Abstract
Background and Objectives
Surgical management of colorectal cancer liver metastases continues to evolve to optimize oncologic outcomes while maximizing parenchymal preservation. Long-term data after intraoperative microwave ablation are limited. This study investigates outcomes and patterns of recurrence in patients who underwent intraoperative microwave ablation.
Methods
A retrospective analysis of 33 patients who underwent intraoperative microwave ablation of colorectal cancer liver metastases from 2009 to 2013 at our institution was performed. Perioperative and long-term data were reviewed to determine outcomes and patterns of recurrence.
Results
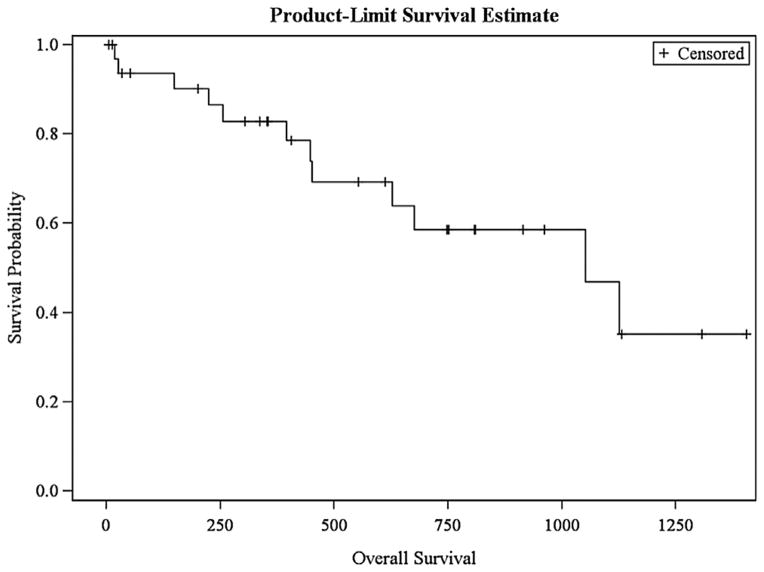
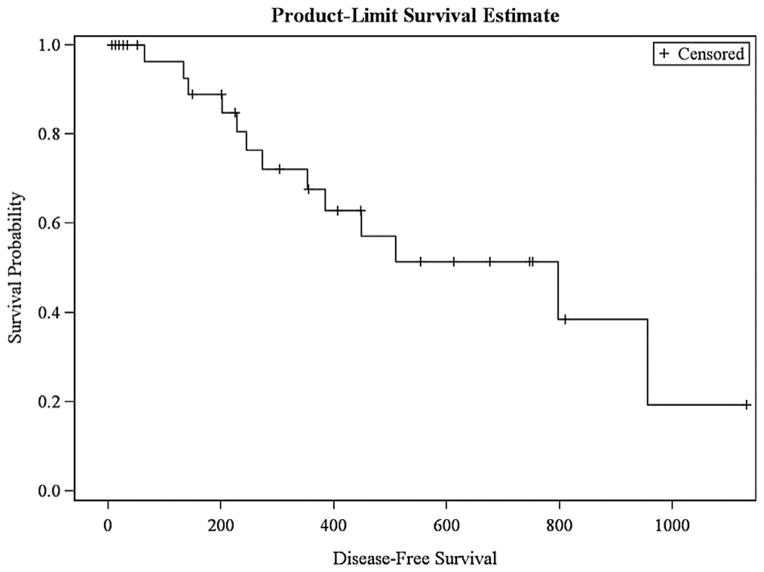
A total of 49 tumors were treated, ranging 0.5–5.5 cm in size. Median Clavien–Dindo classification was one. Median follow-up was 531 days, with 13 (39.4%) patients presenting with a recurrence. Median time to first recurrence was 364 days. In those patients, 1 (7.8%) presented with an isolated local recurrence in the liver. Only 1 of 7 ablated tumors greater than 3 cm recurred (14.3%). Overall survival was 35.2% at 4 years, with a 19.3% disease-free survival at 3.5 years. No perioperative variables predicted systemic or local recurrence.
Conclusion
Intraoperative microwave ablation is a safe and effective modality for use in the treatment of colorectal cancer liver metastases in tumors as large as 5.5 cm in size.
