor songs (revTUT) were created by making a complete mirror-image, such that the temporal order of the entire song was reversed. Tutor songs in which the order of individual syllables was reversed [reverse order (roTUT)] were created by cutting out each syllable and intersyllable interval and placing them in reverse order. This resulted in a song in which each syllable was preserved in forward order, but individual syllables were presented in reverse order.
To assess how the response of NCM neurons changed as juvenile birds gained experience with tutor song (i.e., between 20 and 35 days), we also played back both familiar and unfamiliar conspecific songs as additional controls. Juvenile birds were removed from their home aviary immediately prior to testing and thus had equal exposure to the tutor song and to the songs of other adult males in that aviary. We presented each juvenile bird with three familiar conspecific songs (famCON, songs from adult males in the same aviary in which the juvenile was raised) and three unfamiliar conspecific songs (unfamCON, songs from adult males located at least two aviaries away). The response to different conspecific songs varies, and thus the response strength reported for both familiar and unfamiliar conspecific songs was averaged across the three songs presented to each bird. In addition to these six conspecific songs, juvenile birds were presented with tutor song, reverse tutor song and reverse order tutor song (maximum number of stimuli presented = 9).
Adult birds were presented with all three versions of tutor song (forward, reverse, and reverse order) and with three unfamiliar conspecific songs. A subset of eight adult birds was also presented with conspecific songs of adult birds from the same aviary in which they had been raised; these songs were heard concurrently with tutor song when these adult birds were juveniles residing in a breeding aviary. Neither the tutor song nor the conspecific song from the same aviary had been heard for >40 days. Thus these songs were not considered to be “familiar,” in the sense that they had not been heard immediately prior to testing.
Electrophysiology
Birds were removed directly from the aviary, anesthetized with isoflurane (1.5%, inhalation) and placed in a stereotaxic apparatus. A stainless steel post was glued to the rostral skull with dental cement (Lang Dental) and cyanoacrylate. Birds were then placed in individual cages and returned to the main aviary; 20- and 35-day-old birds were placed in cages with their fathers. Juvenile birds were tested between 2 and 4 h after head-posting (to allow for recovery from anesthetic) and adult birds were tested up to 4 days after head-posting. During recording sessions, awake birds were comfortably restrained by being wrapped in a cloth jacket and placed in a plastic tube in the stereotaxic apparatus; the head was immobilized via the steel post. Birds were awake for the entire time of testing. A craniotomy was made over NCM using stereotaxic coordinates: anterior 0.6–0.8 mm, lateral 0.4 mm, depth 0.8–1.5 mm. This small area encompasses the most dorso-caudal region of NCM (Fig. 1), in which stimulus-specific habituation to complex auditory stimuli has been reported to be robust (Chew et al. 1995, 1996; Terleph et al. 2006). Multiunit recordings were made using single electrodes (0.5–1.0 MΩ, Carbostar, Kation Scientific). Spike data were amplified, band-pass filtered between 300 and 3,000 Hz (DAM 80 amplifier, World Precision Instruments), and digitized at 20 kHz using Spike 2 software (Cambridge Electronic Design). Spikes were defined by manually setting a threshold that was clearly above background activity; any waveform that crossed this threshold was included in our measure of multiunit activity (spikes/s). Only one recording site was made per bird since the stimuli were no longer novel following one test and hence would not show habituation in a new recording location.
Fig. 1.
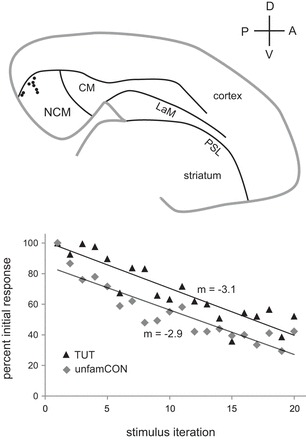
Top: parasagittal schematic at ∼0.5 mm lateral to midline showing location of caudo-medial nidopallium (NCM) in caudal telencephalon. Black dots represent the location of 8 representative recording sites (n = three adults, three 35-day-old and two 20-day-old birds) in a small area encompassing the most dorso-caudal region of NCM; all recording sites within this region showed robust habituation to song playback. CM, caudal mesopallium; LaM, mesopallial lamina; PSL, pallial-subpallial lamina; D, dorsal; V, ventral; P, posterior; A, anterior. Bottom: response habituation for a single recording site in NCM. Examples from an adult bird of response strength during 20 iterations of tutor song (TUT; triangles) and unfamiliar conspecific song (unfamCON; diamonds), normalized to the first trial, are shown. The slope of the linear regression of these normalized functions was used as a measure of the rate of habituation that could be compared across birds and song types.
An unfamiliar conspecific song was used as a search stimulus to ensure that the electrode was within NCM (spikes of medium, heterogeneous amplitude with a brisk response throughout the song presentation). Most birds heard fewer than 5–10 presentations of this search stimulus. The search stimulus was different from any of the tutor or conspecific songs used in the experiment. Immediately prior to playback of song stimuli in this study, birds had been presented with synthetic call stimuli for another experiment (Miller-Sims and Bottjer 2012). Each song stimulus was presented 20 times at an interstimulus interval of 10 ± 2 s; awake birds sometimes move during recording, and we removed any trials with noise artifact. Only awake birds show robust response decrements in NCM over repeated stimulus presentations (David Vicario, personal communication). Song stimuli were presented in blocks of six to nine different stimuli (the stimulus set for different birds ranged from 6 to 9); the order of stimuli within a block was random (without replacement).
At the end of the experiment, a small electrolytic lesion was made by applying 8–12 μA for 5–10 s to mark the electrode location. Birds were deeply anesthetized with Equithesin (0.04 ml/10 g) and perfused through the heart with 0.7% saline followed by 10% formaldehyde. Brains were postfixed, frozen-sectioned in the coronal plane at a thickness of 50 μm, and Nissl stained to verify the location of the recording site.
Data Analysis
Response strength and selectivity.
Both the firing rate during song stimuli and the firing rate during a baseline period were measured as spikes/s. Raw response strength was measured by subtracting the firing rate during a 1-s baseline period immediately before stimulus onset from the firing rate during each stimulus presentation. To compare differences in firing rates across age groups, a standardized response strength (RS) was calculated as:
where S is mean firing rate during the stimulus, and B is mean firing rate during baseline. This measure is similar to a z-score, except that the distribution is centered on the baseline response rate rather than the mean response rate over all stimuli (cf. Thompson and Gentner 2010).
We assessed selectivity of response to tutor song vs. other song stimuli in two ways. A d′ statistic was computed as the difference in raw response strength (firing rate during stimulus minus firing rate during baseline) for two different songs (e.g., TUT vs. revTUT), normalized by their standard deviation:
We calculated d′ scores for selectivity of the neural response in NCM to tutor song against revTUT, roTUT, unfamCON, and famCON. A d′ of 0 indicates no difference in the response to two songs; positive d′ indicate a preference for tutor song. A d′ value of ≥0.5 was taken as a significant preference for tutor song; this value represents a significant difference (P = 0.03, paired t-test) when 20 repetitions of each stimulus are presented (cf. Solis and Doupe 1997). Selectivity of the response of NCM neurons was also assessed using a selectivity index: SI = RSTUT/(RSTUT + RSSONG2) where RSTUT is the raw response strength to tutor song, and RSSONG2 is the raw response strength to the second song (e.g., revTUT) (Solis and Doupe 1997). A SI of 0.5 indicates an equal preference for the two songs. Both response strength and selectivity scores were averaged over 20 iterations of each song type for each bird.
