Abstract
The renal tubular epithelial cells produce more endothelin-1 (ET-1) than any other cell type in the body. Moving down the nephron, the amount of ET-1 produced appears fairly consistent until reaching the inner medullary collecting duct, which produces at least 10 times more ET-1 than any other segment. ET-1 inhibits Na+ transport in all parts of the nephron through activation of the ETB receptor, and to a minor extent, the ETA receptor. These effects are most prominent in the collecting duct where ETB receptor activation inhibits activity of the epithelial Na+ channel. Effects in other parts of the nephron include inhibition of Na+/H+ exchange in the proximal tubule and the Na+, K+, 2Cl− co-transporter in the thick ascending limb. In general, the renal epithelial ET-1 system is an integral part of the body’s response to a high salt intake in order to maintain homeostasis and normal blood pressure. Loss of ETB receptor function results in salt sensitive hypertension. The goal of this article is to review the role of renal ET-1 and how it affects Na+ and water transport throughout the nephron.
Keywords: Endothelin, kidney, sodium transport
In 1988, Masashi Yanagisawa published his PhD thesis research in the journal Nature.1 This work created an explosion of research that has contributed to well over 25,000 publications, very successful bi-annual international research conferences, and at least two novel drug therapies for the treatment of pulmonary hypertension, and most likely, several other important drug applications in the not too distant future. The speed at which the pharmaceutical industry moved into this field was quite remarkable and continues to this day, albeit at a very focused level. The complexity and diversity of this field is demonstrated by scientific investigation ranging from basic science areas such as vascular biology, neuroscience, and renal physiology to pathobiology of the gastrointestinal tract, heart failure, pain, cancer, hypertension, diabetes and chronic kidney disease just to name a few. All this effort was triggered by Yanagisawa’s work related to a small 21 amino acid peptide that he named endothelin (ET).2
The ET family of peptides, ET-1, ET-2, and ET-3, has an incredibly diverse range of actions in virtually every organ system in cell types such as endothelium, epithelium, muscle and nerves. The ET system includes specific isoenzymes known as endothelin converting enzymes (ECE) that are responsible for the conversion of relatively inactive precursor peptides, big ET-1, big ET-2, and big-ET-3, to the active peptides. The biological actions of these peptides are mediated through ETA and ETB receptors (referred to hereafter as ETA and ETB). The kidney contains a very large number of these receptors on both endothelial and epithelial structures.
The actions of the ET peptides depend in large measure on the cell types on which they are located. For example, ETA on vascular smooth muscle cells mediate most of the vasoconstrictor effects of ET-1, but some vascular tissue also contain ETB that can result in vasoconstriction. In general, ETB are primarily located on vascular endothelium and account for endothelial-dependent vasorelaxation that opposes the ETA mediated effects.
While most of the attention has focused on the vascular actions of the ET system, it is important to recognize that this paracrine system is active within many other cell types throughout the body including neuronal and epithelial cells. Studies over the past decade or more have provided powerful evidence that an important physiological role of ET-1 is to regulate the excretion of Na+ and water by the kidney. It is somewhat surprising, perhaps, that this powerful vasoconstrictor actually functions to enhance the excretion of dietary Na+ by reducing renal tubular Na+ reabsorption through direct effects on ion transport, as well as favorable effects on renal blood flow, glomerular filtration rate, and medullary blood flow. This review will focus on the autocrine function of ET-1 within the renal tubule and its direct effects on Na+ and water transport along the nephron, while other aspects are reviewed in the accompanying papers.
Proximal tubule
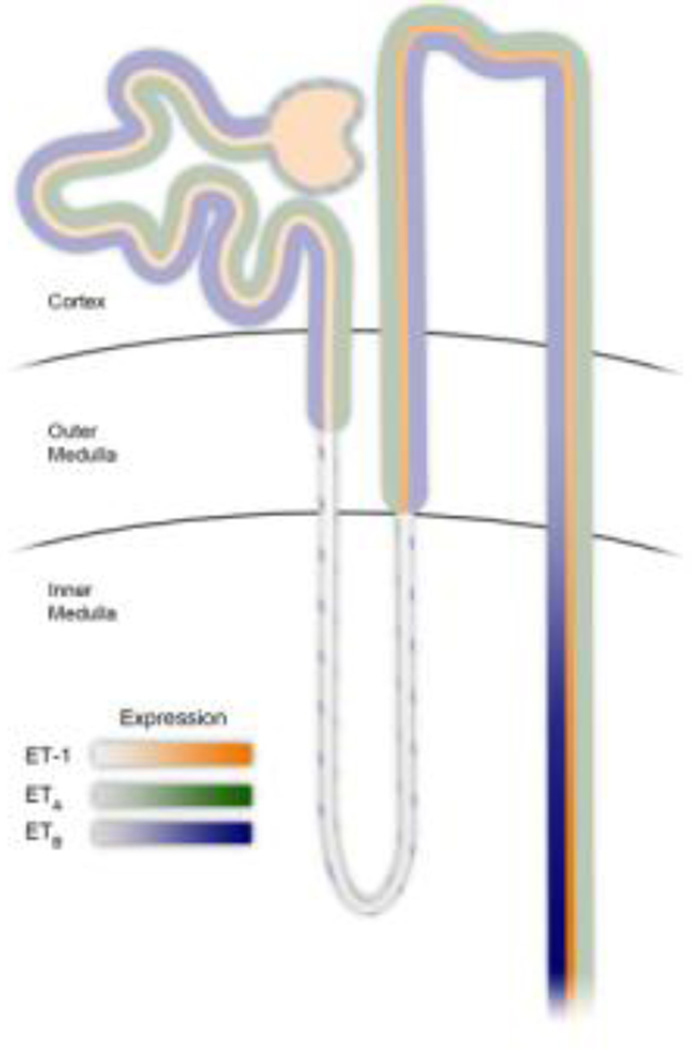
All nephron segments produce ET-1 and also express ET receptors (Figure 1). The proximal tubule (PT) is no exception with synthesis occurring throughout the length of the PT, although expression levels are much lower compared to the thick ascending limb (TAL) and collecting duct (CD). Mechanisms that regulate ET-1 production in the PT under normal physiological conditions are not known. However, a variety of pathophysiological factors such as hypoxia, metabolic acidosis, and inflammation, mediated by tumor necrosis factor alpha (TNFα) and interleukin-1 beta (IL-1β), can increase ET-1 in the PT.
