Abstract
Objective
to determine the relationship between inflammatory (IL-6 and hsCRP) and coagulation (D-dimer) biomarkers and the presence and type of anemia among HIV+ individuals.
Design
cross-sectional study
Methods
cART-treated adults participating in an international HIV trial with hemoglobin and mean corpuscular volume (MCV) measurements at entry were categorized by presence of anemia (hemoglobin ≤ 14 g/dL in men and ≤ 12 g/dl in women) and, for those with anemia, by type (microcytic [MCV< 80 fL], normocytic [80–100], macrocytic [>100]). We analyzed the association between inflammation (IL-6 and hsCRP) and coagulation (D-dimer) and hemoglobin, controlling for demographics (age, race, and gender), body mass index, HIV plasma RNA levels, CD4+ T cell counts (nadir and baseline), Karnofsky score, previous AIDS diagnosis, hepatitis B/C co-infection and use of zidovudine.
Results
Among 1,410 participants, 313 (22.2%) had anemia. Of these, 4.1%, 27.2% and 68.7% had microcytic, normocytic and macrocytic anemia, respectively. When compared with participants with normal hemoglobin values, those with anemia were more likely to be older, black, male and on zidovudine. They also had lower baseline CD4+ T cell counts and lower Karnofsky scores. Adjusted relative odds of anemia per two fold higher biomarker levels were 1.22 (P= 0.007) for IL-6, 0.99 for hsCRP (P= 0.86) and 1.35 (P< 0.001) for D-dimer. Similar associations were seen in those with normal and high MCV values.
Conclusions
Persistent inflammation and hypercoagulation appear to be associated with anemia. Routine measurements of hemoglobin might provide insights into the inflammatory state of treated HIV infection.
Keywords: HIV, coagulation, D-dimer, CRP, inflammation, IL-6, anemia
Introduction
Anemia is the most common hematological abnormality in HIV disease [1,2] and is associated with increased morbidity and mortality [3–8]. Even minor decreases in hemoglobin levels are clinically relevant: a 1 g/dL decrease in hemoglobin was found to be independently correlated with a 57% higher risk of death in those with HIV [3].
The morphological assessment of red cell size based on mean corpuscular volume (MCV) is helpful for assessment of the etiology of anemia. Whereas microcytic (MCV <80 fL) and macrocytic (MCV >100 fL) anemias are often caused by deficiencies of iron and folic acid/cobalamin, respectively, normocytic anemia (MCV 80–100 fL) is predominately seen in patients with chronic disease [9]. In the general population, there is mounting evidence that enhanced inflammation contributes to the development of normocytic anemia via interleukin-6 (IL-6) dependent pathways [10]. IL-6 induces the formation of hepcidin, a hepatic hormone that interferes with iron absorption and promotes iron uptake by the reticuloendothelial system, ultimately leading to anemia [11,12].
In this study, we set out to determine the relationship between inflammatory biomarkers (IL-6 and hsCRP [high-sensitivity C-reactive protein]) and the presence and type of baseline anemia among HIV+ individuals participating in an international HIV treatment trial. Given the close link between inflammation and coagulation in HIV disease, we also determined the association between D-dimer levels and anemia.
Methods
The design and results of the Subcutaneous Recombinant, Human Interleukin-2 in HIV-Infected Patients with Low CD4+ Counts under Active Antiretroviral Therapy (SILCAAT) trial have been described elsewhere [13]. Briefly, the SILCAAT trial compared IL-2 plus combination antiretroviral therapy (cART) with cART alone in 1,695 individuals with entry CD4+ T cell counts between 50 and 299 cells/mm3. All participants with biomarker levels, hemoglobin and mean corpuscular volume (MCV) measurements at entry were included in this study.
We defined anemia as a hemoglobin ≤14 g/dL in men and ≤ 12 g/dL in women [3,5,8]. Anemia was classified as microcytic, normocytic, or macrocytic based on MCV values below 80, between 80–100, and over 100 fL, respectively. Based on strong associations of IL-6, hsCRP and D-dimer with all-cause mortality [14] and the observation that these biomarkers were elevated in treated HIV+ individuals compared to the general population [15], IL-6, hsCRP and D-dimer were measured on stored plasma at baseline for all consenting SILCAAT participants. IL-6 and hsCRP were measured using the Chemiluminescent Sandwich immunoassay (R&D Systems) and D-dimer was measured using the VIDAS immunoassay (BioMerieux Inc.). Measurements were performed by SAIC-Frederick and lower limits of detection for IL-6, hsCRP and D-dimer were 0.156 pg/mL, 0.078 μg/mL and 0.045 μg/mL, respectively.