Habituation.
To assess patterns of habituation, the raw response strength to each of the 20 iterations of each song stimulus was calculated. Responses to each stimulus iteration were then normalized as a percentage of the response on the first presentation and plotted as a function of stimulus iteration. Qualitative examination of these functions indicated that they appeared linear in the vast majority of birds (Fig. 1). The slope of the linear regression of these normalized values over all 20 repetitions was calculated. These slope values provide a measure of the rate of habituation for each song type in each bird. Previous work by Chew et al. (1995, 1996) has shown that this habituation rate assesses the degree to which different songs are novel vs. familiar: the rate of habituation is steeper for novel stimuli than for familiar stimuli. In addition, the rate and specificity of habituation to a repeated song stimulus within a session are not influenced by the numbers of songs presented (i.e., stimulus-specific habituation occurs regardless of whether each stimulus is interleaved with other stimuli or not).
Phan et al. (2006) computed a familiarity index to compare the relative habituation rate of NCM neurons for tutor song to that for unfamiliar conspecific songs by dividing the slope of the normalized regression function for novel CON songs by that for TUT song. A familiarity index greater than 1.0 indicates that the tutor song is more familiar (shows less habituation) than a novel conspecific song. We calculated the familiarity index of unfamCON, famCON, and revTUT compared with TUT for each juvenile bird. For adults only unfamCON and revTUT were calculated (since they did not hear a familiar CON song).
We also calculated an additional measure of habituation for two reasons: 1) a possible lack of linearity in the slope values for some birds might influence the rates of habituation; 2) the normalized slope values do not provide any information regarding absolute response strengths over the course of repeated stimulus presentations. We therefore compared the median value of the raw response strength on the first five stimulus presentations for each song to the median value on the last five stimulus presentations for each bird. We then calculated a percent difference score by subtracting the last five median value from the first five median value and dividing by the first five median value; this yielded a percent decrease score for each bird normalized to response strength during initial stimulus presentations. As demonstrated below, this measure of habituation yielded essentially the same pattern as that obtained by using the slope (linear regression line) of the normalized response values (see results).
Statistics
Nonparametric statistics were used to assess significance in all cases, since group data were not normally distributed in many instances. Kruskal-Wallis tests were used to assess the effect of age on each metric followed by Mann-Whitney tests to make individual comparisons between age groups. Friedman tests for repeated measures were used to test the effect of song type within each age group, and individual comparisons were tested with Wilcoxon signed-ranks tests. One-sample Wilcoxon tests were used to determine whether various dependent measures deviated from a specific value (i.e., from 0 for d′, 0.5 for the SI, or 1.0 for the familiarity index). Kolmogorov-Smirnov tests were used to test whether the cumulative distribution of d′ values showed an effect of age. All values throughout the results are given as means ± SE.
RESULTS
Adult NCM Neurons Show Higher Average Response Strength Than Juvenile NCM Neurons and Respond Most Strongly to Tutor Song Syllables
Because spontaneous firing rates can influence response strength (and other measures presented below), we asked whether baseline firing rates (spikes/s) varied as a function of age. The mean baseline firing rates during a 1-s interval prior to stimulus presentations in adult, 35-day-old, and 20-day-old birds were 41.5 ± 5.8, 30.5, ± 4.3, and 33.2 ± 2.3 (means ± SE), which were not significantly different (Kruskal-Wallis, P = 0.42). However, because there was some tendency for adult birds to have higher spontaneous rates of activity, we measured differences in song-evoked firing rate across age groups by calculating standardized response strengths of NCM neurons to playback of each song stimulus (see methods). Figure 2 shows that standardized response strength was higher in NCM of adults compared with juveniles across song types, whereas the two groups of juvenile birds (20 vs. 35 days) did not differ from each other. A significant effect of age was obtained for each of the four song types that were played to all age groups, reflecting the higher response strength in adult birds (TUT, tutor song; revTUT, the entire tutor song played as a mirror-image reverse; roTUT, tutor song with the order of syllables reversed; unfamCON, unfamiliar conspecific songs) (Kruskal Wallis tests, P = 0.03 or less in all cases). Individual comparisons between age groups revealed that response strengths were greater in adults than in 35-day-old birds for all four song stimuli (Mann-Whitney, P = 0.007 or less in all cases), whereas adults gave a stronger response than 20-day-old birds only for TUT and roTUT (P = 0.02 or less).
Fig. 2.
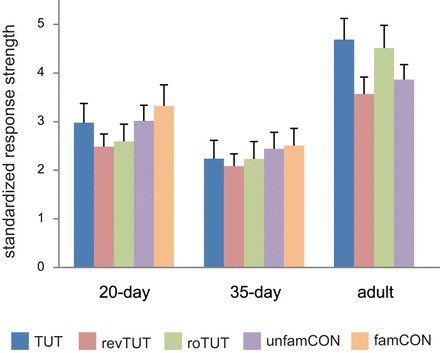
Standardized response strength in dorso-caudal NCM as a function of age and song type. Standardized response strength in each age group averaged over 20 iterations of each song stimulus [TUT; reversed tutor song (revTUT); reverse-order tutor song (roTUT); unfamCON; familiar conspecific song (famCON)]. famCON for juvenile birds were from birds in the breeding aviary in which they were housed up until they were tested; adult birds were removed from their breeding aviary at least 1 mo prior to being tested, and thus there was no comparable famCON song for adults. All values are means + SE.
We also tested the effect of song type within each age group. NCM neurons of adult birds responded strongly to TUT and roTUT, but showed a relatively lower response to revTUT and unfamCON (Fig. 2; Friedman repeated-measures test for main effect of song, P = 0.0005). Individual comparisons showed that the NCM response to tutor song in adults was greater than that to both revTUT and unfamCON (Wilcoxon signed-ranks tests, P < 0.01 or less), but did not differ from roTUT (P = 0.53). In contrast, standardized response strengths in 35-day-old birds did not differ across song stimuli (Friedman test, P = 0.2). Although a significant overall effect of song type was obtained in 20-day-old birds (Friedman test, P = 0.004), the response to tutor song was not different from any other song stimulus (P = 0.09 or higher in all cases). In summary, standardized response strength was greater in adult than in juvenile NCM for all song stimuli, and adult NCM responded more strongly to tutor song than to unfamiliar conspecific songs. Tutor song was an equally effective stimulus in NCM of adults, regardless of whether each syllable was presented in the correct order or in a reversed order, but was less effective when the entire song was played in a mirror-image reverse.
A subset of adult birds were also tested with conspecific songs from the aviary in which they were raised. These songs were heard frequently throughout development but were not considered as “familiar,” in the sense that they had not been heard for at least 40 days prior to the time of testing; in contrast, the familiar conspecific songs played to juveniles were from birds housed in the same aviary up until the day of testing and hence were heard frequently up until testing (see methods). Thus these adult birds had had equal exposure to tutor song and to conspecific songs from the same aviary during development. Although standardized response strength in NCM to these conspecific songs was intermediate between TUT and unfamCON songs, it was not different from either (4.18 ± 0.5 for these conspecific songs compared with 4.68 ± 0.4 for TUT and 3.86 ± 0.3 for unfamCON, P > 0.10 or higher). Thus previous auditory experience with a conspecific song seemed to exert some influence on NCM activity in adult birds, but response strength to such songs was not greater than that to unfamiliar songs.
