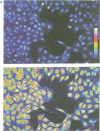
Abstract
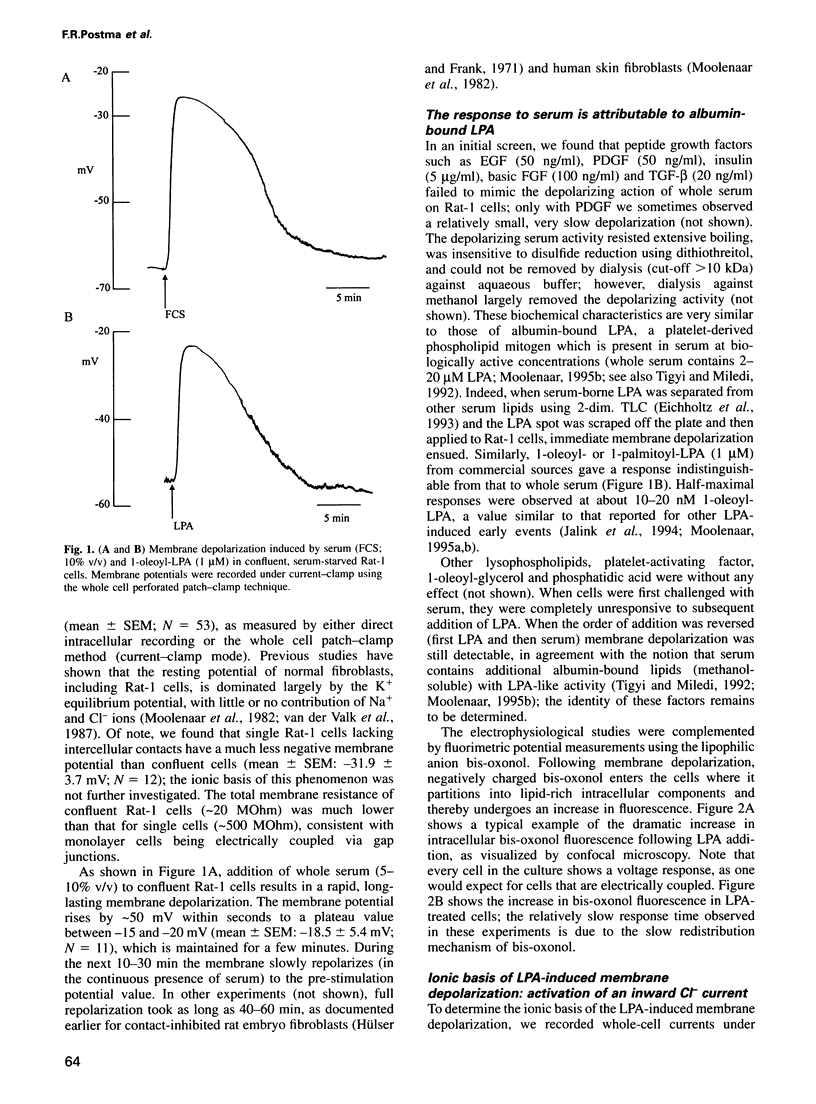
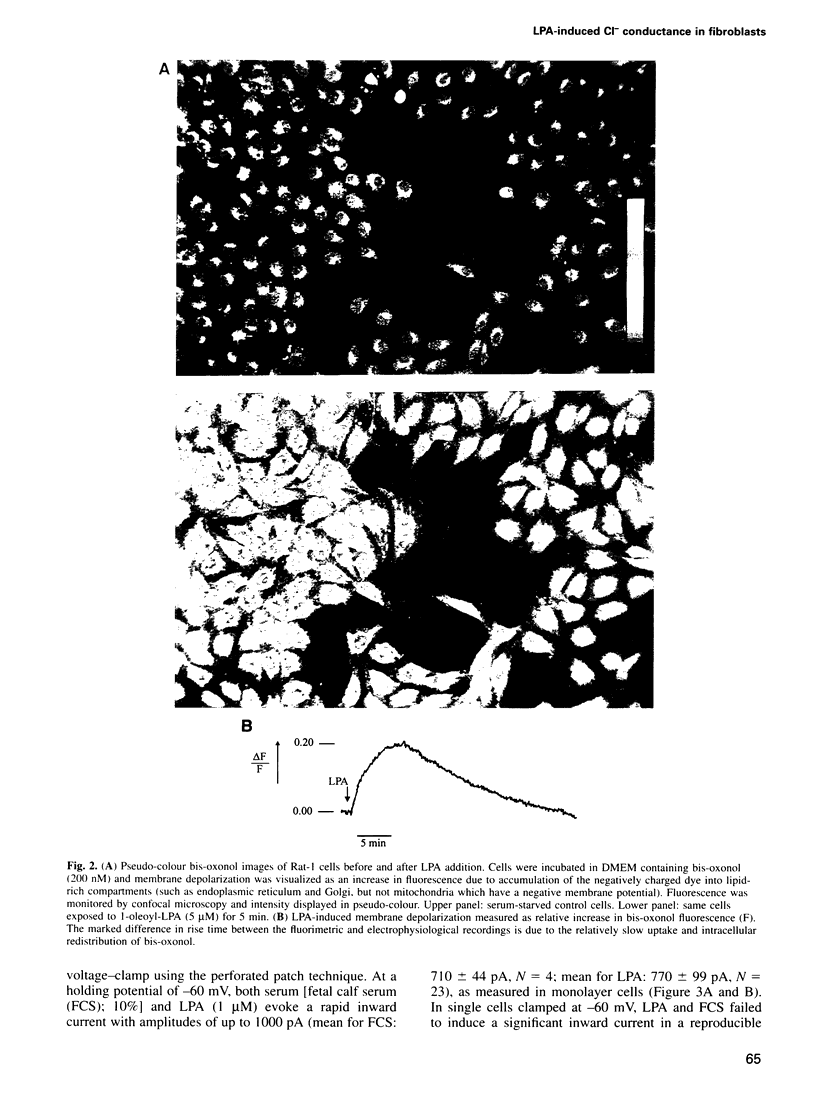
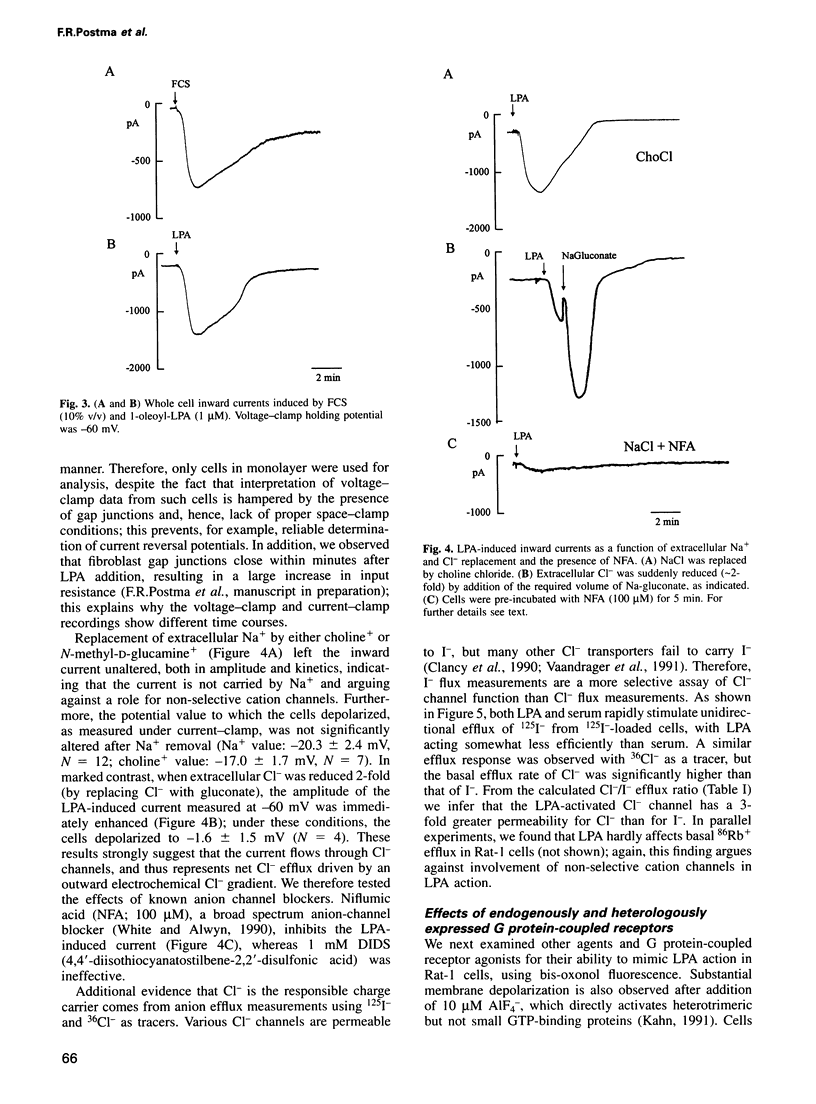
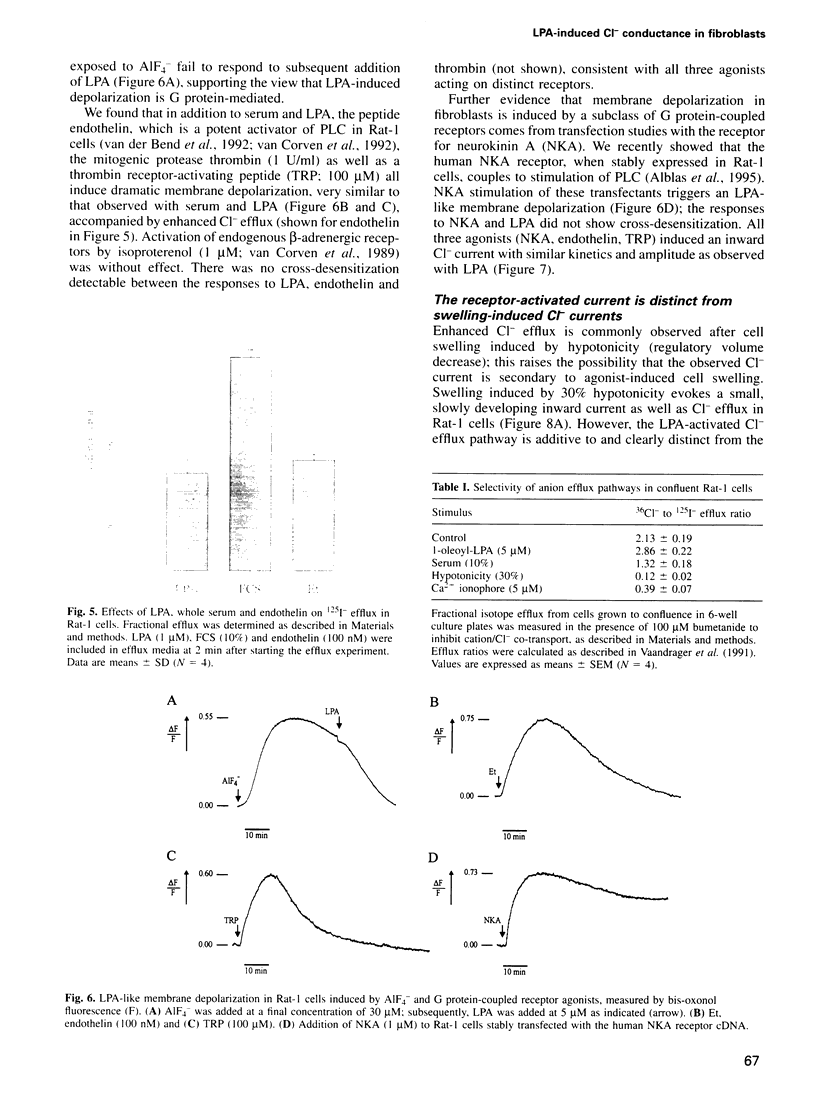
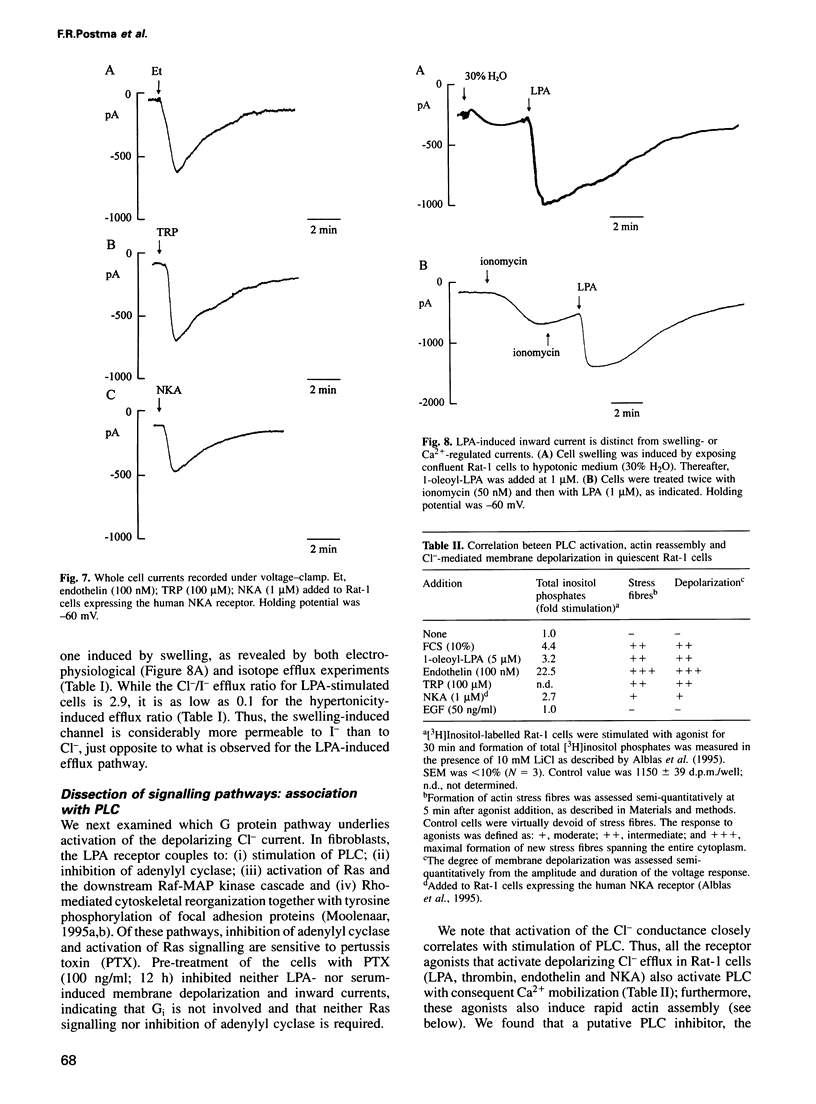
Serum stimulation of quiescent fibroblasts leads to a dramatic depolarization of the plasma membrane; however, the identity of the active serum factor(s) and the underlying mechanism are unknown. We find that this serum activity is attributable to albumin-bound lysophosphatidic acid (LPA) acting on its own G protein-coupled receptor, and that membrane depolarization is due to activation of an anion conductance mediating Cl- efflux. This depolarizing Cl- current can also be activated by thrombin and neuropeptide receptors; it is distinct from volume-regulated Cl- currents. Activation of the Cl- current consistently follows stimulation of phospholipase C and coincides with remodelling of the actin cytoskeleton, which is regulated by the Ras-related GTPase Rho. However, the response is not due to Ca2+/protein kinase C signalling and requires neither Rho nor Ras activation. The results indicate that in quiescent fibroblasts, LPA and other G protein-coupled receptor agonists evoke membrane depolarization by activating a new type of Cl- channel through a signalling pathway that is closely associated with phosphoinositide hydrolysis, yet independent of known second messengers.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alblas J., van Etten I., Khanum A., Moolenaar W. H. C-terminal truncation of the neurokinin-2 receptor causes enhanced and sustained agonist-induced signaling. Role of receptor phosphorylation in signal attenuation. J Biol Chem. 1995 Apr 14;270(15):8944–8951. doi: 10.1074/jbc.270.15.8944. [DOI] [PubMed] [Google Scholar]
