Abstract
The functional impact of altered drug transport protein expression on the systemic pharmacokinetics of morphine, hepatically-derived morphine glucuronide (morphine-3- and morphine-6-glucuronide), and fasting bile acids was evaluated in patients with biopsy-confirmed non-alcoholic steatohepatitis (NASH) compared to healthy subjects. The maximum concentration (Cmax) and area under the concentration-time curve (AUC0-last) of morphine glucuronide in serum were increased in NASH patients (343 vs. 225nM and 58.8 vs. 37.2μM*min, respectively; P≤0.005); morphine pharmacokinetics did not differ between groups. Linear regression analyses detected an association of NASH severity with increased morphine glucuronide Cmax and AUC0-last (P<0.001). Fasting serum glycocholate, taurocholate and total bile acid concentrations were associated with NASH severity (P<0.006). Increased hepatic basolateral efflux of morphine glucuronide and bile acids is consistent with altered hepatic transport protein expression in patients with NASH and may partially explain differences in efficacy and/or toxicity of some highly transported anionic drugs/metabolites in this patient population.
Keywords: non-alcoholic steatohepatitis, multidrug resistance-associated protein 3, morphine, pharmacokinetics, drug transport
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a progressive liver disease ranging from simple steatosis to cirrhosis of the liver. Non-alcoholic steatohepatitis (NASH) is an intermediate pathologic state on the pathway from simple steatosis to cirrhosis that is characterized by hepatocyte steatosis, ballooning, inflammation and fibrosis.(1) NASH is strongly associated with insulin resistance and metabolic syndrome. NAFLD is believed to be the most common cause of chronic liver disease in the Western world and is recognized as a major public health concern.(2) Equally concerning is the fact that many patients are unaware of their disease until progression to advanced stages has occurred.(3) Left untreated, NAFLD and NASH can progress to advanced fibrosis, cirrhosis and hepatocellular carcinoma, ultimately requiring liver transplantation in some patients.(1) Medications such as metformin, pioglitazone, rosiglitazone and rosuvastatin have been used to improve insulin resistance to reverse the inflammation associated with NAFLD and prevent fibrosis.(4-7) Although modest reductions in serum liver function markers were observed, histologically, the progression of fibrosis was not reliably prevented or did not regress for any tested pharmacologic intervention. The major site of action of these medications is within hepatocytes; thus, diminished activity may be partially due to decreased hepatocellular concentrations.(8)
The dynamic interplay between hepatic sinusoidal uptake transporters, drug metabolizing enzymes, and basolateral and/or canalicular efflux transporters regulates hepatocellular concentrations and exposure to medications and their metabolites.(9, 10) Modest changes in one or more of these processes can impact systemic and/or hepatocellular concentrations.(11, 12) Bile acid concentrations are regulated by these factors, as well as de novo synthesis within hepatocytes; intestinal hydrolysis and transporters, which facilitate reabsorption, also contribute to bile acid exposure.(13) Recently, expression of many uptake and efflux transporters and drug metabolizing enzymes was evaluated in liver samples from patients with NASH compared to healthy subjects. In general, NASH down-regulated transport proteins responsible for hepatic uptake of drugs and bile acids (e.g., organic anion transporting polypeptides [OATPs], sodium-taurocholate cotransporting polypeptide [NTCP]) and up-regulated hepatic excretory proteins (e.g., multidrug resistance-associated protein [MRP]3, MRP4, breast cancer resistance protein [BCRP], MRP2).(14-17) However, the impact of the observed up-regulation in MRP2 protein is difficult to predict due to altered localization of this canalicular protein. In contrast to the predictable nature of the changes observed with transporters (i.e., decreased uptake and increased efflux), cytochrome P450s, uridine-5’-diphospho-glucuronosyltransferases (UGTs), and sulfotransferases generally were unchanged with few exceptions.(16) Altered transporter expression in NASH patients is expected to impair hepatic uptake and shift excretion of anionic drugs/hepatically-derived metabolites from bile to sinusoidal blood, resulting in decreased liver exposure and increased systemic concentrations/exposure. These changes may decrease activity or efficacy of highly transported bioactive endogenous substrates, medications and/or hepatically-derived metabolites with an intrahepatic site of action.(4-7)
Despite a general understanding of the impact of decreased transporter-mediated hepatic uptake of medications on systemic and hepatic concentrations(11, 18), the importance of altered basolateral efflux is underappreciated. The functional impact of altered uptake and/or efflux transporter expression has not been evaluated in NASH patients.(9, 10, 19) Therefore, the primary objective of this study was to assess the functional impact of altered hepatic transporter expression in patients with biopsy-confirmed NASH compared to healthy subjects. Hepatically-derived glucuronide conjugates of morphine were selected as a phenotypic probe to evaluate MRP3 function for several reasons: 1) morphine enters the hepatocyte via a combination of passive diffusion and active transport by organic cation transporter 1 (OCT1)(20) and is unaffected by changes in uptake transporters known to be altered in NASH(17); 2) hepatic expression of UGT2B7, which metabolizes morphine to morphine-3- and -6-glucuronide, is not altered in NASH(9, 10, 16, 21); 3) morphine is eliminated almost exclusively as morphine glucuronide in the urine, suggesting that basolateral efflux is important in the systemic and hepatic disposition; and 4) MRP3 appears to be the only protein that transports hepatically-derived morphine glucuronide into sinusoidal blood.(22, 23) Additionally, Mrp3 protein expression positively correlated with basolateral efflux clearance of the Mrp3 substrate acetaminophen glucuronide in rat, and human UGT2B7 polymorphisms that exhibit decreased function appear to have minimal impact on the systemic concentrations of morphine glucuronide.(24, 25) Therefore, systemic morphine glucuronide concentrations should increase in patients with NASH due to MRP3 up-regulation compared to healthy subjects, whereas the pharmacokinetics of parent morphine should be unaffected. Altered transporter expression in patients with NASH could have implications for other MRP3-transported substrates such as endogenous glycocholate and taurocholate.(26) Although bile acids are formed in the liver and generally undergo efficient enterohepatic recirculation(27), increased serum concentrations under fasting conditions would be consistent with decreased hepatic uptake and increased basolateral efflux. Therefore, the secondary objective of this study was to evaluate fasting serum concentrations of total bile acids, glycocholate and taurocholate in patients with NASH compared to healthy subjects.