Figure 1.
Relative ET-1 production and receptor density along the nephron.
The actions of ET-1 in the PT are not as clear as in other segments of the nephron. ET-1 affects Na+ transport in the PT through interactions with both the Na+/K+ ATPase and the Na+/H+ exchanger (NHE3).3–5 In contrast to other tubular segments, it appears that ET-1 increases reabsorption of Na+ and water in the PT at very low (sub-picomolar) concentrations, possibly through increasing NHE3 activity. However, higher concentrations (picomolar) appear to reduce reabsorption through inhibition of the Na+/K+ ATPase.6 Adding to the uncertainty is the observation by Romano et. al. that microinjection of nanomolar ET-1 concentrations into Bowman’s capsule increased reabsorption by the proximal tubule.7 However, this effect may be unrelated to Na+ transport in the proximal tubule, as glomerular filtration was significantly increased, possibly suggesting that the increase in reabsorption was simply related to the increased filtered load. The mechanism that lead to the hyper-filtration observed by Romano et. al. is not clear. Regardless of these findings, the effects of ET-1 in the PT appear to be mediated primarily by ETB, although some actions have been ascribed to ETA. The physiological conditions that lead to the dispirit actions of ET-1 on PT Na+ transport have yet to be resolved.
Effects on Na+/K+ ATPase
The ability of ET-1 to inhibit the Na+/K+ ATPase in the PT has been well documented. The primary receptor mediating this response is ETB. One of the first demonstrations of this effect showed that ET-1 reduces oxygen consumption and inhibits ouabain sensitive 86Rb+ uptake by rabbit PT suspensions, both markers of Na+/K+ ATPase activity.3 Garvin et. al. then reported that ET-1 inhibits fluid and bicarbonate reabsorption in isolated rat PT that was attributed to a 20 percent reduction in Na+/K+ ATPase activity. This effect was shown to be mediated by a number of factors including phosphokinase C and enzymes that facilitate arachidonic acid metabolism, such as cyclooxygenase and lipoxygenase.6 Furthermore, Liu et. al observed that inhibition of Na+/K+ ATPase by ET-1 is dependent on increases in intracellular Ca2+.8
Effects on NHE3
ET-1 stimulates NHE3 activity in the PT, as evidenced by a number of in vitro models. The earliest studies demonstrate activation of NHE3 by ET-1 in rabbit and rat cortical slices and opossum kidney cells through a protein kinase C (PKC) dependent mechanism.5, 9, 10 In vivo evidence suggests that this mechanism is important in acid/base regulation. Laghmani et. al. demonstrated that mice lacking functional ETB everywhere except dopamine β-hydroxylase expressing cells have a significantly greater reduction in plasma [HCO3−] than control mice in response to chronic acid feeding. This was associated with an increase in proximal tubular NHE3 activity in control mice in response to the high acidity diet, while no increase was observed in the ETB deficient mice. Further, chronic acidosis stimulated ET-1 production by the glomerulus and PT of c57Bl/6 mice, enhancing activity of the NHE3 and the Na+/citrate co-transporter in the PT, both mediated by ETB.11–13 Since these initial observations, the study of endothelin in the regulation of acid/base balance has slowed dramatically. More recently, however, somewhat conflicting data in humans suggest that blockade of ET-1 receptors with the dual ETA/ETB blocker, bosentan, stimulates ammonia generation and increases net acid excretion in patients with metabolic acidosis, but only when dietary Na+ is restricted.14 Since this study was performed with a dual ETA/ETB antagonist, the only FDA approved ET antagonist at the time, further studies are needed to fully understand this pathway and its importance in human physiology.
Work from Jose’s laboratory has revealed a number of potentially important regulatory mechanisms that influence ET-1 receptor function in the PT. There appears to be an interaction between the ETB and dopamine D3 receptors, perhaps heterodimerization, in the PT.15 In addition to direct interaction with ETB, activation of dopamine D3 receptors increases expression of ETB. Dysfunction of the dopamine D3/ETB interaction may contribute to hypertension in the spontaneously hypertensive rat (SHR).16, 17 In addition to dopamine D3 receptors, there is also evidence that the angiotensin type 1 receptor (AT1R) positively stimulates ETB function in the proximal tubule. Acute activation of the AT1R stimulates an increase in cell surface localization of ETB, while long-term activation increases total ETB expression.17, 18 It is thought that this increased expression most likely reduces NHE3 activity, since AT1R/ETB interaction is absent in SHR rats.