Keywords: microwave ablation, colorectal cancer, liver metastases
INTRODUCTION
Roughly half of patients with colorectal cancer develop liver metastases, and the management of liver metastases has continued to evolve over the past several decades, with treatment options ranging systemic and regional chemotherapy to a multitude of liver-directed therapies [1]. Liver-directed therapies consist of resection, ablation, embolization (bland, chemo, radio) and external beam radiation (SBRT). Surgical resection with curative intent has slowly become the standard of care, with 10-year survival rates reportedly as high as 25–26% [2,3]. However, surgical resection is a viable option in only approximately 20% of patients [4]. For various reasons due to patient factors (age, co-morbidities) and/or tumor factors (size, number, or location) resection is not possible, and alternate liver-directed therapies are employed [4,5]. This has provided the impetus for the development of the aforementioned treatment modalities. These techniques are aimed at optimizing oncologic outcomes while maximizing parenchymal preservation, and can be performed percutaneously, surgically, and/or in conjunction with concomitant hepatic resection [6].
Long-term results after radiofrequency ablation (RFA) are well reported, and RFA is currently the most commonly used modality among RFA, microwave, and cryotherapy [7]. Recurrence rates from RFA have been shown to be approximately three times lower than that of cryotherapy, but approximately three times higher compared to resection when used as a first-line treatment in patients with resectable disease [8–10]. RFA is safe and effective both percutaneously and surgically, but its limitations include increased impedance as temperatures reach 100 degrees Celsius, a small zone of active heating, decreased effectiveness with charring, and the inability to use multiple antennae simultaneously [11,12]. Microwave ablation (MWA), alternatively, does not rely on conduction of electricity and is not limited by charring. It also remains effective in temperatures above 100 degrees Celsius, allows for the use of multiple antennae, provides a potentially larger ablation zone, and can be performed more quickly [11,12].
The existing literature on outcomes following intraoperative MWA of colorectal cancer liver metastases is sparse. One retrospective series, however, has demonstrated MWA to be safe and effective at local disease control in patients with technically unresectable colorectal liver metastases. In this series of 43 patients, three-year overall survival was 32% in patients undergoing MWA alone and 45% in patients undergoing combined ablation/resection. Three-year disease-free survival rates were 32% and 8% in the two groups respectively [13]. Results from a French Phase II study of patients undergoing combined resection/ablation with RFA have shown a 1-year local disease-free survival of 46% and 5-year overall survival of 43% [14]. Recently, one retrospective series has compared intraoperative RFA versus MWA, with Kaplan–Meier estimates of local recurrence rates at two years of 18% and 7%, respectively (P =0.01) [15].
Our study reports on outcomes and patterns of local and systemic recurrence in patients who underwent intraoperative MWA of colorectal cancer liver metastases at our institution. We hypothesize that MWA is safe and effective at local control, either alone or with concomitant hepatectomy. Furthermore, we attempt to identify factors associated with local and/or systemic recurrence.
METHODS
Patient Selection
A retrospective review was conducted on data acquired from institutional review board-approved, retrospectively acquired electronic medical records at Robert Wood Johnson University Hospital (New Brunswick, NJ). Patients who underwent intraoperative MWA with or without concomitant hepatectomy of colorectal cancer metastases from 2009 to 2013 at our tertiary care center were identified. Three surgeons performed all of the operations over this time period. Preoperative patient characteristics such as age, gender, carcinoembryonic antigen (CEA) level, history of diabetes mellitus, coronary artery disease, pulmonary disease, and smoking were recorded and analyzed, as well as whether preoperative or postoperative chemotherapy and/or radiation was administered. Perioperative outcomes such as 30-day mortality, severity of complications (using the Clavien–Dindo classification system), and length of hospital stay were analyzed.
Operative Technique
Preoperative assessment of tumors consisted of one or more of the following: computed tomography (CT) scan of the chest/abdomen/pelvis, magnetic resonance imaging (MRI), and/or positron emission tomography (PET) CT. Intraoperative ultrasound was used to identify and confirm lesions, as well as to aid in operative planning. MWAs were performed with the Valley Lab© system.
Follow-up and Recurrence
Follow-up imaging was performed roughly 3–6 months postoperatively but varied among individual patients. Recurrences were identified via postoperative imaging, both systemic and/or local. Local recurrence was defined by a radiologically suspicious lesion at or adjacent to the prior site of ablation. Time to recurrence was calculated from the time of ablation to the time of radiologic identification.
