Abstract
Background
To determine the normal ranges of serum age-related prostate-specific antigen (PSA) level in men from Beijing area without cancer.
Methods
In this cross sectional study, form April 2010 to October 2011, 1611 healthy men undergoing a routine health check-up in our hospital and all men received three examinations including serum PSA test, digital rectal ex-amination and transrectal ultrasound. Men with any two abnormal results of the three examinations were undergone a prostate biopsy. Men with any two normal results of the three examinations or with negative biopsy were defined as men without cancer. Men with a prior history of prostate cancer/surgery or with urinary tract infection/obstruction were excluded. 1572 men without cancer were recruited into the study finally and were stratified into 10-year age groups: 40 to 49, 50 to 59, 60 to 69, 70 to 79, and older than 80.
Results
The median PSA value (95th percentile range) was 0.506(1.565), 1.04(2.920), 1.16(4.113), 1.34(5.561)and 2.975 (7.285) for each age group respectively, and the 25th percentile to 75 percentile was 0.343 to 0.923, 0.663 to 1.580, 0.693 to 2.203, 0.789 to 2.368 and 1.188 to 4.295 respectively. The serum PSA value is directly correlated with age (r=0.314, P<0.001).
Conclusions
Use the age-related range for PSA increases the sensitivity in younger men and decreases the biopsy rate in older patients.
Keywords: Age, Prostate cancer, Prostate-specific antigen
Introduction
Prostate specific antigen (PSA) is a serine protease secreted by all types of prostatic tissue - normal, benign hyperplastic and cancerous, and not the prostate cancer (PCa) specific antigen. Under normal conditions, the epithelial layer, the basal cell layer, and the basement membrane separate the intraductal contents from the lymphatic system and the PSA level in the prostatic fluid are a million times higher than in the serum. When tumor or disease interferes with this barrier, PSA enters the lymph and then the circulatory system. So, as a blood marker, PSA has some practical utilities in detecting early prostate cancer and monitoring response to therapy. Nevertheless, because it is produced by all types of prostatic tissue, it lacks sufficient sensitivity and specificity to be the ‘perfect’ tumor marker for the detection of early prostate cancer (1, 2). About 75% of men with PSA levels greater than 4.0ng/ml do not have PCa (3), and PSA has an extremely low positive predictive value (PPV) in diagnosing prostate cancer (4). A serious of conditions can lead the elevation of serum PSA level(1,2), such as benign diseases including benign prostate hyperplasia (BPH), prostatitis, and trauma/necrosis of the prostate gland, and operating on prostate gland including digital rectal examination (DRE) and transrectal ultrasound (TRUS) et al. In addition, race and patient’s age can affect the PSA level as well.
To further improve the utility of PSA as a screening tool for prostate cancer, Oesterling et al.(5) developed, for white Americans of the United States, age-related reference ranges for serum PSA. They demonstrated that the serum PSA concen-trations correlated directly with age and the recommended upper limit of normal serum PSA levels for white men are: 2.5 ng/ml for 40-49 years, 3.5 ng/ml for 50-59 years, 4.5ng/ml for 60-69 years, and 6.5ng/ml for 70-79 years. After that, some articles (6–8) reported the recommended reference ranges for serum PSA for men from different races. Even in the same race, however, the reference ranges for serum PSA has some differences, such as studies on Chinese men from Taiwan, Shaanxi, Shanghai, and Shandong (9–12).
Weinrich et al. (13) compared three studies from different areas of the United States, and suggested that using age- and race-related reference ranges for normal PSA level should be specific to the population where the norms are derived. The serum PSA levels increased with age and the baseline PSA and PSA velocity in young Chinese men without prostate cancer differ from those of African American and Caucasian American men. (14)
The aim of this study was to establish the reference ranges for PSA in men from Beijing area without prostate cancer, and spin-offs include comparisons with white Americans and other Chinese men of different geographic regions.