Neural Activity Is Selective for Tutor Song Syllables Only in Adult NCM
We first assessed the selectivity of neuronal responses to tutor vs. other songs using d′ scores; a value of zero indicates no preference, and values >0.5 indicate a significant preference for tutor song (see methods). Mean d′ scores showed that adult birds responded selectively to TUT compared with revTUT and unfamCON (d′TUT-revTUT, P = 0.01, d′TUT-unfamCON, P = 0.04; significantly different from zero by one-sample Wilcoxon test), but responded equally to TUT and roTUT (d′TUT-roTUT, P > 0.4) (Fig. 3, top). In contrast, mean d′ scores of juvenile birds never showed a selective response to tutor song compared with other songs (P = 0.17 or higher). Despite the fact that significant selectivity for tutor song vs. revTUT and unfamCON was observed only in adult but not juvenile NCM, Kruskal-Wallis tests revealed no effect of age for these two song comparisons (P = 0.10 or higher), indicating that mean d′ scores were not significantly higher in adult NCM compared with that of juveniles.
Fig. 3.
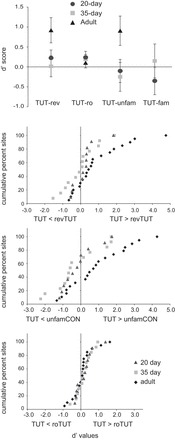
d′ measures of TUT selectivity. Top: mean d′ scores for tutor vs. revTUT, roTUT, unfamCON, and famCON. Bottom: cumulative distributions of individual d′ scores including TUT vs. revTUT; TUT vs. unfamCON; TUT vs. roTUT.
The bottom panels of Fig. 3 show cumulative distribution functions of individual d′ scores for tutor song vs. revTUT, unfamCON, and roTUT in each age group. These distributions were shifted to the right (greater selectivity for tutor song) in adults compared with juveniles for TUT vs. revTUT and unfamCON, indicating that more recording sites in adult NCM showed a selective response to tutor song for these two comparisons. However, Kolmogorov-Smirnov tests failed to show a significant effect of age (P > 0.19 or higher), confirming the absence of a strong age difference in selectivity to TUT relative to revTUT and unfamCON. All three age distributions for TUT vs. roTUT showed that the majority of scores fell in the range around zero (i.e., between ±0.5), indicating an equal preference for these two songs, as described above.
To further examine differences in selectivity of NCM neurons, we plotted the proportion of neural responses with d′ scores greater than 0.5 as a function of age and song stimulus comparison (Fig. 4, top). This analysis showed that a large proportion of recording sites in adult birds was selective for TUT vs. revTUT and unfamCON, but not for TUT vs. roTUT. In contrast, a much lower proportion of recording sites in juvenile birds had d′ scores >0.5, and these did not differ as a function of stimulus comparison. z-Tests for a difference between two proportions showed that adult birds had significantly more d′ scores greater than 0.5 compared with 20- and 35-day-old birds for both TUT-revTUT and TUT-unfamCON comparisons (P < 0.001 in all cases). Thus the overall pattern of results for d′ indicates that the neural response of 20- and 35-day NCM was not selectively tuned to tutor song compared with other song types. In contrast, neural responsivity in NCM of adult birds was stronger to TUT than to both revTUT and unfamCON but not to roTUT, again reflecting the absence of selectivity to syllable order in adult NCM.
Fig. 4.
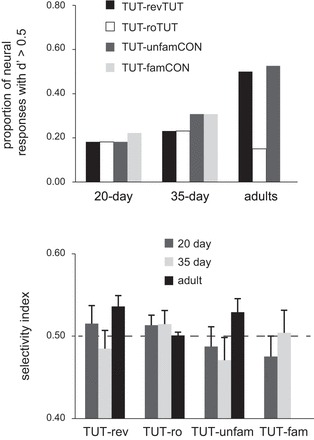
Top: proportion of recording sites with d′ score >0.5 for tutor vs. other songs. Bottom: mean selectivity scores for tutor vs. other songs. See results.
A possible disadvantage of the d′ statistic in this experiment is that it is based on response strength, which is correlated with baseline firing rates, such that higher firing rates may yield larger d′ values. As indicated above, baseline firing rates did not vary significantly as a function of age. However, given that spontaneous firing rates were slightly higher in adults, we also calculated a selectivity index (Solis and Doupe 1997); this ratio measure expresses the response strength to tutor song as a proportion of the combined response to tutor plus a comparison song [e.g., TUT/(TUT + revTUT)]; a value of 0.5 indicates an equal preference for the two songs. Figure 4 (bottom) shows a similar pattern to that obtained with d′ scores: NCM of adults showed a selective response to TUT compared with revTUT (P = 0.02), and a trend toward a response preference for TUT over unfamCON (P = 0.08). NCM neurons of adults did not respond selectively to tutor song compared with roTUT. Selectivity scores for NCM of juvenile birds did not show a preference for tutor song compared with any other song. In addition, Kruskal-Wallis tests revealed no effect of age for any song comparison (P > 0.12 or higher), as indicated above for d′ scores.
To assess whether learning the tutor song could predict neural selectivity, we measured the percentage of syllables copied from the tutor (father) vs. other birds for six experimental birds in the adult group (see methods). This analysis showed that the adult pupil birds learned ∼75% of their song syllables from their father. One bird did not learn any syllables from the father (and few or none from other birds) and was excluded from further analysis. The remaining five birds copied 43–100% of their syllables from the father (77 ± 25%). In contrast, the same birds copied few or no syllables from breeder birds in their aviary that were not their fathers (9 ± 5%). In every case, the syllables that were judged to match those of “non-father” birds were generic introductory-type notes or simple harmonic stacks. Hence the experimental birds never copied complex syllables from other males, indicating that they learned all of the complex song syllables in their repertoire specifically from their father. In summary, these data show that juvenile males reared under the semi-naturalistic conditions of our breeding aviaries selectively copy the song of their father. Furthermore, the mean percentage of the father's song learned is highly similar to a variety of reports from the literature of tutor copying for juvenile birds raised across different rearing conditions (Böhner 1983; Bottjer and Altenau 2010; Clayton 1987; Deregnaucourt et al. 2013; Eales 1985; Roper and Zann 2006; Tchernichovski and Nottebohm 1998). Interestingly, the degree to which pupil birds copied syllables from their father correlated with their neural selectivity index to TUT: a correlation analysis showed that better learners (birds that copied more song syllables from their father) showed a greater degree of neural selectivity in NCM for that song (R = 0.82, n = 5). This pattern is consistent with the idea that better learning of the tutor song establishes a stronger memory of that acoustic pattern in NCM neurons. One caveat is that, since we did not test playback to each BOS, the present data cannot assess whether neural selectivity to the tutor song is partly attributable to tuning to the bird's own song. However, previous studies have reported comparable responsivity to BOS and CON, indicating that NCM neurons do not respond selectively to BOS (but have not tested TUT) (Chew et al. 1995, 1996; Stripling et al. 1997, 2001).