Statistical Analysis
Kaplan–Meier survival estimates were performed to assess both overall and disease-free survival. Disease-free survival was defined as the time to first recurrence. Overall survival was based on the total length of patient follow-up. Univariate analyses were performed using systemic and local recurrence as dependent variables. Statistical significance was accepted at a level of P <0.05.
RESULTS
Patient Demographics and Tumor Characteristics
From January 2009 to April 2013, 89 patients underwent operative management of hepatic colorectal cancer metastases at our institution. MWA was performed in 37% (33) of these patients over this time period. Patient demographics are shown in Table I. Seventy percent (23) of patients underwent ablation in conjunction with a concomitant hepatectomy. In the patient cohort, 27% (9) were female, and the median age was 61 (range 42–89). Median preablation CEA level was 4 ng/mL (range 0.7–9.6). Nearly all patients received either preoperative (32, 97%) and/or postoperative (30, 91%) chemotherapy.
TABLE I.
Patient Demographics
| Patient demographics | ||
|---|---|---|
| N | 33 | |
| Median age, y (range) | 61 | (42–89) |
| Female, n (%) | 9 | (27.3) |
| DM, n (%) | 5 | (15.2) |
| CAD, n (%) | 5 | (15.2) |
| COPD/OSA/asthma, n (%) | 4 | (12.1) |
| History of smoking, n (%) | 12 | (36.4) |
| Median T stage (range) | 3 | (0–3) |
| Median N stage (range) | 1 | (0–2) |
| Median preablation CEA, ng/mL (range) | 4 | (0.7–9.6) |
| Preoperative chemotherapy, n (%) | 32 | (97) |
| Patients with concurrent procedure, n (%) | 28 | (84.8) |
| Hepatic resection, n (%) | 23 | (69.7) |
| Postoperative chemotherapy, n (%) | 30 | (90.9) |
Tumor characteristics are illustrated in Table II. A total of 49 tumors were treated with ablation (an average of 1.5 tumors per patient). Most tumors were ≤3 cm (42, 86%). Tumors >3 cm in size ranged 3.5–5.5 cm. Tumor location analysis was based on Couinaud segment grouping related to ablation approaches. The majority of tumors were located in segments 4a/7/8 (21%).
TABLE II.
Tumor Characteristics and Recurrence Information
| Patient and tumor recurrence | ||
|---|---|---|
| Total ablated tumors, n | 49 | |
| Ablated tumors per patient, n (range) | 1.5 | (1–4) |
| Patients with recurrence, n (%) | 13 | (39.4) |
| Local only | 1 | (7.8) |
| Local and distant | 9 | (69.2) |
| Distant only | 3 | (23.1) |
| Median time to recurrence, d (range) | 364 | (64–956) |
| Median follow-up | 531 | (6–1405) |
| Total | Recurrence | % | |
|---|---|---|---|
| Tumor size (cm) | |||
| ≤1.0 | 15 | 9 | 60.0 |
| 1.1–2 | 15 | 5 | 33.3 |
| 2.1–3 | 12 | 6 | 50.0 |
| >3 | 7 | 1 | 14.3 |
| Tumor location | |||
| Caudate | 2 | 0 | 0 |
| Segments 4a/7/8 | 21 | 11 | 52.4 |
| Segments 2/3/4b | 10 | 4 | 40.0 |
| Segments 5/6 | 16 | 6 | 37.5 |
Perioperative Outcomes and Recurrence Patterns
As shown in Table III, median length of stay was 6 days (range 1–32). Fifteen (46%) patients experienced no complications. Median Clavien–Dindo classification was one (range 1–5). In the eight patients who experienced major complications (Clavien–Dindo classification of 3–5), the most common complication was an intra-abdominalabscess requiring interventional radiology-guided drainage (four patients, 50%). Of note, all of the patients who experienced major complications underwent a concurrent surgical resection. Two patients experienced complications directly related to MWA. In one patient, perihepatic abscesses developed postoperatively near the ablated areas requiring interventional radiology-guided drainage. In the second patient, biliary drainage from one of the MWA probe sites was observed and controlled with a suture ligature, hemostatic agents, and an omental patch. Unfortunately, however, the patient developed persistent bilious drainage postoperatively into a drain left intraoperatively at this area. As a result, the patient underwent endoscopic retrograde cholangiopancreatography, which successfully treated the bile leak.
Table III.
