Abstract
Background
Studies of patients presenting for catheter ablation suggest that premature ventricular contractions (PVCs) are a modifiable risk factor for congestive heart failure (CHF). The relationship between PVC frequency, incident CHF, and mortality in the general population remains unknown.
Objectives
The goal of this study was to determine whether PVC frequency ascertained using a 24-h Holter monitor is a predictor of LVEF decline, incident CHF, and death in a population-based cohort.
Methods
We studied the 1,139 Cardiovascular Health Study (CHS) participants randomly assigned to 24-h ambulatory electrocardiography (Holter) monitoring with a normal left ventricular ejection fraction (LVEF) and no history of CHF. PVC frequency was quantified using Holter studies, and LVEF was measured from baseline and 5-year echocardiograms. Participants were followed for incident CHF and death.
Results
Those in the upper quartile versus the lowest quartile of PVC frequency had a multivariable adjusted 3-fold greater odds of a 5-year LVEF decline (OR: 3.10, 95% CI: 1.42 to 6.77, p = 0.005), and, over a median follow-up >13 years, a 48% increased risk of incident CHF (HR: 1.48, 95% CI: 1.08 to 2.04, p = 0.02), and a 31% increased risk of death (HR: 1.31, 95% CI: 1.06 to 1.63, p = 0.01). Similar statistically significant results were observed for PVCs analyzed as a continuous variable. The specificity for the 15-year risk of CHF exceeded 90% when PVCs comprised at least 0.7% of ventricular beats. The population-level risk for incident CHF attributed to PVCs was 8.1% (95% CI: 1.2 to14.9%).
Conclusions
In a population-based sample, a higher frequency of PVCs was associated with LVEF decline, increased incident CHF, and increased mortality. Given the capacity to prevent PVCs through medical or ablation therapy, PVCs may represent a modifiable risk factor for CHF and death.
Keywords: Arrhythmia, Mortality, Premature Ventricular Contractions
The effect of PVC frequency on left ventricular (LV) systolic function, incident congestive heart failure (CHF), or mortality in the general population remains unknown. Because the diurnal distribution of ectopic beats can vary, 24-h Holter monitoring is essential to accurately assess periodic events that contribute to the true PVC burden (1,2). Given the pervasiveness of PVCs in the general population and the number of “idiopathic” CHF patients that contribute to significant healthcare resource utilization (3,4), it is important to understand the association between PVC frequency and myocardial function in the general population. Therefore, we sought to investigate PVC frequency ascertained using a 24-h Holter monitor as a predictor of a decline in the left ventricular ejection fraction (LVEF), incident CHF, and death in a population-based cohort study.
Methods
Study Design
The Cardiovascular Health Study (CHS) is a prospective, community-based cohort study sponsored by the National Heart, Lung, and Blood Institute. Details regarding eligibility, enrollment, and follow-up have been previously published (5–7). Briefly, 5,201 individuals 65 years of age or older were recruited between 1989 and 1990 from a random sample of Medicare beneficiaries by 4 academic centers (Johns Hopkins University, Wake Forest University, University of Pittsburgh, and University of California, Davis). An additional 687 black patients were recruited between 1992 and 1993. All participants underwent a medical history, physical exam, laboratory testing, and 12-lead electrocardiogram (ECG) at enrollment. Participants were then followed with annual clinic visits and semiannual telephone contact for 10 years, with telephone contact continued every 6 months thereafter.
Study Cohort
Our analysis was restricted to the subset of 1,429 individuals that were randomly assigned to 24-h ambulatory ECG (Holter) monitoring during their initial assessment and who were part of the initial recruitment cohort (those recruited between 1989 and 1990). Patients without a normal LVEF, as determined by the baseline echocardiogram, or with prevalent CHF were excluded from the study cohort.
Holter Assessment
Holter data were analyzed at the Washington University School of Medicine Heart Rate Variability Laboratory using a MARS 8000 Holter scanner (GE Medical Systems, Milwaukee, Wisconsin) and all PVC, atrial fibrillation (AF), and ventricular tachycardia (VT) episodes were identified. The results were then manually reviewed to ensure accuracy. The percentage of PVCs was determined by dividing the total number of ventricular ectopic beats by the total number of beats recorded during Holter monitoring.
Echocardiographic Evaluation
The echocardiographic assessment of participants in the CHS has been described previously (8). In brief, 2-dimensional (2D) echocardiography, 2D targeted M-mode, and Doppler imaging were performed on each participant at baseline using Toshiba SSH-160A echocardiogram machines (Toshiba Medical Systems, Tustin, California), equipped with 2.5 MHz and 3.75 MHz transducers. Imaging was performed at the highest MHz that provided adequate tissue penetration for 2D imaging. Images were recorded and stored on Super-VHS videotape at the recruitment sites and then transferred to the University of California, Irvine for central interpretation. LV function was qualitatively assessed from the 2D imaging views where at least 80% of the myocardium was visualized. Function was subjectively categorized as normal, borderline, or abnormal, with 94% inter-reader agreement and 98% intrareader agreement of paired studies (9). LV end-diastolic diameter and LV mass were derived from M-mode measurements, using leading-edge-to-leading-edge methodologies as per American Society of Echocardiography standards (10). LV mass was calculated using the Devereux formula and indexed by dividing by the body surface area. A second echocardiogram was performed 5 years after enrollment (8).