Neural Activity in NCM Habituates More Slowly in Adults Than in Juveniles; 35-Day NCM Shows a High Rate of Habituation to Tutor Song
We also measured the rate at which NCM activity habituated to repeated presentations of different song stimuli, to assess the degree to which different song stimuli were recognized as familiar (Chew et al. 1995, 1996). Figure 5 shows normalized habituation curves to playback of TUT, revTUT, and unfamCON for each age group. The rate of habituation was expressed as the slope (linear regression) of normalized response strength over 20 repeated presentations of each song stimulus (see methods). For each song type, neural habituation in NCM of adult birds had a shallower slope compared with juveniles, indicating a lower rate of habituation; this pattern suggests that all song types were treated as “more familiar” in adult NCM. Kruskal-Wallis tests of the mean slope values (Fig. 5, bottom right) revealed a significant effect of age for TUT, revTUT, and unfamCON (P = 0.003 or less), and a marginally significant effect for roTUT (P = 0.05). Comparisons between individual age groups showed that slope values for adult NCM were lower than those for both 20- and 35-day NCM for all three song types (P = 0.04 or less); in addition, the slope of the habituation function for roTUT was lower in adult than in 35-day NCM (P = 0.02). Habituation slopes of NCM activity were never different between 20- and 35-day-old birds (P = 0.28 or higher). In summary, the evoked response in NCM habituated during the course of hearing repeated song stimuli in all age groups, with neural activity showing higher levels of habituation in juvenile than in adult NCM. The overall pattern demonstrates a more persistent response over repeated stimulus presentations in adult than in juvenile NCM for all song types presented.
Fig. 5.
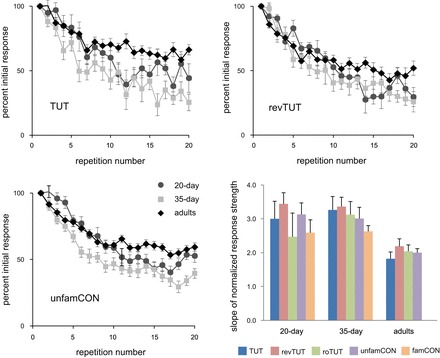
Rate of habituation to different song types as a function of age. Top right and left and bottom left: mean ± SE normalized response strength for each stimulus iteration for TUT, revTUT, and unfamCON, respectively. Bottom right: mean + SE slope of linear regression between normalized response strength and iteration number for each song type in each age group.
The influence of song type on rate of habituation varied within each age group (Fig. 5). Rates of habituation did not vary as a function of song type in adult NCM (Friedman test on mean slope values, P = 0.64), although tutor song tended to have a lower rate of habituation than did revTUT (P = 0.07). Although the mean habituation slope for tutor song was somewhat less than that for unfamCON in adult NCM, this difference did not approach significance (P = 0.41). Song type exerted a marginally significant effect on rate of habituation in 35-day NCM (P = 0.05). Individual comparisons revealed a difference only for TUT vs. famCON (P = 0.02) at 35 days, showing that the rate of habituation to TUT was higher than that to famCON song. This unexpected result suggests that tutor song is not treated as a typical familiar stimulus in NCM of 35-day-old birds (see below). Rate of habituation in 20-day NCM was influenced by song type (P = 0.03), although individual comparisons revealed no significant difference between TUT and any other stimulus (P > 0.13 or higher). There was a significant difference in slope for famCON vs. unfamCON (P = 0.04), reflecting a higher rate of habituation to unfamiliar songs in 20-day NCM. In summary, adult and 20-day NCM showed no difference in rate of habituation to tutor vs. other song types, whereas 35-day NCM showed a higher rate of habituation to tutor song vs. famCON, suggesting that tutor song was treated as somehow relatively “less familiar” than familiar songs of other birds.
Higher Response Strength in Adult NCM Is Due to Less Habituation; Habituation Is Greater for Tutor Song Than Familiar Conspecific Song Only in 35-Day NCM
Does the decreased habituation of song-evoked activity in adult NCM explain the higher overall response strengths (averaged over 20 iterations) in adult compared with juvenile NCM? We addressed this question by comparing the median raw response strength of the first five vs. the last five trials for each bird (see methods). Although raw response strengths were slightly higher in adult than in juvenile NCM during the first five stimulus presentations, no effect of age was obtained (Fig. 6, top) (Kruskal-Wallis, P > 0.15 for all song types except for roTUT, which was higher in adult than in 35-day NCM, P = 0.03). In contrast, raw response strength was higher in adult NCM compared with juvenile NCM during the last five stimulus presentations across all song types (Kruskal-Wallis, P < 0.04 or less). We also calculated percent difference scores between raw response strength on the median first five vs. last five trials (Fig. 6, bottom) as an alternate measure of habituation. This measure revealed that adult NCM showed a smaller decrease over stimulus iterations than did juveniles for TUT, roTUT, and unfamCON (P = 0.02 or less; the age difference for revTUT was not significant, P = 0.08). Interestingly, the main effect of age for TUT, roTUT, and unfamCON was due to the fact that 35-day NCM neurons had significantly larger difference scores (greater habituation) compared with adults (P = 0.004 or less), whereas 20-day and adult NCM did not differ (P = 0.08 or higher). Percent difference scores did not differ between 20- and 35-day NCM, except in the case of TUT (P = 0.03), due to greater habituation to tutor song in 35-day NCM (cf. Fig. 5). Individual age comparisons for revTUT were never significant (P = 0.07 or higher), indicating that NCM neurons habituated to revTUT at the same rate across ages.
Fig. 6.
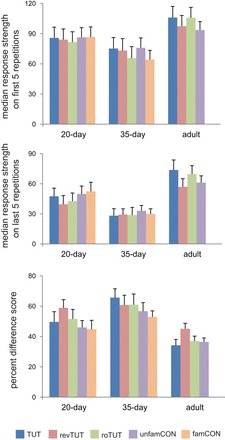
Raw response strength in dorso-caudal NCM on the first five vs. the last five stimulus iterations. Top and middle: median raw response strength of the first five (top) and last five (middle) stimulus repetitions of each song type. Bottom: normalized difference score showing the percent decrease between the first five and the last five stimulus repetitions for each song type. All values are means + SE.
The decrease in neural activity in adult NCM showed a significant effect of song type (P = 0.008), which was entirely due to a lower level of habituation to TUT compared with revTUT (P = 0.002; TUT vs. roTUT and unfamCON, both P = 0.60). In accord with the slope data shown in Fig. 5, neural activity in 35-day NCM showed a greater percent decrease for TUT than for famCON (P = 0.02); this measure also showed a greater decrease for TUT compared with unfamCON that was marginally significant (P = 0.05), indicating a particularly high rate of habituation to TUT in 35-day NCM.