Results
Study Subjects
Twenty-one volunteers (14 healthy subjects and 7 patients with biopsy-confirmed NASH) were studied (Table 1; see Supplementary Figure 1 for a summary of volunteer recruitment, enrollment and completion). In selecting healthy subjects, effort was made to recruit a control cohort comparable to the NASH cohort with respect to age, sex, race, and ethnicity. As expected, patients with NASH weighed more and had a higher body mass index (BMI) than the healthy subjects. Serum creatinine was within normal limits for both groups (Table 2), resulting in estimated glomerular filtration rates within normal limits for all subjects. Compared to healthy subjects, patients with NASH had higher serum alanine aminotransferase (ALT), alkaline phosphatase (ALP), triglycerides, and insulin resistance as demonstrated by increased fasting glucose and insulin levels, as well as homeostasis model for assessing insulin resistance (HOMA-IR) scores, and decreased HDL (Table 2). Biopsy results indicated that all NASH patients had a NAFLD activity score (NAS) of at least 4, with a maximum score of 6 and minimal to moderate (F0 to F3) fibrosis (Table 2).
Table 1.
Demographic characteristics of study participants.
| Parameter | Healthy (n=14) | NASH (n=7) | |
|---|---|---|---|
| Sex | |||
| Men | 7 | 3 | |
| Women | 7 | 4 | |
| Ethnicity | Hispanic | 1 | 1 |
| Non-Hispanic | 13 | 6 | |
| Race | |||
| White | 12 | 7 | |
| Black | 2 | 0 | |
| Age (years) | 42 (13) | 48 (10) | |
| Body weight (kg) | 76 (14) | 93 * (17) | |
| Body mass index (kg/m2) | 26 (2.7) | 32 * (5.2) | |
| Waist circumference (cm) | 90 (7.8) | 118 * (23.5) | |
| Hip circumference (cm) | 103 (6.4) | 117 * (16.4) | |
| Waist-to-hip ratio | All subjects | 0.87 (0.05) | 1.03 * (0.26) |
| Men | 0.90 (0.03) | 0.96 * (0.01) | |
| Women | 0.84 (0.06) | 1.08 (0.36) | |
Data presented as mean (SD)
P<0.05, Student's two-tailed t-test comparing healthy subjects to patients with NASH
Table 2.
Serum chemistries, insulin resistance and liver biopsy grade.
| Clinical Parameter | Healthy | NASH |
|---|---|---|
| Creatinine (mg/dL) | 0.82 (0.14) | 0.80 (0.22) |
| ALT (U/L) | 33 (11) | 75 * (36) |
| ALP (U/L) | 63 (13) | 80 * (14) |
| Albumin (g/dL) | 4.3 (0.3) | 4.5 (0.4) |
| Total Bilirubin (mg/dL) | 0.64 (0.22) | 0.81 (0.29) |
| Cholesterol (mg/dL) | 189 (40) | 190 (48) |
| Triglycerides (mg/dL) | 91 (46) | 253 * (98) |
| HDL (mg/dL) | 68 (26) | 37 * (5) |
| LDL, calculated (mg/dL) | 102 (34) | 101 (47) |
| Fasting Glucose (mg/dL) | 86 (8) | 124 * (16) |
| Serum Insulin (μIU/mL) | 8 (3) | 40 * (27) |
| HOMA-IR | 2 (1) | 12 * (9) |
| Total NAS Score | N/A | 5 (4-6) |
| Steatosis | N/A | 2 (1-3) |
| Hepatocyte Ballooning | N/A | 2 (1-3) |
| Inflammation | N/A | 1 (0-2) |
| Fibrosis | N/A | 1 (0-3) |
| NAS + Fibrosis | N/A | 7 (4-8) |
Data presented as mean (SD); biopsy scoring presented as median (range)
ALT; alanine aminotransferase
ALP; alkaline phosphatase
HDL; high-density lipoprotein cholesterol
LDL; low-density lipoprotein cholesterol
NAS: non-alcoholic fatty liver disease activity score
HOMA-IR; homeostasis model for assessing insulin resistance
N/A; not applicable
P<0.05, Student's two-tailed t-test comparing healthy subjects to patients with NASH
Morphine Pharmacokinetics
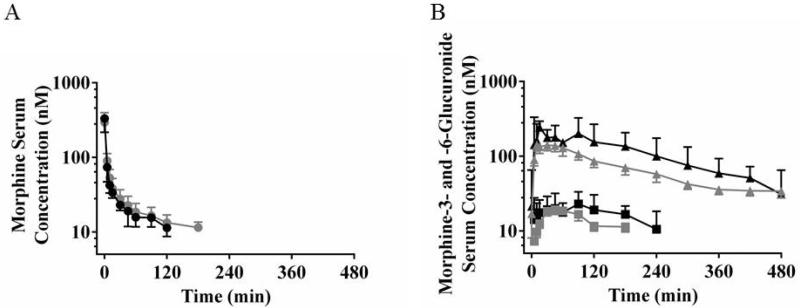
The presence of NASH had minimal impact on the pharmacokinetics of intravenously administered morphine (Figure 1A and Table 3). No statistically significant mean differences were observed between subject groups for maximum serum concentration (Cmax), area under the serum concentration-time curve to the last measured timepoint (AUC0-last), AUC extrapolated to infinity (AUC0-∞), total mass of morphine excreted in urine over the 8-hr collection interval (Xurine), terminal volume of distribution (Vz), total body clearance (CL), or half-life (t1/2). Cmax for each subject was always at the timepoint immediately following the end of the infusion. The geometric mean Cmax and AUC0-last for NASH patients were within 12% and 17%, respectively, of the corresponding values for healthy subjects (Table 3).
Figure 1.
Morphine (circles, A), morphine-3-glucuronide (triangles, B), and morphine-6-glucuronide (squares, B) serum concentration vs. time profiles in healthy subjects (grey) and patients with NASH (black). Data are presented as geometric mean and 95% confidence intervals.
Table 3.
Pharmacokinetic parameters for morphine and morphine glucuronide determined by non-compartmental analysis in healthy subjects and patients with NASH.