- Bartoli F., Lin H. K., Ghomashchi F., Gelb M. H., Jain M. K., Apitz-Castro R. Tight binding inhibitors of 85-kDa phospholipase A2 but not 14-kDa phospholipase A2 inhibit release of free arachidonate in thrombin-stimulated human platelets. J Biol Chem. 1994 Jun 3;269(22):15625–15630. [PubMed] [Google Scholar]
- Cao G., Kuhn A., Dalbey R. E. The translocation of negatively charged residues across the membrane is driven by the electrochemical potential: evidence for an electrophoresis-like membrane transfer mechanism. EMBO J. 1995 Mar 1;14(5):866–875. doi: 10.1002/j.1460-2075.1995.tb07068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. F., Corbley M. J., Roberts T. M., Hess P. Voltage-sensitive calcium channels in normal and transformed 3T3 fibroblasts. Science. 1988 Feb 26;239(4843):1024–1026. doi: 10.1126/science.2449730. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M., Burridge K. Tyrosine phosphorylation is involved in reorganization of the actin cytoskeleton in response to serum or LPA stimulation. J Cell Sci. 1994 Dec;107(Pt 12):3643–3654. doi: 10.1242/jcs.107.12.3643. [DOI] [PubMed] [Google Scholar]
- Clancy J. P., McCann J. D., Li M., Welsh M. J. Calcium-dependent regulation of airway epithelial chloride channels. Am J Physiol. 1990 Feb;258(2 Pt 1):L25–L32. doi: 10.1152/ajplung.1990.258.2.L25. [DOI] [PubMed] [Google Scholar]