This pattern confirms that adult NCM neurons showed less habituation across song types than did those in juvenile NCM, suggesting that songs are treated as being more familiar overall in adult than in juvenile NCM. Furthermore, these data show that the higher average response strength seen in adult NCM (Fig. 2) is primarily attributable to decreased habituation in adult NCM over the course of hearing repeated song presentations, whereas the relatively higher levels of habituation in juvenile NCM neurons contribute to lower average response strength. In addition, the stronger response to tutor song in adult birds is partly attributable to a somewhat lower rate of habituation to TUT (although this was significant only for TUT vs. revTUT; Fig. 6, bottom), which results in relatively more firing to TUT relative to other songs during the last five stimulus iterations (Fig. 6, middle). Strikingly, neural activity in 35-day NCM showed a relatively high level of habituation, especially to tutor song. In contrast to both adult and 20-day NCM, 35-day NCM showed a higher level of habituation to tutor song relative to both familiar and unfamiliar conspecific song, indicating that the response to tutor song decreases rapidly at the developmental time point when birds are in the final stages of actively acquiring a memory of this song (cf. Figs. 5 and 6). This high rate of habituation paradoxically suggests that tutor song is treated as “less familiar” in NCM of 35-day-old birds.
NCM Activity in Adults Habituates Equally to Tutor Song and Unfamiliar Conspecific Song
Although Phan et al. (2006) reported a higher rate of habituation to tutor song in adult NCM than to an unfamiliar conspecific song, the present data indicate a similar rate of habituation to TUT and unfamCON in adult NCM (Figs. 5 and 6). As another means of assessing whether habituation to tutor song in NCM was greater relative to other songs, we calculated a “familiarity index” in which the habituation slope for unfamCON was divided by that for TUT (see Phan et al. 2006). Values greater than 1.0 indicate a lower rate of habituation to tutor song due to greater familiarity based on prior auditory experience (Fig. 7, top). One-sample Wilcoxon tests of whether the unfamCON/TUT ratios were different from 1.0 did not achieve significance for any age (P = 0.13 or higher in all cases). We also computed ratios of habituation slopes for revTUT over TUT; this ratio was also not significantly greater than 1.0, except in 20-day NCM (P = 0.04) and marginally in adult NCM (P = 0.05). Thus, although we obtained a tendency toward a lower rate of habituation for tutor song in adult birds, this trend was not significant. This result is consistent with those shown in Figs. 5 and 6, indicating that the rate of habituation in adult NCM did not vary across different song types played in the forward direction.
Fig. 7.
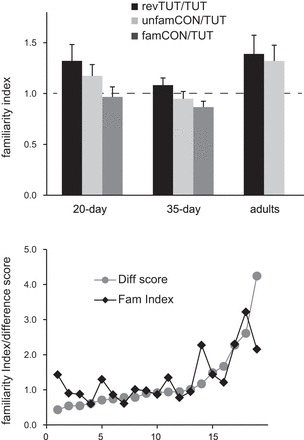
Measures comparing rate of habituation for control songs relative to TUT. Top: familiarity index represents the ratio of the habituation slopes for a given control song to TUT for each age group (mean + SE). See results. Bottom: comparison of familiarity index with a score based on the difference between the normalized median response strength for the first five vs. the last five stimulus iterations; scores are presented as cumulative distributions for individual adult birds (n = 19), so that the two types of score can be compared for each bird.
As an alternative method of assessing whether habituation of the neural response to tutor song in NCM was different from that to unfamCON in adult birds, we computed difference scores for the median response strength (normalized to the first trial) of the first five vs. the last five responses for both TUT and unfamCON songs; these difference scores were expressed as unfamCON/TUT ratios to compare them with the familiarity index based on slope values. Fig. 7 (bottom) shows cumulative distributions for both types of ratios in individual adult birds; these data show that both measures agree well in most individual birds. The mean ratio value based on slopes was 1.32 ± 0.16 (as shown in Fig. 7, top) compared with 1.23 ± 0.21 for the ratio based on difference scores (Wilcoxon one-sample test for median difference scores, P = 0.87). The majority of birds had ratios around 1.0, indicating no difference in relative degree of habituation to tutor song vs. that to novel conspecific songs. This pattern shows that under the conditions of our experiment the habituation functions for NCM activity in adult birds were not different for tutor vs. unfamiliar conspecific songs.
NCM Activity Habituates Rapidly to Tutor Song in 35-Day-Old Birds
We also computed the ratio of famCON to TUT for juvenile birds, to further analyze the unexpectedly high rate of habituation to tutor song seen in 35-day-old birds (Fig. 7, top). Although auditory exposure to famCON vs. TUT songs would be equal for 20- and 35-day-old birds, we had hypothesized that learning the tutor song would cause it to evoke a more familiar response (decreased rate of habituation) in NCM by 35 days. If so, then the famCON/TUT ratio would be greater than 1.0 in 35-, but not 20-day-old, birds, due to learning that is unique to the tutor song. However, although the famCON/TUT ratio was not different from 1.0 in 20-day-old birds (P = 0.52), it was significantly lower than 1.0 in 35-day-old birds (P = 0.05) due to a relatively high rate of habituation to tutor song compared with famCON song. As shown above (Figs. 5 and 6), the rate of habituation to TUT relative to famCON was higher in 35-day-old birds than in 20-day-old birds (the absolute value of habituation slopes for TUT at 35 vs. 20 days did not differ, P = 0.28; however, the percent decrease to TUT was greater at 35 days than at 20 days, P = 0.03, as reported above for Fig. 6). The greater habituation to tutor than to familiar conspecific song in NCM of a 35-day-old bird was evident in both raw traces and peristimulus time histograms (PSTHs) (Fig. 8, top). The bottom panels of Fig. 8 show the decrease in normalized response strength across stimulus presentations for TUT, famCON and unfamCON in both 20- and 35-day NCM. Thus NCM neurons in 35-day-old birds show a higher level of habituation to tutor song, despite the fact that it has been learned and hence is highly familiar at this age. This pattern indicates that NCM of 35-day-old birds does not respond to tutor song in the same way as a familiar conspecific song; the higher relative rate of habituation to tutor song in 35-day-old birds may indicate that tutor song is treated as a familiar but salient stimulus at this age.
Fig. 8.
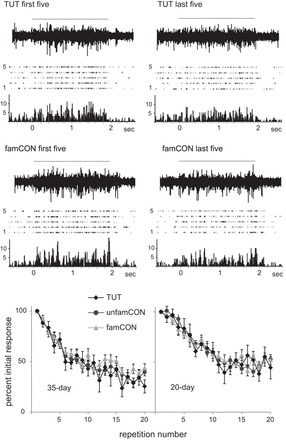
Comparison of habituation to TUT vs. famCON in NCM of a 35-day-old bird. Top: each panel shows, from top to bottom, an example of a raw trace, a raster of 5 trials, and a peristimulus time histogram (PSTH). Top two panels show decrease in response strength from first five to last five repetitions for TUT; middle two panels show decrease in response strength from first five to last five repetitions for familiar CON song. Gray lines at top show timing of song playback; duration of both TUT and famCON was 2 s; bin size for PSTHs was 12.5 ms. Bottom: rate of habituation to TUT, famCON, and unfamCON in 35- (left) and 20-day-old (right) birds. Values are means ± SE normalized response strength for each stimulus iteration.