| Morphine | Morphine Glucuronidea | |||
|---|---|---|---|---|
| Metric | Healthy | NASH | Healthy | NASH |
| Cmax (nM) | 296 (237-369) | 332 (200-551) | 225 (194-261) | 343 * (284-413) |
| Tmaxb (min) | 0 (0-0) | 0 (0-0) | 38 (5-240) | 15 (5-90) |
| AUC0-last (μM*min) | 4.1 (3.1-5.3) | 3.5 (2.4-5.0) | 37.2 (31.6-43.7) | 58.8 * (41.6-83.0) |
| AUC0-∞ (μM*min) | 5.5 (4.3-6.9) | 5.2 (3.7-7.4) | 45.2 (37.5-54.5) | 67.8 * (46.7-98.5) |
| Vz (L) | 153 (125-188) | 173 (113-264) | N/D | N/D |
| CL (L/min) | 1.2 (1.0-1.5) | 1.3 (0.9-1.8) | N/D | N/D |
| Half-life (min) | 88 (66-117) | 95 (49-182) | 187 (153-229) | 146 (104-205) |
| Xurinec (μmol) | 0.89 (0.73-1.1) | 0.64 (0.52-0.78) | 5.4 (4.3-6.8) | 6.2 (4.3-9.1) |
Data presented as geometric mean (95% CI)
Sum of molar concentrations of morphine-3- and morphine-6-glucuronide
Median (range)
Total mass excreted over 8-hr collection interval
P<0.05, Student's two-tailed t-test of log transformed data comparing healthy subjects to patients with NASH
N/D: not determined
Morphine Glucuronide Pharmacokinetics
The geometric mean morphine-3-glucuonide AUC0-∞ was approximately 10-fold higher than morphine-6-glucuronide AUC0-∞ in healthy subjects (Figure 1), consistent with literature reports.(28, 29) The percent difference in Cmax, time to reach Cmax (Tmax), AUC0-last, AUC0-∞, and t1/2 for morphine-3-glucuronide in both subject groups (Figure 1) exhibited similar trends as morphine-6-glucuronide when evaluated separately. Therefore, for brevity, morphine-3-glucuronide and morphine-6-glucuronide molar concentrations were combined for statistical analysis and reported as “morphine glucuronide”.
Morphine glucuronide geometric mean Cmax and AUC0-last were 52% and 58% higher, respectively, in patients with NASH compared to healthy subjects following intravenous morphine administration (P = 0.001 and P = 0.005, respectively; Figure 1B and Table 3). From controls to NASH patients, the median Tmax shifted by 61% from 38 min to 15 min (P = 0.427). Although patients with NASH had slightly higher morphine glucuronide Xurine, this difference was small (0.85 μmol) and the t-test was inconclusive (P = 0.437; Table 3). The difference in morphine glucuronide t1/2 also was small (-41 minutes) and the t-test was inconclusive (P = 0.146; Table 3).
Fasting Bile Acids Serum Concentrations
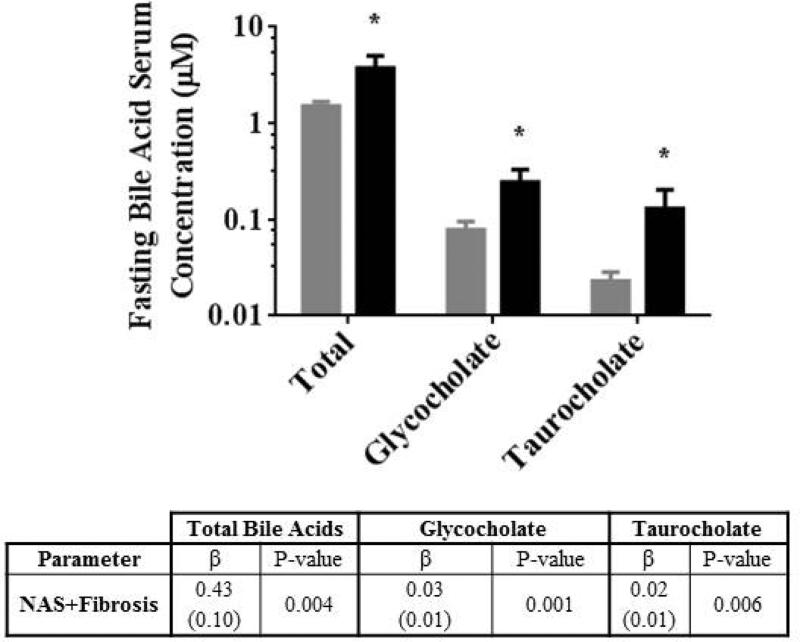
Fasting total bile acids, glycocholate and taurocholate serum concentrations in healthy subjects were 1.5±0.6, 0.08±0.06 and 0.02±0.02 μM, respectively, similar to previous reports.(30) Total bile acid, glycocholate and taurocholate serum concentrations in patients with NASH were 2.5-, 3.1-, and 5.7-fold higher, respectively, compared to healthy subjects (P = 0.022, P = 0.017, P = 0.047, respectively; Figure 2).
Figure 2.
Fasting total bile acids, glycocholate and taurocholate serum concentrations in healthy subjects (grey bars) and patients with NASH (black bars). Data are presented as mean ± SE; * P<0.05. Results of univariate linear regression analysis of NASH severity (NAS+Fibrosis; sum of non-alcoholic fatty liver disease activity and fibrosis scores) with bile acid serum concentrations are included. Data are presented as the regression parameter estimate (β) with SE of the parameter estimate in parentheses.
Linear Regression
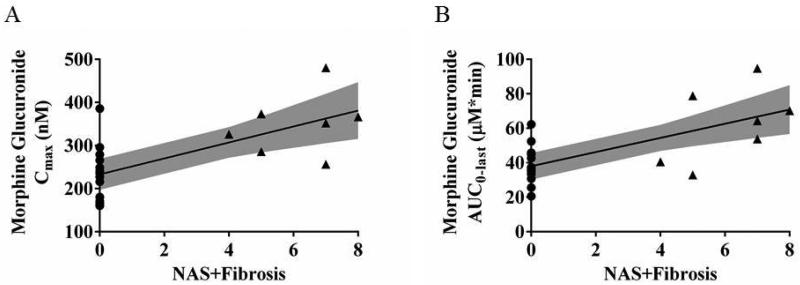
Linear regression models detected an association between the NASH severity score (NAS+Fibrosis scores) and fasting total bile acid, glycocholate and taurocholate serum concentrations (Figure 3). Linear regression models also were used to identify clinical predictors of morphine glucuronide disposition. Considered individually, the clinical predictors identified included NASH severity score, waist circumference, waist-to-hip circumference ratio, ALP, fasting glucose, triglycerides, HDL, insulin and HOMA-IR (Table 4). When these candidate clinical predictors of morphine glucuronide were included together in multivariable regression models for Cmax and AUC0-last, only NASH severity remained statistically significantly (Figure 3 and Table 4).
Figure 3.
Association of the NASH severity score (NAS+Fibrosis severity scores) with morphine glucuronide Cmax (A) and AUC0-last (B). Values for patients with NASH (triangles) and for healthy subjects (circles) are shown with the linear regression and its 95% confidence limits (shaded area). The NASH severity score is the sum of the NAS and fibrosis biopsy scores; for healthy subjects, an imputed value of zero was assumed.
Table 4.
