Abstract
BACKGROUND
The burden of post-traumatic stress disorder (PTSD) symptoms may be associated with worse outcomes after transplantation. Little is known about the prevalence and correlates of PTSD symptoms in lung transplant recipients.
METHODS
We conducted a cross-sectional study of lung transplant recipients between April 2008 and February 2010 at a single center. The PTSD Checklist was used to determine the burden of PTSD symptomatology (total score) and percent of subjects with a provisional PTSD diagnosis (validated algorithms). We assessed the relationship between PTSD symptom burden and patient characteristics with multivariable logistic modeling.
RESULTS
We enrolled 210 subjects (response rate 91%). Most patients were female (50%), and Caucasian (89%). The median age was 59 (interquartile range [IQR] 48 to 63) years and the median time between transplant and follow-up was 2.4 (IQR 0.7 to 5.3) years. Clinically significant PTSD symptomatology was observed in 12.6% (8.4% to 17.9%) of subjects. Subjects were more likely to endorse symptoms of re-experiencing (29.5%) and arousal (33.8%) than avoidant symptoms (18.4%). Multivariable linear regression showed higher PTSD symptom scores among recipients who were: younger (p < 0.001); without private insurance (p = 0.001); exposed to trauma (p < 0.001); or diagnosed with bronchiolitis obliterans syndrome (p = 0.005).
CONCLUSIONS
Overall prevalence of PTSD (12.6%) in our study was two times higher than the general population. Patient characteristics found to be associated with an increased burden of PTSD symptoms may be useful to consider in future interventions designed to reduce this comorbidity.
Keywords: post traumatic stress disorder, PTSD, lung transplantation, prevalence, symptoms, PCL, risk factors
Post-traumatic stress disorder (PTSD) encompasses feelings of re-experiencing a traumatic event in addition to having avoidant and hyperarousal symptoms, which last for periods of at least 1 month.1 Lung transplant recipients may be at a high risk for developing symptoms of PTSD because they may be exposed to several traumatic events such as: (1) having experienced a life-threatening exacerbation of their underlying lung disease and its associated dyspnea prior to transplantation; (2) undergoing transplant surgery; (3) staying in an intensive care unit (ICU) after transplantation where they may experience hallucinations and delusions associated with delirium; and (4) experiencing episodes of life-threatening infections, rejection or bronchiolitis obliterans syndrome.2–6
Studies of PTSD in other types of solid-organ transplant recipients have revealed a significantly higher prevalence of PTSD symptoms in comparison to the general population.7–13 Few studies have assessed PTSD in lung transplant recipients, although a recent study of 178 lung transplant recipients revealed a prevalence of PTSD related to the transplant as high as 15% at the end of the first year post-transplantation.14
A high prevalence of PTSD in lung recipients is of particular concern, because, as illustrated in other types of solid-organ transplant recipients, PTSD may lower medical adherence and may predispose recipients to a higher morbidity and mortality risk.15,16 Because PTSD may increase the risk for poor outcomes in lung transplant recipients, it is also important to understand the prevalence and potential correlates of this condition so that those who may develop PTSD can be identified.
In this study we sought to assess the prevalence of subjects with a provisional PTSD diagnosis and explored what specific types of PTSD symptoms (re-experience, hyper-arousal and avoidant) were most prominent in these subjects. Exploration of specific types of PTSD symptoms has never been assessed in the transplant population and may be important to define, particularly because studies have suggested that patients with a higher burden of hyperarousal symptoms have a lower resolution of symptoms over time and therefore may need an increased intervention. In addition, we examined potential clinical characteristics associated with an increased burden of PTSD symptomatology in order to better understand which patients might be at a higher risk for this condition. In particular, we assessed variables that had been identified in the literature as potential risk factors in other chronic conditions. Furthermore, we hypothesized that life-threatening complications of lung transplantation, such as a history of acute rejection, bronchiolitis obliterans syndrome and severity of disease, may be associated with the development of PTSD, and we evaluated these variables.
Methods
Design
A cross-sectional, observational, survey-based study was conducted between April 2008 and February 2010 at the University of Washington (UW) Lung Transplant Center.
Study participants and clinical management
All adult patients (aged ≥18 years) receiving lung transplantation and/or follow-up care at the UW were identified for potential inclusion. Ambulatory appointment logs and medical records were reviewed by the research coordinator to determine subject eligibilityfor the study, which included being at least 18 years of age and English speaking. As acute PTSD can occur up to 6 months after the event, patients were also required to be at least 6 months post-transplant. Gender, race and ethnicity were collected on participants and non-participants. Subjects completed the questionnaire in a private setting at their regularly scheduled clinic appointment. Institutional review board approval (IRB) was obtained for all activities and subjects provided written informed consent (IRB No. 32823).
Routine post-transplant clinical assessments included pulmonary function testing weekly for the first month, bi-monthly for the second month, and then every 3 months for at least the first 2 years. Routine surveillance biopsies were not performed. Biopsies were performed when subjects demonstrated any of the following: (1) a 10% drop in either forced expiratory volumes in 1 second (FEV1) or forced vital capacity (FVC); (2) symptoms such as shortness of breath or cough; or (3) lower-than-expected values on pulmonary function tests (PFTs) after transplantation.