- Davis P. D., Hill C. H., Keech E., Lawton G., Nixon J. S., Sedgwick A. D., Wadsworth J., Westmacott D., Wilkinson S. E. Potent selective inhibitors of protein kinase C. FEBS Lett. 1989 Dec 18;259(1):61–63. doi: 10.1016/0014-5793(89)81494-2. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Lew P. D., Andersson T., Pozzan T. Plasma membrane potential modulates chemotactic peptide-stimulated cytosolic free Ca2+ changes in human neutrophils. J Biol Chem. 1987 Apr 5;262(10):4574–4579. [PubMed] [Google Scholar]
- Eichholtz T., Jalink K., Fahrenfort I., Moolenaar W. H. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993 May 1;291(Pt 3):677–680. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Gresser M., Young R. N. 5-Lipoxygenase. Annu Rev Biochem. 1994;63:383–417. doi: 10.1146/annurev.bi.63.070194.002123. [DOI] [PubMed] [Google Scholar]
- Frace A. M., Gargus J. J. Activation of single-channel currents in mouse fibroblasts by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2511–2515. doi: 10.1073/pnas.86.7.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslberger A., Romanin C., Koerber R. Membrane potential modulates release of tumor necrosis factor in lipopolysaccharide-stimulated mouse macrophages. Mol Biol Cell. 1992 Apr;3(4):451–460. doi: 10.1091/mbc.3.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. P., Lehmann-Horn F., Rüdel R. Overexcited or inactive: ion channels in muscle disease. Cell. 1995 Mar 10;80(5):681–686. doi: 10.1016/0092-8674(95)90345-3. [DOI] [PubMed] [Google Scholar]
