Abstract
Introduction.
Urolithiasis is a multifactorial disease. Changes in social and economic living conditions have generated changes in chemical composition of urolith too. Although calcium is a predominant crystalline constituent of kidney stones in 80% of cases, metabolic disorders are not the main reason for their formation. Hyperparathyroidism may be a cause of occurrence of calcium lithiasis, however, the biggest number of its occurrence is not a consequence of elevated values of parathormone. Acid uric has a pervasive presence in all body fluids. The serum level of acid uric is determined by its rate of synthesis, rate of excretion by kidney and gastrointestinal tract, and metabolism.
Goal.
The goal of our study is to determine a correlation of calcium lithiasis of the upper part of the urinary tract with the parathormone values and the concomitant values of acidum uricum.
Material and methods.
The study was prospective and included 120 patients with calcium lithiasis of the upper part of urinary tract, divided in three age categories, 20-40 years, 40-60 years and older than 60 years. The diagnosis of calcium lithiasis of the upper part of the urinary tract was made on the basis of urinary tract ultrasonography, and kidney-ureter-bladder radiography (KUB) /intravenous urography (IVU), urine culture and chemical analysis of stone with patients who had a spontaneous emission of stone or following some of the methods for active removal of stone; with some patients non-contrast (NCCT) was carried out too. All patients were subjected to the laboratory analysis of the serum level of acidum uricum and parathormone.
Results.
With observed 120 patients suffering from calcium urolithiasis, who belonged to adult population, no patient had an elevated value of parathormone, while three patients (2.5%) had the values of acidum uricum higher than the reference values. The average value (for both parameters) was the lowest with the youngest patients and vice versa, and only in the group of 40 to 60 years of age there were patients whose values of the acidum uricum parameter was outside the interval of reference values; the other age groups did not have such values. Based on the analysis of the variance, as a statistical method, it was determined that the average values of acidum uricum in different age groups were statistically significantly different, which is not the case for the parameter parathormone. (p>0,05).
Conclusion.
The biggest number of nephrolithiasis is not a consequence of elevated values of parathormone. Hyperuricosemia may be present with calcium urolithiasis, without participation in forming kidney stones, most probably as an indirect sign of the existence of the initial insulin resistance and metabolic disease.
Keywords: calcium lithiasis, parathormone, acidum uricum
1. INTRODUCTION
Urinary stones may be classified with regards to the size, localization, X-ray characteristics, etiology, composition, the ‘risk of recurrence’ (1-3, 4). Stones and stone fragments should be analyzed to determine their structure. The leading analytic procedures include x-ray crystallography and infrared spectroscopy. Every patient should have at least one stone analyzed, whenever possible. If the stones are not analyzed, the conclusions about their structure may be based on other observations, i.e. qualitative cystine test with sodium nitroprusside, bacteriuria or urine culture test, showing of crystals of struvite or cystine in urine sediment, serum urates (when the uric acid stones or urate stones are suspected), pH of urine (it is low with the patients with stones from uric acid, and high with the patients with infective stones), and radiographic appearance of stones (4). Based on classification of stones with regards to X-ray and on the basis of kidney-ureter-bladder (KUB) radiography, stones are divided into “radiopaque, poor radiopacity and radiolucent”.
“Radiopaque” includes calcium stones (calcium oxalate dihydrate, calcium oxalate monohydrate, calcium phosphate), “poor radiopacity” magnesium ammonium phosphate, apatite and cystine, while “radiolucent” includes urine acid, ammonium urate, xanthine, 2,8-dihydroxyadenine and drug-stones (5). The sensitivity and specificity of KUB radiography is 44-77% and 80-87% (6). KUB radiography should not be performed if NCCT is considered (7). However, it is helpful in differentiating between radiolucent and radiopaque stones.
The pathogenesis of kidney stones includes many factors: age, sex, obesity, hypertension, hyperparathyroidism, gastrointestinal diseases, genetically caused urolithiasis, medicines and anatomic anomalies associated with the occurrence of urolith, as well as different external factors such as occupation, physical activity, diet, geographical location of living (4,8-12). Hyperparathyroidism may be a cause of occurrence of calcium lithiasis, however the biggest number of it is not a consequence of increased values of parathormone (PTH). Acid uric (ac. uricum) has a pervasive presence in all body fluids. The serum level of acid uric is determined by its rate of synthesis, the rate of excretion by kidney and gastrointestinal tract, and metabolism (13).
The goal of our study was to determine the correlation of calcium lithiasis of the upper part of the urinary tract with the values of parathormone and concomitant values of acidum uricum.