Perioperative Outcomes
| Perioperative outcomes | ||
|---|---|---|
| Median Clavien–Dindo classification (range) | 1 | (0–5) |
| 0, n (%) | 15 | (45.5) |
| 1 to 2, n (%) | 10 | (30.3) |
| 3 to 5, n (%) | 8 | (24.2) |
| Median length of stay, d (range) | 6 | (1–5) |
Median follow-up was 531 days (range 6–1,405), with 13 (39.4%) patients presenting with a recurrence, as shown in Table II. Median time to first recurrence was 364 days (range 64–956). In those patients, only one (7.8%) presented with an isolated local recurrence in the liver. The majority of recurrences included either a local and distant component (9, 69.2%) or distant disease alone (3, 23.1%). Fifty-two percent of tumor recurrences occurred in segments 4a/7/8, and 57.1% of local recurrences were in tumors greater than 1 cm in size. Yet, only one of seven ablated tumors greater than 3 cm recurred (14.3%).
Figures 1 and 2 demonstrate survival curves based on Kaplan–Meier estimates. In our cohort, estimated overall survival was 35.2% at 4 years, with a 19.3% estimated disease-free survival at 3.5 years. On univariate analyses to assess factors associated with recurrence, perioperative variables included tumor demographics (including size) and patient staging, preablation CEA, and patient co-morbidities as independent variables. There were no perioperative variables to predict systemic or local recurrence.
Fig. 1.
Overall survival.
Fig. 2.
Disease-free survival.
DISCUSSION
While outcomes of hepatic resection for liver metastases from colorectal cancer have been reported, treatment modalities continue to evolve to include historically unresectable disease [2,3,7]. The paradigm of operative techniques has gravitated towards more parenchymal-sparing approaches, from wedge resections to ablations. In fact, the shift from major hepatectomies to parenchymal-sparing approaches has been associated with less mortality while providing similar disease-specific survival [6].
RFA has been the most-studied ablation technique in the literature. Abdalla et al. reported on 418 patients who underwent either hepatic resection with or without RFA, or chemotherapy alone [16]. However, RFA was only performed in patients who could not undergo complete resection of disease. They concluded that while hepatectomy remained the best treatment for overall and disease-free survival, RFA did provide a survival benefit when compared to chemotherapy alone in patients with unresectable disease [16]. One prospective trial randomizing patients with unresectable disease to systemic treatment or systemic treatment plus RFA found systemic treatment plus RFA to result in longer progression-free survival [17]. In patients with resectable disease, local recurrence rates in one meta-analysis were described to be 3.6% for tumors ≤3 cm [18]. Evrard et al performed a prospective, multicenter phase II study examining RFA with or without concurrent resection in patients who unresectable colorectal cancer liver metastases. In this cohort of 52 patients, the 1-year local disease-free survival rate was 46%, and the 3-year disease-free survival rate was 19%. Overall survivals at 1 and 5 years were 94% and 43% respectively [14]. Despite the various studies published in literature, the heterogeneity of patient populations and paucity of randomized trials has made incorporating RFA into treatment algorithms quite challenging [4]. Limitations of RFA include its dependence on the conduction of electric energy into the body, diminished effect with charring, small zone of active heating, and the inability to use multiple antennae at once. As its effects depend on thermal conduction, its effects decrease exponentially away from the RFA source. The tissue-vessel interface actually produces a protective effect called the “heat-sink effect,” which is thought to be responsible for many recurrences from RFA [11,19].
Other ablation modalities, such as MWA and cryotherapy, have not been as frequently reported in the literature. Yet, MWA and cryotherapy have been shown to be associated with improved survival in patients with unresectable disease when compared to chemotherapy alone [20]. Intraoperative cryotherapy, however, has been found to be associated with significantly higher recurrence rates than either RFA or MWA [7]. Percutaneous MWA is a safe and effective method at treating unresectable hepatic malignancies, both primary (hepatocellular carcinoma) and/or metastatic [21]. MWA offers several advantages to RFA, as it does not rely on electrical conduction, is not limited by charring, allows for multiple antennae, and can be performed more quickly than RFA. MWA also provides a larger, more homogenous and uniform area of treatment [11]. Percutaneous RFA and MWA have been compared in the treatment of hepatocellular carcinoma, with some studies showing no significant difference in outcomes, and another demonstrating a slight improvement in disease-free survival in the RFA group (authors attributed this difference to a greater number of MWA tumors >3.0 cm) [11,22,23].