Measures and data collection procedures
Post-traumatic Stress Disorder Checklist—Civilian Version (PCL)
The PCL is a 17-item self-report questionnaire that assesses symptoms of PTSD over the last month. Responses range from 1 (“not at all”) through 3 (“moderately”) to 5 (“extremely).”17 The PCL includes the 3 symptom clusters of PTSD described in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV): re-experiencing (5 items); avoidant (7 items); and hyper arousal (5 items). A score of ≥3 on any question item indicates clinically significant distress. The PCL can be scored with an algorithm to meet criteria for a provisional diagnosis of PTSD, or it can be scored continuously to report the burden of symptoms.17 To identify patients that have a provisional diagnosis of PTSD, we used an algorithm described by Blanchard et al. First, it was determined whether participants met criteria for any of the symptom clusters of the PCL. Participants were identified as having re-experiencing symptoms if they had clinically significant distress (score of ≥3) on 1 of the 5 questions that asked about re-experiencing symptoms. Similarly, participants were identified as having avoidant symptoms if they had clinically significant distress on 3 of the 7 of the questions about avoidant symptoms. Finally, participants were identified as having hyperarousal symptoms if they had clinically significant distress on 2 of the 5 questions that asked about arousal symptoms. To meet criteria for a provisional diagnosis of PTSD, participants had to meet criteria in each of the symptom clusters.17 We did not ask specifically if the symptoms were related to the lung transplant process. Continuous scores on the PCL can range between 17 and 85. A higher PCL score indicates a higher burden of PTSD symptomatology. The measure has been shown to be reliable and has demonstrated criterion and construct validity in chronic disease populations.17–23 We used both approaches to scoring (provisional PTSD diagnoses and burden of PTSD symptomatology) in our analyses.
Structured Clinical Interview for DSM-IV (SCID) PTSD trauma screen
The SCID PTSD trauma screen is a single question asking about a respondent’s lifetime exposure to traumatic events: “Sometimes things happen to people that are extremely upsetting—things like being in a life threatening situation like a major disaster, very serious accident or fire; being physically assaulted or raped; seeing another person killed or dead or badly hurt, or hearing about something horrible that has happened to someone that you are close to. At any time during your life have any of these kinds of things happened to you?”24 This screening item is widely used in research.24 The rationale for the screen is that previous exposure to traumatic events may increase an individual’s risk of PTSD. The SCID trauma screen has been shown to be 84% sensitive and 100% specific in detecting a trauma history.25
Medical record review
Characteristics of the sample were abstracted from the medical record by trained reviewers; these included: pre-transplant clinical characteristics (underlying lung disease, oxygen requirements, PFTs, and 6-minute walk test [6MWT]) and post-transplant characteristics (early acute rejection, bronchiolitis obliterans syndrome [BOS], most recent PFTs, and current oxygen requirements). BOS scores were determined as described by accepted algorithms using PFT data.26 Abstractors were blinded to the PTSD diagnosis. Data were entered onto a form and then entered into a database. Ten percent of randomly selected records were reviewed to ensure 95% agreement between abstractors.
Statistical analysis
Descriptive statistics were calculated (e.g., means, standard deviations, ranges and distributions). When a subject skipped a single item from the PCL, the missing value was imputed by substituting the mean value for the items completed by that individual on that symptom cluster. Patients who missed more than 1 question on the PCL were excluded from the analysis. The percentages of those participants who met provisional criteria for a diagnosis of PTSD as well as those considered to have clinically significant symptoms in each of the 3 PTSD symptom clusters (and the associated 95% confidence intervals [CIs] around these estimates) were calculated. Internal consistency for the PCL score was determined to be strong (Cronbach’s α = 0.92).
To examine the association between PTSD symptomatology levels (using the total PCL score) and subject characteristics, a 2-step procedure was used. First, simple linear regression analyses were performed. Then, a multivariable regression model was constructed by including subject demographic and clinical variables found to have a p < 0.2527,28 in the bivariate analysis. Linear regression model assumptions were evaluated prior to fitting the model. Collinearity was examined between variables. Because the total PCL score was not normally distributed, it was transformed (using an inverse transformation) prior to these analyses. Significance level was set at p < 0.05 for the final regression analysis. Statistical software (STATA SE, version 11; StataCorp, College Station, TX) was used for all analyses.