Covariate Ascertainment
Self-identified race was categorized as white, black, Asian/Pacific Islander, and other. Self-identified sex was classified as male or female. Hypertension was defined as either a reported history of physician-diagnosed hypertension along with the use of antihypertensive medications or a baseline study visit systolic blood pressure ≥140 mm Hg or diastolic pressure ≥90 mm Hg. Diabetes was defined as a reported use of an antihyperglycemic medication at baseline or a fasting glucose level ≥126 mmol/liter. CHF and myocardial infarction (MI) were identified by participant self-report and were confirmed by medical record verification (7). Coronary artery disease (CAD) was defined as angina, previous MI, previous coronary artery bypass graft surgery, or previous angioplasty. Baseline beta-blocker use was ascertained using an in-home medication inventory, and was defined as a current prescription filled by a pharmacist or physician, and taken by the patient in the previous 2 weeks (11).
Event Ascertainment
Medical records were obtained for all hospitalizations after study enrollment. Potential incident CHF and MI events, hospitalized and outpatient, were investigated in detail on the basis of initial identification through International Classification of Diseases (ICD) diagnostic codes, or mention of an endpoint on the hospital face sheet, discharge summary, or outpatient procedure report. Adjudication of incident CHF and MI events was performed by the CHS Cardiac Events Subcommittee (6). For each incident CHF event, all medical records 2 weeks prior to and 30 days after the event were reviewed for any LVEF assessments. The CHF event was considered to be associated with systolic dysfunction if the LVEF assessment closest in time was either documented as qualitatively below normal or if the quantified LVEF was <45%. Death was ascertained by reviewing medical records, death certificates, autopsy exams, coroner’s reports, obituaries, and search of the National Death Index (5).
Statistical Analysis
Continuous variables with a normal distribution are presented as means ± standard deviation (SD) and were compared using Student t tests. Non-normally distributed continuous variables are presented as medians with interquartile ranges (IQRs) and were compared using Kruskal-Wallis tests. The association between categorical variables was determined using chi-square tests.
Continuous echocardiographic variables were analyzed using linear regression, both before and after adjusting for confounders identified a priori. A reduction in LVEF from baseline to 5 years post-enrollment was dichotomized into the presence or absence of any LVEF reduction (any change from normal to borderline or reduced function). Both unadjusted and adjusted analyses were performed using logistic regression.
Incident CHF and mortality outcomes were analyzed using unadjusted and adjusted Cox proportional hazard models. The baseline covariates used in the adjusted analyses were age, sex, race, body mass index (BMI), and a history of hypertension, diabetes, CAD, beta-blocker use, Holter-based AF, and number of Holter-based VT episodes. As all patients with VT also had PVCs, the number of VT episodes was used to better capture the effect of VT burden. A separate multivariable Cox Proportional Hazards model adjusting for time-updated MI was performed. We used log base 2 and cubic spline transformations of the PVC count to meet model linearity assumptions. As the spline transformed PVC counts did not significantly change the log-likelihood, all analyses except for the percent attributable risk assessment were performed using the log transformations. Given the observation that the mean LV mass index was larger in participants with PVC percentages above (vs. below) the median, an additional analysis adjusting for LV mass index was performed. For mediation analyses, we calculated the “proportion of effect explained” as the percentage reduction in the adjusted regression coefficient after additional adjustment for the candidate mediator, with a 95% bias-corrected percentile bootstrap confidence interval (CI).
Cox proportional hazards models incorporating log-transformed percent PVC counts were used to estimate the 15-year CHF risk. Three models were examined: the first using PVC percentage alone; the second using baseline covariates as previously described; and the third using both the baseline covariates and PVC percentage. Predicted risks from the first model were plotted against the percent PVC count, and sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for various percent PVC thresholds. Discrimination of each of the 3 models was assessed using the cross-validated C-index; bias-corrected 95% bootstrap percentile CIs were used to assess differences in the C-index between the 3 models.
The population-attributable risk for incident CHF was calculated for the following covariates using a counterfactual approach (12): PVCs, BMI, hypertension, age, and CAD. Population-attributable risk was estimated by calculating the ratio of the total excess risk associated with the exposure of interest to the total observed risk. The percent PVCs were modeled using cubic splines, with a reference level of the lower quartile of percent PVCs within the cohort. For CAD and hypertension, the reference levels were the absence of the respective disease. The reference BMI was 23 kg/m2 (based on the center of normal), and the reference age was 70 years (the mean of the cohort). CIs for population-attributable risk estimates were obtained using bootstrap resampling with 500 repetitions.
Data were analyzed using Stata 12 (StataCorp, College Station, Texas). A 2-tailed p < 0.05 was considered statistically significant.
Results
At baseline, 1,139 CHS participants had Holter monitoring, echocardiograms demonstrating normal LV systolic function, and no prevalent CHF. Over a median 22.2 h duration (IQR: 21.7 to 22.8 h) of Holter-monitoring, PVCs represented a median 0.011% of all heartbeats (IQR: 0.002 to 0.123%). The maximum recorded PVC percentage was 17.7%. The baseline characteristics of these participants stratified relative to the median PVC burden are shown in Table 1. Other Holter monitor findings included 3 participants (0.26%) with episodes of AF and 63 participants (5.5%) with runs of nonsustained VT (range 0 to 106 episodes). No episodes of sustained VT were noted and all participants with VT also had PVCs.