Patients and Methods
Patients Selection
In this cross sectional study, from April 2010 to October 2011, 1611 Chinese men, aged 40 to 91 years, undergoing a routine health check-up in our hospital were recruited into the study. All the subjects were stratified into 10-year age groups: 40 to 49 years, 50 to 59 years, 60 to 69 years, 70 to 79 years, and older than 80 years. The Institutional Review Board committee approved the research protocols, and all patients provided informed consent. Men were excluded if they presented with a prior history of prostate cancer/surgery, urinary tract infection/obstruction, ejaculation 48 hours before the PSA test, or had a serum PSA level greater than 20ng/ml.
Study Design
All the subjects received three examinations including serum PSA test, DRE and TRUS to determine their health status. The abnormal PSA levels were defined as the concentration>4.0 ng/ml, the abnormal DRE findings were defined as palpable induration, nodularity, irregularity, or asymmetry, and the abnormal TRUS findings were defined as capsular irregularity, deformation, or existence of a hypoechoic region/nodule (15).
We set up the criteria for biopsy as below: Men with any two abnormal results of the upper three examinations. Men with any two normal results or although had two abnormal results but with a negative biopsy were regarded as men without cancer.
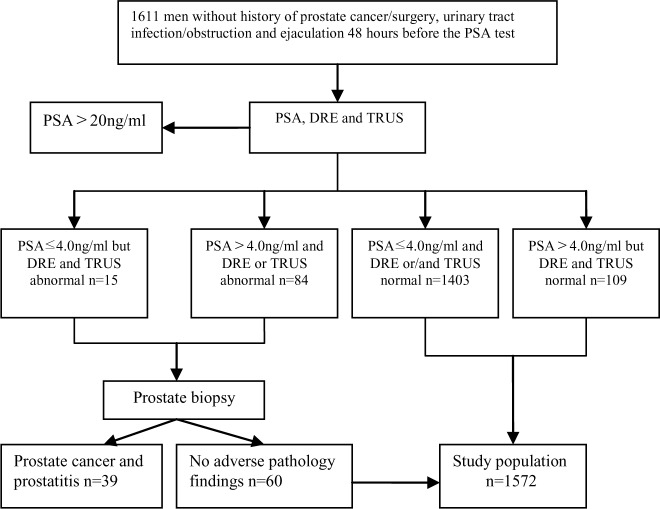
Serum PSA levels were measured using the Elecsys 2010 immunoassay (Roche Diagnostics Gmbh, Mannheim, Germany). TRUS were performed with Acuson Sequoia512 (Siemens Medical Solutions USA, Inc, Mountain View, California, USA) and IU22 (Philips Ultrasound, Bothell, Washington, USA) scanners. TRUS-guided sextant biopsies were performed, and, if TRUS or DRE revealed abnormal findings, we performed additional one to two biopsies in the suspicious areas (Fig. 1).
Fig. 1:
Flow chart of this study
Eligibility Criteria
Men with any two normal results of the three examinations (PSA test, DRE and TRUS) or although had two abnormal results but with a negative biopsy were recruited in this study.
Statistical Analysis
Descriptive statistics including the mean, median, 5th, 25th, 75th, and 95th percentiles of the PSA level were calculated for each 10-year age groups. Spearman rank correlation coefficients were calculated to measure the association between serum PSA and age. The 95th percentile was determined as the upper limit of normal (reference range) for the midpoint of each 10-year age group for the serum PSA level. For all analyses, a P-value<0.05 was considered statistically significant. Analysis was performed using the Statistical Package for Social Sciences software program (SPSS for Windows Ver.11.5).
Results
Of the 1611subjects, 99 were biopsied and 37 cases of PCa, two cases of prostatitis, and 60 cases of BPH and prostatic intraepithelial neoplasia (PIN) were diagnosed. 1572 subjects were included in this study finally. Table 1 shows the proportion of men with various serum PSA levels according to Age.
Table 1:
Proportion of men without prostate cancer with various serum PSA levels according to age
| Age(yr) | N | Serum PSA levels | (ng/ml) | (%) |
|---|---|---|---|---|
| <4 | 4~10 | 10~20 | ||
| 40~49 | 46 | 46(100) | 0(0) | 0(0) |
| 50~59 | 312 | 308(98.7) | 4(1.3) | 0(0) |
| 60~69 | 536 | 505(94.2) | 29(5.4) | 2(0.4) |
| 70~79 | 414 | 370(89.4) | 38(9.2) | 6(1.4) |
| 80~ | 264 | 187(70.8) | 73(27.7) | 4(1.5) |
Abbreviation: PSA: prostate-specific antigen.