Results
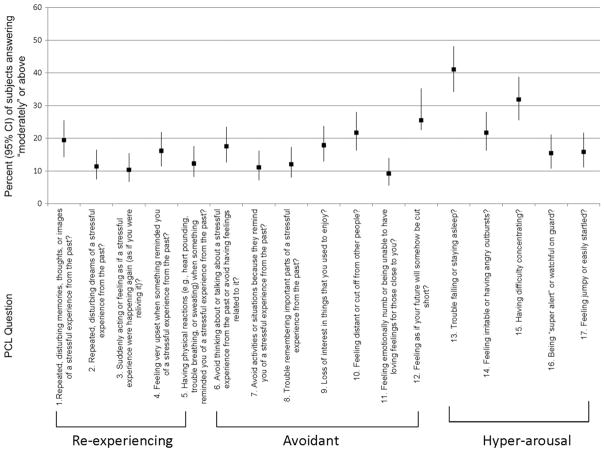
Among the 232 eligible subjects, 7 (3%) refused to participate and 13 (6%) did not complete the survey. The final sample was 210 subjects (91% response rate). Characteristics of the participants are reported in Table 1 Of those diagnosed with a provisional diagnosis of PTSD, 88% (95% CI 68.8% to 97.5%) had an extreme trauma compared with 67.6% patients reporting an extreme trauma (95% CI 60.1% to 74.5%, data not shown). The percentage of lung transplant patients who met provisional criteria for PTSD disease was 12.6% (95% CI 8.3% to 17.9%; Table 2). Figure 1 shows the percentage of subjects who answered “moderately” or above for each PCL question. Patients were most likely to report that they had trouble falling and staying asleep, had trouble concentrating, and believed their future would be cut short. The percentages meeting diagnostic criteria for distress in each of these 3 symptom domains reflect a similar pattern in Table 2. Trouble falling or staying asleep was a prominent symptom among lung transplant recipients (41.1%, interquartile range [IQR] 34.2% to 48.1%; Figure 1).
Table 1.
Baseline Characteristics of Sample (n = 210) and Degree of Missingnessa
| Demographic characteristics | Missing (%) | |
|---|---|---|
| Median age (years) at time of survey (IQR) | 58.7 (48.5–63.6) years | 0 |
| Female (n) | 50.5% (106) | 0 |
| Caucasian race (n) | 89.1% (187) | 0 |
| Private/commercial (primary insurance) (n) | 49.5% (104) | 0 |
| Income (n) | 7.1 | |
| Less than $10,000 | 13.3% (26) | |
| $10–24,999 | 16.9% (33) | |
| $25–49,999 | 25.1% (49) | |
| $50–74,999 | 17.4% (34) | |
| $75–99,999 | 14.4% (28) | |
| $100–149,999 | 8.2% (16) | |
| More than $150,000 | 4.6% (9) | |
| Education (n) | 1.4 | |
| Some high school or less | 8.7% (18) | |
| High school diploma or college/graduate degree | 66.2% (137) | |
| 4-year college or graduate degree | 25.1% (52) | |
| Married or engaged (n) | 63.8% (132) | 1.4 |
| History of traumatic event ever in life (n) | 70.0% (140) | 4.8 |
| Pre-transplant characteristics | ||
| Underlying disease (n) | 0 | |
| COPD/α1-anti-trypsin deficiency | 37.1% (78) | |
| Idiopathic pulmonary fibrosis | 28.6% (60) | |
| Cystic fibrosis | 15.2% (32) | |
| PAH | 5.2% (11) | |
| Other | 13.8% (29) | |
| Median time on waiting list (IQR) | 88.5% (33–188) days | 3.8 |
| Most recent PFTs prior to transplant | ||
| Median FVC, % predicted (IQR) | 48.5% (39–57.5%) | 8.6 |
| Median FEV1, % predicted (IQR) | 27% (18.5–44%) | 6.7 |
| Most recent oxygen requirement prior to transplant (IQR) | 3 (2–4) liters/ 10.9 min | |
| Post-transplant characteristics | ||
| Bilateral lung transplant (n) | 76.2% (160) | 0 |
| Most recent PFTs prior to survey date | ||
| Median FVC, % predicted (IQR) | 73 (59–88) | 1.4 |
| Median FEV1, % predicted (IQR) | 69 (52–87) | 0.1 |
| Median time since transplant (IQR) | 2.4 (0.7–5.3) years | |
| Current supplemental oxygen use (n) | 7.4% (15) | 3.3 |
| Episode of acute rejection (n) | 25.2% (52) | 1.9 |
| Bronchiolitis obliterans syndrome (n) | 18.0% (36) | 4.8 |
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity IQR, interquartile range; PAH, pulmonary arterial hypertension; PFTs, pulmonary function tests.
Percent of cases missing data on each variable.
Table 2.
Subjects Meeting Criteriaa for Provisional Diagnosis of PTSD and Criteria for Presence of Clinically Significant Symptoms Within Each Symptom Clusterb
| Prevalence (95% CI) | n (total = 210) | |
|---|---|---|
| Provisional diagnosis of PTSD (% yes) | 12.6 (8.3–17.9) | 207 |
| Clinically significant symptoms within each PTSD symptom cluster: | ||
| Re-experiencing | 29.5 (23.4–36.2) | 210 |
| Avoidantc | 18.4 (13.3–24.3) | 207 |
| Arousalc | 33.8 (27.4–40.6) | 207 |
Subjects met criteria for PTSD if they had met criteria in each PTSD symptom cluster.
Subjects met clinically significant symptom criteria for symptom clusters by answering “moderately” or higher on 1 of 5 re-experiencing questions, 3 of 7 of avoidance questions or 2 of 5 of hyperarousal questions.
Missing 3 responses.
Figure 1.
Subject responses to PCL questionnaire.
