Abstract
Objective
We conducted a systematic review to answer three questions: 1) Do advance care planning and palliative care interventions lead to a reduction in ICU admissions for adult patients with life-limiting illnesses? 2) Do these interventions reduce ICU length of stay? and 3) Is it possible to provide estimates of the magnitude of these effects?
Data Sources
We searched MEDLINE, EMBASE, Cochrane Controlled Clinical Trials, and Cumulative Index to Nursing and Allied Health Literature databases from 1995 through March 2014.
Study Selection
We included studies that reported controlled trials (randomized and nonrandomized) assessing the impact of advance care planning and both primary and specialty palliative care interventions on ICU admissions and ICU length of stay for critically ill adult patients.
Data Extraction
Nine randomized controlled trials and 13 nonrandomized controlled trials were selected from 216 references.
Data Synthesis
Nineteen of these studies were used to provide estimates of the magnitude of effect of palliative care interventions and advance care planning on ICU admission and length of stay. Three studies reporting on ICU admissions suggest that advance care planning interventions reduce the relative risk of ICU admission for patients at high risk of death by 37% (sd, 23%). For trials evaluating palliative care interventions in the ICU setting, we found a 26% (sd, 23%) relative risk reduction in length of stay with these interventions.
Conclusions
Despite wide variation in study type and quality, patients who received advance care planning or palliative care interventions consistently showed a pattern toward decreased ICU admissions and reduced ICU length of stay. Although sds are wide and study quality varied, the magnitude of the effect is possible to estimate and provides a basis for modeling impact on healthcare costs.
Keywords: advance care planning, critical care, end-of-life, intensive care unit utilization, length of stay, palliative care
In the United States, a significant proportion of healthcare resources are spent on care for critically ill patients. In 2005, critical care costs were estimated to be $82 billion, accounting for 13% of inpatient hospital costs (1, 2). The United States spends more hospital resources on critical care than any other country, as evidenced by the highest ratios of ICU bed-to-population (20 ICU beds per 100K people) and ICU-to-hospital bed (nine ICU beds per 100 hospital beds) in the world (2). Furthermore, a significant and rising portion of these expenses are for patients who die. According to a study of Medicare claims data, ICU use in the last 30 days of life increased 5% between 2000 and 2009 (3).
Importantly, these high-technology treatments may not be targeting outcomes that are consistent with patient values and preferences (4). Interventions that clarify patients’ goals of care and whether ICU care is consistent with these goals may reduce the intensity of end-of-life care. For example, ICU admissions and length of stay (LOS) may be reduced by ensuring that patients do not receive unwanted ICU care; such interventions may incorporate diverse approaches including advance care planning, palliative care consultation, or ethics consultation (4–8). Systematic reviews (5, 9) of the impact of advance care planning or palliative care or ethics interventions on ICU resource utilization suggest decreases in resource utilization. However, none have included interventions both before and in the ICU setting, including primary and specialty palliative care, and none have estimated the magnitude of effects on ICU admission and LOS. Importantly, the primary reason for implementing palliative care should be to improve quality of care and patient and family outcomes. Nonetheless, estimates of the magnitude of these effects may be used for future studies modeling the potential impact of such interventions on healthcare costs (10, 11) and may inform future interventions and policy development.
We conducted a systematic review to answer the following questions: 1) Do advance care planning interventions lead to a reduction in ICU admissions for adult patients with life-limiting illnesses when compared to usual care? 2) Do advance care planning and palliative care interventions reduce ICU LOS in this population when compared to usual care? and 3) Is it possible to provide estimates of the magnitude of these effects?
METHODS
Data Sources
This systematic review includes published controlled trials (randomized and nonrandomized) reporting on the effect of advance care planning and palliative care interventions on ICU admissions and ICU LOS. We refer to ICU admissions and ICU LOS as “ICU utilization.” We excluded studies published prior to 1995 because of the impact the landmark Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments trial had on end-of-life care (12). We searched MEDLINE, EMBASE, Cochrane Controlled Clinical Trials, and Cumulative Index to Nursing and Allied Health Literature databases from 1995 through March 2014. In addition, we reviewed reference lists.
We defined relevant interventions as inclusive of advance care planning, primary palliative care (palliative care or communication interventions provided by nonpalliative care specialists such as family meetings), specialty palliative care (palliative care provided by palliative care specialists), and ethics consultation that include a focus on communication about the goals of care. We included interventions conducted in the outpatient, acute care, and ICU settings. We focused on adults (age ≥ 18) because interventions and outcomes in the neonatal ICU and PICU are likely to be very different.
Search Terms
Our search strategy used a list of terms grouped under three main subject headings: palliative care AND intensive care AND resource utilization (full list of search terms in e-Table 1, Supplemental Digital Content 1, http://links.lww.com/CCM/B162). A research librarian at the University of Washington Health Sciences library assisted with development and execution of our search strategy.
Study Selection, Data Extraction, and Quality Assessment
Two researchers independently screened all titles and reviewed selected abstracts and full-text articles (N.K., E.K.K.: practicing intensivists and researchers focusing on palliative care). Both researchers also independently extracted quantitative and other critical data from included studies. All titles were reviewed. Titles were excluded on the basis of four criteria: 1) no specific focus on palliative or end-of-life care, 2) no relevance to ICU utilization, 3) editorials or narrative reviews, or 4) focus exclusively on pediatric populations. Abstracts for the retained titles were reviewed, and the full-text article was retrieved for any abstract considered potentially relevant. In the full review, we retained articles that met the following criteria: 1) adult patient population, 2) randomized controlled trials (RCTs) or nonrandomized controlled trials (non-RCTs), and 3) ICU LOS and/or ICU admission included as an outcome.