Table 1.
Baseline Characteristics of Participants Stratified by Median Percent PVCs
| Variable | Below or Equal to the Median of Percent PVCs (n = 587) |
Above the Median of Percent PVCs (n = 552) |
p Value |
|---|---|---|---|
| Age in yrs (IQR) | 70 (68–74) | 71 (68–74) | 0.25 |
| Female (%) | 374 (66%) | 283 (50%) | <0.001 |
| Race (%) | |||
| White | 548 (96%) | 535 (94%) | 0.24 |
| Black | 20 (4%) | 31 (5%) | |
| Asian/Pacific Islander | 1 (0.2%) | 0 (0%) | |
| Other | 1 (0.2%) | 3 (0.6%) | |
| Mean BMI (kg/m2) ± SD | 26.6 ± 4.3 | 26.6 ± 3.9 | 0.85 |
| Hypertension (%) | 298 (52%) | 316 (56%) | 0.27 |
| Coronary Artery Disease (%) | 80 (14%) | 103 (18%) | 0.06 |
| Diabetes (%) | 75 (13%) | 89 (16%) | 0.21 |
| Mean LV Diastolic Diameter Index* (cm/m2) ± SD | 2.77 ± 0.3 | 2.82 ± 0.3 | 0.07 |
| Mean LV Mass Index* (g/m2) ± SD | 80.0 ± 19.6 | 84.7 ± 23.6 | 0.02 |
Index measurements are divided by body surface area
BMI = body mass index; IQR = interquartile range; LV = left ventricular; PVC = premature ventricular contraction.
Echocardiographic Changes
Eight hundred and forty-two individuals (74%) underwent the year 5 follow-up echocardiogram. Those that did not undergo the year 5 echocardiogram exhibited several statistically significantly differences (Online Table 1).
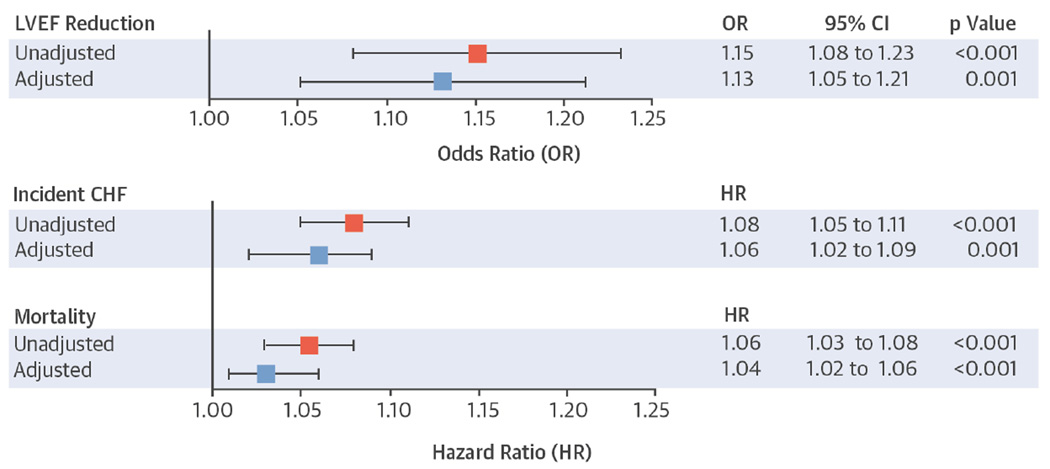
Over 5 years, each doubling of the baseline PVC percentage was associated with a statistically significantly greater odds of a decrease in the LVEF in both the unadjusted and adjusted analyses (Central Illustration). Similar statistically significant associations were observed when PVC percentages were analyzed as quartiles (Table 2). There was also a graded relationship between the baseline mean PVC count in those who demonstrated no change in LVEF (0.002% PVCs), a change from normal to borderline LVEF (0.03% PVCs), and a change from normal to abnormal LVEF (0.3% PVCs), p < 0.001. There were no significant associations between log-transformed PVC percentages or PVC percentage quartiles with either the 5-year change in the diastolic diameter index or LV mass index.
Central Illustration. Associations between Baseline Percent PVCs and 5-year Reduction in LVEF, Incident CHF, and Mortality.
Squares represent unadjusted (red) and adjusted (blue) ORs for log base 2-transformed percent PVCs as a predictor of any reduction in qualitative LVEF between the baseline and 5-year echocardiograms or HRs for incident CHF and mortality. Multivariable models included adjustment for age, sex, race, body mass index, and a history of hypertension, diabetes, coronary artery disease, beta-blocker use, Holter-based AF, and number of Holter-based ventricular tachycardia episodes. Error bars indicate 95% CIs. Hazard ratios express the increase in risk per doubling of the percent PVCs. CHF = congestive heart failure; CI = confidence interval; HR = hazard ratio; LVEF = left ventricular ejection fraction; OR = odds ratio; PVC = premature ventricular contraction.
Table 2.