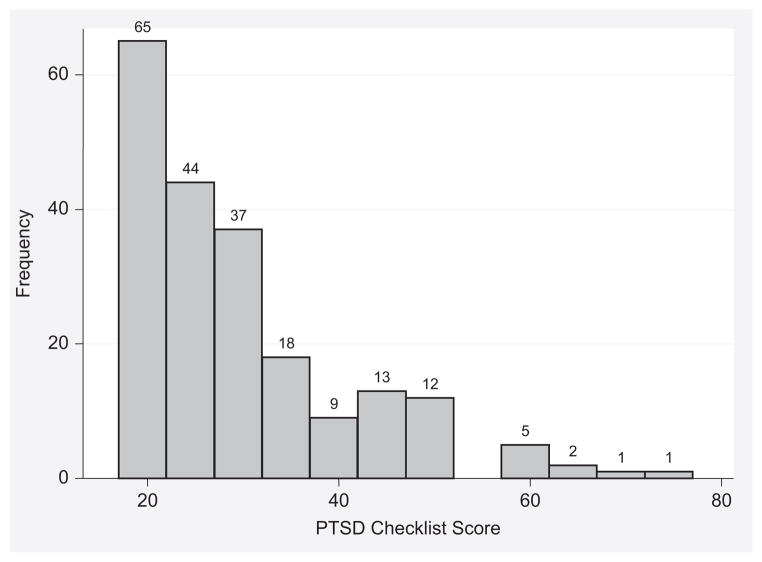
Total scores on the PCL, reflecting the burden of PTSD symptomatology, ranged from 17 to 73, with a median of 25 (IQR 21 to 34). The distribution of scores was skewed, with the majority of subjects having a score of <40 (Figure 2).
Figure 2.
Distribution of PTSD Checklist scores. PCL as a continuous variable. Each bar equals 5 points on the PCL. Range of possible scores: 17 to 85; range of scores in sample: 17 to 73.
On univariable analysis, a higher burden of PTSD symptomatology was significantly associated with being younger (p < 0.001), having a lower income (p = 0.001), being unmarried (p = 0.001), having a previous history of a traumatic event (p = 0.003), having a lower FVC or FEV1 (p < 0.001) at the time of the survey, having an episode of acute cellular rejection (p < 0.001), and having a diagnosis of BOS (p = 0.012; Table 3). There was no difference in prevalence of PTSD by time since transplant (p = 0.63; Figure 3). In comparison to patients with a pre-transplant diagnosis of chronic obstructive pulmonary disease (COPD), those with idiopathic pulmonary fibrosis had a lower burden of PTSD symptomatology (p = 0.046; Table 3). Because the value of the most recent post-transplant PFT results was collinear with having BOS, we chose to include BOS in our model instead of the PFT values, as the BOS variable was dichotomous and would be more useful as a screening tool for clinicians when assessing a subject’s risk for psychologic symptoms. Similarly, as insurance and income were collinear, we chose to include the insurance rather than income in the model, as most clinicians would not be aware of their subjects’ reported income. No other variables were found to be collinear.
Table 3.
Median (IQR) PCL Scores by Patient Characteristica
| Variable | PCL score median (IQR) |
|---|---|
| Demographics | |
| Survey agea | |
| Young (≤58.7) | 29.0 (22.0–40.5) |
| Old (>58.7) | 25.0 (21.0–34.0) |
| Gender | |
| Male | 24.0 (20.0–32.0) |
| Female | 28.0 (21.0–36.0) |
| White | |
| Yes | 25.0 (21.0–33.6) |
| No | 28.0 (21.0–36.0) |
| Private/commercial insurance (primary insurance) | |
| Yes | 23.0 (20.0–30.0) |
| No | 29.0 (22.5–39.5) |
| Income | |
| Less than $10,000 | 32.0 (22.0–45.7) |
| $10–24,999 | 27.0 (21.0–40.0) |
| $25–49,999 | 28.0 (21.0–32.0) |
| $50–74,999 | 26.0 (23.0–36.0) |
| $75–99,999 | 23.0 (20.5–29.0) |
| $100–149,999 | 20.5 (18.0–28.5) |
| More than $150,000 | 21.0 (21.0–26.0) |
| Education | |
| Some HS or less | 28.5 (20.0–33.3) |
| HS degree or some college | 25.0 (21.0–34.0) |
| 4-year college or grad school | 24.5 (21.0–35.0) |
| Married or engaged | |
| Yes | 24.0 (20.0–30.0) |
| No | 29.0 (22.0–42.0) |
| History of traumatic event ever in life | |
| Yes | 28.0 (21.0–36.0) |
| No | 22.0 (19.0–30.0) |
| Pre-/peri-transplant characteristics | |
| Pre-transplant Diagnosis | |
| COPD | 27.0 (21.0–37.0) |
| Cystic fibrosis | 26.5 (21.5–41.5) |
| Idiopathic pulmonary fibrosis | 24.0 (20.5–29.0) |
| PAH | 30.0 (25.0–42.0) |
| Other | 27.0 (20.0–36.0) |
| Days on waitlista | |
| ≤88.5 | 25.0 (21.0–32.0) |
| >88.5 | 26.0 (20.5–36.0) |
| Pulmonary function tests | |
| FVC, % predicteda | |
| ≤48.5 | 24.0 (20.0–35.0) |
| >48.5 | 26.0 (21.0–32.5) |
| FEV1, % predicteda | |
| ≤27 | 25.5 (20.5–36.5) |
| >27 | 25.0 (21.0–32.0) |
| Median most recent oxygen requirement prior to transplant, liters/min (IQR)a | |
| ≤3 | 26.0 (20.0–34.0) |
| >3 | 25.0 (21.0–33.0) |
| Six-minute walk test, feeta | |
| ≤1,057.5 | 25.5 (21.0–34.0) |
| >1,057.5 | 25.0 (21.0–34.0) |
| Transplant type | |
| Bilateral | 27.0 (21.0–34.0) |