Thin ascending limb
Few studies have been carried out investigating ET-1 action in the thin ascending of the loop of Henle. Conflicting reports suggest that ET-1 may or may not be produced by this portion of the nephron. Autoradiography binding studies have found no ET-1 receptor binding in the thin limb; however one report has suggested a possible physiological role of ET receptors in the thin limb. ET-1 was shown to increase intracellular Ca2+ in isolated thin limbs, an ETB -mediated process.19 It is still unknown if ET-1 affects water movement in this nephron segment.
Thick ascending limb
The actions of ET peptides in the thick ascending limb (TAL) are generally believed to be physiologically significant in overall fluid-electrolyte balance, due to both the important role the TAL in urine concentration as well as its ability to influence significant Na+ and water transport. Not only is ET-1 produced by the TAL, expression of both ETA and ETB has been observed in rat TAL.20 Unlike work discussed later for the CD, there are no cell specific knockouts for this nephron segment. However, several lines of evidence suggest that ET-1 has dramatic effects on electrolyte movement in the TAL. The regulation of TAL ET-1 production is closely associated with increased Na+ intake, which increases tubular flow and medullary tonicity21. Both flow and tonicity are believed to be important stimuli for increasing renal tubular ET-1 production.21
Effects on Na+/K+/2Cl− cotransporter
De Jesus Ferreira et al. first demonstrated that ET-1 reduces Cl− transport in isolated mouse TAL, an effect that was blocked by PKC inhibitors and was independent of increases in intracellular calcium concentration ([Ca+]i).22, 23 Plato et al. went on to describe that ET-1 inhibition of Na+/K+/2Cl− co-transporter occurs through the production of nitric oxide (NO), a well-described inhibitor of tubular Na+ transport.24 The same group showed that increases in dietary Na+ increase NOS3 expression in the TAL, which is attenuated by ETB blockade.21 In addition, this occurs though activation of phosphatidylinositol 3-kinase, which stimulates protein kinase B (Akt) activity, thus activating NOS3.25
Another possible mediator of ET-1 action in the TAL is 20-hydroxyeicosatetraeonic acid (20-HETE), a natriuretic metabolite of arachidonic acid metabolism by CYP-450 enzymes.26 Outer medullary 20-HETE levels are elevated in rats in response to chronic high salt feeding. The increase in 20-HETE is abolished in rats treated with an ETB antagonist directly into the renal outer medulla.27
Cortical Collecting Duct
The CD produces significantly more ET-1 than earlier nephron segments. The level of production is particularly high in the inner medullary CD. Synthesis of ET-1 by the CD is stimulated by a number a factors, but most prominently by increased flow and osmolarity that occur as salt intake is elevated.28 Benzamil blocks flow and high osmolality induced ET-1 production, and thus these mechanisms appear to be related to Na+ delivery and uptake by ENaC. Interestingly, ET-1 production appears to be suppressed by the circadian transcription factor Period 1, suggesting a role for ET-1 in circadian blood pressure rhythms that may serve as a counter-regulatory system for the salt-retaining effects of aldosterone.29
Effects on epithelial Na+ channel (ENaC)
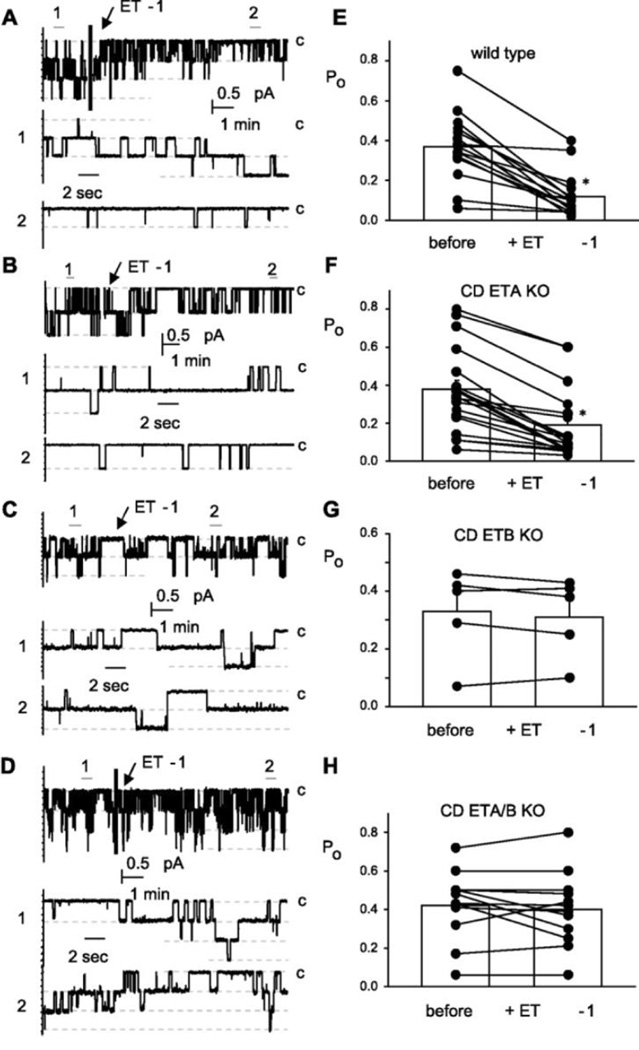
Patch clamp studies from the Stockand laboratory indicate that ENaC is prominently expressed in the cortical collecting duct (CCD), and ET-1 directly inhibits open probability of the ENaC in isolated CCD of mice (Figure 2). This effect was completely absent in CD ETB knockout mice, but not CD ETA knockouts.30 Furthermore, ENaC open probability was reduced in response to high salt feeding in wild type and CD ETA knockout mice, but not CD ETB knockout mice. Collectively, these results suggest that inhibition of ENaC by ET-1 is mediated through ETB. In contrast to studies by Stockand, a recent finding by Lynch et. al. has suggested that inhibition of ENaC by ET-1 is mediated by both ETA and ETB. In isolated perfused CCD from mice, ET-1 significantly inhibited benzamil sensitive Na+ transport an effect that was abolished by ETA or ETB blockade.31 Since aldosterone stimulates ET-1 production by the cortical CD, ET-1 inhibition of ENaC may be to serve as a negative feedback mechanism for the renal actions of aldosterone.