Association Between Quartile of Percent PVC Count, LVEF Reduction, Incident CHF, and Mortality
| Variable | Unadjusted | 95% CI | p Value | Adjusted* | 95% CI | p Value |
|---|---|---|---|---|---|---|
| LVEF Reduction | ||||||
| Quartile 1 | Reference | Reference | ||||
| Quartile 2 | OR: 1.46 | 0.64 to 3.33 | 0.37 | OR: 1.18 | 0.50 to 2.77 | 0.71 |
| Quartile 3 | OR: 1.86 | 0.85 to 4.10 | 0.12 | OR: 1.48 | 0.64 to 3.39 | 0.41 |
| Quartile 4 | OR: 4.02 | 1.91 to 8.45 | < 0.001 | OR: 3.10 | 1.42 to 6.77 | 0.005 |
| Test of Trend | < 0.001 | 0.004 | ||||
| Incident CHF | ||||||
| Quartile 1 | Reference | Reference | ||||
| Quartile 2 | HR: 1.04 | 0.74 to 1.45 | 0.83 | HR: 0.89 | 0.63 to 1.25 | 0.50 |
| Quartile 3 | HR: 1.16 | 0.83 to 1.60 | 0.39 | HR: 0.89 | 0.64 to 1.26 | 0.52 |
| Quartile 4 | HR: 1.77 | 1.30 to 2.41 | <0.001 | HR: 1.48 | 1.08 to 2.04 | 0.02 |
| Test of Trend | <0.001 | 0.02 | ||||
| Mortality | ||||||
| Quartile 1 | Reference | Reference | ||||
| Quartile 2 | OR: 1.25 | 1.01 to 1.55 | 0.04 | HR 1.01 | 0.81 to 1.26 | 0.95 |
| Quartile 3 | OR: 1.38 | 1.12 to 1.71 | 0.003 | HR 1.12 | 0.90 to 1.40 | 0.29 |
| Quartile 4 | OR: 1.60 | 1.30 to 1.98 | <0.001 | HR 1.31 | 1.06 to 1.63 | 0.01 |
| Test of Trend | <0.001 | 0.007 | ||||
Quartiles 1 through 4 represent PVC burdens of 0%–0.002%, 0.002%–0.011%, 0.011%–0.123%, and 0.123%–17.7%, respectively.
Adjusted for age, sex, race, BMI, and history of hypertension, diabetes, coronary artery disease, beta-blocker use, Holter-based atrial fibrillation, and number of Holter-based ventricular tachycardia episodes.
CHF = congestive heart failure; CI = confidence interval; HR = hazard ratio; LVEF = left ventricular ejection fraction; OR = odds ratio. Other abbreviations as in Table 1.
Incident CHF
Over a median follow-up of 13.7 years (IQR: 8.0 to 18.2 years), 308 participants (27%) developed incident CHF. The baseline characteristics of those that did and did not develop incident CHF are shown in Online Table 2.
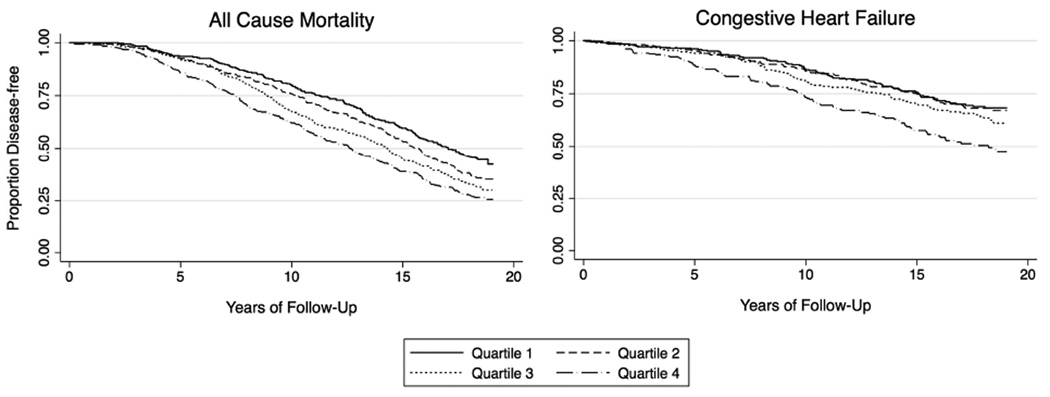
PVC percentage was associated with incident CHF in both unadjusted and multivariable-adjusted analyses (Central Illustration). Adjustment for time-updated incident MI did not substantively change these results. Similarly, after adjustment for LV mass index (restricted to the 778 participants with Holter data and this measurement available from baseline echocardiographic data), no meaningful differences were observed. PVC percentage analyzed as quartiles was also associated with incident CHF (Table 2, Figure 1). Both Holter-identified AF and VT were independently associated with incident CHF before and after multivariate adjustment (Online Table 3).
Figure 1. Rates of CHF and All-Cause Mortality by PVC Quartiles.
Unadjusted Kaplan-Meier estimates depicting incident congestive heart failure and mortaility, as stratified by percent PVC count quartiles. Quartiles 1 through 4 represent PVC burdens of 0% to 0.002%, 0.002% to 0.011%, 0.011% to 0.123%, and 0.123% to 17.7%, respectively. CHF = congestive heart failure; PVC = premature ventricular contraction.