2. MATERIAL AND METHODS
The study was prospective and was carried out at the Clinical Center in Banja Luka, at the Urology Clinic during the period from 01 April 2012 to 01 January 2013. It included 120 patients with calcium lithiasis of the upper part of the urinary tract. The patients were divided into three age categories, 20-40 years, 40-60 years and older than 60 years. The diagnosis of calcium lithiasis of the upper part of the urinary tract was made on the basis of ultrasonography of urinary tract and kidney-ureter-bladder radiography (KUB) /intravenous urography (IVU), urine culture and chemical analysis of the stone with the patients who had a spontaneous emission of the stone or after some of the methods for active stone removal. With a number of patients, a non-contrast CT (NCCT) was done too. The ultrasound examinations were done by using LOGIQ 5 PRO sonograph, with transabdominal 3.5 MHz probe. Generally speaking, an ultrasound examination can detect any stone, regardless of its chemical composition. Pathognomonic sign of calcium urolithiasis on KUB implies the presence of the mineral shadow of calcium intensity. Intravenous urography, besides the pathognomonic sign of calcium urolithiasis on the x-ray image, provided insight in the functional kidney status, as well as in the morphological presentation of pyelocaliceal system of kidney, ureter and urinary bladder with defining whether there was an obstruction in urine derivation in the sense of anatomic or functional obstruction that may have caused the occurrence of urolithiasis. Sterile urinary culture was used to exclude the stones with infection. NCCT, which can determine stone density as well as the internal structure of the stone, was carried out with some patients. All patients were subjected to the laboratory analysis of acidum uricum and parathormone at the Clinical Center Central Laboratory. In order to determine the above parameters, 3 ml of blood (1-2 ml of serum) were taken, with vacuumized test tubes and treated with COBAS 6000 device. The normal values of serum acidum uricum for adults older than 18 years on the mentioned devices were at the level 142-339, and of parathormone at the level of 15-65.
3. RESULTS
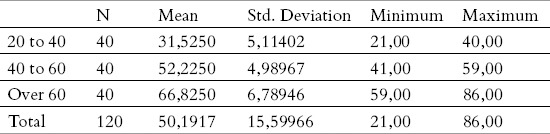
From a demographic point of view, there were 120 patients in the working sample, were grouped in three age categories: 20-40, 40-60 and over 60 years. Each age group consisted of 40 subjects.
The average age was 50.19 years, with standard deviation of 15.60, while, on the other hand, the sex structure was not quite even, as there were 52.5% men and 47.5% women.
The main descriptive measures, on the total sample, and depending on the age category (average, minimum, maximum, deviation) are given in Tables 1 and 2.
Table 1.
Demographic characteristics of the total sample
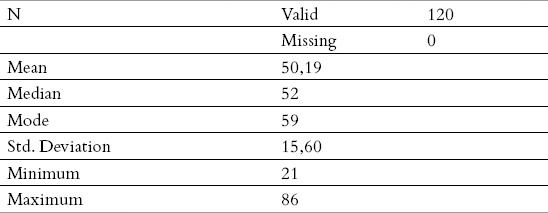
Table 2.
Demographic characteristics of the sample by age categories.
The values of acid uric and parathormone in relation to the age category are shown in Tables 3 and 4. Something that is characteristic for both parameters is that a very small number of patients were outside the allowed intervals, i.e. three patients (2,5%) had the value of acid uricum higher than the reference values, while for PTH parameter, there were no patients at all with the values outside the allowed interval.
Table 3.
Descriptive measures for the parameters acidum uricum and PTH
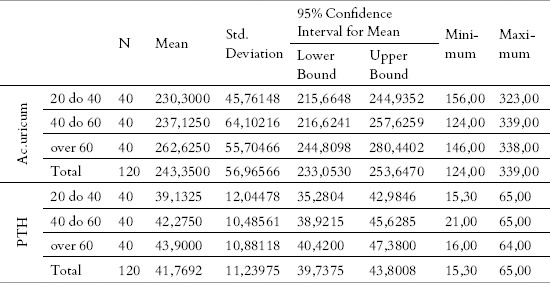
Table 4.
ANOVA for the parameters Ac.uricum and PTH
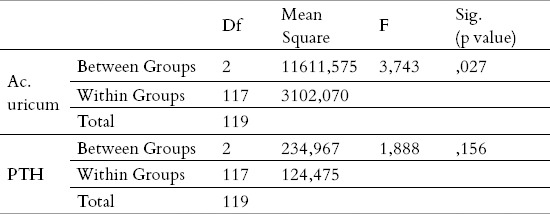
In order to conclude whether the average values within the three age groups differ, from the point of view of these two parameters, we used a statistical method – the analysis of variance (ANOVA). It can be seen in Table 3 that the average value within the groups is different, concerning both parameters; in order to be able to say whether this difference is statistically important too, using the stated test, the results are presented in Table 4. Something that can be noticed is that the average value was the lowest (for both parameters) with the youngest patients, and vice versa. In the last columns (Table 3) it is obvious that only in the group from 40 to 60 years there were patients who had the value of the parameter of acidum uricum outside the interval which is tolerant, while the other age groups did not have such values.