Among the 1572 subjects, 1416 (90.1%) had a serum PSA level less than 4ng/ml, 144 (9.2%) greater than 4ng/ml but less than 10ng/ml, and only 12 (0.7%) greater than 10 ng/ml.
Figure 2 shows the frequency of PSA levels in men from Beijing area. The serum PSA levels among each age groups had statistics significant (between group 40-49 and group 50-59, P<0.05; among other groups, P<0.01),and over the entire age range, the serum PSA level directly correlated with age (r=0.314,P<0.001). Table 2 shows the mean, median and percentiles of serum PSA levels according to Age.
Fig. 2:
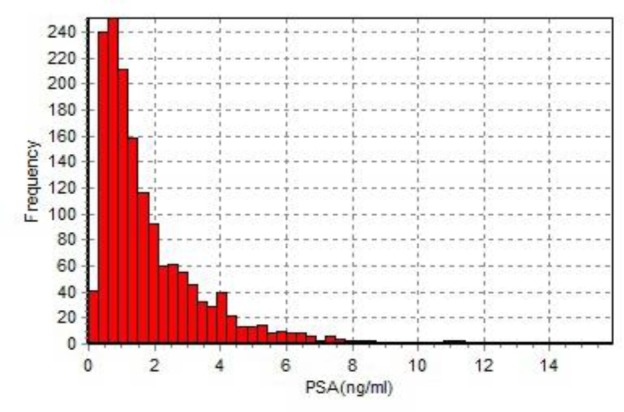
Frequency of prostate-specific antigen (PSA) in Chinese men without prostate cancer
Table 2:
The mean, median and percentiles of serum PSA according to age
| Serum PSA (ng/ml) Percentiles value | |||||||
|---|---|---|---|---|---|---|---|
| Age(yr) | N | Mean | 5th | 25th | median | 75th | 95th |
| 40~49* | 46 | 0.555 | 0.149 | 0.343 | 0.506 | 0.923 | 1.565 |
| 50~59 | 312 | 1.053 | 0.428 | 0.663 | 1.040 | 1.580 | 2.920 |
| 60~69 | 536 | 1.214 | 0.356 | 0.693 | 1.160 | 2.203 | 4.113 |
| 70~79 | 414 | 1.365 | 0.374 | 0.789 | 1.340 | 2.368 | 5.561 |
| 80~ | 264 | 2.242 | 0.417 | 1.188 | 2.975 | 4.295 | 7.285 |
Abbreviation: PSA: prostate-specific antigen/*P<0.05 between group 40-49 and group 50-59; among other groups, P<0.01.
Discussion
Since PSA had been discovered by Ablin (16) in 1970, it has been the most widely used tumor marker nowadays for detecting early prostate cancer and monitoring response to therapy. Therefore, to determine the normal range for serum PSA level is important, and 4.0ng/ml as the upper limit of serum PSA level has been widely accepted nowadays. However, a meta-analysis (3) showed that among men whose serum PSA level greater than 4.0ng/ml, more than 70 percent did not have PCa. As man ages, the prostate gland enlarges gradually. So, for the younger men without BPH or with slight BPH, the notion of “normal range” for serum PSA level should be different from the older ones with heavy BPH.