DISCUSSION
The present results, based on multiunit activity in dorso-caudal NCM of awake male zebra finches, show that 1) NCM of juvenile birds does not respond selectively to tutor song; 2) tutor song selectivity in NCM emerges during vocal development; and 3) evoked responses in NCM of juveniles that are completing formation of a memory of their tutor song show greater habituation to the tutor song than to familiar conspecific song. Figure 2 shows that the average strength of evoked activity in NCM is higher across different song types in adult birds that are producing a stable song pattern than in both fledglings and older juvenile birds. However, this pattern is primarily due to decreased habituation in adult NCM (Figs. 5 and 6). That is, the emergence of a more persistent response over repeated stimulus iterations in dorso-caudal NCM during the course of vocal development causes a broad increase in song-evoked activity. Two additional factors other than decreased habituation contribute to selectivity to tutor song in adult NCM. First, “pre-habituation” response strength across all song types is slightly (but nonsignificantly) higher in adult birds compared with juveniles, especially for TUT and roTUT (i.e., during the first five stimulus presentations; Fig. 6, top). In addition, habituation to tutor song tends to be somewhat less relative to other songs within adult NCM (although this trend was significant only for TUT vs. revTUT; Fig. 6, bottom). These two trends contribute to a stronger average response to tutor song that is maintained when the order of syllables is reversed, as evidenced by the higher response strength in adult NCM neurons for these two song stimuli than for tutor song played as a mirror-image reverse or an unfamiliar conspecific song (Figs. 2 and 6, middle). The stronger average response to TUT and roTUT causes adult NCM neurons to respond selectively to tutor song syllables, regardless of order (Figs. 3 and 4). The absence of selectivity for TUT vs. roTUT in NCM of adult birds that are producing a stereotyped sequence suggests that NCM neurons become tuned to individual song elements, but not their sequence.
Previous comparisons of neural responses to song in 37-day-old vs. adult urethane-anesthetized zebra finches in primary auditory cortex (field L) and a higher-level region of auditory cortex (caudal mesopallium) reported a substantial increase in response strength in adults, suggesting that increased responsivity to complex auditory stimuli may be a general maturational feature of auditory telencephalon (Amin et al. 2007; Grace et al. 2003). Because neural activity in primary auditory cortex does not habituate, this pattern suggests that an actual increase in firing rate may contribute to increased response strength in some brain regions. Both the current results (Figs. 2 and 6) and those of Stripling et al. (2001) indicate that song-evoked activity in NCM of awake zebra finches is comparable during the first 2 wk of vocal learning between ∼20–35 days of age, which represents the major period for memorization of a tutor song (Böhner 1990; Roper and Zann 2006). Thus the decreased habituation in adult NCM that underlies the developmental increase in response strength occurs after the major period of sensory learning based on auditory experience with a tutor song. Juvenile birds in our breeding population begin to produce song-related vocalizations at ∼36 days of age, indicating that more persistent song-evoked activity occurs after birds begin producing song vocalizations, and raising the possibility that auditory-vocal feedback during sensorimotor integration contributes to this type of increase in neural activity in NCM.
Although neural activity in adult NCM was selective for tutor song (both TUT and roTUT) compared with revTUT and unfamCON, NCM of juvenile birds did not show a selective response to tutor song compared with any other song. However, direct tests of whether the tutor-selective response in adults was higher compared with juveniles were not significant for the most part, indicating that, although NCM neurons of adult birds show a selective response to tutor song syllables, it is not a particularly strong preference when measured across the population. Adult zebra finch males are known to prefer the song of their tutor to unfamiliar conspecific songs, as indicated by behavioral approach measures, and lesions of NCM abolish this preference for tutor song (Gobes and Bolhuis 2007; Remage-Healey et al. 2010; Riebel 2000). Thus the stronger auditory response of adult NCM neurons to hearing tutor song syllables may be part of a neural circuit that mediates recognition and behavioral approach. If so, then the present results suggest that behavioral preference for tutor song would be the same, regardless of the order in which song syllables are presented (e.g., for both TUT and roTUT songs).
NCM of 35-day-old birds did not show a stronger response to tutor song compared with other songs (Figs. 2 and 6), despite the fact that birds exposed to a tutor only from 20 to 35 days are able to form a good copy of that song as adults (Böhner 1990; Roper and Zann 2006). In addition, although 35-day-old birds tend not to respond in preference (approach) tests, those that do respond prefer tutor over novel CON songs (Clayton 1988). This pattern suggests that, although a memory of the tutor song may be established by 35 days, it is not evident at that point in terms of multiunit response strength of NCM neurons or in terms of a well-developed behavioral preference for tutor song. Selectivity in most regions of auditory cortex may develop after 35 days (Gehr et al. 2000), suggesting that experience with the tutor song during early vocal learning does not act to drive increases in responsivity across NCM neurons, and that song production and sensorimotor integration may be necessary for the development of a selective response. In accord with this idea, neural tuning in NCM mirrors the behavioral ability to discriminate vocal calls of varying frequencies during vocal development, with adult performance showing significant improvement after 35 days (Miller-Sims and Bottjer 2012).
The extent to which neural activity in higher-level regions of auditory cortex in zebra finches is selective for different song stimuli is not well understood in general. Surprisingly, no previous studies have tested selectivity of response strength for tutor song in NCM. However, studies that have tested the response of NCM neurons in awake birds (as in this study) have reported comparable levels of responsivity to diverse sounds. For example, Stripling et al. (1997) reported a lack of selectivity across the population of NCM single neurons in adult male zebra finches for BOS, conspecific songs, and heterospecific songs (HET). Chew et al. (1996) reported similar levels of multiunit activity in NCM to BOS, conspecific song, and human speech, although the response to CON was higher than that to different heterospecific songs. In accord with this lack of selectivity, we did not observe differential response strength to reversed tutor song vs. novel CON songs; however, we did observe stronger responsivity to TUT and roTUT. One caveat is that we did not test neural responsivity to each BOS in this study, and hence we cannot evaluate whether the higher response to tutor song we observed reflects a partial contribution from similarity of the bird's own song to tutor song. However, the lack of selectivity to BOS reported by previous studies suggests that NCM neurons may develop a selective representation of tutor song that is distinct from their own self-produced song. Interestingly, although Chew et al. (1995) observed comparable rates of habituation to CON, HET, and human speech, habituation was retained for much longer to CON songs (20–40 h) than to HET or speech (4 h) (Chew et al. 1995), indicating that retention of habituation may be a more sensitive measure of memory traces for different song stimuli than is response strength. We did not test for retention of habituation to different song stimuli following training, but our results suggest the possibility that retention of habituation to tutor song might be greater 24–48 h later than that for conspecific song.
The premise of the study by Phan et al. (2006) was that exposure to and imitation of a tutor song during the period of vocal learning in juveniles would result in a memory trace conferring greater familiarity to tutor song, reflected as a decreased rate of habituation in NCM of adult birds. Although we observed a trend in this direction, it was not significant. Several differences in procedure could account for this apparent discrepancy. Chief among these may be that our birds were raised in a large colony, including several group aviaries that each contained six breeding pairs (see methods), whereas birds in the Phan et al. study were isolated from adult male songs until being placed in a key-peck tutoring paradigm that provided controlled exposure to only one tutor song from ∼45–85 days. Thus auditory experience during the period of vocal learning for birds in the Phan et al. study was restricted to playback of tutor song and self-produced song, whereas birds in the present study were exposed to a rich diversity of conspecific sounds (from both juveniles and adults) in the context of being raised by a tutor (their father). We did observe stronger neural selectivity to tutor song in adult birds that copied more syllables from their tutor's song, a finding that is consistent with the idea that NCM neurons encode a memory of the tutor song.