Data from selected articles were abstracted using a standardized instrument. A quality checklist was created using previously reported quality metrics (5) and recommendations from the Consolidated Standards for Reporting Trials group (13, 14). In addition, criteria for non-RCTs from the Transparent Reporting of Evaluations with Nonrandomized Designs were included (15). Our final quality metric checklist is a modified version of a previously used checklist and included type of controls, determination of sample size, data quality, prespecification of outcome measures, and intervention adherence (5).
Data Synthesis and Analysis
Studies selected for inclusion were grouped by our outcomes— ICU admissions and ICU LOS; within these subgroups, studies were further grouped by RCTs and non-RCTs. For trials reporting LOS, studies were additionally categorized by intervention setting.
In order to estimate the magnitude of effect of advance care planning and palliative care interventions on ICU utilization, we calculated the mean relative risk reduction of ICU admission and ICU LOS for each study, when applicable. We then aggregated these values and determined the mean relative risk reduction in ICU admission and ICU LOS among all relevant studies. For ICU LOS, we categorized studies according to the setting in which the intervention took place and provided separate estimates for interventions taking place in the ICU, acute care, and outpatient settings.
When calculating LOS estimates, we excluded five studies for the following reasons: 1) two studies where intervention setting was not clear (16, 17); 2) one study not designed to study the effect of the intervention on resource utilization because patients were recruited if expected to die within a few days (18); 3) one study with a very small sample size (n = 10/2009 eligible) (19); and 4) one study confounded by indication bias that did not report adjusted estimates (20). For studies that reported separate estimates for decedents (21, 22), we included estimates for decedents in the primary analysis because the primary mechanism of action for reducing ICU LOS is likely to be earlier decisions to limit life-sustaining therapies for patients who will die irrespective of duration of life-sustaining therapy. We conducted sensitivity analyses using results for survivors and the entire cohort and found results were similar. These data have been included in e-Table 2 (Supplemental Digital Content 2, http://links.lww.com/CCM/B163).
RESULTS
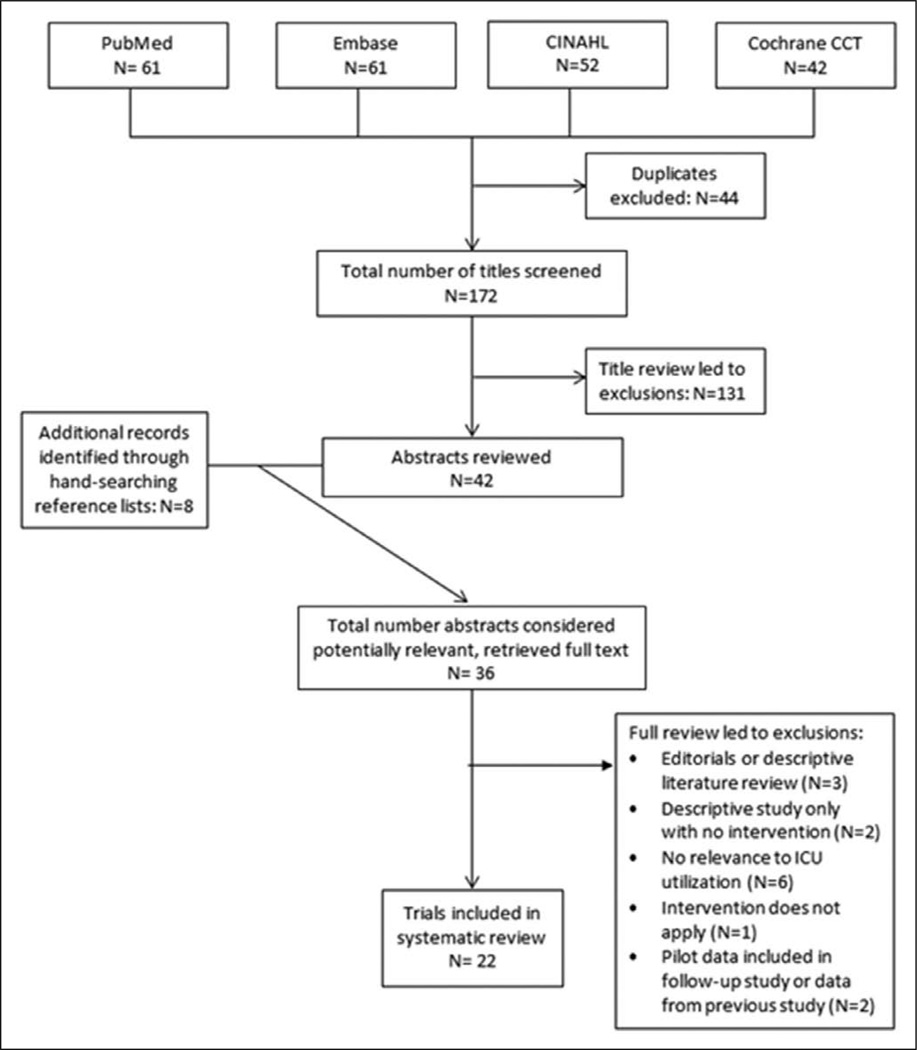
A total of 216 articles were identified; 44 duplicates were excluded, leaving 172 titles to be screened. Title review led to the exclusion of 131 articles. Of the 42 remaining abstracts and eight additional abstracts identified by hand-searching reference lists, full-text articles were retrieved for 36 studies. Of these 36 articles, 22 met our inclusion criteria (Fig. 1). All 22 studies compared an advance care planning or palliative care intervention (as defined above) to usual care in adult patient populations with ICU admissions and/or ICU LOS as an outcome. Results of the overall methodological quality are displayed in Tables 1 and 2. A variety of interventions at the patient or system level were studied. Although patient populations varied, all were patients considered to be at high risk of death. Studies included in estimating the magnitude of intervention effects are listed in Tables 3 and 4. Heterogeneity in study interventions, study design, and study populations precluded us from conducting a quantitative meta-analysis.
Figure 1.