| Unilateral | 23.0 (20.5–33.6) |
| Post-transplant characteristics | |
| Pulmonary function tests | |
| FVC, % predicteda | |
| ≤73 | 26.0 (22.0–36.0) |
| >73 | 24.0 (19.5–31.5) |
| FEV1, % predicteda | |
| ≤69 | 26.0 (22.0–36.0) |
| >69 | 24.0 (19.0–31.0) |
| Time since transplant, yearsa | |
| ≤2.4 | 28.0 (21.0–34.0) |
| >2.4 | 24.0 (20.0–34.0) |
| Current supplemental oxygen use | |
| Yes | 25.0 (22.0–46.0) |
| No | 25.0 (20.0–32.5) |
| Acute cellular rejection | |
| Yes | 28.0 (22.0–40.0) |
| No | 24.5 (20.0–32.0) |
| Bronchiolitis obliterans syndrome | |
| Yes | 30.0 (21.5–42.9) |
| No | 24.0 (20.0–32.0) |
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PAH, pulmonary arterial hypertension.
Continuous variables were dichotomized at their median for descriptive purposes only. They were analyzed as continuous variables
Figure 3.
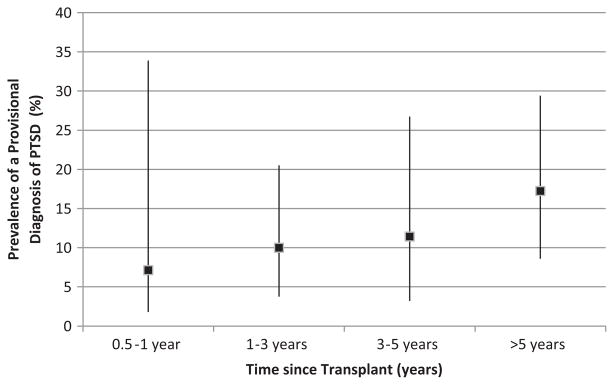
Cross-sectional prevalence (95% CI) of PTSD provisional diagnosis grouped by time from transplantation.
Our final multivariable model revealed that the level of PTSD symptomatology was associated with younger age (p < 0.001), lack of private insurance (p = 0.001), a previous history of a traumatic event (p < 0.001), and having BOS (p = 0.005) (Table 4). These factors accounted for 29% of the variance in PTSD symptomatology [overall model, F(12, 169) = 7.72, p < 0.001].
Table 4.
Multivariate Associations Between Level of PTSD Symptomatology and Sample Characteristicsa
| Variable | Standardized β-coefficients | p-value |
|---|---|---|
| Age at time of survey (years) | 0.349 | <0.001 |
| Female gender | −0.120 | 0.10 |
| Pre-transplant diagnosis | ||
| COPD | Reference | --- |
| Idiopathic pulmonary fibrosis | 0.0162 | 0.84 |
| Cystic fibrosis | 0.0937 | 0.32 |
| Pulmonary arterial hypertension | 0.0054 | 0.94 |
| Other | 0.0345 | 0.65 |
| Private/commercial (primary insurance) | 0.227 | 0.001 |
| Married | 0.115 | 0.11 |
| History of lifetime traumatic exposure | −0.252 | <0.001 |
| Acute rejection | −0.104 | 0.10 |
| Bronchiolitis obliterans syndrome | −0.185 | 0.005 |
PTSD symptom scores underwent inverse transformation so that a negative β-coefficient indicates a higher level of symptoms.
Discussion
In this study we found that the prevalence of clinically significant PTSD symptomatology (i.e., meeting criteria for a provisional diagnosis of PTSD) in our sample of lung transplant recipients (12.6%) was at least twice as high as the rates of PTSD reported in the general population (3.5% to 6%).10–13 Our findings of an elevated level of PTSD symptomatology, as defined by a higher PCL total score, are consistent with those previously reported in lung transplant patients, cardiac transplant recipients29 and adolescent liver transplant recipients.30 In aggregate, these findings suggest that there may be something about the organ transplant process that increases one’s risk of PTSD.
There are several plausible mechanisms for a high burden of PTSD symptoms among organ transplant recipients. One potential traumatic event in the transplant process may be the ICU experience. The prevalence of PTSD among ICU survivors has been reported to be as high as 14% to 27%.31,32 Several studies have supported the association between hallucinations and delusional memories in ICU and post-ICU symptomatology.33–36 This association has also been seen in transplant recipients. DiMartini et al3 presented 4 cases of transplant recipients who were clinically diagnosed with PTSD and who reported re-experiencing delusions and/or hallucinations that they had during the peri-transplant period.