Demographics and clinical data from participants with and without anemia and those with normocytic and macrocytic anemia were compared. The association between inflammatory (IL-6 and hsCRP) and coagulation (D-dimer) biomarkers and anemia was assessed by multivariable logistic regression. IL-6, hsCRP and D-dimer levels were log2-transformed because their distributions were right-skewed. With this approach, a one log2 higher level of a biomarker corresponds to a two-fold higher level of the marker. Variables adjusted for were chosen on the basis of their epidemiological importance and biological plausibility and included: demographics (age, race, and gender), body mass index (BMI), HIV plasma RNA levels, CD4+ T cell counts (nadir and baseline), Karnofsky score, previous AIDS diagnosis, hepatitis B (defined as hepatitis B surface antigen [HBsAg] seropositivity by ELISA) and hepatitis C (defined as seropositivity for anti-hepatitis-C antibodies by EIA) co-infection and use of zidovudine. Log2-transformed levels of IL-6, hsCRP and D-dimer were also entered into the logistic regression models and were thus adjusted for simultaneously.
As the best hemoglobin cut-off to define anemia may be debatable, a multivariable linear regression model, which was adjusted for the aforementioned covariates, was deployed to further explore associations between biomarkers and hemoglobin. Continuous hemoglobin levels were modeled as the outcome. Regression coefficients (RCs) with 95% confidence intervals (CI) were calculated to assess the independent contribution of IL-6, hsCRP and D-dimer to the variance of hemoglobin levels.
Because macrocytic anemia can be a manifestation of zidovudine toxicity [16,17], analyses were repeated excluding participants receiving zidovudine. Statistical analyses were performed using SAS (version 9.2)
Results
Baseline hemoglobin, MCV and biomarker levels were available in 1,410/1,695 individuals enrolled in SILCAAT. Among the 1,410 participants in the present study, the median (IQR) age was 41 (36, 48) years; 84.2% were men, 81.1% were Caucasian, 8.3% had hepatitis B and 25.6% had hepatitis C co-infection. Study participants received cART for a median (IQR) of 4.4 (2.0, 7.8) years. The median (IQR) baseline CD4+ T cell count was 202 (149, 255) cells/mm3. Most (81.8%) had a HIV RNA lower than 500 copies/mL plasma. A total of 285 participants lacked biomarker measurements at baseline and were excluded. Data were missing mainly among participants from Argentine, Brazilian and Belgian study sites, wherein blood samples were not routinely collected. Relative to the studied cohort, these subjects had received cART for a shorter time, were younger, were more likely be on zidovudine and less likely to be Caucasian, but had otherwise similar demographic and clinical characteristics (data not shown).
A total of 313 (22.2%) participants had anemia. When compared with participants with normal hemoglobin levels, those with anemia were more likely to be older, black, male and on zidovudine. They also had lower baseline CD4+ T cell counts and lower Karnofsky scores (Table 1). D-dimer, hsCRP and IL-6 levels were significantly higher in those with anemia compared with those without anemia (Table 1).
Table 1.
