Abstract
Introduction
Electrical impedance myography (EIM) quantifies muscle health and is used as a biomarker of muscle abnormalities in neurogenic and myopathic diseases. EIM has yet to be evaluated in the tongue musculature in people with amyotrophic lateral sclerosis (ALS), who often show clinical bulbar signs.
Methods
The lingual musculature of 19 subjects with motor neuron disease and 21 of normal participants were assessed using EIM, strength and endurance testing, and clinical observation.
Results
Tongue musculature in the ALS group was characterized by significantly smaller phase (Ph) and larger resistance (R) when compared to the healthy cohort. Ph and tongue endurance were correlated in the ALS group.
Discussion
EIM of tongue musculature could distinguish people with ALS from healthy controls. The demonstrated relationship between tongue function and Ph supports further testing of EIM of the tongue as a potential biomarker in ALS.
Keywords: electrical impedance myography, tongue musculature, bulbar dysfunction, amyotrophic lateral sclerosis, motor neuron disease
Introduction
Amyotrophic Lateral Sclerosis (ALS) is a neurodegenerative disease characterized by loss of motor neurons in the brain and spinal cord, resulting in spasticity, weakness, and atrophy of voluntary muscles. Limb and trunk muscles, as well as speech and swallowing (bulbar) muscles may be involved. Despite differences in the site of disease onset (e.g., limbs, respiratory, or bulbar), the majority of patients demonstrate signs of bulbar impairment as the disease progresses 1-3. When the bulbar region is involved, the tongue often shows the earliest and most profound changes relative to the jaw and lips 4-7. This pattern of differential motor neuron impairment is also observed among people with symptoms confined to the limbs 4, suggesting an involvement of hypoglossal motor neurons even at a presymptomatic stage of bulbar disease.
The diagnosis of ALS is based primarily on clinical judgments, with electrophysiological evaluation (electromyography, EMG) providing additional support. Diagnosis of bulbar-onset ALS is particularly challenging, given that strength testing and EMG of the region are limited 4, 8-10. In ALS research, strength, function, and respiratory function are tracked over time and used as trial outcome measures, but none of these capture bulbar function well. Considering the diagnostic challenges in ALS and clinical trial outcome needs 11, there is substantial value in developing a technique to diagnose and monitor changes in the tongue musculature with high sensitivity and reproducibility.
Recently, electrical impedance myography (EIM) has been developed and evaluated as a biomarker of muscle abnormalities in neurogenic and myopathic diseases 12. EIM provides quantitative data on muscle health by measuring localized tissue impedance 13. Changes in the composition and architecture of a muscle affect the impedance values. The common pathological accompaniments of neuromuscular disease (e.g., muscle fiber atrophy, increased endomysial connective tissue, increased fat) alter tissue impedance.
Tissue impedance is expressed with respect to electrical resistance (R, measured in ohms; Ω), reactance (X, also measured in ohms; Ω), and phase (Ph, measured in degrees; °), where Ph = arctan (X/R).
Studies that have identified disease-related differences in limb muscles of healthy people and those with neuromuscular diseases have reported smaller than normal X and larger than normal R, resulting in smaller than normal Ph 14-16.
Existing work on this novel technique has demonstrated the value of EIM for assessment of neuromuscular disease states14, 17, sensitivity to muscle abnormalities16, and overall utility as a sensitive biomarker in clinical trials13. Tarulli et al. demonstrated a relationship between Ph and muscle strength in quadriceps muscle (2005), suggesting that EIM may also be related to muscle function. To date, however, EIM testing has not been performed on human tongue muscles.