Another potential explanation that may be specific to lung transplantation is that PTSD symptoms may be linked to patients’ experience of end-stage lung disease prior to transplant, including dyspnea and/or life-threatening exacerbations of illness during the wait for transplant. Although few data exist regarding the prevalence of PTSD symptomatology in patients with advanced cystic fibrosis or idiopathic pulmonary fibrosis, PTSD has been reported to be elevated in patients with severe COPD.37 Furthermore, in a community-based study of 1,772 adults, subjects with PTSD were more likely to have significant airflow obstruction than those without PTSD.4 Longitudinal studies that assess the difference in PTSD symptoms before and after lung transplantation are necessary to further elucidate this relationship.
In addition, we found that lung recipients were most likely to report symptoms of hyperarousal and re-experiencing of a traumatic event than avoidant symptoms. In particular, sleep problems were prominent in our sample. Although symptoms of re-experiencing are unique to PTSD, symptoms of hyperalertness and sleep disturbances can be present in both the general population and in individuals with other anxiety disorders.38 Therefore, it is possible that some subjects in our study may have been having anxiety or sleep disorders other than or in addition to PTSD. It will be important in future work to explore the wider spectrum of anxiety disorders in lung recipients. Furthermore, additional studies that explore sleeping difficulties and potential etiologies of this disorder are needed.
Our multivariable analyses identified several correlates of PTSD total symptom severity similar to those previously reported in the general population.39–41 In particular, subjects with a lower socioeconomic status (as defined by using governmental insurance or insurance other than that of a private or commercial source) and history of a lifetime traumatic event were more likely to have a higher level of PTSD symptomatology. However, unlike previous findings, we found an association between the burden of PTSD symptomatology and younger rather than older age. Furthermore, we did not find an association between female gender and having a higher burden of PTSD symptomatology.14,40 The prevalence of PTSD among women has been demonstrated to vary by the type of traumatic event; therefore, the trauma experienced in previous studies may not be generalizable to the transplant process.13,42
In addition, we found that, after controlling for other factors, subjects with more severe PTSD symptomatology were more likely to have had BOS, as defined by PFT criteria. As our study is retrospective, we were unable to assess PTSD symptom burden prior to transplant or prior to the development of BOS, and therefore we cannot determine the causal nature of this relationship or even definitively determine the sequence. Few studies have evaluated the relationship between PTSD and post-transplant medical morbidities in any solid-organ transplant recipient. One study of liver transplant recipients showed a positive correlation between having symptoms of PTSD and having episodes of acute rejection.43 Dew et al demonstrated that heart transplant recipients with PTSD were at increased risk for subsequent mortality, but they did not find that PTSD was related to either acute or chronic rejection.15 Further studies are needed to determine the mechanism of the relationship between PTSD and BOS.
This study has several limitations. First, the PCL only measures symptoms consistent with PTSD; therefore, we do not know if patients actually had PTSD or if the symptoms they were experiencing were related to PTSD and not some other transplant-related characteristic, such as immunosuppression medication. Second, we do not know if the lung transplant process itself—or what specific element of the process—is the provoking event for the PTSD symptoms that we observed. In addition, similar to the general population, a high percentage of our subjects reported at least one exposure to a traumatic event during their lifetime. We do not know any further details of the traumatic event, but it may have been the provoking factor. Regardless of the cause, our findings suggest that symptoms of PTSD may have clinical implications for lung recipients’ well-being and for their development of post-transplant morbidities. Further studies are needed to evaluate these relationships. Second, as subjects did not receive surveillance bronchoscopies, we may have missed asymptomatic acute rejection. Moreover, we may have overestimated the diagnosis of BOS as we based the criteria entirely upon PFTs and did not consider any other clinical data that could explain the decline, such as an infection, persistent pleural effusion, airway stenosis or acute rejection. In addition, although we did not find an association between PTSD symptoms and time on the waiting list, we did not collect information on the lung allocation score (LAS). It is possible that the LAS can predict symptoms of PTSD; therefore, further studies that address this issue are warranted. Finally, generalizability may be reduced as this study was performed at single lung transplant center, which was a cystic fibrosis and idiopathic pulmonary fibrosis referral base. In addition, the subjects in our study were predominantly Caucasian and therefore our results may not generalize to patients of other races or ethnicities.
In conclusion, our findings suggest that lung transplant recipients are at higher risk for having PTSD symptomatology than the general population. Clinicians who care for these patients should be aware of this potential significant morbidity. Although we cannot identify whether or not the lung transplant process caused the PTSD symptomatology, our findings suggest several factors that increase the likelihood of experiencing symptoms of PTSD in the setting of lung transplantation. In addition, given the significant burden of PTSD symptoms and potential implications, clinicians should consider including the assessment and treatment of PTSD in their management of lung transplantation. Furthermore, we have highlighted the need for studies to better elucidate the mechanisms that may contribute to PTSD symptomatology as well as the relationship of PTSD, adherence and outcomes after transplantation.