A particularly interesting feature of the present results is that 35-day-old birds showed an increased level of habituation to tutor song relative to a familiar conspecific song (Figs. 5 and 6). Auditory experience with these two song types should be comparable, since both were drawn from the aviary in which 35-day-old juveniles were reared. Despite this fact, the relatively high rate of habituation to tutor song suggests that birds have learned to distinguish tutor song by 35 days, and that it is not treated as a typical “familiar” song. Perhaps increased attentional or motivational factors are influencing the response to tutor song, since it is being learned as a template for subsequent auditory-motor integration. Social factors are extremely important for vocal learning in both humans and songbirds (Brainard and Doupe 2013; Deregnaucourt et al. 2013; Goldstein et al. 2003; Jones et al. 1996; Kuhl 2010; 2007; Nelson and Marler 1994). For example, juvenile zebra finches learn the song of a foster father (Bengalese finch) who cares for them, even if their genetic father is housed in an adjacent cage (Immelmann 1969). Zebra finch parents typically feed and groom their young up until at least 30–35 days of age, which encompasses the major period of auditory learning from fledging (20 days) to memorization of the tutor song (∼35 days). One possible interpretation of the higher rate of habituation to tutor song at 35 days is that juvenile birds have learned the acoustic pattern of tutor song, and that it is a highly salient stimulus that commands increased attention. If so, working memory for the tutor song may persist for longer periods of time, and thereby facilitate habituation of the neural response due to higher short-term “familiarity.” Attentional and task demands are known to modulate response properties of cortical neurons (Bao et al. 2004; Crist et al. 2001; Fritz et al. 2005a, 2005b; Knudsen and Gentner 2013; Polley et al. 2006). The influence of social and motivational factors on vocal learning are likely mediated in part by neuromodulators such as norepinephrine and dopamine, which is thought to provide a reinforcement signal that drives cortical plasticity (Bao et al. 2001; Velho et al. 2012). Indeed, neurons in the ventral tegmental area of 35-day-old zebra finches show enhanced induction of Fos in response to tutor song relative to a conspecific song (Nordeen et al. 2009), which could contribute to the greater habituation seen here in NCM.
Regardless of the mechanism by which tutor song evokes greater habituation, the present results clearly demonstrate a difference in how tutor song is processed by NCM neurons once it has been memorized (i.e., between 20 and 35 days). How does this result bear on the question of how and where the memory of tutor song is encoded? It seems possible that the relatively greater habituation to tutor song in NCM of 35-day-old birds could somehow facilitate vocal learning, especially if it indicates, as suggested above, that the representation of tutor song is being actively maintained in working memory. In any case, this neural signature of differential processing of tutor song suggests that early auditory experience with the tutor song is exerting some developmentally limited effect on NCM neurons. This could mean that NCM encodes some transient representation of the tutor song (which is gone by adulthood, at least as measured by differential habituation) and/or that NCM neurons forward information to sensorimotor vocal-control regions [such as the high vocal center (HVC) and lateral part of the magnocellular nucleus of anterior nidopallium (LMAN)] to influence the manner in which they encode or process tutor song information. The neural representation of tutor song is likely to be highly distributed, and evidence for tutor-tuned neurons has been found in HVC and LMAN of juvenile birds and in HVC of adult birds (Adret et al. 2012; Nick and Konishi 2005; Prather et al. 2010), raising the possibility that top-down influences or feedback to NCM from sensorimotor vocal-control regions could contribute to the neural representation of tutor song (Roberts et al. 2012; Suga and Ma 2003). Interestingly, neurons selectively tuned to tutor song in LMANSHELL of juvenile zebra finches are abundant at 45 days of age, but scarce by 60 days (Achiro and Bottjer 2013). Furthermore, few if any neurons in LMAN respond to song playback at all in 30-day-old birds (J. Achiro and S. Bottjer, unpublished data), indicating that specific song-evoked responses emerge in LMAN sometime after 30 days. This pattern raises the possibility that processing of tutor song in NCM unique to the time when it is being memorized influences the subsequent development of selective tuning in LMAN (and other vocal-control regions), to guide the development of a distributed representation of tutor song necessary for sensorimotor integration.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.C.M.-S. and S.W.B. conception and design of research; V.C.M.-S. performed experiments; V.C.M.-S. and S.W.B. analyzed data; V.C.M.-S. and S.W.B. interpreted results of experiments; V.C.M.-S. and S.W.B. edited and revised manuscript; V.C.M.-S. and S.W.B. approved final version of manuscript; S.W.B. prepared figures; S.W.B. drafted manuscript.
ACKNOWLEDGMENTS
We thank Vandana Suresh and Jenny Achiro for assistance with Matlab programming for data analysis, and Jenny Achiro for comments on the manuscript.
REFERENCES
- Achiro JM, Bottjer SW. Neural representation of a target auditory memory in a cortico-basal ganglia pathway. J Neurosci 33: 14475–14488, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adret P, Meliza CD, Margoliash D. Song tutoring in presinging zebra finch juveniles biases a small population of higher-order song-selective neurons toward the tutor song. J Neurophysiol 108: 1977–1987, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin N, Doupe A, Theunissen FE. Development of selectivity for natural sounds in the songbird auditory forebrain. J Neurophysiol 97: 3517–3531, 2007. [DOI] [PubMed] [Google Scholar]
- Ayala YA, Malmierca MS. Stimulus-specific adaptation and deviance detection in the inferior colliculus. Front Neural circuits 6: 89, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature 412: 79–83, 2001. [DOI] [PubMed] [Google Scholar]
- Bao S, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci 7: 974–981, 2004. [DOI] [PubMed] [Google Scholar]
- Basham ME, Nordeen EJ, Nordeen KW. Blockade of NMDA receptors in the anterior forebrain impairs sensory acquisition in the zebra finch (Poephila guttata). Neurobiol Learn Mem 66: 295–304, 1996. [DOI] [PubMed] [Google Scholar]
- Böhner J. Early acquisition of song in the zebra finch, Taeniopygia guttata. Anim Behav 39: 369–374, 1990. [Google Scholar]
- Böhner J. Song learning in the zebra finch (Taeniopygia guttata): selectivity in the choice of a tutor and accuracy of song copies. Anim Behav 31: 231–237, 1983. [Google Scholar]
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci 7: 347–357, 2006. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Altenau B. Parallel pathways for vocal learning in basal ganglia of songbirds. Nat Neurosci 13: 153–155, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer SW, Arnold AP. Developmental plasticity in neural circuits for a learned behavior. Annu Rev Neurosci 20: 459–481, 1997. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Translating birdsong: songbirds as a model for basic and applied medical research. Annu Rev Neurosci 36: 489–517, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchpole C, Slater PJB. Bird Song: Biological Themes and Variations. Cambridge, UK: Cambridge University Press, 1995. [Google Scholar]
- Chew SJ, Mello C, Nottebohm F, Jarvis E, Vicario DS. Decrements in auditory responses to a repeated conspecific song are long-lasting and require two periods of protein synthesis in the songbird forebrain. Proc Natl Acad Sci U S A 92: 3406–3410, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SJ, Vicario DS, Nottebohm F. A large-capacity memory system that recognizes the calls and songs of individual birds. Proc Natl Acad Sci U S A 93: 1950–1955, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton NS. Song discrimination learning in zebra finches. Behaviour 36: 1016–1024, 1988. [Google Scholar]