EIM variables are also sensitive to age-related changes in healthy limb muscles 18-20. Specifically, people over age 60 years have a significant reduction in Ph compared to their younger counterparts. The age-dependent changes are linked to sarcopenia and to changes in muscle related to the increasingly sedentary lifestyle in this age group 18, 21-23. Considering that ALS is most commonly diagnosed in middle and late adulthood with a mean age of onset of 65 years 24, there is a need to account for potential age-related effects on tongue musculature when investigating disease-related changes in EIM measures. EIM performed on healthy limb muscles has also shown gender and age interactions in 1 study, with men having a more pronounced decrease in X and R with age when compared to women 20. There is, therefore, a need to investigate the effect of gender on EIM measures of the tongue.
The objectives of this pilot study were to: (1) examine age and gender effects on EIM measures of the healthy tongue; (2) compare EIM measures between groups of people with ALS and healthy controls; and (3) determine if there is an association between EIM measures and standard clinical measures of bulbar disease and tongue function (e.g., tongue strength and endurance). Based on existing limb studies, we hypothesized that healthy tongue muscles would show age and gender effects. Also, participants would show a decrease in Ph and X and an increase in R, when compared to healthy controls. We hypothesized that there would be a relationship between Ph and standard clinical measures of disease progression and tongue function.
MATERIALS AND METHODS
Participants
Seventeen individuals with ALS (9 men, 8 women), 1 with primary lateral sclerosis (PLS; man), and 1 with Kennedy disease (KD; man) were investigated. The ALS participants were selected based on the presence of clinical signs of bulbar involvement including tongue fasciculations, atrophy, and weakness as determined by a neurologist (Author L.Z.). The control group consisted of 21 participants: 13 were age-matched to the clinical group (C>60; 7 men, 6 women), and 8 were under age 30 (C<30; 2 men, 6 women). The study was approved by the Research Ethics Boards at the Sunnybrook Research Institute in Toronto, Canada and at the University of Nebraska-Lincoln, USA. The procedures followed the standards of the Helsinki Declaration of 1975. All participants signed informed consents.
Procedures
EIM
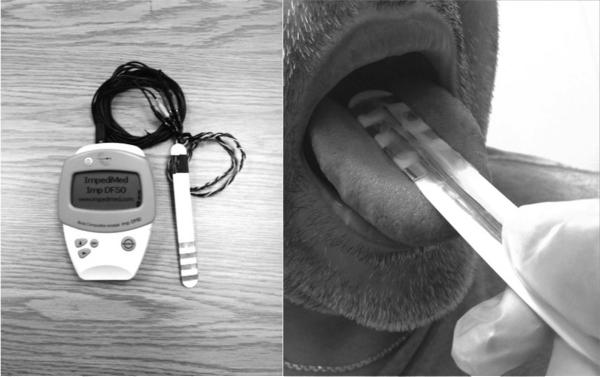
The 50kHz electrical impedance of the tongue was measured using the DF50 (Impedimed, Inc, San Diego, CA). The choice to use this single frequency was motivated the fact that most other EIM work to date had demonstrated that data obtained at this frequency were very sensitive to disease states 25. A custom 4-electrode array was designed for tongue application 26 (see Figure 1, left). This electrode arrangement was believed to be the simplest for application to the tongue, putting aside the complex nature of the underlying muscle fiber structure of that organ 27.
FIGURE 1.
The ImpediMed DF50 and tongue probe equipped with four-electrode array used to measure EIM measures (left). The typical probe placement at the midline of the tongue (right).
Figure 1 (on the right) shows the placement of the array at the tongue midline. Prior to the placement in this position, participants were asked to swallow excessive saliva. After the array was placed on the tongue surface, the participants were instructed to return their tongues to a relaxed position inside the oral cavity with the mouth almost completely closed.
The device outputs the values of EIM phase (Ph), reactance (X), and resistance (R), which were recorded manually after each trial. Five to ten trials were obtained for all participants depending on tolerance. Brief rest breaks occurred between each repetition. The first repetitions were discarded. Individual mean values were computed based on an average of the remaining trials.