Tang et al. (17) recently reported that Patients’ age, PSA, prostate volume and DRE status were the independent variables to predict a positive initial prostate biopsy, while TRUS was not. In our study, we set up the criteria for biopsy as men with any two abnormal results of the three tests (DRE/TRUS/PSA) for some reasons. Firstly, evidence from autopsies study showed that the prevalence of prostate cancer was 15 to 60 percent among men 60 to 90 years old and increased with age (18), and considering that a man’s risk of death from prostate cancer is 3 to 4 percent and his lifetime risk of the diagnosis of prostate cancer is 16.7 percent, it is apparent that many prostate cancers detected in routine practice may be clinically unimportant, and lowing the cutoff would increase the risks of overdiagnosing and overtreating clinically unimportant disease (19). Secondly, the detection rate of abnormal findings on DRE and TRUS was 14.4% and 9.5% respectively with PSA levels of 4.0ng/ml or less, adding TRUS to DRE in the screening program of subjects with PSA levels of 2.0 to 4.0 ng/mL increased the detection rate of prostate cancer to 30.8% (15). Thirdly, 72% to 82% of patients who undergo biopsy based on DRE findings will not have prostate cancer (20). Frothily, TRUS has not been used in the first-line screening examination for prostate cancer because of it lacks the ability to diagnose the prostate cancer in early stage, while it has been widely used as a screening and following-up tool for prostate cancer in China, because it is much cheaper. Finally, Papers have been reported that even some patients with prostate cancer cannot be treated immediately at the time it was diagnosed, and they can undergo follow-ups with a specific surveillance program, called Active surveillance (AS), which is the practical way of avoiding possible overtreatment of prostate cancer (21, 22). In our study, more than 90 percent of the patients’ PSA levels were lower than 4.0ng/ml, and for the subjects with only one positive finding out of the three tests (DRE/TRUS /PSA), we take them in a following-up program, monitoring the PSA level, DRE and TRUS periodically, which may avoid the unnecessarily biopsies and not miss the prostate cancers.
In current study, the proportion of men with PSA level less than 4.0g/ml was 100% for 40-49 year-old age group, 98.7% for 50-59 year-old age group, and the upper limit (95th percentile) for the two groups was 1.565 and 2.920 respectively. If we still use 4.0ng/ml as the upper limit of serum PSA level, then we can image the sensitivity will be very low in these two groups in the PCa screening test. So, lower the PSA cutoff value for the relatively young men is necessary. Similarly, the upper limits for the three groups old than 60 years were higher than 4.0 ng/ml, and the traditional cutoff of 4.0 ng/ml would cause too many unnecessarily biopsies. Compared with the results for White Americans (5) and Chinese men from different regions, the upper limit of normal serum PSA levels for Chinese men in this study were lower than in White men and in Taiwanese (9), higher than in Chinese Shaanxi (10), and similar to that in Chinese Shanghai (11) and Shandong (12) (Table 3).
Table 3:
Comparison of Serum PSA Reference Ranges among White Americans and Chinese from Different Regions
| 95th Percentiles Value for Serum PSA | ||||||
|---|---|---|---|---|---|---|
| Chinese men from Different Regions | ||||||
| Age(yr) | White Americans (5) | Taiwanese (9) (7803) |
Shaanxi (10) (1096) |
Shanghai (11) (8422) |
Shandong (12) (9358) |
Current Study(1572) |
| 20↓ | - | 1.712(17) | - | - | - | - |
| 20~29 | - | 1.796(360) | 1.20(77) | - | - | - |
| 30~39 | - | 1.836(1442) | 1.21(189) | - | 1.89(1135) | - |
| 40~49 | 2.5 | 2.167(2333) | 1.23(233) | 2.15(1880) | 2.19(3700) | 1.565(46) |
| 50~59 | 3.5 | 3.329(2258) | 2.35(177) | 3.20(2255) | 2.88(2859) | 2.920(312) |
| 60~69 | 4.5 | 5.114(880) | 3.20(265) | 4.10(1800) | 4.42(1075) | 4.113(536) |
| 70~79 | 6.5 | 6.237(440) | 3.39(155) | 5.37(797) | 6.52(589) | 5.711(414) |
| 80~ | - | 6.613(93) | - | - | - | 7.285(264) |
Abbreviation: PSA: prostate-specific antigen.