Sixty-nine (22%) of the incident CHF events were associated with evidence of systolic dysfunction, 110 (36%) exhibited preserved systolic function, and 129 (42%) had insufficient medical records to assess concomitant systolic function. Excluding participants without known systolic function at the time of their incident CHF event, PVC percentage was associated with incident heart failure with systolic dysfunction in both the unadjusted (HR: 1.10, 95% CI: 1.03 to 1.17, p = 0.003) and adjusted (HR: 1.08, 95% CI: 1.01 to 1.15, p = 0.02) analyses. In contrast, PVC percentage failed to predict incident heart failure with preserved systolic function (unadjusted HR: 1.04, 95% CI: 0.99 to 1.10, p = 0.118; adjusted HR: 1.01, 95% CI: 0.96 to 1.07, p = 0.62).
Mortality
Over a median follow-up of 15.2 years (IQR: 9.6 to 18.4 years), a total of 729 deaths (64%) were identified. Whether analyzed as a continuous variable (Central Illustration) or as quartiles (Table 2, Figure 1), a higher PVC percentage was associated with increased mortality in both unadjusted and multivariable adjusted analyses. In order to assess how much incident CHF might explain the PVC-mortality association, a mediation analysis adjusting for incident CHF attenuated the relationship between percentage PVCs and mortality by 26.8% (percent treatment explained 95% CI: 0.9 to 67.9%).
Test Characteristics
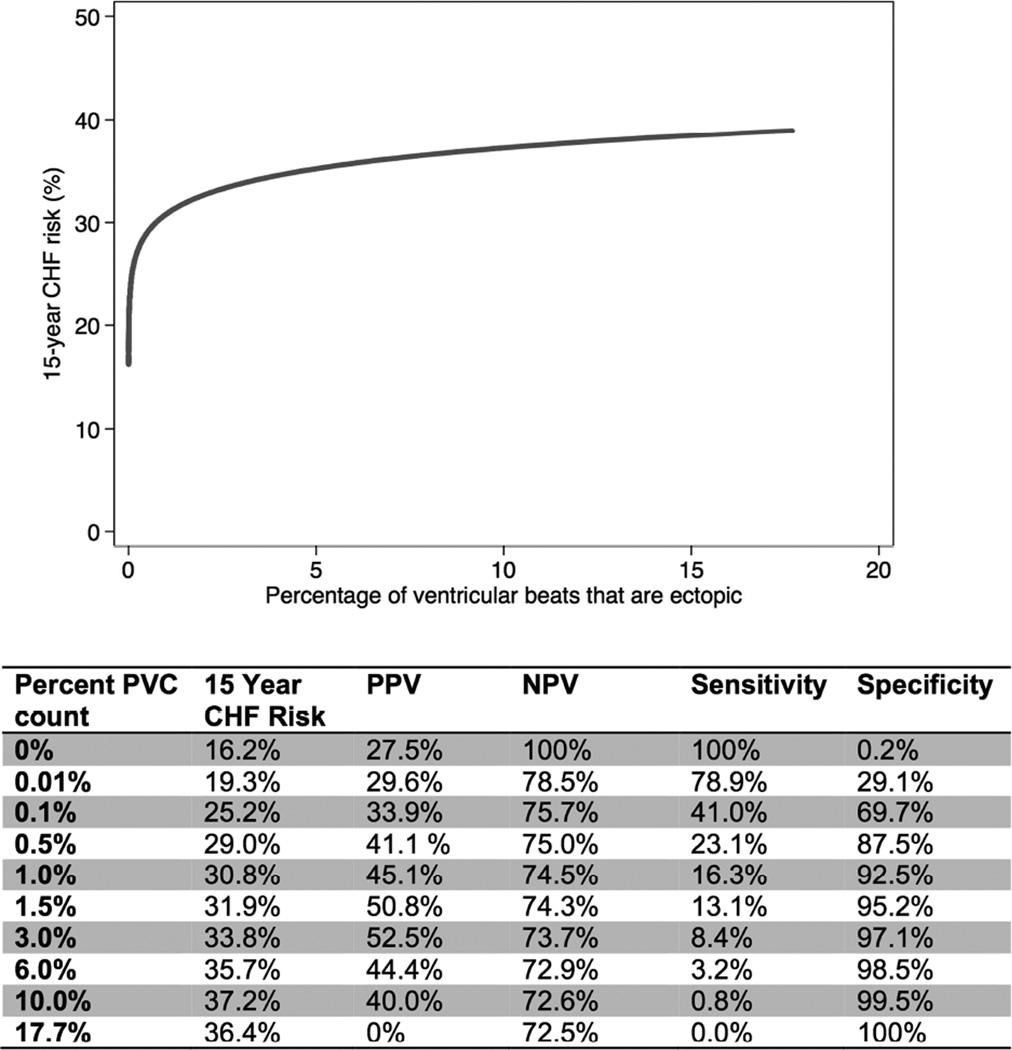
In the CHF prediction model using PVC percentage alone, the 15-year risk for CHF rose abruptly as the percentage of PVCs increased between 0% and 0.5%, with tapering of the risk curve for PVC percentages >10% (Figure 2). The specificity for CHF prediction exceeded 90% when PVCs comprised at least 0.7% of total ventricular beats. The positive predictive value for the 15-year risk of incident CHF was >50% for PVC percentages between 1.24% and 3.55%. Cross-validated C-indexes were 0.566 for the Cox model using PVC percentage alone, 0.587 for the model using baseline covariates alone, and 0.594 for the model using both. The 95% bootstrap confidence interval for the increase in the C-index achieved by adding PVCs to the model using covariates only included 0 (95% CI: −0.37 to 0.50).