Tongue function
The Iowa Oral Performance Instrument (IOPI 28, 29) was used to record the maximum tongue strength and endurance in the participants with ALS. Control data was not collected, as normative values have been reported in the past 30. For the IOPI recordings, a small rubber bulb was attached to a pressure transducer. Pressure exerted by the tongue against the bulb positioned against the hard palate was measured in kilopascals (kPa). Maximum strength was determined by calculating an average of three maximal force generation efforts, each of approximately one-second in duration. Endurance was measured in seconds by asking participants to maintain 50% of their maximal pressure for as long as possible. Participants were instructed to generate enough pressure to activate a light source, serving to provide visual feedback.
Bulbar Severity Measures
The severity of disease and its bulbar manifestation were assessed using the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R31). Both total scores and bulbar subscores were obtained. The ALSFRS-R bulbar function subscore included three questions related to speech, swallowing and salivation functions, with a maximum score of 12 indicating intact bulbar function. Values below 12 indicated bulbar impairment in one, or all, bulbar functions. Speaking rate, the number of words produced per minute (WPM), was estimated using the Sentence Intelligibility Test (SIT 32) and used to evaluate severity of bulbar impairment in relation to speech function. Speaking rate has been shown to be sensitive to bulbar disease progression and specifically tongue dysfunction in ALS 33.
All participants were also evaluated for clinical LMN signs in the tongue, e.g., muscle atrophy, weakness and fasciculations, by a neurologist (Author 7). Atrophy and weakness were rated on a 4-point Likert Scale from normal (scored 0) to severe (scored 3), and the presence of fasciculations was noted (1/0). The sum of these scores served as the severity index of LMN impairment and was estimated by adding these scores. A maximum score of 7 points indicated the most severe state of muscle weakness and atrophy, with the presence of fasciculations.
Additionally, the severity of hypersalivation was assessed using the Oral Secretion Scale (OSS34), as excessive saliva, being a highly conductive substance, might affect EIM values. Saliva retention was evaluated using a 5-point scale from very severe (scored 0) to normal (scored 4).
Statistical Analysis
All statistical analyses were conducted using IBM SPSS Statistics Version 20. The data from patients with KD and PLS were combined with ALS data, because they fell within the range of those obtained for the ALS group. Reproducibility was assessed for younger and older healthy controls by calculating intraclass correlation coefficients (ICC) between the first and last measurable trials. To address objective 1, differences in age groups (>60 years and <30 years) and gender among healthy individuals for EIM measures were tested using a 2-way Analysis of Variance (ANOVA). To address objective 2, the existence of statistical differences between patients and healthy controls for the EIM measures were analyzed using a 2-way ANOVA with gender and group as factors. To address objective 3, Pearson Correlation Coefficients (r) determined the associations between EIM measures and measures of tongue function and bulbar severity for the ALS group. The assumptions for the relevant statistical tests were met. An alpha level of .05 was used for all statistical tests.
RESULTS
All healthy controls and many individuals with ALS tolerated procedures associated with EIM data collection well. The length of the probe restricted patient recruitment to those without an abnormally sensitive gag reflex. The width of the probe restricted EIM measurements to the midline of the tongue.
Summary of demographic information and bulbar severity measures are shown in Table 1.
Table 1.
Participant demographics and disease severity scores, mean (SD), by group and gender. C>60 = Age-matched healthy controls, C<30 =healthy controls under the age of 30. WPM= words per minute. LMN = Lower Motor Neuron. OSS= Oral Secretion Scale.