The analysis of variance, as a statistical method, was used in order to infer whether the average values were (un)even between the groups, and since these were three equal groups (n=40), the requirements for the application of the mentioned test were met. In this case we obtained different values for these two parameters, because the average values of acidum uricum were statistically significantly different from one another, which is not the case for the parameter PTH (p>0,05).
4. DISCUSSION
The epidemiology of urolithiasis differs depending on geographic regions, sex, age, chemical composition and the localization of urolith. Such differences may be explained in the context of the race, diets and climate conditions. Changes of socio-economic living conditions have also resulted in the changes in the chemical composition of urolith. (14). According to literature, the incidence of renal stones of all race groups is similar speaking of metabolic abnormalities, suggesting that the influences of the external environment and the diet, i.e. the living habits are very important in the etiology of stone disease (15). Although calcium is a predominant crystalline constituent of kidney stones in 80% of cases, metabolic disorders are not the prevailing reason for their occurrence (16). Hyperparathyroidism may be a cause of occurrence of calcium lithiasis, however the biggest number of it is not a consequence of elevated values of parathormone and most patients have idiopathic hypercalciuria without hypercalcemia. However, it should be underlined that the stones of the upper part of the urinary tract with children are more often associated with metabolic disorders than with the urinary tract anomalies and infections (17). Acid uric has a pervasive presence in all body fluids. The serum level of acid uric is determined by its rate of synthesis, rate of excretion by kidney and gastrointestinal tract, and metabolism. Speaking of ammonium acid urate calculi (AAU), they are a rare type of urolithiasis in developed countries, while in the developing countries they are endemic. AAU are observed with 0.38% of the Japanese with urinary stones (18). Pure AAU occur predominantly with young, thin women, while the laboratory signs besides the elevated values of acidum uricum exhibit also a low level of serum proteins and potassium and urine pH, although hyperuricosuria is not always associated with hyperuricosemia either (18). The mixed AAU more frequently occurs with middle-age men. (18). Type 2 diabetes, obesity and hypertension may be associated with nephrolithiasis, in particular diabetes may be a factor in the development of uric acid stones (19). Insulin resistance, elevated values of ac. uricum with a predisposition of forming acidum uric stone may precede the diagnosis of diabetes in the following decades (20). Interestingly, hyperuricosuria is also associated with increased calcium containing stone formation, and is believed to be related to the uric acid crystals acting as a nidus on which calcium oxalate and calcium phosphate can precipitate (21).
The results of our study have shown that, of observed 120 patients with calcium urolithiasis, who belonged to the adult population, no patient had an elevated value of parathormone, while three patients (2.5%) had a value of acidum uricum higher than the reference values. The average value (for both parameters) was the lowest with the youngest patients and vice versa, while only in the group of age from 40 to 60 years, there were the patients who had the value of acidum uricum parameter outside the interval of reference values, while the other age groups did not have such values. Based on the analysis of variance, as a statistical method, the average values of acidum uricum in different age groups significantly differ statistically, which is not the case for the parameter PTH (p>0,05). Concomitantly elevated value of acidum uricum in the age group of 40-60 years was not a cause of occurrence of urate lithiasis, but may quite certainly be associated with insulin resistance, as well as with other characteristics of metabolic syndrome, the incidence of which rises with an increase in age.
5. CONCLUSION
The biggest number of occurrence of calcium nephrolithiasis is not a consequence of elevated values of parathormone. Hyperuricosemia may be present with calcium urolithiasis, without participation in forming kidney stones, most probably as an indirect sign of the existence of initial insulin resistance and metabolic syndrome.
Footnotes
CONFLICT OF INTERESTS: NONE DECLARED.