Acknowledgments
This study was supported by the Institute of Translational Research (1 UL1 RR 025014-01), the National Institutes of Health/National Center for Research Resources (NIH/NCRR), and the Cystic Fibrosis Foundation Program for Adult Care Excellence. R.C. was supported by a K24 Award from the National Heart, Lung, and Blood Institute, NCRR, a component of the NIH (K24HL68593), and the NIH Roadmap for Medical Research (5KL2RR025015).
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- 1.American Psychiatric Association. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.Dobbels F, Wernli-Fluri C, Denhaerynck K, et al. Comparison of perceived health status among solid organ transplant candidates. Clin Transplant. 2010;24:660–8. doi: 10.1111/j.1399-0012.2009.01163.x. [DOI] [PubMed] [Google Scholar]
- 3.DiMartini A, Dew MA, Kormos R, et al. Posttraumatic stress disorder caused by hallucinations and delusions experienced in delirium. Psychosomatics. 2007;48:436–9. doi: 10.1176/appi.psy.48.5.436. [DOI] [PubMed] [Google Scholar]
- 4.Spitzer C, Koch B, Grabe HJ, et al. Association of airflow limitation with trauma exposure and post-traumatic stress disorder. Eur Respir J. 2011;37:1068–75. doi: 10.1183/09031936.00028010. [DOI] [PubMed] [Google Scholar]
- 5.Fusar-Poli P, Lazzaretti M, Ceruti M, et al. Depression after lung transplantation: causes and treatment. Lung. 2007;185:55–65. doi: 10.1007/s00408-006-0093-1. [DOI] [PubMed] [Google Scholar]
- 6.Stilley CS, Dew MA, Stukas AA, et al. Psychological symptom levels and their correlates in lung and heart–lung transplant recipients. Psychosomatics. 1999;40:503–9. doi: 10.1016/s0033-3182(99)71189-8. [DOI] [PubMed] [Google Scholar]
- 7.Dew MA, Kormos RL, DiMartini AF, et al. Prevalence and risk of depression and anxiety-related disorders during the first three years after heart transplantation. Psychosomatics. 2001;42:300–13. doi: 10.1176/appi.psy.42.4.300. [DOI] [PubMed] [Google Scholar]
- 8.Grant BF, Hasin DS, Stinson FS, et al. The epidemiology of DSM-IV panic disorder and agoraphobia in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:363–74. doi: 10.4088/jcp.v67n0305. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin RD, Faravelli C, Rosi S, et al. The epidemiology of panic disorder and agoraphobia in Europe. Eur Neuropsychopharmacol. 2005;15:435–43. doi: 10.1016/j.euroneuro.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Resnick HS, Kilpatrick DG, Dansky BS, et al. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61:984–91. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- 11.Norris FH. Epidemiology of trauma: frequency and impact of different potentially traumatic events on different demographic groups. J Consult Clin Psychol. 1992;60:409–18. doi: 10.1037//0022-006x.60.3.409. [DOI] [PubMed] [Google Scholar]
- 12.Mehnert A, Koch U. Prevalence of acute and post-traumatic stress disorder and comorbid mental disorders in breast cancer patients during primary cancer care: a prospective study. Psychooncology. 2007;16:181–8. doi: 10.1002/pon.1057. [DOI] [PubMed] [Google Scholar]
- 13.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 14.Dew MA, DiMartini AF, Devito Dabbs AD, et al. Onset and risk factors for anxiety and depression during the first 2 years after lung transplantation. Gen Hosp Psychiatry. 2012;34:127–38. doi: 10.1016/j.genhosppsych.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dew MA, Kormos RL, Roth LH, et al. Early post-transplant medical compliance and mental health predict physical morbidity and mortality one to three years after heart transplantation. J Heart Lung Transplant. 1999;18:549–62. doi: 10.1016/s1053-2498(98)00044-8. [DOI] [PubMed] [Google Scholar]
- 16.Favaro A, Gerosa G, Caforio AL, et al. Posttraumatic stress disorder and depression in heart transplantation recipients: the relationship with outcome and adherence to medical treatment. Gen Hosp Psychiatry. 2011;33:1–7. doi: 10.1016/j.genhosppsych.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Blanchard EB, Jones-Alexander J, Buckley TC, et al. Psychometric properties of the PTSD Checklist (PCL) Behav Res Ther. 1996;34:669–73. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 18.Zatzick D, Russo J, Grossman DC, et al. Posttraumatic stress and depressive symptoms, alcohol use, and recurrent traumatic life events in a representative sample of hospitalized injured adolescents and their parents. J Pediatr Psychol. 2006;31:377–87. doi: 10.1093/jpepsy/jsj056. [DOI] [PubMed] [Google Scholar]
- 19.Zatzick DF, Kang SM, Muller HG, et al. Predicting posttraumatic distress in hospitalized trauma survivors with acute injuries. Am J Psychiatry. 2002;159:941–6. doi: 10.1176/appi.ajp.159.6.941. [DOI] [PubMed] [Google Scholar]