Clinical predictors of morphine glucuronide Cmax and AUC0-last.
| Morphine Glucuronidea | ||||
|---|---|---|---|---|
| Cmax | AUC0-last | |||
| Parameter | β | P-value | β | P-value |
| NASH Severity | 18.51 (4.72) | 0.001 | 4.11 (1.02) | 0.001 |
| Waist Circumference | 1.96 (0.87) | 0.036 | 0.40 (0.19) | 0.051 |
| Waist-to-Hip Ratio | 157 (110) | 0.170 | 48.86 (22.62) | 0.044 |
| ALP | 3.14 (1.07) | 0.008 | 0.59 (0.25) | 0.027 |
| Fasting Glucose | 2.27 (0.75) | 0.007 | 0.45 (0.17) | 0.017 |
| Triglycerides | 0.42 (0.17) | 0.019 | 0.09 (0.04) | 0.024 |
| HDL | −1.75 (0.62) | 0.011 | −0.20 (0.16) | 0.216 |
| Insulin | 2.05 (0.78) | 0.017 | 0.38 (0.18) | 0.049 |
| HOMA-IR | 6.20 (2.35) | 0.016 | 1.15 (0.54) | 0.046 |
Data are presented as the regression parameter estimate (β) (standard error [SE] of the parameter estimate).
Sum of molar concentrations of morphine-3- and morphine-6-glucuronide
b Only NASH Severity remained statistically significant when all significant predictors were included in a multivariable regression model subject to backward elimination
ALP; alkaline phosphatase
NAS+Fibrosis; sum of non-alcoholic fatty liver disease activity and fibrosis scores
HOMA-IR; homeostasis model for assessing insulin resistance
Discussion
To the authors’ knowledge, this study is the first to assess the impact of a disease-associated increase in hepatic basolateral efflux transporter expression on drug disposition in humans. This study demonstrated that patients with NASH, a liver disease associated with increased protein expression of the hepatic basolateral efflux transporter MRP3, exhibited increased systemic concentrations of morphine glucuronide.(15) The primary endpoint, Cmax of hepatically-derived morphine glucuronide, which was selected based on the mathematical model developed a priori, was higher in patients with NASH compared to healthy subjects (Figure 1; Table 3). Increased morphine glucuronide Cmax in NASH likely reflects increased hepatic basolateral efflux clearance mediated by MRP3, which transports glucuronide conjugates from hepatocytes to blood.(15, 22) Multivariable regression analysis of clinical liver function markers, insulin resistance metrics and biopsy results indicated that only biopsy-determined NASH severity (NAS+Fibrosis) independently predicted increased morphine glucuronide Cmax and AUC0-last (Figure 3; Table 4). The secondary endpoint, fasting bile acid serum concentrations, was statistically significantly higher in patients with NASH compared to healthy volunteers and positively correlated with NASH severity based on biopsy scores (Figure 2). Specifically, serum concentrations of glycocholate and taurocholate, bile acids that are MRP3 substrates(26), were increased in NASH patients with varying degrees of disease progression/inflammation. These findings suggest that in the progressive inflammatory states investigated in this study, increasing NASH severity may further increase MRP3-mediated efflux clearance.
Recently, altered drug metabolizing enzyme and transporter expression was evaluated in liver biopsies from healthy subjects and from patients exhibiting varying degrees of NAFLD, from simple steatosis to steatohepatitis.(14-17) Through what appears to be an adaptive mechanism to prevent further hepatic damage, uptake transporters are generally down-regulated, whereas efflux transporters are up-regulated. Although these changes in expression patterns may protect the hepatocyte from toxins (e.g., increased bile acid concentrations), such alterations may have unanticipated effects on the efficacy of medications that rely on these transporters for hepatic entry and retention. Results from this study and prior pharmacokinetic modeling of morphine/morphine glucuronide hepatic exposure are consistent with this notion.(31)
Although systematic, comprehensive investigations of altered drug metabolism and/or transport have not been conducted for most hepatic disease states, clearance of choline following oral administration to patients with NASH was decreased compared to healthy volunteers.(32) The authors proposed that the increased systemic choline concentrations were due to decreased metabolism secondary to reduced microsomal triglyceride transfer protein function in patients with NASH. Alternatively, reduced hepatic uptake and/or increased basolateral efflux could contribute to increased systemic choline concentrations. (33) Altered hepatic drug transport was observed in rodent models of NASH.(34) For example, increased systemic and decreased hepatic exposure to simvastatin acid were reported in rats with diet-induced NASH compared to control.(34) Additionally, bile acid concentrations were increased in rodent models of steatosis.(35) Clearly, a growing body of literature demonstrates the importance of altered hepatic uptake and efflux on the systemic and hepatic disposition of many drugs, metabolites, and endogenous substrates.(32, 34, 35)
Altered hepatic transport due to disease or drug-drug interactions that shifts the predominant excretion pathway of drugs/metabolites can have unexpected consequences on systemic and hepatic disposition and ultimately, therapeutic efficacy and/or toxicity. For example, coadministration of cyclosporine, a potent inhibitor of hepatic transport proteins, with mycophenolate mofetil in transplant patients unexpectedly decreased mycophenolic acid systemic exposure.(36) Further investigation revealed that inhibition of canalicular transport likely shifted the excretion profile of mycophenolic acid glucuronide from predominantly biliary, with subsequent enterohepatic recycling of mycophenolic acid, to hepatic basolateral efflux and ultimately urinary excretion.(37) The recent failure of rosuvastatin to decrease histologic severity or prevent worsening fibrosis in patients with NASH(7) may be an example of altered transporter-mediated pharmacokinetics; decreased hepatic uptake(17, 34), with a shift toward basolateral excretion and ultimately terminal urinary elimination(38, 39), potentially decreased the hepatic exposure and therapeutic efficacy of rosuvastatin. These examples emphasize the dynamic interplay between MRP3 and MRP2 and the need to better understand how altered hepatic transport impacts the systemic and hepatic disposition of both endogenous and exogenous compounds.
Altered hepatic transporter expression may influence the disposition of endogenous compounds (e.g., bile acids) in addition to drugs/metabolites. Bile acids are formed in the liver and undergo efficient enterohepatic recirculation, resulting in low systemic serum concentrations.(30) Fasting serum concentrations of total bile acids, glycocholate, and taurocholate were increased 2.5- to 5.7-fold in patients with NASH compared to healthy controls. These changes correlate with previous reports of intrahepatic bile acid concentrations in liver tissue from patients with NASH compared to healthy subjects.(40) In addition to MRP3 up-regulation, and down-regulation of NTCP and OATPs, taurine conjugation is increased.(40) Studies are ongoing to investigate the influence of altered bile acid profiles in NASH patients on drug disposition, efficacy and toxicity.