Demographic and Clinical Variables by Presence of Anemia at Baseline
| Anemia at Baselinea | |||
|---|---|---|---|
| Yes | No | ||
| (N=313) | (N=1097) | ||
| Baseline variable | Median (IQR) | Median (IQR) | P-valueb |
| Hemoglobin (g/dL) | 13.3 (12.4, 13.7) | 15.2 (14.5, 15.9) | 0.001 |
| Age (years) | 43 (38, 51) | 40 (36, 47) | 0.001 |
| Black race (%) | 15.7 | 6.9 | 0.001 |
| Female (%) | 10.2 | 17.4 | 0.002 |
| BMI (Kg/m2) | 23.5 (21.3, 25.8) | 23.9 (21.8, 26.1) | 0.09 |
| HIV RNA ≤ 500 copies/mL (%) | 80.8 | 82.0 | 0.62 |
| Baseline CD4+ T cell count (cells/mm3) | 192 (136, 241) | 205 (153, 257) | 0.001 |
| Nadir CD4+ T cell count (cells/mm3) | 50 (24, 101) | 60 (27, 104) | 0.13 |
| Prior AIDS diagnosis (%) | 33.5 | 30.9 | 0.38 |
| Hepatitis B co-infection (%) | 9.0 | 8.1 | 0.82 |
| Hepatitis C co-infection (%) | 25.1 | 25.7 | 0.62 |
| Karnofsky score | 100 (90, 100) | 100 (100, 100) | 0.11 |
| On PI (%) | 72.8 | 66.3 | 0.03 |
| On NNRTI (%) | 41.5 | 45.6 | 0.20 |
| On zidovudine (%) | 60.1 | 38.3 | 0.001 |
| On stavudine (%) | 58.1 | 38.8 | 0.001 |
| D-dimer (μg/ml) | 0.3 (0.2, 0.5) | 0.2 (0.2, 0.4) | 0.001 |
| IL-6 (pg/ml) | 2.0 (1.3, 3.3) | 1.7 (1.2, 2.6) | 0.001 |
| hsCRP (μg/mL) | 1.7 (0.7, 4.6) | 1.4 (0.6, 3.1) | 0.02 |
| MCV (fl) | 107 (98, 114) | 105 (99, 111) | 0.37 |
| MCV < 80 (%) | 4.2 | 0.4 | 0.001 |
| 80 ≤ MCV ≤ 100 (%) | 27.2 | 31.5 | |
| MCV > 100 (%) | 68.7 | 68.1 | |
BMI: Body mass index; hsCRP: high-sensitivity C-reactive protein; IL-6: interleukin-6; MCV: mean corpuscular volume; NNRTI: non-nucleoside reverse-transcriptase inhibitors; PI: protease inhibitors
Anemia is defined as hemoglobin ≤ 14 mg/dl for men and ≤ 12 mg/dl for women.
P-values are from t-test. For D-dimer, hsCRP and IL-6, log2 transformed values were used.
P-value for comparing MCV categories (< 80, 80–100, > 100) are from chi-squared test.
Of the 313 anemic patients, 13 (4.1%), 85 (27.2%) and 215 (68.7%) had microcytic, normocytic and macrocytic anemia, respectively (supplementary Table 1). As expected, zidovudine use was significantly more common in participants with macrocytic anemia than in those with normocytic anemia (78.6% versus 14.1%, respectively; P< 0.001). Furthermore, those with macrocytic anemia were more likely to be black, male, and to be virologically suppressed than those with normocytic anemia. The levels of IL-6, hsCRP and D-dimer did not differ significantly between participants with normocytic and macrocytic anemia (Supplementary Table 1).
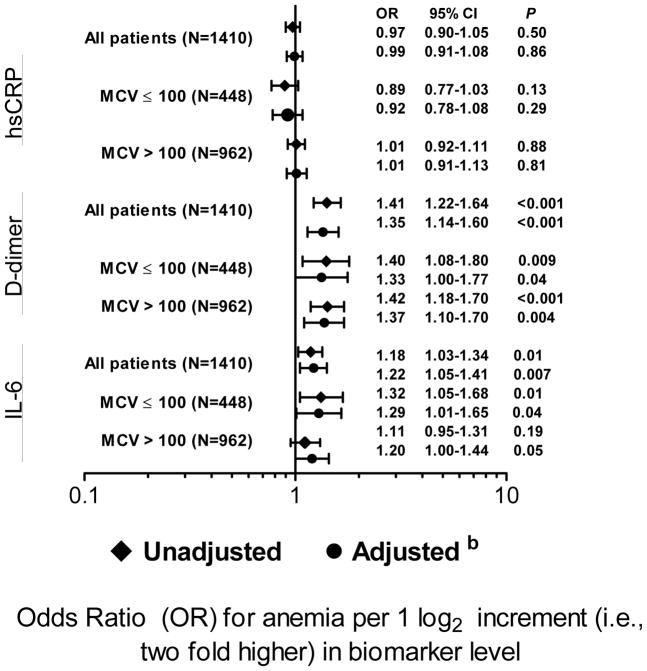
Considering all participants, higher levels of IL-6 and D-dimer, but not hsCRP, were found to be associated with anemia in univariate and adjusted analyses (Figure 1). Adjusted relative odds of anemia per two fold higher biomarker levels were 1.35 (P< 0.001) for D-dimer, 0.99 for hsCRP (P= 0.86) and 1.22 (P= 0.007) for IL-6. Similar associations were seen in those with normal and high MCV values (Figure 1). The percent of patients with anemia increased with higher quintiles of D-dimer and IL-6 levels (supplementary Figures 1 and 2). The presence of anemia did not appear to be linearly associated with activated coagulation, as it was much higher in the top two D-dimer quintiles and similar in the bottom three quintiles (supplementary Figure 1).