Figure 2. Test Characteristics of PVCs for 15-year CHF Risk.
The predicted 15-year risk for CHF (using the log base 2-transformed PVC model) is plotted against the percentage of PVCs. The PPV, NPV, sensitivity and specificity for the diagnosis of CHF at 15 years for an individual participant are listed for various unadjusted PVC cutoff values. NPV = negative predictive value; PPV = positive predictive value. Other abbreviations as in Figure 1.
Population-Attributable Risk
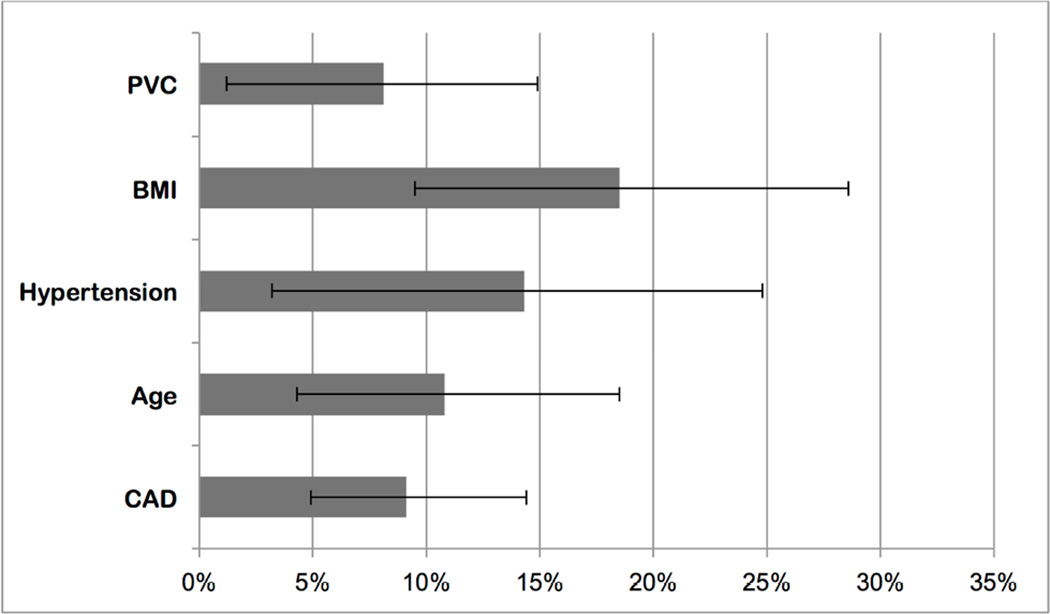
The risk of incident CHF that could be attributed to PVCs was 8.1% (95% CI: 1.2 to 14.9%) when compared to a reference population with percent PVCs equal to the lower quartile of the cohort (Figure 3). This was of a similar magnitude to the risk of CHF that could be attributed to BMI, hypertension, age, and CAD.
Figure 3. Population-Attributable Risk of PVCs for Incident CHF.
Each bar represents the population-attributable risk for the listed covariates, with error bars denoting 95% CIs.
BMI = body mass index (weight in kg divided by the square of the height in meters); CAD = coronary artery disease. Other abbreviations as in Figure 1.
Discussion
In a population-based cohort of more than 1,100 participants over the age of 65, a higher frequency of PVCs was associated with an LVEF reduction, incident CHF, and increased mortality both before and after multivariable adjustment. The increase in mortality appeared to be partly explained by incident CHF. Percent PVCs were highly specific for the 15-year risk of CHF and yielded a clinically meaningful PPV. The percent attributable risk of PVCs for incident CHF was comparable to other CHF risk factors, such as BMI, hypertension, age, and CAD. These findings suggest that PVCs may exhibit an important association with heart failure in the general population and that future research investigating the potential benefits of PVC modification or eradication in select individuals may be warranted.
CHF currently affects more than 5 million Americans, and its prevalence is expected to increase by 25% within the next 15 years (13). Up to 50% of CHF cases have no known etiology (4). Among the established risk factors for CHF, such as obesity, diabetes, hypertension, and CAD (14), few, if any, are readily reversible.
Recent studies arising from the electrophysiology laboratory have demonstrated that systolic dysfunction may improve and even normalize after successful ablation of high-burden PVCs (generally >10% PVCs) (15–19). These data suggest that PVCs could be a significant and modifiable risk factor for incident CHF. However, because these observations are limited to patients already diagnosed with heart failure, these studies are unable to provide the denominator of individuals at risk—to do this, we require a population-based study with long-term follow-up, as is provided by the CHS.
No previous population-based study has quantified the relationship between PVC frequency and heart failure. Using 24-h Holter monitoring (considered the reference standard for PVC quantification) (1), echocardiography, and incident CHF and mortality data, we provide the first evidence that PVC percentage predicts new systolic dysfunction, as well as clinically-diagnosed CHF and overall mortality. These data are also the first to quantify those relationships.