Flow diagram of article inclusion. CCT = Controlled Clinical Trials, CINAHL = Cumulative Index to Nursing and Allied Health Literature.
TABLE 1.
Trials Reporting on ICU Admissions
| Author (Reference) |
Year | Type of Study |
Sample Size: for RCT, Number Randomized to Intervention (Control); for Non-RCT, Number in Each Group |
Sample Size for ICU Admissions Outcome: Intervention (Control) |
Data Quality (Monitored Accuracy or Reliability) |
Primary Outcome Measures Prespecified |
Percent Randomized to Intervention Who Received Intervention |
|---|---|---|---|---|---|---|---|
| Gade et al (23) | 2008 | RCT | 280 (237) | 230 (218) | Yes | Yes | 98.2 |
| Penrod et al (16) | 2006 | Retrospective, observational | 82 (232) | 82 (232) | No | Yes | NA |
| Penrod et al (17) | 2010 | Retrospective, observational | 606 (2715) | 824 (5,771)a | No | Yes | NA |
RCT = randomized controlled trial, NA = not applicable.
Represents number of hospitalizations.
TABLE 2.
Trials Reporting on ICU Length of Stay
| Author (Reference) | Year | Type of Study | Sample Size: for RCT, Number Randomized to Intervention (Control); for Non- RCT, Number in Intervention Group (Usual Care) |
Sample Size for ICU Length of Stay Outcome: Intervention (Control) |
Data Quality (Monitored Accuracy or Reliability) |
Primary Outcome Measures Prespecified |
Percent Randomized to Intervention Who Received Intervention |
|---|---|---|---|---|---|---|---|
| RCTs | |||||||
| Schneiderman et al (4) | 2003 | Multicenter RCT | 278 (273) | 173 (156) | No | Yes | 75 |
| Lautrette et al (18) | 2007 | Multicenter RCT | 63 (63) | 63 (63) | No | Yes | 100 |
| Bakitas et al (25) | 2009 | Multicenter RCT | 161 (161) | 145 (134) | Yes | Yes | 89 |
| Cheung et al (19) | 2010 | RCT | 10 (10) | 10 (10) | No | Yes | 100 |
| Detering et al (24) | 2010 | RCT | 154 (155) | 16 (14)a | No | Yes | 82 |
| Curtis et al (27) | 2011 | Multicenter, cluster RCT | 1,183 (1,135) | 1,166 (1,072) | Yes | Yes | Unknown |
| Andereck et al (26) | 2014 | RCT | 240 (238) | 56 (52) | No | Yes | 73 |
| Schneiderman et al (7) | 2000 | RCT | 35 (35) | 21 (21) | No | Yes | 66 |
| Nonrandomized trials, observational studies | |||||||
| Dowdy et al (21) | 1998 | Non-RCT | 31 (68) | Survivors: 10 (16); Decedents 21 (15) | No | Yes | NA |
| Ahrens et al (28) | 2003 | Non-RCT | 43 (108) | 43 (108) | No | No | NA |
| Campbell and Guzman et al (29) | 2003 | Historical controls | 20 (18) with GCI after CPR; 21 (22) with MOSF | 20 (18) with GCI after CPR; 21 (22) with MOSF | No | No | NA |
| Lilly et al (33) | 2003 | Pre-post | 396 (134) | 396 (134) | No | No | NA |
| Campbell and Guzman et al (30) | 2004 | Historical controls | 26 (26) | 26 (26) | No | No | NA |
| Penrod et al (16) | 2006 | Retrospective, observational | 82 (232) | 82 (232) | No | Yes | NA |
| Norton et al (22) | 2007 | Pre-post | 126 (65) | 126 (65) | No | Yes | NA |
| Curtis et al (31) | 2008 | Pre-post | 337 (253) | 150 (125) | No | Yes | NA |
| Mosenthal et al (32) | 2008 | Pre-post | 367 (266) | 52 (42) | No | No | NA |
| Shelton et al (34) | 2010 | Pre-post | 113 (114) | 113 (114) | No | No | NA |
| Daly et al (35) | 2010 | Pre-post | 354 (135) | 346 (135) | Yes | Yes | NA |
| Penrod et al (17) | 2010 | Retrospective, observational | 606 (2,715) | 824 (5,771)b | No | Yes | NA |
| Kim et al (20) | 2014 | Retrospective, observational | 42 (80) | 41 (80) | No | No | NA |
RCT = randomized controlled trial, NA = not applicable, GCI = global cerebral ischemia, CPR = cardiopulmonary resuscitation, MOSF = multiple organ system failure.
Data provided by authors.
Represents number of hospitalizations.
TABLE 3.
Eligibility Criteria, Intervention, and Outcomes for Trials Reporting on ICU Admissions Included in Estimates of Effect
| Author/Article | Type of Study |
Patient Population |
Intervention (All Patient Targets) |
ICU Admissions | Percent Relative Risk Reduction (Mean) |
Decedent Analysis |
|---|---|---|---|---|---|---|
| Gade et al (23) | Multicenter randomized controlled trial | Adult patients hospitalized with at least one life-limiting diagnosis and whose attending physician indicated they “would not be surprised if the patient died within 1 yr” | Consultative, interdisciplinary palliative care service | 5.2% for palliative care intervention vs 9.6% for usual care, p = 0.04 | 50 | No |
| Penrod et al (16) | Retrospective, observational, controlled by propensity score matching | Veterans who died after a hospitalization of > 3 d | Palliative care consult | 33% for palliative care consultation vs 68% for usual care, p < 0.0001 Absolute risk reduction = 35% Adjusted risk reduction = 42% (95% CI, −56%, −31%)a |
51 | Yes |
| Penrod et al (17) | Retrospective, observational, controlled by instrumental variable approach | Veterans admitted to an acute care facility with at least one advanced disease (specific criteria listed); excluded if hospital length of stay < 48 hr | Palliative care consult | 33% for palliative care consultation vs 37% for usual careb Absolute risk reduction = 4% Adjusted risk reduction = 44% (95% CI, 49%, 39%; p < 0.001)c |
11 | No |
Calculated from a multivariable probit regression model adjusting for age, principle diagnosis, comorbidity score, service, and center.