Some limitations are associated with this work. First, this study enrolled a relatively small number of patients with NASH. However, low enrollment was balanced by increased precision obtained by recruiting 2:1 healthy subjects to NASH patients. Second, to define a homogenous population of healthy subjects, only subjects without insulin resistance and with normal liver enzymes were enrolled in an effort to exclude subjects with prevalent steatosis. Patients with probable steatosis were excluded for two reasons: 1) since biopsies were not obtained as part of this study and the prevalence of undiagnosed NAFLD/NASH is high in the overall population, differentiation of non-NAFLD healthy subjects with insulin resistance vs. prevalent simple steatosis or NASH was not possible, and 2) changes in transporter-mediated drug/metabolite disposition are only expected following onset of inflammation (i.e., NAS>3) when efflux transporter expression is up-regulated.(15, 17) Although clinical liver biopsy results were used to identify patients with NASH, quantification of transport protein expression in these biopsies was not possible, and ethical considerations precluded an additional biopsy. Hepatic MRP3 and MRP2 protein expression were shown previously to be increased in patients with NASH; however, MRP2 protein appeared to be internalized.(15) Although MRP2 function in NASH patients is unknown, mathematical modeling suggested that a ±2-fold change in MRP2-mediated clearance should not meaningfully impact morphine glucuronide Cmax or confound our interpretation of the data that changes in systemic concentrations were due to increased MRP3-mediated efflux clearance. Nevertheless, the possibility that altered MRP2 function influenced morphine glucuronide systemic concentrations cannot be dismissed.(31)
In conclusion, patients with NASH exhibited increased morphine glucuronide Cmax and AUC0-last, as well as fasting serum concentrations of total bile acids, glycocholate, and taurocholate compared to healthy volunteers. These changes are consistent with NASH-associated alterations in expression of hepatic transport proteins reported previously (e.g., increased MRP3). Increased Cmax and exposure to morphine glucuronide conjugates, as well as bile acids, positively correlated with biopsy-determined NASH severity. Given the increasing incidence of NASH, changes in hepatic transport proteins contributing to altered disposition of drugs/metabolites and bile acids may have a major impact on drug efficacy and/or toxicity. Understanding the consequences of alterations in transporter-mediated disposition is paramount for new medications that will be administered to patients who may have underlying, undiagnosed liver disease.
Patients and Methods
Patients
Healthy subjects and patients with biopsy-confirmed NASH, between 18 and 65 years of age and of any race and ethnicity who reported drinking less than 20 g/day of alcohol, were enrolled in the study. General inclusion criteria for both groups were normal serum creatinine and total bilirubin levels; nonreactive HIV, hepatitis B antigen and hepatitis C antibody; no history of gastrointestinal surgery, autoimmune disease or other GI/liver disease. Subjects were excluded if they were unable to pass a urine drug screen for opiates, marijuana and barbiturates; female subjects were excluded if they were, or were trying to become, pregnant. Healthy volunteers were eligible if all of the following applied: ALP and ALT levels within normal limits; HOMA-IR score < 2.5; BMI ≤ 30 kg/m2; not taking any concomitant medications other than birth control or a standard multivitamin. In selecting healthy subjects, effort was made to recruit a control cohort comparable to the NASH cohort with respect to age, sex, race, and ethnicity. Patients with NASH were recruited from the University of North Carolina at Chapel Hill (UNC-CH) hepatology clinic and were eligible for enrollment if all of the following applied: biopsy confirmed non-cirrhotic NASH with non-alcoholic fatty liver disease activity score (NAS) >3 (41); BMI ≤ 45 kg/m2; no treatment for type 2 diabetes other than metformin; no milk thistle products or high dose antioxidant treatment during the prior 30 days; absence of prior treatment with NASH-inducing drugs (i.e., tamoxifen, amiodarone, valproate, methotrexate). Written informed consent was obtained from all subjects. This study was approved by the UNC Biomedical Institutional Review Board and published in ClinicalTrials.gov (NCT01766960).
Study Design
This single center, comparative cohort, proof-of-concept study evaluated morphine and morphine glucuronide pharmacokinetics in healthy subjects and patients with NASH. Subjects and patients who met all inclusion/exclusion criteria fasted overnight prior to presenting to the Clinical and Translational Research Center at UNC-CH Hospitals. Following admission, hip and waist circumferences were measured, vital signs were assessed, and an i.v. catheter was placed in each arm delivering 50 mL/hr of either normal saline or lactated Ringer's solution. Patients ate a standardized breakfast sandwich containing 23.9 g of fat and finished within 30 min. Two hours after completion of the meal, a 5 mg i.v. dose of morphine sulfate was infused over 5 min. Blood (5 mL) was collected in glass tubes pre-dose, end of infusion (0), and 5, 10, 15, 30, 45, 60, 90, 120, 180, 240, 300, 360, 420, and 480 min after the end of the infusion; urine was collected at 4-hr intervals after the end of the infusion. Blood samples were allowed to clot for 30 to 60 min, and serum was separated, divided into aliquots and frozen. Serum and urine samples were stored at -80°C until analysis. Following discontinuation of both i.v. lines, patients were administered a safety questionnaire and discharged to a waiting third party to be driven home.
Sample Processing and Analysis
Morphine serum and urine samples were analyzed in batches every three months according to a validated bioanalytical method (see supplementary materials).(42) Briefly, aliquots (100 μL) of serum and urine samples were transferred to a 96-well plate insert; proteins were precipitated with 600 μL acetonitrile containing morphine-d3, morphine-3-glucuronide-d3 and morphine-6-glucuronide-d3 (100 ng/mL) as internal standards (Lipomed, Cambridge, MA). Samples were mixed by vortex for 2.5 min and centrifuged (3,000g for 20 min at 4° C). Supernatant (500 μL) was transferred to a separate 96-well plate insert, dried under nitrogen, and 100 μL of 50:50 water:methanol were added to each insert. Calibration solutions and quality controls containing all three analytes (Lipomed) were prepared similarly using pooled blank plasma or urine.
Morphine and morphine glucuronide concentrations were measured by high resolution liquid chromatography coupled with an ABSciex 5600 TripleTOF mass spectrometer (LC-MS/MS) as described previously.(42) For morphine analysis, the product of 286.14 with an exact m/z of 286.1200 was monitored. Deuterated morphine, morphine-3- and -6-glucuronide, and deuterated internal standards for the glucuronides were monitored using exact m/z's of 289.1600, 462.1700, and 465.1900, respectively. The two morphine glucuronides were separated chromatographically; insource fragmentation from morphine glucuronide to morphine was negligible.