- 20.Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- 21.Marshall GN, Schell TL. Reappraising the link between peritraumatic dissociation and PTSD symptom severity: evidence from a longitudinal study of community violence survivors. J Abnorm Psychol. 2002;111:626–36. doi: 10.1037//0021-843x.111.4.626. [DOI] [PubMed] [Google Scholar]
- 22.Cordova MJ, Studts JL, Hann DM, et al. Symptom structure of PTSD following breast cancer. J Trauma Stress. 2000;13:301–19. doi: 10.1023/A:1007762812848. [DOI] [PubMed] [Google Scholar]
- 23.Conybeare D, Behar E, Solomon A, et al. The PTSD Checklist—Civilian Version: reliability, validity, and factor structure in a nonclinical sample. J Clin Psychol. 2012;68:699–713. doi: 10.1002/jclp.21845. [DOI] [PubMed] [Google Scholar]
- 24.Elhai JD, Franklin CL, Gray MJ. The SCID PTSD module’s trauma screen: validity with two samples in detecting trauma history. Depress Anxiety. 2008;25:737–41. doi: 10.1002/da.20318. [DOI] [PubMed] [Google Scholar]
- 25.Franklin CL, Sheeran T, Zimmerman M. Screening for trauma histories, posttraumatic stress disorder (PTSD), and subthreshold PTSD in psychiatric outpatients. Psychol Assess. 2002;14:467–71. doi: 10.1037//1040-3590.14.4.467. [DOI] [PubMed] [Google Scholar]
- 26.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 27.Bendel RB, Afifi AA. comparison of stopping rules in forward “stepwise” regression. J Am Stat Assoc. 1977;72:46–53. [Google Scholar]
- 28.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 29.Dew MA, Roth LH, Thompson ME, et al. Medical compliance and its predictors in the first year after heart transplantation. J Heart Lung Transplant. 1996;15:631–45. [PubMed] [Google Scholar]
- 30.Mintzer LL, Stuber ML, Seacord D, et al. Traumatic stress symptoms in adolescent organ transplant recipients. Pediatrics. 2005;115:1640–4. doi: 10.1542/peds.2004-0118. [DOI] [PubMed] [Google Scholar]
- 31.Cuthbertson BH, Hull A, Strachan M, et al. Post-traumatic stress disorder after critical illness requiring general intensive care. Intensive Care Med. 2004;30:450–5. doi: 10.1007/s00134-003-2004-8. [DOI] [PubMed] [Google Scholar]
- 32.Scragg P, Jones A, Fauvel N. Psychological problems following ICU treatment. Anaesthesia. 2001;56:9–14. doi: 10.1046/j.1365-2044.2001.01714.x. [DOI] [PubMed] [Google Scholar]
- 33.Jones C, Griffiths RD, Humphris G, et al. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med. 2001 Mar;29:573–80. doi: 10.1097/00003246-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 34.Jones C, Backman C, Capuzzo M, et al. Precipitants of post-traumatic stress disorder following intensive care: a hypothesis generating study of diversity in care. Intensive Care Med. 2007;33:978–85. doi: 10.1007/s00134-007-0600-8. [DOI] [PubMed] [Google Scholar]
- 35.Weinert CR, Sprenkle M. Post-ICU consequences of patient wakeful-ness and sedative exposure during mechanical ventilation. Intensive Care Med. 2008;34:82–90. doi: 10.1007/s00134-007-0829-2. [DOI] [PubMed] [Google Scholar]
- 36.Granja C, Gomes E, Amaro A, et al. Understanding posttraumatic stress disorder-related symptoms after critical care: the early illness amnesia hypothesis. Crit Care Med. 2008;36:2801–9. doi: 10.1097/CCM.0b013e318186a3e7. [DOI] [PubMed] [Google Scholar]
- 37.Felker B, Bush KR, Harel O, et al. Added burden of mental disorders on health status among patients with chronic obstructive pulmonary disease. Prim Care Companion J Clin Psychiatry. 2010;12:4. doi: 10.4088/PCC.09m00858gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helzer JE, Robins LN, McEvoy L. Post-traumatic stress disorder in the general population. Findings of the epidemiologic catchment area survey. N Engl J Med. 1987;317:1630–4. doi: 10.1056/NEJM198712243172604. [DOI] [PubMed] [Google Scholar]
- 39.Bisson JI. Post-traumatic stress disorder. BMJ. 2007;334:789–93. doi: 10.1136/bmj.39162.538553.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vieweg WV, Julius DA, Fernandez A, et al. Posttraumatic stress disorder: clinical features, pathophysiology, and treatment. Am J Med. 2006;119:383–90. doi: 10.1016/j.amjmed.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 41.Liebschutz J, Saitz R, Brower V, et al. PTSD in urban primary care: high prevalence and low physician recognition. J Gen Intern Med. 2007;22:719–26. doi: 10.1007/s11606-007-0161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breslau N, Davis GC, Andreski P, et al. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry. 1991;48:216–22. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- 43.Rothenhausler HB, Ehrentraut S, Kapfhammer HP, et al. Psychiatric and psychosocial outcome of orthotopic liver transplantation. Psychother Psychosom. 2002;71:285–97. doi: 10.1159/000064811. [DOI] [PubMed] [Google Scholar]