Represents percentage of hospitalizations that included an ICU stay.
Calculated from a multivariate probit regression model adjusting for advanced disease conditions, age, marital status, race, Hispanic ethnicity, and principle diagnosis.
Table 4.
Trials Reporting on ICU Length of Stay Included in Estimates of Effect by Intervention Target
| Author (Reference) | Difference in ICU Length of Stay for Intervention Minus Control |
Percent Relative Risk Reduction for Intervention Group Compared With Control |
Decedent Analysis |
|---|---|---|---|
| Intervention target: Patients in tde acute care setting | |||
| Detering et al (24)a | Mean: −5.7 d | 52 | No |
| Median: −8 d | |||
| Intervention target: Patients in the outpatient setting | |||
| Bakitas et al (25) | No change | 0 | No |
| Intervention target: Patients and/or providers in the ICU | |||
| Schneiderman et al (7) | −9 d | 68 | Yes |
| Schneiderman et al (4) | −1.4 d | 18 | Yes |
| Ahrens et al (28) | −3.4 d | 36 | No |
| Campbell and Guzman et al (29) | Global cerebral ischemia: −3.4 d | 48 | No |
| Multisystem organ failure: No change | |||
| Campbell and Guzman et al (30) | −3.3 d | 49 | No |
| Dowdy et al (21) | Survivors: −6 d | Survivors: 28 | Yes |
| Decedents: −13 d | Decedents: 30 | ||
| Lilly et al (33) | −1 d | 25 (median) | No |
| Norton et al (22) | Overall: −7.3 d | Entire cohort: 45; decedents: 60 | Yes |
| Medical ICU decedents: −8.4 d | |||
| Andereck et al (26) | No change | 0 | Yes |
| Daly et al (35) | No change | 0 | No |
| Intervention target: System ± patient in ICU setting | |||
| Curtis et al (31) | Mean: −1.4 d | 19 mean | Yes |
| Median: −0.8 d | 21 median | ||
| Mosenthal et al (32) | Mean: −1.5 d | 19 mean; 67 median | Yes |
| Median: −2 d | |||
| Curtis et al (27) | No change | 0 | Yes |
| Shelton et al (34) | No change | 0 | No |
For additional information, see e-Table 3 (Supplemental Digital Content 3, http://links.lww.com/CCM/B164).
Data provided by authors.
Trials Evaluating ICU Admissions
For all studies with ICU admissions as an outcome, the intervention was focused at the patient level (Tables 1 and 3).
RCTs
In a multicenter RCT, Gade et al (23) examined the effect of routine palliative care consultation among hospitalized patients and reported future ICU admissions as an outcome. In this trial, 275 patients hospitalized with a life-limiting illness received the intervention; 237 received usual care. Patients receiving the palliative care intervention had fewer ICU admissions upon subsequent hospital admission (5% vs 10% in usual care; relative risk reduction for all patients 50%; p = 0.04).
Non-RCTs
Penrod et al (16) conducted two retrospective observational studies evaluating the impact of palliative care consultation on ICU admissions. In the first study, published in 2006 and including consecutive veterans who died after hospitalization greater than 3 days, 33% of patients receiving a palliative care consultation had an ICU admission compared to 68% of patients receiving usual care (p < 0.001). Using multivariate probit regression to adjust for patient characteristics, the authors found an adjusted risk reduction of 42% (95% CI, −56%, −31%) in ICU admissions for patients who received a palliative care consultation. A subsequent, larger retrospective study consisting of 3,321 veterans hospitalized with advanced disease found that patients receiving palliative care consultation had an adjusted risk reduction of being admitted to the ICU during the same hospitalization of 44% (95% CI, 49%, 39%; p < 0.001) (17). In this cohort, including both survivors and decedents, 33% of hospitalizations for patients receiving palliative care involved an ICU stay, whereas 37% of hospitalizations for patients in the usual care group involved an ICU stay. The mean relative risk reduction for all three studies combined was 37% (sd, 23%) (Table 5).
Table 5.
Estimates of Intervention Effect Size
| Category | Percent Relative Risk Reductiona |
|---|---|
| Trials reporting on ICU admissions (n = 3) | |
| Mean (sd) | 37 (23) |
| Median (IQR) | 50 (11, 51) |
| Trials reporting on ICU length of stay | |
| Intervention in acute care setting (n = 1) | 52 |
| Intervention in outpatient setting (n = 1) | No change |
| Intervention in ICU settingb (n = 14) | |
| Mean (sd) | 26 (23) |
| Median (IQR) | 22 (48, 0) |
| Intervention in ICU setting restricted to patient/provider targets (n = 10) | |
| Mean (sd) | 33 (23) |
| Median (IQR) | 33 (49, 18) |
IQR = interquartile range.
Mean relative risk reduction value used from each trial except for Lilly trial (33), which only reported a median reduction in length of stay.
For trials with separate values for survivors and decedents, we selected the relative risk reduction for decedents.
Trials Evaluating ICU LOS
Interventions in the Acute Care and Outpatient Setting: RCTs
Two randomized trials involved patient-centered interventions taking place outside of the ICU setting—one enrolled medical inpatients 80 years old and older for an advance care planning intervention (24) and one enrolled outpatients with a new diagnosis of life-limiting cancer for a routine palliative care consultation (25) (Tables 2 and 4). In the first trial, Detering et al (24) assessed the impact of advance care planning on end-of-life care. They randomized eligible patients admitted under internal medicine, cardiology medicine, or respiratory medicine in a large university hospital. Upon request from the authors, we were able to obtain ICU LOS data as this outcome was not originally reported; mean LOS was 10.9 days in the control arm and 5.3 days in the intervention arm (relative risk reduction for all patients 52%; no p-value reported) (24). The second trial, Project Educate, Nurture, Advise Before Life Ends II, was designed to improve palliative care for patients with advanced cancer in the outpatient setting. This study randomized patients to a multicomponent palliative care intervention consisting of four weekly educational sessions: ICU LOS did not differ when compared to the control group receiving usual care (0.06 d for both groups; p = 1) (25).