| Group | Gender | Age | ALSFRS-R Total | ALSFRS-R Bulbar | Speaking Rate (WPM) | LMN Score (0-7) | OSS (0-4) |
|---|---|---|---|---|---|---|---|
| ALS | M (n=9) | 56.81 (12.16) | 32.9 (9.31) | 8.9 (2.73) | 110.58 (58.25) | 5 (± 1.75) | 3.25 (0.46) |
| W (n=8) | 62.67 (10.23) | 35.80 (7.16) | 8.2 (3.03) | 85.33 (57.87) | 3.71 (1.92) | 3.00 (0.63) | |
| PLS | M (n=1) | 45 | 46 | 11 | 128 | 1 | 4.00 |
| KD | M (n=1) | 46 | 24 | 9 | 154 | 5 | 3.00 |
| C>60 | M (n=7) | 65.42 (8.63) | - | - | - | - | - |
| W (n=6) | 60.5 (8.01) | - | - | - | - | - | |
| C<30 | M (n=2) | - | - | - | - | - | - |
| W(n=6) | - | - | - | - | - | - |
EIM values of healthy individuals
Summary statistics for EIM measures by gender and age for the 2 groups (healthy controls and ALS) are shown in Table 2. The analysis indicated that Ph, R, and X values were not significantly different between men and women, or younger and older individuals in the healthy group. The age by gender interactions were also non-significant. Therefore, the data from the 2 healthy control groups were combined for further analyses.
Table 2.
Descriptive statistics, mean (SD), for EIM measures, Phase (Ph), Reactance (X), and Resistance (R) for healthy controls and participants with ALS by gender and age.
| Variables | Controls (n=21) | ALS (n=19) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Ph (°) | Xc (Ω) | R (Ω) | n | Ph (°) | Xc (Ω) | R (Ω) | ||
| Gender | M | 9 | 13.92 (1.88) | 8.39 (1.99) | 33.94 (4.07) | 11 | 11.01 (3.24) | 7.64 (2.34) | 40.36 (10.94) |
| W | 12 | 13.05 (1.78) | 8.39 (1.35) | 35.07 (5.76) | 8 | 10.24 (4.52) | 7.86 (6.03) | 43.58 (12.98) | |
| Age | >60 | 13 | 13.79 (2.19) | 8.75 (1.91) | 35.73 (5.55) | 19 | 10.68 (3.74) | 7.74 (4.14) | 41.72 (11.60) |
| <30 | 8 | 12.88 (0.91) | 7.81 (0.74) | 32.72 (3.57) | - | - | - | - | |
Reproducibility
The immediate reproducibility analysis of the Ph, X, and R measures obtained for the control participants revealed moderate to high reproducibility with ICCs of 0.790, 0.733, and 0.911, respectively. See Figure 2 for the corresponding test-retest plots for 2 consecutive trials.
FIGURE 2.
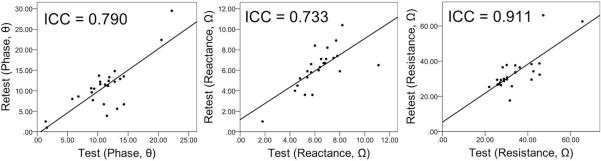
The test-retest plots of phase (°), reactance (Ω), and resistance (Ω) at 50 kHz for healthy individuals. The ICC values are shown; n= 21.
Group differences in EIM measures
The bar graphs shown in Figure 3 revealed a pattern of group (ALS versus healthy controls) differences for 2 of 3 EIM measurements. The mean Ph was reduced significantly in individuals with ALS compared with healthy controls (P = 0.007). The main effect of gender and group by gender interaction were not significant. The mean R was significantly greater for individuals with ALS than for healthy controls (P = 0.010). The main effect of gender and the group by gender interaction were also not significant in this model. The X values were not significantly different between healthy controls and individuals with ALS, neither were the main effect of gender and the group by gender interaction.
FIGURE 3.
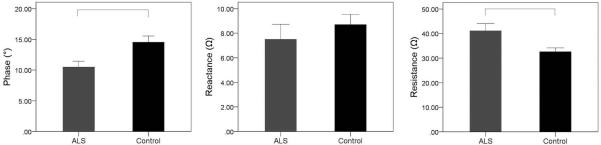
Mean values (±SEM) for EIM measures, phase (°), reactance (Ω), and resistance (Ω) by group; participants with ALS and healthy controls. Square brackets represent significant group differences (P<0.05).