In current study, the reference ranges was lower than in White Americans (5), this must be caused by the differences among races, dietary and environment factors. Interestingly, the reference ranges for PSA level in Chinese men in Taiwan, Shanghai, Shandong and Beijing (current study) are all higher than in Shaanxi, and geographically, the former four locate in the east of China and the latter one locates in the west of China. The environment differences between east and west of China are conspicuous, and this phenomenon may suggest that geography and the environmental factors might play an important role in the development of PCa. Moreover, the dietary among the five regions has some differences, and which will be another factor in the development of PCa. In addition, although China is a country with multiple nationalities, but more than 90 percent of the populations are Han nationality, moreover, because of the dynasties alternation, wars and marriages between different nationalities in the past thousands of years, the ethnical disparity inside Chinese are vague now. So the reference ranges for PSA level caused by different nationalities among the five studies maybe very small and can be ignored. Obviously,the norm of reference ranges founded by Oesterling et al. (5) cannot fit to Chinese men. At the same time, the reference ranges for PSA level for Chinese men in different regions should be different, just like table 3 shows. The goal of screening is to detect clinically significant prostate cancers at a stage when intervention reduces morbidity and mortality. This study shows that over the entire age range, the serum PSA level directly correlated with age (r=0.314,P<0.001), coincidences with prior studies (6–12). But not only age can cause the elevation of PSA level, a serious of conditions can lead the elevation of serum PSA level (1, 2), as we mentioned before, and all these factors should be considered in screening. In addition, the family history of prostate cancer and previous negative biopsies should be involved also. Even so, it is more appropriate to practice the age-related reference ranges rather than use the single cutoff of 4.0ng/ml for men of all age groups. The current study had some limitations. First, the data of current study were not derived from a community-based population, but came from the same hospital. Yet, in this study cohort, all subjects were the healthy people who came to our hospital for routine health examination instead of the patients from the out-patient or in-patient department, and we also excluded the patients with urinary tract infection or obstruction, so the bias were decreased to the minimum. Second, the definition of men without cancer is men with any two negative results of the three examinations (PSA, DRE and TRUS) or had two abnormal results but with a negative biopsy, which may miss some PCa cases, and also the sextant biopsies would miss some PCa cases (23). To avoid such bias, all the subjects should be biopsied and undergo more than 10 to 12 biopsies, which may increase the risks of overdiagnosing and overtreating clinically unimportant disease (3), and it cannot be easily accomplished also.
Conclusions
The aim to determine the reference ranges for age-related PSA levels is to improve the diagnostic accuracy rate in the PCa screening test, i.e., increase the sensitivity for the relatively young men who may be diagnosed earlier and benefit from aggressive treatment intervention, and increase the specificity for the older men to avoid the unnecessarily biopsies which can decrease the patient suffering from the biopsies and alleviate the burden on the patient’s mind and economy.
Our study shows that the serum PSA level increases as man ages and use the age-related range for PSA increases the sensitivity in younger men and decreases the biopsy rate in older patients.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
The study received no financial support. The authors declare that there is no conflict of interest.
References
- 1.Arcangeli CG, Ornstein DK, Keetch KW, Andriole GL (1997). Prostate-specific antigen as a screening test for prostate cancer: The United States experience. Urol Clin North Am, 24(2):299–306. [DOI] [PubMed] [Google Scholar]
- 2.Balk SP, Ko YJ, Bubley GJ (2003). Biology of prostate-specific antigen. J Clin Oncol, 21: 383–391. [DOI] [PubMed] [Google Scholar]