Interventions in the ICU Setting: RCTs
Four RCTs evaluated the effect of ethics or palliative care consultations in the ICU on ICU LOS; for three RCTs, the intervention was focused on individual patients (4, 7, 26) and one was focused at the hospital level (27). Two separate trials conducted by Schneiderman et al (4, 7) examined the effect of routine ethics consultation, enrolling patients in whom value-related treatment conflicts arose. A study conducted by Andereck et al (26) also examined the effect of routine ethics consultation, but targeted patients who were in the ICU for at least 5 days. Curtis et al (27) examined the effect of a multifaceted quality improvement intervention to improve palliative care skills of ICU clinicians, and they identified families as participants for outcome assessment from all adult patients who died in the ICU.
Both of the studies of routine ethics consultation in the ICU by Schneiderman et al (4, 7) demonstrated significant reductions in ICU LOS for decedents in the intervention group compared with usual care. In their single-center study, ICU LOS was 4.2 days in the intervention group versus 13.2 days with usual care (relative risk reduction of 68%; p = 0.03) (7). The impact on LOS among decedents was lower in the larger, seven-center trial, although still statistically significant (6.4 d for intervention vs 7.9 d in usual care; relative risk reduction of 18%; p = 0.03) (4). Neither trial found a difference in ICU LOS for patients who survived to hospital discharge.
By contrast, the two RCTs assessing ICU LOS as an outcome found negative results. Andereck et al (26) failed to find any difference in ICU LOS attributable to an ethics consultation intervention; both intervention and control patients had the same LOS (11 d; p = 0.91). Similarly, the quality improvement intervention by Curtis et al (27) targeted at hospitals and clinicians to integrate palliative care in the ICU did not result in a significant decrease in LOS (5 vs 6 d, intervention vs control, respectively; p = 0.07).
Interventions in the ICU Setting: Non-RCTs
Ten non-RCTs reported ICU LOS as a study outcome. For seven, the interventions targeted patients and three targeted the ICU. Eight studies reported a decrease in ICU LOS associated with a palliative care intervention. Ahrens et al (28) evaluated the impact of a communication team consisting of a physician and clinical nurse specialist with predefined roles aimed at addressing barriers to communication; patients in the intervention group had shorter LOS compared with the control group (6.1 d vs 9.5 d; relative risk reduction for all patients 36%; p < 0.01). The study of palliative care consults for patients with global cerebral ischemia after cardiopulmonary resuscitation using historical controls by Campbell and Guzman (29) found a decrease in LOS (3.7 d vs 7.1 d; relative risk reduction for all patients 48%; p < 0.01). In this same study, however, patients with multisystem organ failure did not spend a significantly longer time in the ICU when compared with historical controls receiving usual care (p = 0.74). The following year, Campbell and Guzman (30) published another study using historical controls and found that proactive palliative care consultation led to a significant reduction in ICU LOS for patients with advanced dementia (3.5 d vs 6.8 d; relative risk reduction for all patients 49%; p < 0.01). Proactive case finding involved screening the medical ICU (MICU) census daily for any patient meeting study criteria. Using nonrandomized controls, Dowdy et al (21) reported a 6-day reduction in LOS when the ethics service intervened proactively after patients received more than 96 hours of continuous mechanical ventilation (relative risk reduction for decedents 30%; no p-value reported).
Four of the eight studies that found a reduction in ICU LOS were pre-post in design. Of these, two focused on ICU system change. In a single-center study, Curtis et al (31) evaluated the impact of a quality improvement intervention targeted to ICU personnel and designed to integrate palliative care into the ICU; median ICU LOS was shorter in the postimplementation period (3 d vs 4 d; relative risk reduction for all patients 19%; p = 0.01). As described above, this difference was not observed in the follow-up multicenter cluster RCT (27). Mosenthal et al (32) evaluated the impact of integrating, into standard care in a trauma ICU, a structured palliative care intervention consisting of assessment of patient prognosis and preferences, an interdisciplinary family meeting, and family bereavement support. Among decedents, median LOS decreased from 3 days to 1 day in the postimplementation period for relative risk reduction of 19%. Results of hypothesis testing were not reported.
Among the patient-focused interventions, Lilly et al (33) evaluated the impact of a multidisciplinary family meeting held within 72 hours of admission to the MICU; they found a reduction in median LOS in the postimplementation phase when compared with the baseline period (3 d vs 4 d; relative risk reduction for all patients 25%; no p-value reported). Similarly, Norton et al (22) evaluated the impact of proactive palliative care consultation for patients admitted to the MICU and identified to be at high risk of death. This study, using a pre/post nonequivalent control group design, found a significant reduction in ICU LOS when compared to usual care (9 d vs 16 d; relative risk reduction for decedents 60%; p < 0.01).
Lastly, two of these studies did not identify a difference in ICU LOS with the intervention. Shelton et al (34) evaluated the effect of adding a full-time family support coordinator to a surgical ICU team in a pre-post study design; no differences in LOS were observed in the pre versus postimplementation period (11.8 d vs 11.4 d, respectively, p = 0.89). In a patient-targeted intervention, Daly et al (35) enrolled patients from five different ICUs and evaluated the effectiveness of an intensive communication strategy in a pre-post design; there were no significant differences in ICU LOS (13.4 d vs 14.4 d, pre vs post, respectively; p = 0.16).