Figure 1.
Associations between anemia and biomarkers across MCV levelsa
aThe number of anemic patients with MCV< 80 (N=11) was too low to calculate ORs. Adjusted ORs for patients with normocytic anemia (MCV 80–100) were 1.27 (P= 0.09) for D-dimer and 1.18 (P= 0.17) for IL-6.
bAdjusted for demographics (age, race, gender), BMI, HIV RNA levels, CD4+ cell counts (nadir and baseline), Karnofsky score, previous AIDS diagnosis, hepatitis B and C co-infection and use of zidovudine. Log2-transformed levels of IL-6, hsCRP and D-dimer were adjusted for simultaneously.
In multivariable liner regression models, higher IL-6 and D-dimer levels were found to be independently correlated with lower hemoglobin. Adjusted RCs (95% CI) were −0.13 (−0.21, −0.06), P< 0.001, for IL-6 (i.e., hemoglobin levels were 0.13 g/dl lower on average per two fold higher IL-6 level) and −0.25 (−0.34, −0.16), P< 0.001, for D-dimer. No significant association was observed between hsCRP and hemoglobin (RC [95%CI] = 0.00 [−0.04, 0.04], P= 0.98) in adjusted analyses.
Analyses excluding individuals using zidovudine rendered consistent results (data not shown).
Discussion
In the largest study to date investigating the relationship among inflammation, coagulation and anemia in the setting of antiretroviral-treated HIV infection, we found that, among antiretroviral-treated adults with moderate CD4+ T cells counts, higher levels of both IL-6 and D-dimer are associated with the presence of anemia after adjustment for demographic and clinical variables. The risk for anemia appeared to increase most dramatically in those with the highest levels of IL-6 and D-dimer. This association was not restricted to normocytic anemia, as we initially hypothesized, but was consistently seen across all MCV levels, although it is possible that zidovudine, which was used by the majority of participants with macrocytosis (supplementary Table 1) could have caused macrocytic anemia in patients who otherwise would have had normocytic anemia.
The pathogenesis of anemia in HIV infection is complex and multifactorial. Possible causes include blood loss (i.e., neoplastic disease or gastrointestinal opportunistic infection), bone marrow infiltration or suppression, drug- or infection-related hemolysis, nutritional deficiencies (iron, folic acid or cobalamin), hypogonadism and myelo-suppressive drugs [18]. It is clear that anemia is associated with decreased survival in several other chronic conditions [19, 20]. With HIV infection, however, questions remain as to whether anemia is an epiphenomenon as a consequence of the underlying severe disease or is causally related to morbidity and mortality. Our findings suggest that the previously identified link between anemia and increased morbidity and mortality in HIV+ individuals may reflect in part elevated inflammation and coagulation.
Our data are broadly consistent with the results of the Veterans Aging Cohort Study, which also found higher D-dimer and IL-6 levels in those with lower hemoglobin values [21], although in that study the correlation between hemoglobin and biomarkers was not adjusted for confounding. Furthermore, an earlier study found an association between increasing hemoglobin levels after cART initiation and decreased plasma levels of the inflammatory biomarkers neopterin and TNF-α [22]. Finally, structured cART interruptions resulted in increased levels of inflammatory and coagulation markers [14] and an increased risk of new and worsening anemia [8]. In multivariable models adjusted for all three biomarkers simultaneously, we did not find significant associations between hsCRP and anemia or between hsCRP and hemoglobin levels. Adjustment for a higher number of variables and for other biomarkers had already been shown to significantly reduce associations between hsCRP and clinical outcomes [23]. Among other factors, this may be due to the fact that the hepatic production of hsCRP is determined by IL-6 [24].