These results are supported by data from other population-based studies. The ARIC (Atherosclerosis Risk in Communities) Study demonstrated that the dichotomized presence (vs. absence) of PVCs ascertained from a 2-min recording increased the risk for incident CHF, although, unlike the present study, 24-h PVC frequency and change in systolic function data were not available (20). Other studies have demonstrated an association of mortality with PVCs, either in combined endpoints or specific subgroups. In the Framingham Heart Study, PVCs detected using a 1-h recording were associated with increased mortality only in men without CAD (21). The Copenhagen Holter Study found an association between >30 PVCs/h and a combined endpoint of death or acute MI (22). Among 456 participants in the Men Born in 1914 Study, 24-h Holter monitor-detected PVCs predicted death due specifically to ischemic heart disease (23). Our positive findings regarding overall mortality as a stand-alone endpoint may be due to this being the largest study to utilize 24-h Holter monitoring and with the longest follow-up. Although the pathophysiology underlying the relationship between a greater frequency of PVCs and mortality remains unknown, our mediation analysis suggest that incident CHF may explain at least part of that association.
Consistent with the serial echocardiographic findings on all participants, the CHF events associated with increasing PVCs appeared to be primarily due to systolic dysfunction. The mechanism(s) by which frequent PVCs may lead to systolic dysfunction remains unknown—however, the available evidence favors adverse ventricular remodeling that occurs due to repeated dyssynchrony (24,25) and impaired calcium handling (26), rather than a tachycardia-induced cardiomyopathy (27).
In order to characterize the potential clinical interpretation of these findings, we constructed test characteristics for varying PVC percentages using the 15-year risk of CHF as the reference outcome. Sensitivity was low, reflecting the fact that few patients who develop CHF have high PVC burdens. However, the specificity was quite high, exceeding 90% for PVCs representing as little as 0.7% of total ventricular beats and rising to >99% for PVC percentages ≥10%. More clinically relevant is the PPV: among those with 3% PVCs, more than half would go on to develop heart failure. In light of both the high specificity and PPV, patients with high PVC burdens may warrant special attention. These data also reveal a heightened CHF risk at a far lower PVC percentage than previously recognized (17,19). However, cross-validated C-indexes showed that the discrimination of all 3 of the prediction models was poor to moderate, at best. Furthermore, adding PVC percentage to the prediction model for CHF using clinically available variables did not achieve statistically significant increases in discrimination, although substantial increases of up to 5% could not be excluded. Further research may be indicated to investigate the prognostic utility of adding PVC burden to other established CHF prediction models.
The percentage of incident CHF that could be attributed to increased PVCs was similar to that of other well-established risk factors (13). Unlike age, which is immutable, or BMI, hypertension, and CAD, which may have strong inherited components and may be difficult to modify, PVCs can be readily eradicated with radiofrequency ablation. Therefore, it is interesting to speculate whether prophylactic treatment for PVCs, with either medicines or ablation, might reduce the burden of heart failure in the population. However, future study is required before entertaining this in clinical practice. For example, it is possible that some proportion of this population-based cohort simply manifested PVCs as the first evidence (or as an epiphenomenon) of a cardiomyopathy destined to result in CHF. Because some patients with frequent PVCs may never develop CHF, future studies would need to more fully elucidate other covariates that might identify ideal candidates for treatment and would need to determine the relative risks and benefits of any prophylactic therapies to be considered.
Limitations of this study should be acknowledged. Our participants were predominately elderly and white, which may constrain the generalizability of our results. We did not have data on particular PVC coupling intervals, PVC QRS durations, or PVC morphologies. We also did not examine baseline or interim differences in medication use, which might have biased our results in either direction. Finally, despite data from the electrophysiology laboratory that PVCs cause heart failure in some patients and although we are confident of the association between antecedent PVCs and the development of CHF in our cohort, our observational study does not prove a causal relationship.
Conclusions
An increased percentage of PVCs detected by 24-h Holter monitoring is associated with a subsequent decrease in the LVEF, increased incident CHF, and increased mortality. These effects were observed at a far lower PVC percentage than previously recognized. The relationship between increased PVCs and mortality appears to be at least partly mediated by incident CHF. The specificity of PVCs for the long-term diagnosis of CHF is high, and the risk of incident CHF attributable to PVCs is comparable to that of other CHF risk factors. These findings suggest that PVCs may be an important cause of occult or “idiopathic” cardiomyopathy and may be important determinants of incident CHF amongst those with other established CHF risk factors. Given these observations and the high prevalence of PVCs in the general population, further research into the potential benefits of PVC suppression are warranted.
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge
Premature ventricular contractions (PVC) are associated with a decline in left ventricular function, exacerbation of clinical heart failure (HF) and increased mortality. These relationships exist in patients with low PVC burdens, and risks increase with greater PVC frequency.
Competency in Patient Care
The risk of developing HF attributable to PVCs is comparable to that of other HF risk factors such as body mass index, hypertension, age, and coronary artery disease. Patients with frequent PVCs should be monitored over time for development of left ventricular dysfunction and clinical HF.
Translational Outlook
Additional research is needed to evaluate the potential benefits of PVC suppression to reduce the risk of progressive ventricular dysfunction and incident heart failure.
Acknowledgments
Source of Funding: This work was made possible by grants 12GRNT11780061 from the American Heart Association and the Joseph Drown Foundation to Dr. Marcus, and grant R01HL116747 to Dr. Sotoodehnia from the National Heart, Lung, and Blood Institute (NHLBI); contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the NHLBI, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by AG023629 from the National Institute on Aging. A full list of principal CHS investigators and institutions can be found at CHSNHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Psaty has reported serving on the Data and Safety Monitoring Board for a clinical trial of a device funded by Zoll (LifeCor) and is on the Steering Committee of the Yale Open Data Access Project funded by Medtronic. Dr. Marcus has received research support from Gilead Sciences and Medtronic.