All 30 bile acid standards were obtained from Steraloids Inc. (Newport, RI), and stable isotope-labeled standards were obtained from C/D/N Isotopes Inc. (Quebec, Canada). Sample preparation followed a published method with modifications.(30) Briefly, 150 μL of internal standard was added to 50 μL of serum or standard solution. After centrifugation, the supernatant was transferred to a clean plate and evaporated to dryness. The residue was reconstituted with 20 μL of acetonitrile and 20 μL of water and filtered through a 0.45 μm membrane before injecting 5 μL into the UPLC-MS/MS (ACQUITY UPLC-Xevo TQ-S, Waters Corp., Milford, MA) to quantify 30 bile acids in serum. The optimized instrument settings are described in the supplementary materials. The sum of all measured bile acids is reported as total bile acids.
Mathematical Modeling and Simulation for Sample Size Calculation
A previously published whole body physiologically-based pharmacokinetic (PBPK) model was used as a base model to describe the disposition of morphine and morphine glucuronide.(43) The model was developed using Phoenix® WinNonlin® (v6.3; Certara, St. Louis, MO) and was parameterized with adult reference values.(43, 44) The kinetics of morphine in all tissues was assumed to be described by a flow-limited, well-mixed model; morphine glucuronide distribution was governed by hepatic MRP3-mediated basolateral, and MRP2-mediated canalicular, efflux clearances (CLMRP3 and CLMRP2, respectively). Serum morphine-3- and -6-glucuronide were modeled as a single metabolite (morphine glucuronide). Morphine glucuronide was assumed to be eliminated by the kidney via glomerular filtration. Model optimization was guided by assessing the model output compared to data extracted from published clinical studies.(28, 29, 45-48) The model was used to simulate altered disposition of morphine and metabolites in virtual patients. Patients with NASH were assumed to have a 3-fold higher morphine glucuronide CLMRP3 based on published MRP3 protein expression compared to healthy subjects.(15) Simulations demonstrated a 36% increase in glucuronide Cmax (data not shown).(31) The difference in mean Cmax was selected as the primary endpoint. Based on the modeling output and estimates of variability from two studies using similar analytical techniques(28, 47), the number of subjects recruited (14 healthy subjects, 7 NASH patients) was projected to provide sufficient power to reject the null hypothesis of no difference in Cmax between subject groups.
Pharmacokinetic Data Analysis
Morphine, morphine-3-glucuronide and morphine-6-glucuronide pharmacokinetics were evaluated using non-compartmental analysis (Phoenix® WinNonlin® v6.3). Cmax, time to reach Cmax (Tmax), last measureable concentration (Clast), terminal volume of distribution (Vz, for morphine only) and terminal elimination rate constant (λz) were estimated from the serum concentration-time profile. The area under the concentration-time curve (AUC) from time zero to the last measurable concentration (AUC0-last) was determined using a linear-up log-trapezoidal-down algorithm. Total AUC (AUC0-∞) was calculated as the sum of AUC0-last and Clast/λz. The terminal half-life (t1/2) was calculated as 0.693/λz. Total body clearance (CL) of morphine was calculated as the ratio of dose to AUC0-∞. The total mass of morphine and morphine glucuronide excreted in urine over the 8-hr collection interval (Xurine) was calculated as the product of urine concentration and volume of urine collected. Concentrations below the limit of quantification were excluded from analysis.
Statistical Analysis Strategy
Morphine and morphine glucuronide pharmacokinetics are presented as geometric means and 95% CIs with the exception of Tmax, which is presented as median and range. Log transformed metrics were compared between groups using a two-tailed t-test of size α = 0.05; the Wilcoxon Mann-Whitney test was used to detect cohort differences in median Tmax.
Linear regression models were used to identify clinical predictors of morphine glucuronide AUC0-last and Cmax. Candidate clinical predictors included age, BMI, body weight, waist and hip circumference, waist-to-hip ratio, SCr, ALT, ALP, albumin, total bilirubin, NASH severity score, fasting glucose, triglycerides, HDL and LDL cholesterol, insulin, and HOMA-IR. Separate models for AUC0-last and Cmax were fit for each of these predictors. Clinical variables with statistically significant predictive value were included in a multivariable regression model subject to backward elimination. The criterion for retention in the multivariable model was P ≤ 0.10. NASH severity scores for healthy subjects were assumed to be zero. All statistical computations for the primary analyses were performed using SAS software (v9.3; SAS Institute, Inc., Cary, NC). Unless otherwise stated, tabulations are presented as mean and standard deviation. Cohort comparisons relied on a two-tailed pooled-variance t-test procedure of size α=0.05.
Supplementary Material
Study Highlights.
What is the current knowledge on the topic?
Hepatic transport protein expression is altered in patients with NASH (e.g., MRP3 up-regulation), a prevalent form of liver disease associated with increased mortality. However, functional changes in transporter-mediated drug disposition have not been evaluated in NASH.
What question did the study address?
Hepatically-derived morphine glucuronide and fasting bile acid serum concentrations were evaluated as a phenotypic probe of MRP3 in biopsy-confirmed NASH patients vs. healthy subjects.
What this study adds to our knowledge?
Morphine glucuronide systemic exposure and bile acid serum concentrations were significantly higher in NASH patients vs. healthy subjects. NASH severity was significantly associated with morphine glucuronide and bile acid serum concentrations.
How this might change clinical pharmacology and therapeutics?
NASH patients may exhibit increased systemic exposure to MRP3 substrates (e.g., anionic drugs/metabolites, bile acids). These changes may lead to increased systemic toxicities and decreased hepatic drug efficacy. Understanding the consequences of altered transporter-mediated disposition, including the dynamic interplay between MRP3 and MRP2, is paramount for medications used in NASH. This knowledge may help guide drug/dose selection in NASH.
Acknowledgements
The authors would like to sincerely thank Nathan D. Pfeifer for insightful discussions during the development of this study as well as Mingming Su and Guoxiang Xie for expertise in analysis of the bile acid serum concentrations. This project was supported in part by the National Institutes of Health, National Center for Advancing Translational Sciences (NCATS), through award number 1UL1TR001111, National Institute of General Medical Sciences through award number R01 GM041935 [K.L.R.B], the North Carolina Biotechnology Center Institutional Development Grant #2012-IDG-1008 [A.S.B], an Amgen Predoctoral Fellowship in Pharmacokinetics and Drug Disposition [B.C.F.], and Quintiles Pharmacokinetics/Pharmacodynamics Fellowships [C.K.J. and E.T.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the North Carolina Biotechnology Center, Amgen or Quintiles.
Abbreviations
- BMI
body mass index
- HOMA-IR
homeostasis model for assessing insulin resistance
- Cmax
maximum serum concentration
- MRP
multidrug resistance-associated protein
- NAFLD
non-alcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
non-alcoholic steatohepatitis
- OATPs
organic anion transporting polypeptides
- PBPK
physiologically-based pharmacokinetic
- NTCP
sodium-taurocholate cotransporting polypeptide
- UGTs
uridine 5’-diphospho-glucuronosyltransferases
Footnotes
Conflict of Interest/Disclosure
The authors have no conflict of interest to disclose.