EIM and tongue function measures
Functional measures, such as tongue maximum strength, endurance, OSS scores for individuals in the ALS group are reported in Table 3.
Table 3.
Descriptive statistics, mean (SD), of tongue functional measures for people with ALS: maximum strength (kPa) and endurance (sec).
| Gender | Tongue Strength (kPa) | Tongue Endurance (sec) |
|---|---|---|
| M (n= 11) | 33.42 (19.32) | 21.81 (11.83) |
| W (n= 8) | 21.19 (14.61) | 11.5 (7.5) |
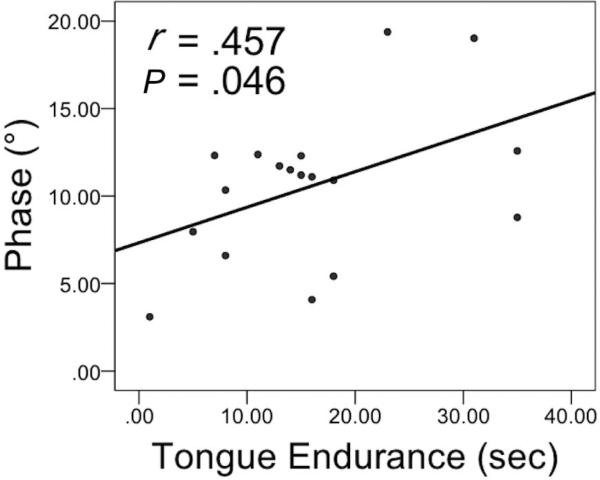
Correlation analyses were performed to delineate possible associations between EIM measures and clinical measures of disease, including ALSFRS-R total and bulbar scores, LMN scores, and speaking rate, as well as measures of tongue function, such as maximum strength and endurance. Correlation analyses were not performed between OSS scores and EIM measures, as most people with ALS exhibited minimal (scored 3) to normal (scored 4) levels of hypersalivation on the OSS with seemingly no effect on the EIM measures. A significant moderate, positive correlation was observed only between Ph (°) values and tongue endurance (s) in the ALS group (P = 0.046) (see Figure 4).
FIGURE 4.
A scatterplot of phase (°) and tongue endurance (sec) in the ALS group (n=18). Pearson Correlation Coefficients (r) and P-values (P) are shown.
DISCUSSION
This study investigated tongue muscle health via electrical impedance myography (EIM). We compared the 50 kHz EIM measures (phase, reactance, and resistance) in the tongue of individuals with ALS and healthy controls, including differences between younger and older healthy individuals. Gender or age did not affect tongue EIM measures in healthy participants. Consistent with previous limb-based findings13-15, phase was significantly reduced among people with ALS when compared to healthy individuals, yet resistance was significantly greater in the ALS group in comparison to the control group. No differences between the 2 groups were observed for reactance. An association between phase and tongue endurance was observed in the ALS group.
EIM in the healthy tongue
In concordance with EIM studies on limb muscles, these results suggest that EIM measures in the tongue are highly reproducible 35.
EIM measures of the tongue did not vary by age in healthy individuals. The age difference for the EIM measures was expected based on existing limb studies, which revealed a significant decrease in phase with increasing age in healthy muscle 18, 36. Existing literature identified aging effects on contractile properties of limb musculature by age 60 30, 37. To date, very few studies have addressed the age-related changes in tongue musculature 23, 30, 38- 40. In contrast to limb studies, they demonstrated that tongue strength is affected only after age 80 years 30, 38. These differences may possibly be due to a relatively constant level of tongue activity throughout life, allowing for maintenance of function 30. Our study population consisted of only 1 individual over age 80 whose performance on all 3 EIM measures did not deviate from normal values (< 1SD). Therefore, further work investigating age-related changes in impedance values of the tongue in elderly subjects is indicated.