Figure 2.
ENaC activity in isolated split open cortical CD using patch clamp techniques. Exogenous ET-1 reduces ENaC open probability in CD from floxed control mice and CD specific ETA KO mice. ET-1 induced reductions in ENaC activity was absent in tubules from CD ETB KO mice (used with permission ref. 28)
There is a much greater appreciation for the physiological role of ET-1 in Na+ transport in the CD compared to other nephron segments. This is mainly due to a series of studies from Kohan et. al. in which his laboratory knocked out various components of the ET system specifically in the CD of mice, and measured blood pressure in response to changes in dietary salt content.32–34 Using cre recombinase driven by the aquaporin 2 promoter, they were able to selectively eliminate expression of ET-1 in the CD. These animals displayed an elevated baseline blood pressure as measured by 24hr telemetry and introduction of a high salt diet produced an additional increase in blood pressure. Interestingly, CD specific knockout of ETB also increased blood pressure in a salt sensitive manner, although to a lesser degree than in the CD ET-1 knockout. CD specific ETA knockout mice had no difference in blood pressure from controls; however, CD knockout of both ETA and ETB fully restored the salt sensitive phenotype observed in CD ET-1 KO mice. Therefore, even though patch clamp studies mentioned earlier suggest that ETA activation has no effect on Na+ transport in the CD, in vivo physiological observations may suggest otherwise. The cause of these apparent contradictions may be due to which part of the CCD was patch clamped.
Inner Medullary Collecting Duct
The inner medullary collecting duct (IMCD) produces more ET-1 and has more ETB than any other tissue in the body (Figure 1).35–39 Even though only a small amount of the filtered load of Na+ is reabsorbed here, it is the final nephron segment where the “fine tuning” of Na+ and water homeostasis occurs, potentially impacting overall Na+ excretion by several fold. Small changes in the amount of Na+ reabsorbed here can have dramatic effects on Na+ balance and blood pressure, therefore tight regulation is required.
Similar to other nephron segments, it is thought that ET-1 release by the IMCD is promoted by increases in flow and osmolality, and becomes more important as salt intake is elevated.40 In fact, similar to the TAL, a high salt diet stimulates both ET-1 mRNA in the inner medulla and increases urinary ET-1 excretion, which is thought to be mainly indicative of IMCD production.32, 41, 42 Selective knockout of ET-1 expression from the CD severely reduces urinary ET-1 excretion suggesting that urinary ET-1 is derived from intrarenal sources and not due to renal clearance.32
Effects on IMCD Na+ transport
ET-1 inhibits Na+ reabsorption by the IMCD through activation of ETB, while very little, if any, ETA expression has been observed (Figure 3).43–49 Soon after the discovery of ET-1, Zeidel et al. demonstrated that ET-1 inhibits ouabain sensitive 86Rb+ uptake and decreases oxygen consumption of intact rabbit IMCD suspensions, suggesting that ET-1 inhibits the Na+/K+ ATPase.3 ET-1 inhibition of transport was blocked by a cyclooxygenase inhibitor and treatment with prostaglandin E2 resulted in inhibition of transport equal to and was not additive to the effects of ET-1.3.
Figure 3.
Autocrine and paracrine effects of inner medullary collecting duct derived ET-1.