Author Contributions:
Ferslew, Paine, Stewart, Jia, Barritt, and Brouwer designed the research
Ferslew, Johnston, Tsakalozou, Barritt, and Bridges performed the research
Ferslew, Johnston, Stewart, Bridges, Jia, Barritt and Brouwer analyzed the data
Ferslew, Johnston, Paine, Stewart, Bridges, Barritt and Brouwer wrote the manuscript
References
- 1.Chalasani N, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Browning JD, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 3.Williams CD, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Torres DM, Jones FJ, Shaw JC, Williams CD, Ward JA, Harrison SA. Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis in humans: a 12-month randomized, prospective, open- label trial. Hepatology. 2011;54:1631–9. doi: 10.1002/hep.24558. [DOI] [PubMed] [Google Scholar]
- 5.Tikkanen MJ, et al. Effect of intensive lipid lowering with atorvastatin on cardiovascular outcomes in coronary heart disease patients with mild-to-moderate baseline elevations in alanine aminotransferase levels. International journal of cardiology. 2013 doi: 10.1016/j.ijcard.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Sanyal AJ, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. The New England journal of medicine. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakahara T, et al. Efficacy of rosuvastatin for the treatment of non-alcoholic steatohepatitis with dyslipidemia: An open-label, pilot study. Hepatology research : the official journal of the Japan Society of Hepatology. 2012;42:1065–72. doi: 10.1111/j.1872-034X.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- 8.Chu X, et al. Intracellular drug concentrations and transporters: measurement, modeling, and implications for the liver. Clinical pharmacology and therapeutics. 2013;94:126–41. doi: 10.1038/clpt.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kock K, Brouwer KLR. A perspective on efflux transport proteins in the liver. Clinical pharmacology and therapeutics. 2012;92:599–612. doi: 10.1038/clpt.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamek-Gliszczynski MJ, Chu X, Polli JW, Paine MF, Galetin A. Understanding the Transport Properties of Metabolites: Case Studies and Considerations for Drug Development. Drug metabolism and disposition: the biological fate of chemicals. 2013 doi: 10.1124/dmd.113.055558. [DOI] [PubMed] [Google Scholar]
- 11.Pfeifer ND, et al. Effect of Ritonavir on (99m)Technetium-Mebrofenin Disposition in Humans: A Semi-PBPK Modeling and In Vitro Approach to Predict Transporter-Mediated DDIs. CPT: pharmacometrics & systems pharmacology. 2013;2:e20. doi: 10.1038/psp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filppula AM, Tornio A, Niemi M, Neuvonen PJ, Backman JT. Gemfibrozil impairs imatinib absorption and inhibits the CYP2C8-mediated formation of its main metabolite. Clinical pharmacology and therapeutics. 2013;94:383–93. doi: 10.1038/clpt.2013.92. [DOI] [PubMed] [Google Scholar]
- 13.Jonker JW, Liddle C, Downes M. FXR and PXR: potential therapeutic targets in cholestasis. The Journal of steroid biochemistry and molecular biology. 2012;130:147–58. doi: 10.1016/j.jsbmb.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher CD, et al. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug metabolism and disposition: the biological fate of chemicals. 2009;37:2087–94. doi: 10.1124/dmd.109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardwick RN, Fisher CD, Canet MJ, Scheffer GL, Cherrington NJ. Variations in ATP-binding cassette transporter regulation during the progression of human nonalcoholic fatty liver disease. Drug metabolism and disposition: the biological fate of chemicals. 2011;39:2395–402. doi: 10.1124/dmd.111.041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardwick RN, et al. Altered UDP-glucuronosyltransferase and sulfotransferase expression and function during progressive stages of human nonalcoholic fatty liver disease. Drug metabolism and disposition: the biological fate of chemicals. 2013;41:554–61. doi: 10.1124/dmd.112.048439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lake AD, et al. Analysis of global and absorption, distribution, metabolism, and elimination gene expression in the progressive stages of human nonalcoholic fatty liver disease. Drug metabolism and disposition: the biological fate of chemicals. 2011;39:1954–60. doi: 10.1124/dmd.111.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe T, Kusuhara H, Maeda K, Shitara Y, Sugiyama Y. Physiologically based pharmacokinetic modeling to predict transporter-mediated clearance and distribution of pravastatin in humans. The Journal of pharmacology and experimental therapeutics. 2009;328:652–62. doi: 10.1124/jpet.108.146647. [DOI] [PubMed] [Google Scholar]
- 19.Pfeifer ND, Hardwick RN, Brouwer KLR. Role of hepatic efflux transporters in regulating systemic and hepatocyte exposure to xenobiotics. Annual review of pharmacology and toxicology. 2014;54:509–35. doi: 10.1146/annurev-pharmtox-011613-140021. [DOI] [PubMed] [Google Scholar]
- 20.Tzvetkov MV, dos Santos Pereira JN, Meineke I, Saadatmand AR, Stingl JC, Brockmoller J. Morphine is a substrate of the organic cation transporter OCT1 and polymorphisms in OCT1 gene affect morphine pharmacokinetics after codeine administration. Biochemical pharmacology. 2013;86:666–78. doi: 10.1016/j.bcp.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Bodenham A, Quinn K, Park GR. Extrahepatic morphine metabolism in man during the anhepatic phase of orthotopic liver transplantation. British journal of anaesthesia. 1989;63:380–4. doi: 10.1093/bja/63.4.380. [DOI] [PubMed] [Google Scholar]
- 22.Zelcer N, et al. Mice lacking multidrug resistance protein 3 show altered morphine pharmacokinetics and morphine-6-glucuronide antinociception. Proc Natl Acad Sci U S A. 2005;102:7274–9. doi: 10.1073/pnas.0502530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Wetering K, et al. Multidrug resistance proteins 2 and 3 provide alternative routes for hepatic excretion of morphine-glucuronides. Molecular pharmacology. 2007;72:387–94. doi: 10.1124/mol.107.035592. [DOI] [PubMed] [Google Scholar]
- 24.Holthe M, et al. Morphine glucuronide-to-morphine plasma ratios are unaffected by the UGT2B7 H268Y and UGT1A1*28 polymorphisms in cancer patients on chronic morphine therapy. European journal of clinical pharmacology. 2002;58:353–6. doi: 10.1007/s00228-002-0490-1. [DOI] [PubMed] [Google Scholar]