- Hordijk P. L., Verlaan I., van Corven E. J., Moolenaar W. H. Protein tyrosine phosphorylation induced by lysophosphatidic acid in Rat-1 fibroblasts. Evidence that phosphorylation of map kinase is mediated by the Gi-p21ras pathway. J Biol Chem. 1994 Jan 7;269(1):645–651. [PubMed] [Google Scholar]
- Jalink K., Hordijk P. L., Moolenaar W. H. Growth factor-like effects of lysophosphatidic acid, a novel lipid mediator. Biochim Biophys Acta. 1994 Dec 30;1198(2-3):185–196. doi: 10.1016/0304-419x(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Jalink K., Moolenaar W. H. Thrombin receptor activation causes rapid neural cell rounding and neurite retraction independent of classic second messengers. J Cell Biol. 1992 Jul;118(2):411–419. doi: 10.1083/jcb.118.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen L. J., Sims S. M. Acetylcholine activates non-selective cation and chloride conductances in canine and guinea-pig tracheal myocytes. J Physiol. 1992;453:197–218. doi: 10.1113/jphysiol.1992.sp019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch T. J. Molecular physiology of anion channels. Curr Opin Cell Biol. 1994 Aug;6(4):600–606. doi: 10.1016/0955-0674(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Kahn R. A. Fluoride is not an activator of the smaller (20-25 kDa) GTP-binding proteins. J Biol Chem. 1991 Aug 25;266(24):15595–15597. [PubMed] [Google Scholar]
- Kremer S. G., Breuer W. V., Skorecki K. L. Vasoconstrictor hormones depolarize renal glomerular mesangial cells by activating chloride channels. J Cell Physiol. 1989 Jan;138(1):97–105. doi: 10.1002/jcp.1041380114. [DOI] [PubMed] [Google Scholar]