Summary Statistics for Reduction in ICU Admissions and ICU LOS
The estimates for mean relative risk reduction by intervention type are displayed in Table 5. The mean relative risk reduction for ICU admissions associated with advance care planning and palliative care interventions was 37% (sd, 23%). The mean relative risk reduction for ICU LOS associated with all palliative care interventions in the ICU setting was 26% (sd, 23%). When restricting to palliative care interventions in the ICU setting that were directly targeted at the level of individual patients, the mean relative risk reduction was 33% (sd, 23%).
DISCUSSION
Our systematic review included 22 studies—nine RCTs and 13 non-RCTs. Interventions were diverse, populations were heterogeneous, and study designs varied. Variability in these dimensions limited our ability to conduct a quantitative meta-analysis. Despite this, two important trends emerged that warrant further investigation: 1) studies targeting ICU admissions suggest that advance care planning and palliative care interventions reduce the number of ICU admissions for patients at high risk of death; and 2) the majority of studies demonstrated a reduced ICU LOS with advance care planning or palliative care interventions.
For the three studies reporting on ICU admissions, the mean relative risk reduction in percentage of admissions seen with palliative care consultations was 37% (sd, 23%). Two of these studies were retrospective studies that used instrumental variable (16) and propensity score matching (17) techniques to control for potential confounding, and one study was an RCT (23). All interventions were targeted directly at patients rather than at the system level. These studies suggest the value of targeting the intervention to appropriate patients and that the effect of an intervention will vary widely based on the “risk” of the target population.
Although the trend for studies reporting ICU LOS favored decreased utilization, significant variability was present. Among the 16 studies used to estimate the magnitude of effect, 11 reported a decrease in LOS and five demonstrated no change. There are several possible reasons for this observed variability. First, baseline characteristics of selected patients may have influenced results. For example, in the trial by Andereck et al (26), any adult patient in the ICU for at least 5 days was included; the lack of restriction on baseline comorbidities or palliative care needs and the fact that patients were already in the ICU for 5 days at enrollment may have made it more difficult for palliative care interventions to affect LOS. Second, intervention location differed among trials (outpatient, acute care, ICU). For example, in the RCT conducted by Bakitas et al (25), the intervention took place in the outpatient setting, potentially reducing the impact on ICU LOS. Third, intervention targets varied between studies (patient vs system). For example, two of the trials reporting no difference in LOS involved system-level interventions with patient-level outcomes, which could potentially attenuate the effect seen on ICU LOS (27, 34). For these reasons, we separated interventions by setting and level of the intervention target.
Even among trials that reported a decrease in LOS, the magnitude of effect varied significantly. One explanation for this may be the alignment of the intervention target and unit of analysis for outcomes. Although some heterogeneity is present, in general, patient-targeted interventions were more successful in reducing utilization than system-level interventions. Perhaps attenuation of measurable impact is more likely when the intervention takes place at a different level than the assessed outcome. Additionally, the degree of impact of advance care planning and palliative care consultation on ICU utilization is dependent on selecting the appropriate patients and tailoring of care to the individual patient.
The observed variability might also be explained by differences associated with studying decedent versus surviving subjects. Researchers should separate decedents and survivors for analyses as implications for reduced LOS are very different for survivors versus decedents (36).
Our review highlights limitations in existing data that make it difficult to provide precise estimates of the effects that advance care planning and palliative care interventions have on reducing ICU admissions and ICU LOS. Future studies are needed in order to address these limitations and provide more accurate assessments of the magnitude of effect on resource utilization. For example, it was difficult to assess whether the strength (“dose”) of interventions was sufficient to be effective and whether residual confounding or selection bias influenced findings. Additionally, while our study questions focused on ICU utilization, consistently reporting hospital LOS along with ICU LOS is important when assessing the true economic effect of a reduced ICU LOS as the marginal benefit between an ICU day saved versus a hospital day saved is a debated topic that requires further evaluation (10, 11, 37). Lastly, although data from RCTs are generally considered more robust, observational data and non-RCT study designs may be more pragmatic in this patient population. Methods to reduce bias such as multivariate regression techniques, propensity score matching, and instrumental variable adjustment should be routinely used in non-RCTs. However, rigorous randomized trials are still needed.
Our systematic review also has several limitations. First, we acknowledge that a major limitation of the estimates provided for reduced ICU admissions and LOS is the wide variability. However, this is a reflection of the current literature, and future studies are needed to increase the precision of these estimates. Second, our review may be limited by publication bias as we did not attempt to identify unpublished studies and articles. Third, our search strategy may have missed pertinent studies. However, because we identified the same list of included publications in multiple databases, reference lists, and other systematic reviews (5, 9, 38), we have considerable confidence in the accuracy and completeness of review. Lastly, our search strategy excluded studies that focused on early palliative care interventions with an outcome of hospital admissions if ICU admission was not a reported outcome. We recognize that not including these studies may lead to an underestimation of the quality of evidence for early palliative care interventions.
Despite the limitations of the existing literature, it is possible to provide estimates of the magnitude of effect of advance care planning and palliative care interventions on ICU admission and ICU LOS for patients with life-limiting illness. Although the target population and the interventions themselves are heterogeneous, assessment of the effect of palliative care interventions across different settings and times provides greater external validity for these estimates (39). Indeed, these compound estimates are more reliable for future studies of the potential effects of palliative care interventions on critically ill patients, regardless of the underlying disease or condition, than using estimates from one RCT involving a specific population. The primary rationale for advance care planning or palliative care interventions is to improve quality of care, ensure patients receive the care they would choose if fully informed, and improve patient and family outcomes. However, understanding and quantifying the potential for reduction in unwanted intensive care at the end of life may have important clinical and economic implications for how we approach end-of-life care in the hospital and ICU settings. These interventions aim to relieve suffering and improve overall quality of life for patients with advanced life-limiting illnesses and their families, yet may be difficult to implement in an effective or cost-effective way. Demonstrating that these interventions have the potential to lead to significant cost savings may guide decisions on resource allocation for end-of-life care in the ICU. In an era focused on cost containment and transition from fee-for-service to the Accountable Care Organization environment, understanding the true effect of advance care planning and palliative care interventions on ICU resource utilization will be increasingly important, and this systematic review provides a useful step in this direction.