- 3.Mistry K, Cable G (2003). Meta-analysis of prostate- specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract, 16(2):95–101. [DOI] [PubMed] [Google Scholar]
- 4.Harvey P, Basuita A, Endersby D, Curtis B, Iacovidou A, Walker M (2009). A systematic review of the diagnostic accuracy of prostate specific antigen. BMC Urol, 9: 14. doi: 10.1186/1471-2490-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oesterling JE, Jacobsen SJ, Chute CG, et al. (1993). Serum prostate-specific antigen in a community-based population of healthy men, Establishment of age-specific reference ranges. JAMA, 270(7):860–864. [PubMed] [Google Scholar]
- 6.Oesterling JE, Kumamoto Y, Tsukamoto T, et al. Serum prostate-specific antigen in a community-based population of healthy Japanese men: lower values than for similarly aged white men. Br J Urol, 1995; 75: 347–353. [DOI] [PubMed] [Google Scholar]
- 7.Morgan TO, Jacobsen SJ, McCarthy WF, Jacobson DJ, McLeod DG, Moul JW (1996). Age-specific reference ranges for prostate-specific antigen in black men. N Engl J Med, 335: 304–310. [DOI] [PubMed] [Google Scholar]
- 8.Lee SE, Kwak C, Park MS, Lee CH, Kang W, Oh SJ (2000). Ethnic differences in the age-related distribution of serum prostate-specific antigen values: a study in a healthy Korean male population. Urology, 56: 1007–1010. [DOI] [PubMed] [Google Scholar]
- 9.Lin KJ, Pang ST, Chang YH, et al. (2010). Age-related Reference Levels of Serum Prostate-specific Antigen among Taiwanese Men without Clinical Evidence of Prostate Cancer. Chang Gung Med J, 33(2):182–187. [PubMed] [Google Scholar]
- 10.He D, Wang M, Chen X, et al. (2004). Ethnic differences in distribution of serum prostate-specific antigen: a study in a healthy Chinese male population. Urology, 63: 722–726. [DOI] [PubMed] [Google Scholar]
- 11.Liu ZY, Sun YH, Xu CL, Xu G, Zhang LM, Ren SC (2009). Age-specific PSA reference ranges in Chinese men without prostate cancer. Asian J Androl, 11: 100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan XD, Dong ZG, Zhang H, et al. (2011). Distribution of serum prostate-specific antigen in Chinese healthy men: a population-based study. Chin Med J, 124(8):1189–1192. [PubMed] [Google Scholar]
- 13.Weinrich MC, Jacobsen SJ, Weinrich SP, et al. (1998). Reference ranges for serum prostate-specific antigen in black and white men without cancer. Urology, 52: 967–973. [DOI] [PubMed] [Google Scholar]
- 14.Tang P, Du W, Xie K, et al. (2012). Characteristics of baseline PSA and PSA velocity in young men without prostate cancer: racial differences. Prostate, 72: 173–180 [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto T, Ito K, Ohi M et al. (2001). Diagnostic Significance of Digital Rectal Examination and Transrectal Ultrasonography in Men with Prostate-Specific Antigen Levels of 4 ng/ml or Less. Urology, 58(6):994–998. [DOI] [PubMed] [Google Scholar]
- 16.Ablin RJ, Soanes WA, Bronson P, Witebsky E (1970). Precipitating Antigens of the Normal Human Prostate. J Repro Fert, 22: 573–574. [DOI] [PubMed] [Google Scholar]
- 17.Tang P, Chen H, Uhlman M, et al. (2013). A nomogram based on age, prostate-specific antigen level, prostate volume and digital rectal examination for predicting risk of prostate cancer. Asian J Androl, 15: 129–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter HB, Piantadosi S, Isaacs JT (1990). Clinical evidence fr and implications of the multistep development of prostate ancer J Urol, 143;742–746. [DOI] [PubMed] [Google Scholar]
- 19.Thompson IM, Pauler DK, Goodman PJ, e.4). Prevalence of prostate cancer among men with a prostate-specific antigen level of 4.0 ng per milliliter. N Engl J Med, 350: 2239–2246. [DOI] [PubMed] [Google Scholar]
- 20.Wilbur J (2008). Prostate cancer screening: the continuing controversy. Am Fam Physician, 78(12); 1377–1384. [PubMed] [Google Scholar]
- 21.Kakehi Y (2012). Active surveillance as a practical strategy to differentiate lethal and non-lethal prostate cancer subtypes. Asian J Androl, 14, 361–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugimoto M, Kakehi Y (2012). Updated results from the European Randomized Study of Prostate-Specific Antigen (PSA) Screening for Prostate Cancer: are Asian countries encouraged to promote PSA screening? Asian J Androl, 14; 522–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart CS, Leibovich BC, Weaver AL, Lieber MM (2001). Prostate cancer diagnosis using as a turation needle biopsy technique after previous negative sextant biopsies. J Urol, 166(1):86–91, discussion 91–92. [PubMed] [Google Scholar]