- Kumagai N., Morii N., Fujisawa K., Nemoto Y., Narumiya S. ADP-ribosylation of rho p21 inhibits lysophosphatidic acid-induced protein tyrosine phosphorylation and phosphatidylinositol 3-kinase activation in cultured Swiss 3T3 cells. J Biol Chem. 1993 Nov 25;268(33):24535–24538. [PubMed] [Google Scholar]
- Lovisolo D., Munaron L., Baccino F. M., Bonelli G. Potassium and calcium currents activated by foetal calf serum in Balb-c 3T3 fibroblasts. Biochim Biophys Acta. 1992 Dec 9;1112(2):241–245. doi: 10.1016/0005-2736(92)90397-5. [DOI] [PubMed] [Google Scholar]
- Lytton J., Westlin M., Hanley M. R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991 Sep 15;266(26):17067–17071. [PubMed] [Google Scholar]
- Moolenaar W. H. Lysophosphatidic acid signalling. Curr Opin Cell Biol. 1995 Apr;7(2):203–210. doi: 10.1016/0955-0674(95)80029-8. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H. Lysophosphatidic acid, a multifunctional phospholipid messenger. J Biol Chem. 1995 Jun 2;270(22):12949–12952. doi: 10.1074/jbc.270.22.12949. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Mummery C. L., van der Saag P. T., de Laat S. W. Rapid ionic events and the initiation of growth in serum-stimulated neuroblastoma cells. Cell. 1981 Mar;23(3):789–798. doi: 10.1016/0092-8674(81)90443-8. [DOI] [PubMed] [Google Scholar]
- Otero A. S., Yi X. B., Gray M. C., Szabo G., Hewlett E. L. Membrane depolarization prevents cell invasion by Bordetella pertussis adenylate cyclase toxin. J Biol Chem. 1995 Apr 28;270(17):9695–9697. doi: 10.1074/jbc.270.17.9695. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Rouzer C. A., Ford-Hutchinson A. W., Morton H. E., Gillard J. W. MK886, a potent and specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore-challenged leukocytes. J Biol Chem. 1990 Jan 25;265(3):1436–1442. [PubMed] [Google Scholar]