ET-1 effects on water reabsorption
Water reabsorption by the IMCD is highly dependent on the number of membrane bound aquaporins, and mounting evidence suggests that ET-1 reduces aquaporin expression and promotes water excretion. Early on, it was demonstrated that intravenous infusion of ET-1 in rats increases free water clearance in the absence of changes in renal hemodynamics.50 Interestingly, ET-1 infusion directly into the renal artery at a dose that reduces renal plasma flow also increased free water clearance in sheep. When treated with an ETA antagonist, the reduction in plasma flow in response intrarenal ET-1 infusion was abolished, but the diuresis was maintained.51 Several reports indicate that these results are explained by ET-1 inhibition of vasopressin (AVP) signaling in the CD. For instance, ET-1 inhibits AVP stimulated water permeability and cAMP accumulation in rat isolated perfused IMCD, and IMCD suspensions, a response recapitulated by an ETB agonist, and blocked by an ETB antagonist.52, 53 Further, since increases in [Ca2+]i are required for AVP signaling in the IMCD and ET-1 simulates an increase in [Ca2+]i in isolated perfused IMCD, it is possible that ET-1 inhibition of water transport requires a spike in [Ca2+]i.53 Finally, it was recently demonstrated that increased [Ca2+]i in response to ET-1 on IMCD is mediated by activation of ETA and is more prominent in male rats vs. female rats.1
Mechanisms of ET-1 induced natriuresis and diuresis
Much of what we know about ET-1 diuretic and natriuretic effects in the IMCD derives from studies in which ET-1 or the ETB agonist, sarafatoxin 6c (S6c) were infused directly into the renal medulla. In a dose dependent manner, acute intramedullary infusion of S6c increases urine volume and urinary Na+ excretion in rats. S6c also increased medullary cGMP, and the natriuretic/diuretic response was blunted by a NOS1 and a protein kinase G inhibitor, and was completely absent in rats lacking functional ETB. These observations suggest that ET-1 inhibition of Na+ and water reabsorption produce significant natriuresis and diuresis through ETB dependent increases in NOS1 signaling through PKG and cAMP.54
Role of salt intake on ETB function
Altering salt content of the diet has major effects on the natriuretic ability of ET-1. The natriuretic actions of ETB receptr stimulation can be enhanced by increasing salt intake (unpublished observations). In contrast, reducing salt intake can inhibit ETB function in male rats.55 These findings can be attributed to actions of angiotensin II because the reduction in ETB function is reversed by treatment with an angiotensin receptor blocker. Further, exogenous angiotensin II inhibits ETB mediated natriuresis.56, 57
Sex differences in ET-1 natriuresis and diuresis
Interestingly, interstitial infusion of ET-1 into the renal medulla produces natriuresis in female, but not male rats. This obvious sex difference is due to a marked decrease in medullary blood flow in male rats, but not females, that can be overcome by gonadectomy.54 These findings further suggest that changes in medullary blood flow have dramatic effects on ETB function in terms of renal tubular transport function. In the same set of experiments Nakano et al. demonstrated that blockade of ETB in female rats inhibited the natriuretic response to intramedullary infusion of ET-1. They went on to demonstrate that intramedullary infusion of ET-1 into ETB deficient rats produced natriuresis and diuresis in female, but not male rats, suggesting females have an ETA contribution to the natriuresis. Further studies indicate that angiotensin II mediated reductions in ETB function described earlier are attenuated in female rats, thus providing a potential explanation for reduced salt-sensitive blood pressure changes in females.
Role of the ETA in the CD
The natriuretic effects of ET-1 produced by the IMCD have mostly been attributed to activation of ETB because early studies found no ETA on IMCD; however, several functional studies report that ETA can influence ET-1 actions on Na+ handling by the IMCD. The most direct indication is from previously mentioned CD knockout studies in mice. Knockout of only ETB in the CD causes salt sensitive hypertension, but not to the degree of CD ET-1 knockout. While specific ETA deletion from the CD had no effect on blood pressure, dual CD ETA/ETB knockout mice exhibited hypertension similar to what was observed in ET-1 knockout mice, which was more than that observed in CD ETB knockout mice.
Interstitial infusion of hypertonic saline into the renal medulla produces natriuresis by an ET-1 dependent mechanism. Interestingly, this can only be blocked when the both ETA and ETB are blocked.58 Stuart et al. recently provided further evidence that ETA can influence fluid transport. Knockout of ETA from the entire nephron of mice resulted in fluid retention, a common side effect of systemic ETA blockade in humans.59 Collectively, these findings suggest an ETA component to the effects of ET-1 on renal tubular transport, although these effects are far less evident compared to the dominant role of ETB.
Summary and Conclusion
It is clear that ET-1 functions in an autocrine role to regulate renal tubular handling of Na+ and water (Table 1). Given that 1) increasing dietary Na+ intake is a powerful stimulus for renal tubular ET-1 production, 2) ETB is dominant in the CD to inhibit Na+ reabsorption, and 3) disruption of the ET system in the nephron results in salt-dependent hypertension, it is clear that this system plays a major role in fluid-electrolyte balance and blood pressure regulation. However, a number of important questions remain to be answered. These include the physiological role of the ET system in the PT and the TAL, the nature of ETA versus ETB function, and the mechanism by which ET-1 production is regulated.
Table 1.
ET-1 inhibition of Na+ transporters along the nephron.