- 25.Xiong H, Suzuki H, Sugiyama Y, Meier PJ, Pollack GM, Brouwer KLR. Mechanisms of impaired biliary excretion of acetaminophen glucuronide after acute phenobarbital treatment or phenobarbital pretreatment. Drug metabolism and disposition: the biological fate of chemicals. 2002;30:962–9. doi: 10.1124/dmd.30.9.962. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi K, Murai T, Yabuuchi H, Kurosawa T. Measurement of transport activities of bile acids in human multidrug resistance-associated protein 3 using liquid chromatography-tandem mass spectrometry. Analytical sciences : the international journal of the Japan Society for Analytical Chemistry. 2010;26:317–23. doi: 10.2116/analsci.26.317. [DOI] [PubMed] [Google Scholar]
- 27.Ahlberg J, Angelin B, Bjorkhem I, Einarsson K. Individual bile acids in portal venous and systemic blood serum of fasting man. Gastroenterology. 1977;73:1377–82. [PubMed] [Google Scholar]
- 28.Osborne R, Joel S, Trew D, Slevin M. Morphine and metabolite behavior after different routes of morphine administration: demonstration of the importance of the active metabolite morphine-6-glucuronide. Clinical pharmacology and therapeutics. 1990;47:12–9. doi: 10.1038/clpt.1990.2. [DOI] [PubMed] [Google Scholar]
- 29.Everts B, Karlson BW, Herlitz J, Hedner T. Morphine use and pharmacokinetics in patients with chest pain due to suspected or definite acute myocardial infarction. Eur J Pain. 1998;2:115–25. doi: 10.1016/s1090-3801(98)90004-0. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Canaveras JC, Donato MT, Castell JV, Lahoz A. Targeted profiling of circulating and hepatic bile acids in human, mouse, and rat using a UPLC-MRM-MS-validated method. Journal of lipid research. 2012;53:2231–41. doi: 10.1194/jlr.D028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston CK, Ferslew BC, Barritt AS, Brouwer KLR. Mathematical Modeling of Systemic and Hepatic Disposition of Morphine and Morphine Glucuronides in Nonalcoholic Steatohepatitis to Inform Study Design. AAPS Journal. 2013 [Google Scholar]
- 32.Imajo K, et al. Oral choline tolerance test as a novel noninvasive method for predicting nonalcoholic steatohepatitis. Journal of gastroenterology. 2014;49:295–304. doi: 10.1007/s00535-013-0776-3. [DOI] [PubMed] [Google Scholar]
- 33.Lockman P, Allen D. The transport of choline. Drug development and industrial pharmacy. 2002;28:749–71. doi: 10.1081/ddc-120005622. [DOI] [PubMed] [Google Scholar]
- 34.Clarke JD, Hardwick RN, Lake AD, Canet MJ, Cherrington NJ. Experimental nonalcoholic steatohepatitis increases exposure to simvastatin hydroxy Acid by decreasing hepatic organic anion transporting polypeptide expression. The Journal of pharmacology and experimental therapeutics. 2014;348:452–8. doi: 10.1124/jpet.113.211284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamazaki M, et al. Perturbation of bile acid homeostasis is an early pathogenesis event of drug induced liver injury in rats. Toxicology and applied pharmacology. 2013;268:79–89. doi: 10.1016/j.taap.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 36.Pou L, et al. Mycophenolic acid plasma concentrations: influence of comedication. Therapeutic drug monitoring. 2001;23:35–8. doi: 10.1097/00007691-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Matsunaga N, Wada S, Nakanishi T, Ikenaga M, Ogawa M, Tamai I. Mathematical Modeling of the in Vitro Hepatic Disposition of Mycophenolic Acid and Its Glucuronide in Sandwich-Cultured Human Hepatocytes. Molecular pharmaceutics. 2013 doi: 10.1021/mp400513k. [DOI] [PubMed] [Google Scholar]
- 38.Pfeifer ND, Bridges AS, Ferslew BC, Hardwick RN, Brouwer KLR. Hepatic basolateral efflux contributes significantly to rosuvastatin disposition II: characterization of hepatic elimination by basolateral, biliary, and metabolic clearance pathways in rat isolated perfused liver. The Journal of pharmacology and experimental therapeutics. 2013;347:737–45. doi: 10.1124/jpet.113.208314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfeifer ND, Yang K, Brouwer KLR. Hepatic Basolateral Efflux Contributes Significantly to Rosuvastatin Disposition: Characterization of Basolateral vs. Biliary Clearance Using a Novel Protocol in Sandwich-Cultured Hepatocytes. The Journal of pharmacology and experimental therapeutics. 2013 doi: 10.1124/jpet.113.207472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lake AD, et al. Decreased hepatotoxic bile acid composition and altered synthesis in progressive human nonalcoholic fatty liver disease. Toxicology and applied pharmacology. 2013;268:132–40. doi: 10.1016/j.taap.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleiner DE, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 42.Clavijo CF, et al. A sensitive assay for the quantification of morphine and its active metabolites in human plasma and dried blood spots using high-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;400:715–28. doi: 10.1007/s00216-011-4775-z. [DOI] [PubMed] [Google Scholar]
- 43.Chen S. Physiologically-Based Pharmacokinetic (PBPK) Models for the Description of Sequential Metabolism of Codeine to Morphine and Morphine 3-Glucuronide (M3G) in Man and Rat. University of Toronto Thesis. 2010 [Google Scholar]
- 44.Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicology and industrial health. 1997;13:407–84. doi: 10.1177/074823379701300401. [DOI] [PubMed] [Google Scholar]
- 45.Hanna MH, Peat SJ, Knibb AA, Fung C. Disposition of morphine-6-glucuronide and morphine in healthy volunteers. British journal of anaesthesia. 1991;66:103–7. doi: 10.1093/bja/66.1.103. [DOI] [PubMed] [Google Scholar]
- 46.Lotsch J, Skarke C, Schmidt H, Liefhold J, Geisslinger G. Pharmacokinetic modeling to predict morphine and morphine-6-glucuronide plasma concentrations in healthy young volunteers. Clinical pharmacology and therapeutics. 2002;72:151–62. doi: 10.1067/mcp.2002.126172. [DOI] [PubMed] [Google Scholar]
- 47.Skarke C, Schmidt H, Geisslinger G, Darimont J, Lotsch J. Pharmacokinetics of morphine are not altered in subjects with Gilbert's syndrome. British journal of clinical pharmacology. 2003;56:228–31. doi: 10.1046/j.1365-2125.2003.01866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murthy BR, Pollack GM, Brouwer KLR. Contribution of morphine-6-glucuronide to antinociception following intravenous administration of morphine to healthy volunteers. Journal of clinical pharmacology. 2002;42:569–76. doi: 10.1177/00912700222011508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.