REFERENCES
- 1.Leusmann DB. Whewellite, weddellite and company: whwrw do all the strange names originate? BJU Int. 2000;862(4):411–413. doi: 10.1046/j.1464-410x.2000.00860.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim Sc, Burns EK, Lingeman JE, et al. Cystine calculi: correaltion of CT-visible structure, CT number and stone morphoplogy with fragmentationby shockwave lithotripsy. Urol Res. 2007;35(6):319–324. doi: 10.1007/s00240-007-0117-1. [DOI] [PubMed] [Google Scholar]
- 3.Hesse A, Brandle E, Wilbert D, et al. Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs. 2000. Eur Urol. 2003;44(6):709–713. doi: 10.1016/s0302-2838(03)00415-9. [DOI] [PubMed] [Google Scholar]
- 4.Hesse AT, Tiselius HG, Siener R, Basel S, Karger AG, et al., editors. 3rd edn. 2009. Urinary Stones, Diagnosis, Treatment and Prevention of Recurrence. ISBN 978-3-8055-9149-2. [Google Scholar]
- 5.Milicevic S, Bijelic R, Krivokuca V, Bojic M, Popovic-Pejicic S, Bojanic N. Correlation of the Body Mass Index and Calcium Nephrolithiasis in Adult Population. Med Arh. 2013 Dec;67(6):393–396. doi: 10.5455/medarh.2013.67.423-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidenreich A, Desgrandschamps F, Terrier F. Modern approach of diagnosis and management of acute flank pain: review of all modalities. Eur Urol. 2002;41(4):351–362. doi: 10.1016/s0302-2838(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 7.Kennish SJ, Bhatnagar P, Wah TM, et al. Is rthe KUBradiograph redundant for inmvestigating acute ureteric colicin rthe non-contrast enhanced computedtomography era? Clin Radiol. 2008;63(10):1131–5. doi: 10.1016/j.crad.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Massimo A. The genetic components of idiopathic nephrolithiasis. Pediatr Nephrol. 2011;26:337–346. doi: 10.1007/s00467-010-1562-6. [DOI] [PubMed] [Google Scholar]
- 9.Keoghane S, Walmsley B, Hodgson D. The natural history of untreated renal tract calculi. BJU Int. 2010;105(12):1627–1629. doi: 10.1111/j.1464-410X.2010.09389.x. [DOI] [PubMed] [Google Scholar]
- 10.Basiri A, Shakhssalim N, Khoshdel AR, et al. Familial relations and recurrence pattern in nephrolithiasis: new words about old subjects. Urol J. 2010;7(2):81–86. [PubMed] [Google Scholar]
- 11.Goldfarb DS, Fischer ME, Keich Y, et al. A twin study of genetic and dietary influences on nephrolithiasis: a report from the Vietnam Era Twin (VET) Registry. Kidney Int. 2005;67(3):1053–1061. doi: 10.1111/j.1523-1755.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 12.Durrani O, Morrisroe S, Jackman S, et al. Analysis of stone disease in morbidly obese patients undergoing gastric bypass surgery. J Endourol. 2006;20(10):749–752. doi: 10.1089/end.2006.20.749. [DOI] [PubMed] [Google Scholar]
- 13.Wiederkehr MR. Uric Acid Nephrolithiasis: A systemastic Metabolic Disorder. Clin Rev Bone Miner Metab. 2011;9(3-4):207–217. doi: 10.1007/s12018-011-9106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Triunchieri A. Epidemiology of urolithiasis: an update. Clin Cases Miner Bone Metab. 2008;5(2):101–106. [PMC free article] [PubMed] [Google Scholar]
- 15.Maloney ME, Springhart WP, Ekeruo WO, Young MD, Enemchukwu CU, Preminger GM. Ethnic background has minimal impact on the etiology of nephrolithiasis. J Urol. 2005;173:2001–2004. doi: 10.1097/01.ju.0000159076.70638.1e. [DOI] [PubMed] [Google Scholar]
- 16.Amanzadeh J, Gitomer WL, Zerwekh JE, et al. Effect of high protein diet on stone-forming propensity and bone loss in rats. Kidney International. 2013;64:2142–2149. doi: 10.1046/j.1523-1755.2003.00309.x. [DOI] [PubMed] [Google Scholar]
- 17.Coward RJ, Peters CJ, Duffy PG, et al. Epidemilogy of paediatric renal stone disease in the UK. Arch Dis Child. 2003;88:962–965. doi: 10.1136/adc.88.11.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuruma H, Arakawa T, Kubo S, et al. Ammonium acid urate urolithiasis in Japan. Int J Urol. 2006;13:498–501. doi: 10.1111/j.1442-2042.2006.01348.x. [DOI] [PubMed] [Google Scholar]
- 19.Lieske JC, de la Vega LS, Gettman MT, et al. Diabetes mellitus and the risk of urinary tract stones: a population-based case-control study. Am J Kidney Dis. 2006;48:897–903. doi: 10.1053/j.ajkd.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68:1230–1235. doi: 10.1111/j.1523-1755.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 21.Fishman MC, Hoffman AR. Lippincott Williams & Wilkins; 2004. Medicine. ISBN:0781725437. [Google Scholar]