Abbreviations
- AF
atrial fibrillation
- BMI
body mass index
- CAD
coronary artery disease
- CHF
congestive heart failure
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- PVC
premature ventricular contraction
- VT
ventricular tachycardia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No other disclosures were reported.
References
- 1.Evenson KR, Welch VL, Cascio WE, et al. Validation of a short rhythm strip compared to ambulatory ECG monitoring for ventricular ectopy. J Clin Epidemiol. 2000;53:491–497. doi: 10.1016/s0895-4356(99)00190-0. [DOI] [PubMed] [Google Scholar]
- 2.Poblete PF, Kennedy HL, Caralis DG. Detection of ventricular ectopy in patients with coronary heart disease and normal subjects by exercise testing and ambulatory electrocardiography. Chest. 1978;74:402–407. doi: 10.1378/chest.74.4.402. [DOI] [PubMed] [Google Scholar]
- 3.Codd MB, Sugrue DD, Gersh BJ, et al. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975–1984. Circulation. 1989;80:564–572. doi: 10.1161/01.cir.80.3.564. [DOI] [PubMed] [Google Scholar]
- 4.Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 5.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 6.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 7.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 8.Gardin JM, Wong ND, Bommer W, et al. Echocardiographic design of a multicenter investigation of free-living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 9.Gardin JM, Siscovick D, Anton-Culver H, et al. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The Cardiovascular Health Study. Circulation. 1995;91:1739–1748. doi: 10.1161/01.cir.91.6.1739. [DOI] [PubMed] [Google Scholar]
- 10.Sahn DJ, DeMaria A, Kisslo J, et al. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 11.Psaty BM, Lee M, Savage PJ, et al. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992;45:683–692. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 12.Kooperberg C, Petitti DB. Using logistic regression to estimate the adjusted attributable risk of low birthweight in an unmatched case-control study. Epidemiology. 1991;2:363–366. doi: 10.1097/00001648-199109000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djoussé L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394–400. doi: 10.1001/jama.2009.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chugh SS, Shen WK, Luria DM, Smith HC. First evidence of premature ventricular complex-induced cardiomyopathy: a potentially reversible cause of heart failure. J Cardiovasc Electrophysiol. 2000;11:328–329. doi: 10.1111/j.1540-8167.2000.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 16.Takemoto M, Yoshimura H, Ohba Y, et al. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol. 2005;45:1259–1265. doi: 10.1016/j.jacc.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 17.Bogun F, Crawford T, Reich S, et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm. 2007;4:863–867. doi: 10.1016/j.hrthm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Ezzat VA, Liew R, Ward DE. Catheter ablation of premature ventricular contraction-induced cardiomyopathy. Nat Clin Pract Cardiovasc Med. 2008;5:289–293. doi: 10.1038/ncpcardio1180. [DOI] [PubMed] [Google Scholar]
- 19.Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–869. doi: 10.1016/j.hrthm.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal SK, Simpson RJ, Jr, Rautaharju P, et al. Relation of ventricular premature complexes to heart failure (from the Atherosclerosis Risk In Communities [ARIC] Study) Am J Cardiol. 2012;109:105–109. doi: 10.1016/j.amjcard.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bikkina M, Larson MG, Levy D. Prognostic implications of asymptomatic ventricular arrhythmias: the Framingham Heart Study. Ann Intern Med. 1992;117:990–996. doi: 10.7326/0003-4819-117-12-990. [DOI] [PubMed] [Google Scholar]
- 22.Sajadieh A, Nielsen OW, Rasmussen V, et al. Ventricular arrhythmias and risk of death and acute myocardial infarction in apparently healthy subjects of age >or=55 years. Am J Cardiol. 2006;97:1351–1357. doi: 10.1016/j.amjcard.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 23.Hedblad B, Janzon L, Johansson BW, et al. Survival and incidence of myocardial infarction in men with ambulatory ECG-detected frequent and complex ventricular arrhythmias. 10 year follow-up of the “Men born 1914” study in Malmö, Sweden. Eur Heart J. 1997;18:1787–1795. doi: 10.1093/oxfordjournals.eurheartj.a015174. [DOI] [PubMed] [Google Scholar]
- 24.Ellis ER, Josephson ME. Heart failure and tachycardia-induced cardiomyopathy. Curr Heart Fail Rep. 2013;10:296–306. doi: 10.1007/s11897-013-0150-z. [DOI] [PubMed] [Google Scholar]
- 25.Delgado V, Tops LF, Trines SA, et al. Acute effects of right ventricular apical pacing on left ventricular synchrony and mechanics. Circ Arrhythm Electrophysiol. 2009;2:135–145. doi: 10.1161/CIRCEP.108.814608. [DOI] [PubMed] [Google Scholar]
- 26.Shinbane JS, Wood MA, Jensen DN, et al. Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol. 1997;29:709–715. doi: 10.1016/s0735-1097(96)00592-x. [DOI] [PubMed] [Google Scholar]
- 27.Olgun H, Yokokawa M, Baman T, et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm. 2011;8:1046–1049. doi: 10.1016/j.hrthm.2011.02.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.