Acknowledgments
Financial support for this work: This work was supported by research grants from the National Heart Lung and Blood Institute (P01 HL095499, P01 HL069999, and U01 HL117684).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure and conflict of interest statement: none
References
- 1.Speed JS, Pollock DM. Endothelin, kidney disease, and hypertension. Hypertension. 2013;61:1142–1145. doi: 10.1161/HYPERTENSIONAHA.113.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J Hypertension. 1988;6:S188–S191. doi: 10.1097/00004872-198812040-00056. [DOI] [PubMed] [Google Scholar]
- 3.Zeidel ML, Brady HR, Kone BC, Gullans SR, Brenner BM. Endothelin, a peptide inhibitor of Na+-K -ATPase in intact renaltubular epithelial cells. Am J Physiol. 1989;257:C1101–C1107. doi: 10.1152/ajpcell.1989.257.6.C1101. [DOI] [PubMed] [Google Scholar]
- 4.Garvin J, Sanders K. Endothelin inhibits fluid and bicarbonate transport in part by reducing Na+/K+ ATPase activity in the rat proximal straight tubule. J Am Soc Nephrol. 1991;2:976–982. doi: 10.1681/ASN.V25976. [DOI] [PubMed] [Google Scholar]
- 5.Eiam-Ong S, Hilden SA, King AJ, Johns CA, Madias NE. Endothelin-1 stimulates the Na+/H+ and Na+/HCO3− transporters in rabbit renal cortex. Kidney Int. 1992;42:18–24. doi: 10.1038/ki.1992.255. [DOI] [PubMed] [Google Scholar]
- 6.Garcia NH, Garvin JL. Endothelin's biphasic effect on fluid absorption in the proximal straight tubule and its inhibitory cascade. J Clin Invest. 1994;93:2572–2577. doi: 10.1172/JCI117268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romano G, Giagu P, Favret G, Bartoli E. Effect of endothelin 1 on proximal reabsorption and tubuloglomerular feedback. Kidney Blood Press Res. 2000;23:360–365. doi: 10.1159/000025984. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Yang J, Ren H, He D, Pascua A, Armando MI, Yang C, Zhou L, Felder RA, Jose PA, Zeng C. Inhibitory effect of ETB receptor on Na+-K+ ATPase activity by extracellular Ca2+ entry and Ca2+ release from the endoplasmic reticulum in renal proximal tubule cells. Hypertension Res. 2009;32:846–852. doi: 10.1038/hr.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walter R, Helmle-Kolb C, Forgo J, Binswanger U, Murer H. Stimulation of Na+/H+ exchange activity by endothelin in opossum kidney cells. Pflugers Archiv. 1995;430:137–144. doi: 10.1007/BF00373849. [DOI] [PubMed] [Google Scholar]
- 10.Guntupalli J, DuBose TD., Jr Effects of endothelin on rat renal proximal tubule Na+-Pi cotransport and Na+/H+ exchange. Am J Physiol. 1994;266:F658–F666. doi: 10.1152/ajprenal.1994.266.4.F658. [DOI] [PubMed] [Google Scholar]
- 11.Licht C, Laghmani K, Yanagisawa M, Preisig PA, Alpern RJ. An autocrine role for endothelin-1 in the regulation of proximal tubule NHE3. Kidney Int. 2004;65:1320–1326. doi: 10.1111/j.1523-1755.2004.00506.x. [DOI] [PubMed] [Google Scholar]
- 12.Laghmani K, Preisig PA, Moe OW, Yanagisawa M, Alpern RJ. Endothelin-1/endothelin-B receptor-mediated increases in NHE3 activity in chronic metabolic acidosis. J Clin Invest. 2001;107:1563–1569. doi: 10.1172/JCI11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Zacchia M, Tian X, Wan L, Sakamoto A, Yanagisawa M, Alpern RJ, Preisig PA. Acid regulation of NaDC-1 requires a functional endothelin B receptor. Kidney Int. 2010;78:895–904. doi: 10.1038/ki.2010.264. [DOI] [PubMed] [Google Scholar]
- 14.Pallini A, Hulter HN, Muser J, Krapf R. Role of endothelin-1 in renal regulation of acid-base equilibrium in acidotic humans. Am J Physiol Renal Physiol. 2012;303:F991–F999. doi: 10.1152/ajprenal.00309.2012. [DOI] [PubMed] [Google Scholar]
- 15.Zeng C, Asico LD, Yu C, Villar VA, Shi W, Luo Y, Wang Z, He D, Liu Y, Huang L, Yang C, Wang X, Hopfer U, Eisner GM, Jose PA. Renal D3 dopamine receptor stimulation induces natriuresis by endothelin B receptor interactions. Kidney Int. 2008;74:750–759. doi: 10.1038/ki.2008.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu C, Yang Z, Ren H, Zhang Y, Han Y, He D, Lu Q, Wang X, Wang X, Yang C, Asico LD, Hopfer U, Eisner GM, Jose PA, Zeng C. D3 dopamine receptor regulation of ETB receptors in renal proximal tubule cells from WKY and SHRs. Am J Hyper. 2009;22:877–883. doi: 10.1038/ajh.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng C, Wang Z, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Aberrant ETB receptor regulation of AT receptors in immortalized renal proximal tubule cells of spontaneously hypertensive rats. Kidney Int. 2005;68:623–631. doi: 10.1111/j.1523-1755.2005.00440.x. [DOI] [PubMed] [Google Scholar]
- 18.Zeng C, Hopfer U, Asico LD, Eisner GM, Felder RA, Jose PA. Altered AT1 receptor regulation of ETB receptors in renal proximal tubule cells of spontaneously hypertensive rats. Hypertension. 2005;46:926–931. doi: 10.1161/01.HYP.0000174595.41637.13. [DOI] [PubMed] [Google Scholar]