Intraoperative MWA, with or without concomitant hepatectomy or other surgical intervention, has been investigated in several studies. Martin et al. reported on a 5-year experience of intraoperative MWA of hepatic tumors, both primary and metastatic. In this retrospective analysis of 100 patients, the majority of whom had colorectal cancer liver metastases, median tumor size was 3.0 cm, with 2% local recurrence and 37% intrahepatic recurrence rates and 29% 90-day morbidity [24]. Shibata et al. randomized 30 patients with resectable metastatic colorectal liver metastases to treatment with MWA or hepatectomy. Patients in this cohort had anywhere from 2 to 9 liver lesions. Mean survival did not differ between the groups, while blood loss was significantly less in the MWA group, leading the authors to conclude that MWA is as effective as hepatic resection [25]. Stattner et al. retrospectively analyzed 43 patients treated with MWA, 65% of whom underwent MWA and resection. Three-year overall survival in the MWA and resection group was 45%, with a disease-free survival of 8% [13]. In our cohort, 84.8% of patients underwent MWA with a concurrent procedure (69.7% of which were hepatic resections). The median Clavien–Dindo classification was one, with only 27% of patients experiencing significant morbidity. Importantly, 90% of local recurrences were associated with distant disease, demonstrating a safe and effective treatment technique. In addition, only one in seven ablated tumors greater than 3 cm recurred, showing the effectiveness of MWA in tumors up to 5.5 cm. The overall and disease-free survival rates in our study were comparable. Our cohort contains the highest percentage of patients with concomitant hepatectomy in the MWA literature, the highest percentage of patients with neoadjuvant therapy, and is the only one to perform a univariate analysis showing survival after MWA is not associated with tumor size. All of these characteristics demonstrate not only the safety and effectiveness of MWA, but in patients who have received neoadjuvant treatment and undergo MWA with concomitant hepatic resection, regardless of the size of lesions ablated. Additionally, what is unique about this manuscript is that we have meticulously outlined, collected, and reported perioperative complications associated with this approach.
The only study to compare intraoperative RFA to MWA was by Correa-Gallego et al., who retrospectively analyzed local recurrence between these patient groups (N =127 tumors per group). Tumor size was not associated with local recurrence. MWA was found to be associated with lower local recurrence rates than RFA (6 vs. 20%, P <0.01). Median follow-up, however, was significantly longer in the RFA group (18 vs. 31 months, P <0.001), and clinical practice patterns at the institution shifted from RFA to MWA over the decade [15].
The limitations of this study include its retrospective design and small cohort of patients. In addition, our limited duration of patient follow-up prevented the analysis of more long-term outcomes. While a historical perspective of this treatment modality at our single center can be obtained, greater-powered, randomized trials are needed to address various questions about the role of intraoperative MWA in treatment of colorectal cancer liver metastases. Additionally, it should be noted that at our institution (as in others), there is an inherent selection bias in patients with presumed favorable disease biology for MWA with or without concomitant hepatectomy.
In conclusion, intraoperative microwave ablation is a safe and effective modality for use in the treatment of colorectal cancer liver metastases. Tumor size was not associated with local recurrence, and tumors up to 5.5 cm in size were treated successfully. Recurrences in these patients most often included distant disease. Therefore, microwave ablation alone or in conjunction with hepatectomy is a reasonable approach for the management of metastatic colorectal cancer to the liver.