Supplementary Material
ACKNOWLEDGMENTS
We recognize the contributions of Sarah Safranek, research librarian at the University of Washington Health Sciences library.
Dr. Khandelwal received support for article research from the National Institutes of Health (NIH). Her institution received grant support from the NIH (supported by training grant: The National Institute of General Medical Sciences [5T32GM086270]). Dr. Kross received support for article research from the NIH. Her institution received grant support from the National Heart, Lung, and Blood Institute (NHLBI)-K23 award (grant support for career development activities including submitted work) and the American Lung Association Social Behavioral Research Award (grant support for other research outside of the submitted work). Dr. Engelberg received support for article research from the NIH. Her institution received grant support from the NIH, the Robert Wood Johnson Foundation, Patient-Centered Outcomes Research Institute, and the National Palliative Care Research Center. Dr. Long received support for article research from the NIH. Her institution received grant support from the NIH-NHLBI. Dr. Curtis received support for article research from the NIH. His institution received grant support from the NIH.
Footnotes
This work was performed at Harborview Medical Center.
Dr. Khandelwal is responsible for the integrity of the work as a whole. Dr. Khandelwal served as primary author, designed the study protocol, developed the search strategy, reviewed all studies, performed data abstraction, analyzed the data, wrote the article and its revisions, and approved the final version of the article. She attests that no undisclosed authors contributed to the article. Dr. Kross reviewed all studies, performed data abstraction, analyzed the data, edited drafts of the article, and approved the final version of the article. Dr. Engelberg designed the study protocol, refined the search strategy, edited drafts of the article, and approved the final version of the article. Dr. Coe analyzed the data, edited drafts of the article, and approved the final version of the article. Dr. Long analyzed the data, edited drafts of the article, and approved the final version of the article. Dr. Curtis designed the study protocol, refined the search strategy, edited drafts of the article, and approved the final version of the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Dr. Coe has disclosed that she does not have any potential conflicts of interest.
REFERENCES
- 1.Halpern NA, Pastores SM. Critical care medicine in the United States 2000–2005: An analysis of bed numbers, occupancy rates, payer mix, costs. Crit Care Med. 2010;38:65–71. doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- 2.Pastores SM, Dakwar J, Halpern NA. Costs of critical care medicine. Crit Care Clin. 2012;28:1–10. doi: 10.1016/j.ccc.2011.10.003. v. [DOI] [PubMed] [Google Scholar]
- 3.Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for Medicare beneficiaries: Site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309:470–477. doi: 10.1001/jama.2012.207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneiderman LJ, Gilmer T, Teetzel HD, et al. Effect of ethics consultations on nonbeneficial life-sustaining treatments in the intensive care setting: A randomized controlled trial. JAMA. 2003;290:1166–1172. doi: 10.1001/jama.290.9.1166. [DOI] [PubMed] [Google Scholar]
- 5.Scheunemann LP, McDevitt M, Carson SS, et al. Randomized, controlled trials of interventions to improve communication in intensive care: A systematic review. Chest. 2011;139:543–554. doi: 10.1378/chest.10-0595. [DOI] [PubMed] [Google Scholar]
- 6.Molloy DW, Guyatt GH, Russo R, et al. Systematic implementation of an advance directive program in nursing homes: A randomized controlled trial. JAMA. 2000;283:1437–1444. doi: 10.1001/jama.283.11.1437. [DOI] [PubMed] [Google Scholar]
- 7.Schneiderman LJ, Gilmer T, Teetzel HD. Impact of ethics consultations in the intensive care setting: A randomized, controlled trial. Crit Care Med. 2000;28:3920–3924. doi: 10.1097/00003246-200012000-00033. [DOI] [PubMed] [Google Scholar]
- 8.Quill TE, Holloway R. Time-limited trials near the end of life. JAMA. 2011;306:1483–1484. doi: 10.1001/jama.2011.1413. [DOI] [PubMed] [Google Scholar]
- 9.Aslakson R, Cheng J, Vollenweider D, et al. Evidence-based palliative care in the intensive care unit: A systematic review of interventions. J Palliat Med. 2014;17:219–235. doi: 10.1089/jpm.2013.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis JR, Engelberg RA, Bensink ME, et al. End-of-life care in the intensive care unit: Can we simultaneously increase quality and reduce costs? Am J Respir Crit Care Med. 2012;186:587–592. doi: 10.1164/rccm.201206-1020CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luce JM, Rubenfeld GD. Can health care costs be reduced by limiting intensive care at the end of life? Am J Respir Crit Care Med. 2002;165:750–754. doi: 10.1164/ajrccm.165.6.2109045. [DOI] [PubMed] [Google Scholar]
- 12.A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). The SUPPORT Principal Investigators Understanding costs and cost-effectiveness in critical care: Report from the second American Thoracic Society workshop on outcomes research. JAMA. 1995;274:1591–1598. [PubMed] [Google Scholar]
- 13.Boutron I, Moher D, Altman DG, et al. CONSORT Group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: Explanation and elaboration. Ann Intern Med. 2008;148:295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