- 19.Bailey MA, Haton C, Orea V, Sassard J, Bailly C, Unwin RJ, Imbert-Teboul M. ETA receptor-mediated Ca2+ signaling in thin descending limbs of Henle's loop: impairment in genetic hypertension. Kidney Int. 2003;63:1276–1284. doi: 10.1046/j.1523-1755.2003.00880.x. [DOI] [PubMed] [Google Scholar]
- 20.Dean R, Zhuo J, Alcorn D, Casley D, Mendelsohn FA. Cellular distribution of 125I-endothelin-1 binding in rat kidney following in vivo labeling. Am J Physiol. 1994;267:F845–F852. doi: 10.1152/ajprenal.1994.267.5.F845. [DOI] [PubMed] [Google Scholar]
- 21.Herrera M, Garvin JL. A high-salt diet stimulates thick ascending limb eNOS expression by raising medullary osmolality and increasing release of endothelin-1. Am J Physiol Renal Physiol. 2005;288:F58–F64. doi: 10.1152/ajprenal.00209.2004. [DOI] [PubMed] [Google Scholar]
- 22.de Jesus Ferreira MC, Bailly C. Luminal and basolateral endothelin inhibit chloride reabsorption in the mouse thick ascending limb via a Ca2+-independent pathway. J Physiol. 1997;505(Pt 3):749–758. doi: 10.1111/j.1469-7793.1997.749ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailly C. Effect of luminal atrial natriuretic peptide on chloride reabsorption in mouse cortical thick ascending limb: inhibition by endothelin. J Am Soc Nephrol. 2000;11:1791–1797. doi: 10.1681/ASN.V11101791. [DOI] [PubMed] [Google Scholar]
- 24.Plato CF, Pollock DM, Garvin JL. Endothelin inhibits thick ascending limb chloride flux via ETB receptor-mediated NO release. Am J Physiol Renal Physiol. 2000;279:F326–F333. doi: 10.1152/ajprenal.2000.279.2.F326. [DOI] [PubMed] [Google Scholar]
- 25.Herrera M, Hong NJ, Ortiz PA, Garvin JL. Endothelin-1 inhibits thick ascending limb transport via Akt-stimulated nitric oxide production. The J Biol Chem. 2009;284:1454–1460. doi: 10.1074/jbc.M804322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito O, Roman RJ. Role of 20-HETE in elevating chloride transport in the thick ascending limb of Dahl SS/Jr rats. Hypertension. 1999;33:419–423. doi: 10.1161/01.hyp.33.1.419. [DOI] [PubMed] [Google Scholar]
- 27.Speed JS, George EM, Arany M, Cockrell K, Granger JP. Role of 20-hydroxyeicosatetraenoic acid in mediating hypertension in response to chronic renal medullary endothelin type B receptor blockade. PloS one. 2011;6:e26063. doi: 10.1371/journal.pone.0026063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandit MM, Strait KA, Matsuda T, Kohan DE. Na delivery and ENaC mediate flow regulation of collecting duct endothelin-1 production. Am J Physiol Renal Physiol. 2012;302:F1325–F1330. doi: 10.1152/ajprenal.00034.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stow LR, Voren GE, Gumz ML, Wingo CS, Cain BD. Dexamethasone stimulates endothelin-1 gene expression in renal collecting duct cells. Steroids. 2012;77:360–366. doi: 10.1016/j.steroids.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bugaj V, Mironova E, Kohan DE, Stockand JD. Collecting duct-specific endothelin B receptor knockout increases ENaC activity. Am J Physiol Cell Physiol. 2012;302:C188–C194. doi: 10.1152/ajpcell.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch IJ, Welch AK, Kohan DE, Cain BD, Wingo CS. Endothelin-1 inhibits sodium reabsorption by ETA and ETB receptors in the mouse cortical collecting duct. Am J Physiol Renal Physiol. 2013;305:F568–F573. doi: 10.1152/ajprenal.00613.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest. 2004;114:504–511. doi: 10.1172/JCI21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ge Y, Stricklett PK, Hughes AK, Yanagisawa M, Kohan DE. Collecting duct-specific knockout of the endothelin A receptor alters renal vasopressin responsiveness, but not sodium excretion or blood pressure. Am J Physiol Renal Physiol. 2005;289:F692–F698. doi: 10.1152/ajprenal.00100.2005. [DOI] [PubMed] [Google Scholar]
- 34.Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol. 2006;291:F1274–F1280. doi: 10.1152/ajprenal.00190.2006. [DOI] [PubMed] [Google Scholar]
- 35.Kohan D. Endothelins in the normal and diseased kidney. Am J Kid Dis. 1997;29:2–26. doi: 10.1016/s0272-6386(97)90004-4. [DOI] [PubMed] [Google Scholar]
- 36.Chen M, Todd-Turla K, Wang W-H, Cao X, Smart A, Brosius FC, Killen PD, Keiser JA, Briggs JP, Schnermann J. Endothlein-1 mRNA in glomerular and epithelial cells of kidney. Am J Physiol. 1993;265:F542–F550. doi: 10.1152/ajprenal.1993.265.4.F542. [DOI] [PubMed] [Google Scholar]
- 37.Kohan DE. Endothelin synthesis by rabbit renal tubule cells. Am J Physiol. 1991;261:F221–F226. doi: 10.1152/ajprenal.1991.261.2.F221. [DOI] [PubMed] [Google Scholar]
- 38.Uchida S, Takemoto F, Ogata E, Kurokawa K. Detection of endothelin-1 mRNA by RT-PCR in isolated rat renal tubules. Biochem Biophys Res Commun. 1992;188:108–113. doi: 10.1016/0006-291x(92)92356-3. [DOI] [PubMed] [Google Scholar]