References
- 1.Bozzetti F, Doci R, Bignami P, et al. Patterns of failure following surgical resection of colorectal cancer liver metastases. Rationale for a multimodal approach. Ann Surg. 1987;205:264–270. doi: 10.1097/00000658-198703000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long- term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 4.Cirocchi R, Trastulli S, Boselli C, et al. Radiofrequency ablation in the treatment of liver metastases from colorectal cancer. Cochrane Database Syst Rev. 2012;6:CD006317. doi: 10.1002/14651858.CD006317.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong SL, Mangu PB, Choti MA, et al. American Society of Clinical Oncology 2009 clinical evidence review on radio-frequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28:493–508. doi: 10.1200/JCO.2009.23.4450. [DOI] [PubMed] [Google Scholar]
- 6.Gold JS, Are C, Kornprat P, et al. Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: trends in treatment over time in 440 patients. Ann Surg. 2008;247:109–117. doi: 10.1097/SLA.0b013e3181557e47. [DOI] [PubMed] [Google Scholar]
- 7.Kingham TP, Tanoue M, Eaton A, et al. Patterns of recurrence after ablation of colorectal cancer liver metastases. Ann Surg Oncol. 2012;19:834–841. doi: 10.1245/s10434-011-2048-x. [DOI] [PubMed] [Google Scholar]
- 8.Adam R, Hagopian EJ, Linhares M, et al. A comparison of percutaneous cryosurgery and percutaneous radiofrequency for unresectable hepatic malignancies. Arch Surg. 2002;137:1332–1339. doi: 10.1001/archsurg.137.12.1332. discussion 1340. [DOI] [PubMed] [Google Scholar]
- 9.Otto G, Duber C, Hoppe-Lotichius M, et al. Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann Surg. 2010;251:796–803. doi: 10.1097/SLA.0b013e3181bc9fae. [DOI] [PubMed] [Google Scholar]
- 10.White RR, Avital I, Sofocleous CT, et al. Rates and patterns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. J Gastro-intest Surg. 2007;11:256–263. doi: 10.1007/s11605-007-0100-8. [DOI] [PubMed] [Google Scholar]
- 11.Wright AS, Sampson LA, Warner TF, et al. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236:132–139. doi: 10.1148/radiol.2361031249. [DOI] [PubMed] [Google Scholar]
- 12.Dupuy DE. Microwave ablation compared with radiofrequency ablation in lung tissue-is microwave not just for popcorn anymore? Radiology. 2009;251:617–618. doi: 10.1148/radiol.2513090129. [DOI] [PubMed] [Google Scholar]
- 13.Stattner S, Jones RP, Yip VS, et al. Microwave ablation with or without resection for colorectal liver metastases. Eur J Surg Oncol. 2013;39:844–849. doi: 10.1016/j.ejso.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Evrard S, Rivoire M, Arnaud J, et al. Unresectable colorectal cancer liver metastases treated by intraoperative radiofrequency ablation with or without resection. Br J Surg. 2012;99:558–565. doi: 10.1002/bjs.8724. [DOI] [PubMed] [Google Scholar]
- 15.Correa-Gallego C, Fong Y, Gonen M, et al. A Retrospective Comparison of Microwave Ablation vs. Radiofrequency Ablation for Colorectal Cancer Hepatic Metastases. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-3817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. discussion 825–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruers T, Punt C, Van Coevorden F, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non- resectable colorectal liver metastases: A randomized EORTC Intergroup phase II study (EORTC 40004) Ann Oncol. 2012;23:2619–2626. doi: 10.1093/annonc/mds053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulier S, Ni Y, Jamart J, et al. Local recurrence after hepatic radiofrequency coagulation: multivariate meta- analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tungjitkusolmun S, Staelin ST, Haemmerich D, et al. Three-Dimensional finite-element analyses for radio-frequency hepatic tumor ablation. IEEE Trans Biomed Eng. 2002;49:3–9. doi: 10.1109/10.972834. [DOI] [PubMed] [Google Scholar]
- 20.Pathak S, Jones R, Tang JM, et al. Ablative therapies for colorectal liver metastases: A systematic review. Colorectal Dis. 2011;13:e252–265. doi: 10.1111/j.1463-1318.2011.02695.x. [DOI] [PubMed] [Google Scholar]
- 21.Livraghi T, Meloni F, Solbiati L, et al. omplications of microwave ablation for liver tumors: Results of a multicenter study. Cardiovasc Intervent Radiol. 2012;35:868–874. doi: 10.1007/s00270-011-0241-8. [DOI] [PubMed] [Google Scholar]
- 22.Lu MD, Xu HX, Xie XY, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: A retrospective comparative study. J Gastroenterol. 2005;40:1054–1060. doi: 10.1007/s00535-005-1671-3. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Wang N, Shen Q, et al. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PLoS ONE. 2013;8:e76119. doi: 10.1371/journal.pone.0076119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: A prospective review of a 5-year experience. Ann Surg Oncol. 2010;17:171–178. doi: 10.1245/s10434-009-0686-z. [DOI] [PubMed] [Google Scholar]
- 25.Shibata T, Niinobu T, Ogata N, Takami M. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer. 2000;89:276–284. [PubMed] [Google Scholar]