- 14.Campbell MK, Elbourne DR, Altman DG CONSORT group. CONSORT statement: Extension to cluster randomised trials. BMJ. 2004;328:702–708. doi: 10.1136/bmj.328.7441.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Des Jarlais DC, Lyles C, Crepaz N TREND Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND statement. Am J Public Health. 2004;94:361–366. doi: 10.2105/ajph.94.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penrod JD, Deb P, Luhrs C, et al. Cost and utilization outcomes of patients receiving hospital-based palliative care consultation. J Palliat Med. 2006;9:855–860. doi: 10.1089/jpm.2006.9.855. [DOI] [PubMed] [Google Scholar]
- 17.Penrod JD, Deb P, Dellenbaugh C, et al. Hospital-based palliative care consultation: Effects on hospital cost. J Palliat Med. 2010;13:973–979. doi: 10.1089/jpm.2010.0038. [DOI] [PubMed] [Google Scholar]
- 18.Lautrette A, Darmon M, Megarbane B, et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med. 2007;356:469–478. doi: 10.1056/NEJMoa063446. [DOI] [PubMed] [Google Scholar]
- 19.Cheung W, Aggarwal G, Fugaccia E, et al. Palliative care teams in the intensive care unit: A randomised, controlled, feasibility study. Crit Care Resusc. 2010;12:28–35. [PubMed] [Google Scholar]
- 20.Hsu-Kim C, Friedman T, Gracely E, et al. Integrating palliative care into critical care: A quality improvement study. J Intensive Care Med. 2014 Mar 5; doi: 10.1177/0885066614523923. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Dowdy MD, Robertson C, Bander JA. A study of proactive ethics consultation for critically and terminally ill patients with extended lengths of stay. Crit Care Med. 1998;26:252–259. doi: 10.1097/00003246-199802000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Norton SA, Hogan LA, Holloway RG, et al. Proactive palliative care in the medical intensive care unit: Effects on length of stay for selected high-risk patients. Crit Care Med. 2007;35:1530–1535. doi: 10.1097/01.CCM.0000266533.06543.0C. [DOI] [PubMed] [Google Scholar]
- 23.Gade G, Venohr I, Conner D, et al. Impact of an inpatient palliative care team: A randomized control trial. J Palliat Med. 2008;11:180–190. doi: 10.1089/jpm.2007.0055. [DOI] [PubMed] [Google Scholar]
- 24.Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: Randomised controlled trial. BMJ. 2010;340:c1345. doi: 10.1136/bmj.c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The Project ENABLE II randomized controlled trial. JAMA. 2009;302:741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andereck WS, McGaughey JW, Schneiderman LJ, et al. Seeking to reduce nonbeneficial treatment in the ICU: An exploratory trial of proactive ethics consultation. Crit Care Med. 2014;42:824–830. doi: 10.1097/CCM.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 27.Curtis JR, Nielsen EL, Treece PD, et al. Effect of a quality-improvement intervention on end-of-life care in the intensive care unit: A randomized trial. Am J Respir Crit Care Med. 2011;183:348–355. doi: 10.1164/rccm.201006-1004OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahrens T, Yancey V, Kollef M. Improving family communications at the end of life: Implications for length of stay in the intensive care unit and resource use. Am J Crit Care. 2003;12:317–323. discussion 324. [PubMed] [Google Scholar]
- 29.Campbell ML, Guzman JA. Impact of a proactive approach to improve end-of-life care in a medical ICU. Chest. 2003;123:266–271. doi: 10.1378/chest.123.1.266. [DOI] [PubMed] [Google Scholar]
- 30.Campbell ML, Guzman JA. A proactive approach to improve end-of-life care in a medical intensive care unit for patients with terminal dementia. Crit Care Med. 2004;32:1839–1843. doi: 10.1097/01.ccm.0000138560.56577.88. [DOI] [PubMed] [Google Scholar]
- 31.Curtis JR, Treece PD, Nielsen EL, et al. Integrating palliative and critical care: Evaluation of a quality-improvement intervention. Am J Respir Crit Care Med. 2008;178:269–275. doi: 10.1164/rccm.200802-272OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosenthal AC, Murphy PA, Barker LK, et al. Changing the culture around end-of-life care in the trauma intensive care unit. J Trauma. 2008;64:1587–1593. doi: 10.1097/TA.0b013e318174f112. [DOI] [PubMed] [Google Scholar]
- 33.Lilly CM, Sonna LA, Haley KJ, et al. Intensive communication: Four-year follow-up from a clinical practice study. Crit Care Med. 2003;31(5 Suppl):S394–S399. doi: 10.1097/01.CCM.0000065279.77449.B4. [DOI] [PubMed] [Google Scholar]
- 34.Shelton W, Moore CD, Socaris S, et al. The effect of a family support intervention on family satisfaction, length-of-stay, and cost of care in the intensive care unit. Crit Care Med. 2010;38:1315–1320. doi: 10.1097/CCM.0b013e3181d9d9fe. [DOI] [PubMed] [Google Scholar]
- 35.Daly BJ, Douglas SL, O’Toole E, et al. Effectiveness trial of an intensive communication structure for families of long-stay ICU patients. Chest. 2010;138:1340–1348. doi: 10.1378/chest.10-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cassel JB, Kerr K, Pantilat S, et al. Palliative care consultation and hospital length of stay. J Palliat Med. 2010;13:761–767. doi: 10.1089/jpm.2009.0379. [DOI] [PubMed] [Google Scholar]
- 37.Kahn JM, Rubenfeld GD, Rohrbach J, et al. Cost savings attributable to reductions in intensive care unit length of stay for mechanically ventilated patients. Med Care. 2008;46:1226–1233. doi: 10.1097/MLR.0b013e31817d9342. [DOI] [PubMed] [Google Scholar]
- 38.Smith S, Brick A, O’Hara S, et al. Evidence on the cost and costeffectiveness of palliative care: A literature review. Palliat Med. 2014;28:130–150. doi: 10.1177/0269216313493466. [DOI] [PubMed] [Google Scholar]
- 39.Biondi-Zoccai G, Lotrionte M, Landoni G, et al. The rough guide to systematic reviews and meta-analyses. HSR Proc Intensive Care Cardiovasc Anesth. 2011;3:161–173. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.