- Seckl M. J., Seufferlein T., Rozengurt E. Lysophosphatidic acid-depleted serum, hepatocyte growth factor and stem cell growth factor stimulate colony growth of small cell lung cancer cells through a calcium-independent pathway. Cancer Res. 1994 Dec 1;54(23):6143–6147. [PubMed] [Google Scholar]
- Seufferlein T., Rozengurt E. Lysophosphatidic acid stimulates tyrosine phosphorylation of focal adhesion kinase, paxillin, and p130. Signaling pathways and cross-talk with platelet-derived growth factor. J Biol Chem. 1994 Mar 25;269(12):9345–9351. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. K., Mostafapour S. P., Denlinger L. C., Bleasdale J. E., Fisher S. K. The aminosteroid U-73122 inhibits muscarinic receptor sequestration and phosphoinositide hydrolysis in SK-N-SH neuroblastoma cells. A role for Gp in receptor compartmentation. J Biol Chem. 1991 Dec 15;266(35):23856–23862. [PubMed] [Google Scholar]
- Tigyi G., Miledi R. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J Biol Chem. 1992 Oct 25;267(30):21360–21367. [PubMed] [Google Scholar]
- Vaandrager A. B., Bajnath R., Groot J. A., Bot A. G., De Jonge H. R. Ca2+ and cAMP activate different chloride efflux pathways in HT-29.cl19A colonic epithelial cell line. Am J Physiol. 1991 Dec;261(6 Pt 1):G958–G965. doi: 10.1152/ajpgi.1991.261.6.G958. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C., Lazdunski M. Endothelin and vasopressin activate low conductance chloride channels in aortic smooth muscle cells. Pflugers Arch. 1993 Oct;425(1-2):156–163. doi: 10.1007/BF00374516. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Smith A. E. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993 Jul 2;73(7):1251–1254. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- White M. M., Aylwin M. Niflumic and flufenamic acids are potent reversible blockers of Ca2(+)-activated Cl- channels in Xenopus oocytes. Mol Pharmacol. 1990 May;37(5):720–724. [PubMed] [Google Scholar]
- van Corven E. J., Groenink A., Jalink K., Eichholtz T., Moolenaar W. H. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989 Oct 6;59(1):45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- van Corven E. J., van Rijswijk A., Jalink K., van der Bend R. L., van Blitterswijk W. J., Moolenaar W. H. Mitogenic action of lysophosphatidic acid and phosphatidic acid on fibroblasts. Dependence on acyl-chain length and inhibition by suramin. Biochem J. 1992 Jan 1;281(Pt 1):163–169. doi: 10.1042/bj2810163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bend R. L., de Widt J., van Corven E. J., Moolenaar W. H., van Blitterswijk W. J. The biologically active phospholipid, lysophosphatidic acid, induces phosphatidylcholine breakdown in fibroblasts via activation of phospholipase D. Comparison with the response to endothelin. Biochem J. 1992 Jul 1;285(Pt 1):235–240. doi: 10.1042/bj2850235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Valk J., Verlaan I., de Laat S. W., Moolenaar W. H. Expression of pp60v-src alters the ionic permeability of the plasma membrane in rat cells. J Biol Chem. 1987 Feb 25;262(6):2431–2434. [PubMed] [Google Scholar]