- 39.Ujiie K, Terada Y, Nonoguchi H, Shinohara M, Tomita K, Marumo F. Messenger RNA expression and synthesis of endothelin-1 along rat nephron segments. J Clin Invest. 1992;90:1043–1048. doi: 10.1172/JCI115918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyon-Roberts B, Strait KA, van Peursem E, Kittikulsuth W, Pollock JS, Pollock DM, Kohan DE. Flow regulation of collecting duct endothelin-1 production. Am J Physiol Renal Physiol. 2011;300:F650–F656. doi: 10.1152/ajprenal.00530.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Speed JS, LaMarca B, Berry H, Cockrell K, George EM, Granger JP. Renal medullary endothelin-1 is decreased in Dahl salt-sensitive rats. Am J Physiol Regu Integr Comp Physiol. 2011;301:R519–R523. doi: 10.1152/ajpregu.00207.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fattal I, Abassi Z, Ovcharenko E, Shimada K, Takahashi M, Hoffman A, Winaver J. Effect of dietary sodium intake on the expression of endothelin-converting enzyme in the renal medulla. Nephron Physiol. 2004;98:89–96. doi: 10.1159/000081557. [DOI] [PubMed] [Google Scholar]
- 43.Cernacek P, Legault L, Stewart DJ, Levy M. Specific endothelin binding sites in renal medullary collecting duct cells: lack of interaction with ANP binding and cGMP signalling. Can J Physiol Pharmacol. 1992;70:1167–1174. doi: 10.1139/y92-162. [DOI] [PubMed] [Google Scholar]
- 44.Omatsu S, Tomoyoshi T. Immunohistochemical study on endothelin in rat kidney. Hinyokika Kiyo. 1997;43:109–114. [PubMed] [Google Scholar]
- 45.Edwards RM, Trizna W. Characterization of 125I-endothelin-1 binding to rat and rabbit renal microvasculature. J Pharmacol Exp Ther. 1995;274:1064–1089. [PubMed] [Google Scholar]
- 46.Yukimura T, Notoya M, Mizojiri K, Mizuhira V, Matsuura T, Ebara T, Miura K, Kim S, Iwao H, Song K. High resolution localization of endothelin receptors in rat renal medulla. Kidney Int. 1996;50:135–147. doi: 10.1038/ki.1996.296. [DOI] [PubMed] [Google Scholar]
- 47.Kohan DE, Hughes AK, Perkins SP. Characterization of endothelin receptors in the inner medullary collecting duct of the rat. J Biol Chem. 1992;267:12336–12340. [PubMed] [Google Scholar]
- 48.Wendel M, Knels L, Kummer W, Koch T. Distribution of endothelin receptor subtypes ETA and ETB in the rat kidney. J Histochem Cytochem. 2006;54:1193–1203. doi: 10.1369/jhc.5A6888.2006. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto T, Suzuki H, Kubo Y, Matsumoto A, Uemura H. Endothelin A receptor-like immunoreactivity on the basal infoldings of rat renal tubules and collecting ducts. Arch Histol Cytol. 2008;71:77–87. doi: 10.1679/aohc.71.77. [DOI] [PubMed] [Google Scholar]
- 50.Schnermann J, Lorenz JN, Briggs JP, Keiser JA. Induction of water diuresis by endothelin in rats. Am J Physiol. 1992;263:F516–F526. doi: 10.1152/ajprenal.1992.263.3.F516. [DOI] [PubMed] [Google Scholar]
- 51.Kamphuis C, Yates NA, McDougall JG. Differential blockade of the renal vasoconstrictor and diuretic responses to endothelin-1 by endothelin antagonist. Clin Exptl Pharmacol Physiol. 1994;21:329–333. doi: 10.1111/j.1440-1681.1994.tb02522.x. [DOI] [PubMed] [Google Scholar]
- 52.Edwards RM, Stack EJ, Pullen M, Nambi P. Endothelin inhibits vasopressin action in rat inner medullary collecting duct via the ETB receptor. J Pharmacol Exptl Therap. 1993;267:1028–1033. [PubMed] [Google Scholar]
- 53.Nadler SP, Zimpelmann JA, Hebert RL. Endothelin inhibits vasopressin-stimulated water permeability in rat terminal inner medullary collecting duct. J Clin Invest. 1992;90:1458–1466. doi: 10.1172/JCI116013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakano D, Pollock JS, Pollock DM. Renal medullary ETB receptors produce diuresis and natriuresis via NOS1. Am J Physiol Renal Physiol. 2008;294:F1205–F1211. doi: 10.1152/ajprenal.00578.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kittikulsuth W, Pollock JS, Pollock DM. Loss of renal medullary endothelin B receptor function during salt deprivation is regulated by angiotensin II. Am J Physiol Renal Physiol. 2012;303:F659–F666. doi: 10.1152/ajprenal.00213.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kittikulsuth W, Pollock JS, Pollock DM. Sex differences in renal medullary endothelin receptor function in angiotensin II hypertensive rats. Hypertension. 2011;58:212–218. doi: 10.1161/HYPERTENSIONAHA.111.172734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kittikulsuth W, Looney SW, Pollock DM. Endothelin ETB receptors contribute to sex differences in blood pressure elevation in angiotensin II hypertensive rats on a high-salt diet. Clin Exptl Pharmacol Physiol. 2013;40:362–370. doi: 10.1111/1440-1681.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boesen EI, Pollock DM. Acute increases of renal medullary osmolality stimulate endothelin release from the kidney. Am J Physiol Renal Physiol. 2007;292:F185–F191. doi: 10.1152/ajprenal.00021.2006. [DOI] [PubMed] [Google Scholar]
- 59.Stuart D, Rees S, Woodward SK, Koesters R, Strait KA, Kohan DE. Disruption of the endothelin A receptor in the nephron causes mild fluid volume expansion. BMC Nephrol. 2012;13:166. doi: 10.1186/1471-2369-13-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol. 2008;295:F1063–F1070. doi: 10.1152/ajprenal.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]