The findings of this study suggest that gender does not affect impedance values of tongue musculature in healthy individuals. Existing literature has been inconclusive in demonstrating whether gender affects EIM measures in the limbs. Previous work has suggested that normal values may be different for healthy older men and women 20; however, more recent studies have not identified gender differences 25. Our study might further suggest that the structural and compositional properties of tongue musculature in healthy individuals do not differ significantly between men and women.
EIM in ALS Tongue
This study identified differences among EIM values in tongue muscles between healthy controls and those with ALS. Specifically, reductions in phase and an increase in resistance among participants with ALS were observed. The primary mechanisms responsible for these differences may be a reduction in muscle size due to atrophy and changes in muscle composition due to fatty infiltration 12. These suggestions are supported by the findings from existing MRI and histopathological studies of the tongue in ALS, which describe abnormalities in size, shape, and internal structure 41-44. Tongue size was reported to decrease by as much as two-thirds of normal with areas of significant fat replacement 41.
Both upper and lower motor neuron (UMN versus LMN) abnormalities underlie structural and functional changes in ALS. EIM measures differ depending on the relative degree of UMN or LMN impairment. Those with UMN predominance may present with disuse muscle states, while those with LMN predominance may present with atrophy muscle states, both of which can be quantified using EIM in limb muscles 36. However, a similar distinction would be challenging in the tongue. Clinically, LMN involvement is assessed through observations of fasciculations and muscle atrophy, as well as lingual EMGs; however, UMN signs in the tongue are difficult to identify 1. Promising techniques for detecting UMN involvement, such as transcranial magnetic stimulation (TMS), are currently under investigation, but are not yet commonly used in the clinical setting. Further work linking EIM with other instrumental techniques such as lingual EMG and TMS is needed.
The tongue structure and function
Disease-related changes in EIM were not associated with either the ALSFRS-R total and bulbar subscores31 or the LMN severity ratings. This lack of association may be explained by the subjective nature of ALS severity measures and their insensitivity to underlying changes in bulbar anatomy. Indeed, a recent study found that the ALSFRS-R bulbar subscores were insensitive to the bulbar decline detected in speech movements (i.e., tongue, lips, and jaw speed) 46.
Disease-related changes in EIM were also not associated with the measure of speaking rate. Speaking rate measures the severity of speech production impairment and is known to decrease significantly with disease progression. 3 However, multiple structures (e.g., tongue, jaw, lips, velum, larynx, and diaphragm) contribute to speech production 45, therefore isolated changes in tongue muscles may not affect speaking rate 47-49.
Disease effects on the tongue have also been evaluated using strength and endurance measures 30, 40, 50, 51. People with ALS consistently demonstrate reduced tongue strength and endurance compared to healthy individuals 4, 5, 39, 52-54 and these changes have effects on speaking 54-56 and swallowing 57-59. In agreement with normative data published elsewhere 30, our patient group showed reduced maximum strength and endurance in the tongue, with a relatively large range of performance among individuals.
Previous investigations of the limbs reported significant associations between EIM measures and muscle strength; however, this association was not found in the tongue. This finding may result from differences in how strength measures are obtained in the limbs as compared to those in the tongue. In contrast to the tongue muscles, limb muscles can be isolated during strength measurements. This is difficult to achieve in the tongue due to the co-contraction of the tongue and jaw musculature during tongue tasks 54. Subsequently, the less affected jaw might compensate for tongue muscle weakness, resulting in poorer correlations with EIM measures. The variation in tongue endurance, however, was explained partially by the variation in phase values among the patient group. This suggests that phase values may be indicative of oral-motor dysfunction, with smaller phase indicating greater severity of tongue impairment.
Technical Issues
This pilot study revealed that technical adjustments are needed to improve device tolerance and accommodate for disease-related changes in tongue structure (e.g., muscle atrophy 60). Specifically, the length of the electrode array should be reduced to minimize the likelihood of stimulating a gag reflex. The width should be reduced to allow data collection bilaterally on the tongue surface in all subjects. A redesign of the probe to accommodate patients with ALS is underway.