- Clayton NS. Song tutor choice in zebra finches. Anim Behav 35: 714–721, 1987. [Google Scholar]
- Crist RE, Li W, Gilbert CD. Learning to see: experience and attention in primary visual cortex. Nat Neurosci 4: 519–525, 2001. [DOI] [PubMed] [Google Scholar]
- Deregnaucourt S, Poirier C, Kant AV, Linden AV, Gahr M. Comparisons of different methods to train a young zebra finch (Taeniopygia guttata) to learn a song. J Physiol (Paris) 107: 210–218, 2013. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci 22: 567–631, 1999. [DOI] [PubMed] [Google Scholar]
- Eales LA. Song learning in zebra finches–some effects of song model availability on what is learnt and when. Anim Behav 33: 1293–1300, 1985. [Google Scholar]
- Foster EF, Bottjer SW. Lesions of a telencephalic nucleus in male zebra finches: influences on vocal behavior in juveniles and adults. J Neurobiol 46: 142–165, 2001. [DOI] [PubMed] [Google Scholar]
- Fritz J, Elhilali M, Shamma S. Active listening: task-dependent plasticity of spectrotemporal receptive fields in primary auditory cortex. Hear Res 206: 159–176, 2005a. [DOI] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, Shamma SA. Differential dynamic plasticity of A1 receptive fields during multiple spectral tasks. J Neurosci 25: 7623–7635, 2005b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehr DD, Hofer SB, Marquardt D, Leppelsack H. Functional changes in field L complex during song development of juvenile male zebra finches. Brain Res Dev Brain Res 125: 153–165, 2000. [DOI] [PubMed] [Google Scholar]
- Gobes SM, Bolhuis JJ. Birdsong memory: a neural dissociation between song recognition and production. Curr Biol 17: 789–793, 2007. [DOI] [PubMed] [Google Scholar]
- Goldstein MH, King AP, West MJ. Social interaction shapes babbling: testing parallels between birdsong and speech. Proc Natl Acad Sci U S A 100: 8030–8035, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace JA, Amin N, Singh NC, Theunissen FE. Selectivity for conspecific song in the zebra finch auditory forebrain. J Neurophysiol 89: 472–487, 2003. [DOI] [PubMed] [Google Scholar]
- Immelmann K. Song development in the zebra finch and other estrildid finches. In: Bird Vocalizations: Their Relations to Current Problems in Biology and Psychology; Essays Presented to W. H. Thorpe, edited by Hinde R. A. London, UK: Cambridge Univ Press, 1969, p. 61–74. [Google Scholar]
- Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc Natl Acad Sci U S A 94: 4097–4102, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AE, TenCate C, Slater PJB. Early experience and plasticity of song in adult male zebra finches (Taeniopygia guttata). J Comp Psychol 110: 354–369, 1996. [Google Scholar]
- Knudsen DP, Gentner TQ. Active recognition enhances the representation of behaviorally relevant information in single auditory forebrain neurons. J Neurophysiol 109: 1690–1703, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK. Brain mechanisms in early language acquisition. Neuron 67: 713–727, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK. Is speech learning “gated” by the social brain? Dev Sci 10: 110–120, 2007. [DOI] [PubMed] [Google Scholar]
- London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nat Neurosci 11: 579–586, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann NI, Slater PJB. Song tutor choice by zebra finches in aviaries. Anim Behav 49: 811–820, 1995. [DOI] [PubMed] [Google Scholar]
- Marler P. Birdsong and speech development: could there be parallels? Am Sci 58: 669–673, 1970. [PubMed] [Google Scholar]
- Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J Neurosci 14: 6652–6666, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Vates GE, Okuhata S, Nottebohm F. Descending auditory pathways in the adult male zebra finch (Taeniopygia guttata). J Comp Neurol 395: 137–160, 1998. [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci U S A 89: 6818–6822, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Sims VC, Bottjer SW. Development of auditory-vocal perceptual skills in songbirds. PLos One 7: e52365, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DA, Marler P. Selection-based learning in bird song development. Proc Natl Acad Sci U S A 91: 10498–10501, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netser S, Zahar Y, Gutfreund Y. Stimulus-specific adaptation: can it be a neural correlate of behavioral habituation? J Neurosci 31: 17811–17820, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick TA, Konishi M. Neural song preference during vocal learning in the zebra finch depends on age and state. J Neurobiol 62: 231–242, 2005. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Holtzman DA, Nordeen KW. Increased Fos expression among midbrain dopaminergic cell groups during birdsong tutoring. Eur J Neurosci 30: 662–670, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci U S A 103: 1088–1093, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci 26: 4970–4982, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Persistent representation of juvenile experience in the adult songbird brain. J Neurosci 30: 10586–10598, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A 107: 3852–3857, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebel K. Early exposure leads to repeatable preferences for male song in female zebra finches. Proc Biol Sci 267: 2553–2558, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TF, Gobes SM, Murugan M, Olveczky BP, Mooney R. Motor circuits are required to encode a sensory model for imitative learning. Nat Neurosci 15: 1454–1459, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper A, Zann R. The onset of song learning and song tutor selection in fledgling zebra finches. Ethology 112: 458–470, 2006. [Google Scholar]
- Solis MM, Doupe AJ. Anterior forebrain neurons develop selectivity by an intermediate stage of birdsong learning. J Neurosci 17: 6447–6462, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripling R, Kruse AA, Clayton DF. Development of song responses in the zebra finch caudomedial neostriatum: role of genomic and electrophysiological activities. J Neurobiol 48: 163–180, 2001. [DOI] [PubMed] [Google Scholar]
- Stripling R, Volman SF, Clayton DF. Response modulation in the zebra finch neostriatum: relationship to nuclear gene regulation. J Neurosci 17: 3883–3893, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci 4: 783–794, 2003. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Nottebohm F. Social inhibition of song imitation among sibling male zebra finches. Proc Natl Acad Sci U S A 95: 8951–8956, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav 59: 1167–1176, 2000. [DOI] [PubMed] [Google Scholar]
- Terleph TA, Mello CV, Vicario DS. Auditory topography and temporal response dynamics of canary caudal telencephalon. J Neurobiol 66: 281–292, 2006. [DOI] [PubMed] [Google Scholar]
- Terpstra NJ, Bolhuis JJ, den Boer-Visser AM. An analysis of the neural representation of birdsong memory. J Neurosci 24: 4971–4977, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JV, Gentner TQ. Song recognition learning and stimulus-specific weakening of neural responses in the avian auditory forebrain. J Neurophysiol 103: 1785–1797, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovsky N, Las L, Nelken I. Processing of low-probability sounds by cortical neurons. Nat Neurosci 6: 391–398, 2003. [DOI] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches. J Comp Neurol 366: 613–642, 1996. [DOI] [PubMed] [Google Scholar]
- Velho TA, Lu K, Ribeiro S, Pinaud R, Vicario D, Mello CV. Noradrenergic control of gene expression and long-term neuronal adaptation evoked by learned vocalizations in songbirds. PLos One 7: e36276, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H. Models for song learning in the zebra finch: fathers or others? Anim Behav 39: 745–757, 1990. [Google Scholar]