Several issues need to be considered when interpreting our results. First, because this is a cross-sectional study we could not establish temporal relationship, and no definitive inference of causality between enhanced inflammation/ coagulation and anemia can be made. Second, we could not adjust for environmental factors or for socio-economic status. Third, we have not explored specific causes of anemia, and information on reticulocyte counts, ferritin, folic acid, cobalamin and hepcidin levels was not routinely collected. Fourth, the number of participants with microcytic anemia was too small to perform statistical analyses. Fifth, we had no data on hepatitis C virus (HCV) RNA and hepatitis B virus (HBV) DNA plasma levels. As participants with detectable or higher HCV-RNA/ HBV- DNA may have had more active hepatic disease, examining differences between them and co-infected participants with undetectable HCV-RNA/HBV-DNA would have been helpful. Finally, SILCAAT recruited participants who did not achieve immunological reconstitution despite cART use for several years. As the bone marrow from immunological nonresponders have impaired clonogenic capability and altered cytokine production [25], which might have contributed to the development of anemia, confirmation of our findings by studies involving cART-treated participants with high CD4+ T counts is warranted. As the occurrence of prior AIDS-defining events was comparable in those with and without anemia, we do not believe that our findings can be explained by concurrent opportunistic infections resulting in anemia and raised levels of inflammatory and coagulation biomarkers.
In conclusion, we have shown that higher levels of IL-6 and D-dimer are associated with anemia in HIV-treated adults. These associations remained strong after adjustment for clinical and demographic covariates and are seen across all MCV levels, a finding that suggests that activation of coagulation and inflammation may contribute to the development of all types of anemia in HIV-infected individuals. Lower hemoglobin levels may provide indirect evidence of activation of coagulation/inflammation among HIV-infected adults. Further studies are needed to explore whether the established link between anemia and increased morbidity and mortality has a causal component related to enhanced inflammation and coagulation.
Supplementary Material
Percent with anemia by D-dimer quintile
Supplementary Figure 2. Percent with anemia by IL-6 quintile
Supplementary Table 1. Demographic and Clinical Variables by Type of Anemia at Baseline (N= 313)
BMI: Body mass index; hsCRP: high-sensitivity C-reactive protein; IL-6: interleukin-6; MCV: mean corpuscular volume; NNRTI: non-nucleoside reverse-transcriptase inhibitors; PI: protease inhibitors aP-values are from t-test the normocytic and macrocytic groups. For D-dimer, hsCRP and IL-6, log2 transformed values were used.
Acknowledgments
Research Support: SILCAAT was supported by grants from Chiron and Novartis. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of this manuscript.
We would like to acknowledge the SILCAAT participants and investigators (see N Engl J Med 2009; 361:1548–1559 for the complete list of investigators).
JVB has received research support from Gilead and ViiV. SGD is supported by the NIH (K24AI069994).
Footnotes
AHB, JDN, JDL and SGD contributed to the conception and design of the study. GC and JDN performed all statistical analyses. AHB drafted the manuscript. All authors contributed to data analysis and interpretation and reviewed the manuscript.
This study was presented in part at the 20th Conference on Retroviruses and Opportunistic Diseases, Atlanta, 3th–6th March, 2013 and at the 30th Annual Meeting of the Nordic Society of Clinical Microbiology and Infectious Diseases, Aarhus, Denmark, 5th–8th September, 2013
Conflicts of Interest:
The authors declare no conflicts of interest.