Conclusions
This study demonstrates that EIM provides a useful method for assessing the effects of ALS on tongue musculature, supporting its application as a potential clinical biomarker for diagnostic and monitoring purposes. Future work is required to determine longitudinal changes in impedance measures as ALS progresses, including changes in early disease stages when bulbar signs and symptoms are not present clinically. The ability to identify pre-symptomatic tongue abnormalities may allow an earlier diagnosis, resulting in an increased capacity to accurately predict changes in the bulbar functions of speech and swallowing as ALS progresses.
Acknowledgements
The authors would like to thank Aaron Pattee, Emily Foutch, Sara Benning, Lori Synhorst, Ashkon Pourheidary, Danielle Thomas, and Madhura Kulkarni for their assistance with this project. They also thank the patients and their families for their participation. This research was supported by NIH-NIDCD grants R01 DC009890 & R01 DC0135470, CIHR Planning Grant # FRN126682, and an ALS Society of Canada Bernice Ramsey Discovery Grant.
Abbreviations
- ALS
Amyotrophic Lateral Sclerosis
- ALSFRS-R
Amyotrophic Lateral Sclerosis Functional Rating Scale- Revised
- C<30
Healthy controls under 30 years of age
- C>60
Healthy controls over 60 years of age
- EIM
Electrical Impedance Myography
- EMG
Electromyography
- ICC
Intraclass correlation
- IOPI
Iowa Oral Performance Instrument
- KD
Kennedy Disease
- LED
Light emitting diode
- LMN
Lower motor neuron
- OSS
Oral Secretion Scale
- Ph
Phase
- PLS
Primary Lateral Sclerosis
- R
Resistance
- SIT
Sentence Intelligibility Test
- UMN
Upper motor neuron
- X
Reactance
References
- 1.Del Aguila MA, Longstreth WT, McGuire V, Koepsell TD, Van Belle G. Prognosis in amyotrophic lateral sclerosis A population-based study. Neurology. 2003;60(5):813–819. doi: 10.1212/01.wnl.0000049472.47709.3b. [DOI] [PubMed] [Google Scholar]
- 2.Armon C, Moses D. Linear estimates of rates of disease progression as predictors of survival in patients with ALS entering clinical trials. Journal of the neurological sciences. 1998;160:S37–S41. doi: 10.1016/s0022-510x(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 3.Yorkston KM, Strand E, Miller R, Hillel A, Smith K. Speech deterioration in amyotrophic lateral sclerosis: Implications for the timing of intervention. Journal of Medical Speech-Language Pathology. 1993;1(1):35–46. [Google Scholar]
- 4.DePaul R, Abbs JH, Caligiuri M, Gracco VL, Brooks BR. Hypoglossal, trigeminal, and facial motoneuron involvement in amyotrophic lateral sclerosis. Neurology. 1988;38(2):281–281. doi: 10.1212/wnl.38.2.281. [DOI] [PubMed] [Google Scholar]
- 5.DePaul R, Brooks BR. Multiple orofacial indices in amyotrophic lateral sclerosis. Journal of Speech, Language and Hearing Research. 1993;36(6):1158. doi: 10.1044/jshr.3606.1158. [DOI] [PubMed] [Google Scholar]
- 6.Hirose H, Kiritani S, Sawashima M. Patterns of dysarthric movement in patients with amyotrophic lateral sclerosis and pseudobulbar palsy. Folia Phoniatrica et Logopaedica. 1982;34(2):106–112. doi: 10.1159/000265636. [DOI] [PubMed] [Google Scholar]
- 7.Langmore SE, Lehman ME. Physiologic deficits in the orofacial system underlying dysarthria in amyotrophic lateral sclerosis. Journal of Speech, Language and Hearing Research. 1994;37(1):28. doi: 10.1044/jshr.3701.28. [DOI] [PubMed] [Google Scholar]