References
- 1.Sullivan PS, Hanson DL, Chu SY, Jones JL, Ward JW. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood. 1998;91:301–308. [PubMed] [Google Scholar]
- 2.Belperio PS, Rhew DC. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. Am J Med. 2004;116:27S–43S. doi: 10.1016/j.amjmed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Mocroft A, Kirk O, Barton SE, Dietrich M, Proenca R, Colebunders R, et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. AIDS. 1999;13:943–950. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- 4.Lundgren JD, Mocroft A, Gatell JM, Ledergerber B, D'Arminio Monforte A, Hermans P, et al. A clinically prognostic scoring system for patients receiving highly active antiretroviral therapy: results from the EuroSIDA study. J Infect Dis. 2002;185:178–187. doi: 10.1086/338267. [DOI] [PubMed] [Google Scholar]
- 5.Berhane K, Karim R, Cohen MH, Masri-Lavine L, Young M, Anastos K, et al. Impact of highly active antiretroviral therapy on anemia and relationship between anemia and surviv al in a large cohort of HIV-infected women: Women's Interagency HIV Study. J Acquir Immune Defic Syndr. 2004;37:1245–1252. doi: 10.1097/01.qai.0000134759.01684.27. [DOI] [PubMed] [Google Scholar]
- 6.Harris RJ, Sterne JA, Abgrall S, Dabis F, Reiss P, Saag M, et al. Prognostic importance of anaemia in HIV type-1-infected patients starting antiretroviral therapy: collaborative analysis of prospective cohort studies. Antivir Ther. 2008;13:959–967. [PMC free article] [PubMed] [Google Scholar]
- 7.Mocroft A, Reiss P, Gasiorowski J, Ledergerber B, Kowalska J, Chiesi A, et al. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. J Acquir Immune Defic Syndr. 2010;55:262–270. doi: 10.1097/QAI.0b013e3181e9be6b. [DOI] [PubMed] [Google Scholar]
- 8.Mocroft A, Lifson AR, Touloumi G, Neuhaus J, Fox Z, Palfreeman A, et al. Haemoglobin and anaemia in the SMART study. Antivir Ther. 2011;16:329–337. doi: 10.3851/IMP1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 10.Raj DS. Role of interleukin-6 in the anemia of chronic disease. Semin Arthritis Rheum. 2009;38:382–388. doi: 10.1016/j.semarthrit.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormonehepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laftah AH, Ramesh B, Simpson RJ, Solanky N, Bahram S, Schümann K, et al. Effect of hepcidin on intestinal iron absorption in mice. Blood. 2004;103:3940–3944. doi: 10.1182/blood-2003-03-0953. [DOI] [PubMed] [Google Scholar]
- 13.Abrams D, Lévy Y, Losso MH, Babiker A, Collins G, Cooper DA, et al. Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 361:1548–1559. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuhaus J, Jacobs DR, Jr, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richman DD, Fischl MA, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, et al. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987;317:192–197. doi: 10.1056/NEJM198707233170402. [DOI] [PubMed] [Google Scholar]
- 17.Romanelli F, Empey K, Pomeroy C. Macrocytosis as an indicator of medication (zidovudine) adherence in patients with HIV infection. AIDS Patient Care STDS. 2002;16:405–411. doi: 10.1089/108729102760330245. [DOI] [PubMed] [Google Scholar]
- 18.Volberding PA, Levine AM, Dieterich D, Mildvan D, Mitsuyasu R, Saag M, et al. Anemia in HIV infection: clinical impact and evidence-based management strategies. Clin Infect Dis. 2004;38:1454–1463. doi: 10.1086/383031. [DOI] [PubMed] [Google Scholar]
- 19.Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91:2214–2221. [PubMed] [Google Scholar]
- 20.Nissenson AR, Goodnough LT, Dubois RW. Anemia Not Just an Innocent Bystander? Arch Intern Med. 2003;163:1400–1404. doi: 10.1001/archinte.163.12.1400. [DOI] [PubMed] [Google Scholar]
- 21.Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54:984–994. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarcletti M, Quirchmair G, Weiss G, Fuchs D, Zangerle R. Increase of haemoglobin levels by anti-retroviral therapy is associated with a decrease in immune activation. Eur J Haematol. 2003;70:17–25. doi: 10.1034/j.1600-0609.2003.02810.x. [DOI] [PubMed] [Google Scholar]
- 23.Hemingway H, Philipson P, Chen R, Fitzpatrick NK, Damant J, et al. Evaluating the quality of research into a single prognostic biomarker: a systematic review and meta-analysis of 83 studies of C-reactive protein in stable coronary artery disease. PLoS Med. 2010;7:e1000286. doi: 10.1371/journal.pmed.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isgrò A, Leti W, De Santis W, Marziali M, Esposito A, Fimiani C, et al. Altered clonogenic capability and stromal cell function characterize bone marrow of HIV-infected subjects with low CD4+ T cell counts despite viral suppression during HAART. Clin Infect Dis. 2008;46:1902–1910. doi: 10.1086/588480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Percent with anemia by D-dimer quintile
Supplementary Figure 2. Percent with anemia by IL-6 quintile
Supplementary Table 1. Demographic and Clinical Variables by Type of Anemia at Baseline (N= 313)
BMI: Body mass index; hsCRP: high-sensitivity C-reactive protein; IL-6: interleukin-6; MCV: mean corpuscular volume; NNRTI: non-nucleoside reverse-transcriptase inhibitors; PI: protease inhibitors aP-values are from t-test the normocytic and macrocytic groups. For D-dimer, hsCRP and IL-6, log2 transformed values were used.