- 8.Andres PL, Hedlund W, Finison L, Conlon T, Felmus M, Munsat TL. Quantitative motor assessment in amyotrophic lateral sclerosis. Neurology. 1986;36(7):937–937. doi: 10.1212/wnl.36.7.937. [DOI] [PubMed] [Google Scholar]
- 9.Andres PL, Thibodeau LM, Finison LJ, Munsat TL. Quantitative assessment of neuromuscular deficit in ALS. Neurologic clinics. 1987;5(1):125–141. [PubMed] [Google Scholar]
- 10.Finsterer J, Erdorf M, Mamoli B, Fuglsang-Frederiksen A. Needle electromyography of bulbar muscles in patients with amyotrophic lateral sclerosis Evidence of subclinical involvement. Neurology. 1998;51(5):1417–1422. doi: 10.1212/wnl.51.5.1417. [DOI] [PubMed] [Google Scholar]
- 11.Brooks BR, Sufit RL, DePaul R, Tan YD, Sanjak M, Robbins J. Design of clinical therapeutic trials in amyotrophic lateral sclerosis. Advances in neurology. 1990;56:521–546. [PubMed] [Google Scholar]
- 12.Rutkove S. Electrical impedance myography as a biomarker for ALS. The Lancet Neurology. 2009;8(3):226. doi: 10.1016/S1474-4422(09)70030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutkove SB, Zhang H, Schoenfeld DA, Raynor EM, Shefner JM, Cudkowicz ME, et al. Electrical impedance myography to assess outcome in amyotrophic lateral sclerosis clinical trials. Clinical Neurophysiology. 2007;118(11):2413–2418. doi: 10.1016/j.clinph.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Rutkove SB. Alteration in surface muscle electrical anisotropy in the rat SOD1 model of amyotrophic lateral sclerosis. Clinical Neurophysiology. 2012;123(1):206–210. doi: 10.1016/j.clinph.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutkove SB, Caress JB, Cartwright MS, Burns TM, Warder J, David WS, et al. Electrical impedance myography as a biomarker to assess ALS progression. Amyotrophic Lateral Sclerosis. 2012;13(5):439–445. doi: 10.3109/17482968.2012.688837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarulli AW, Duggal N, Esper GJ, Garmirian LP, Fogerson PM, Lin CH, et al. Electrical impedance myography in the assessment of disuse atrophy. Archives of physical medicine and rehabilitation. 2009;90(10):1806–1810. doi: 10.1016/j.apmr.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esper GJ, Shiffman CA, Aaron R, Lee KS, Rutkove SB. Assessing neuromuscular disease with multifrequency electrical impedance myography. Muscle & nerve. 2006;34(5):595–602. doi: 10.1002/mus.20626. [DOI] [PubMed] [Google Scholar]
- 18.Aaron R, Esper GJ, Shiffman CA, Bradonjic K, Lee KS, Rutkove SB. Effects of age on muscle as measured by electrical impedance myography. Physiological measurement. 2006;27(10):953. doi: 10.1088/0967-3334/27/10/002. [DOI] [PubMed] [Google Scholar]
- 19.Tarulli AW, Chin AB, Lee KS, Rutkove SB. Impact of skin-subcutaneous fat layer thickness on electrical impedance myography measurements: An initial assessment. Clinical Neurophysiology. 2007;118(11):2393–2397. doi: 10.1016/j.clinph.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kortman HG, Wilder SC, Geisbush TR, Narayanaswami P, Rutkove SB. Age-and gender-associated differences in electrical impedance values of skeletal muscle. Physiological measurement. 2013;34(12):1611. doi: 10.1088/0967-3334/34/12/1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. Journal of the American Geriatrics Society. 2003;51(11):1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 22.Roubenoff R. Sarcopenia: effects on body composition and function. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2003;58(11):M1012–M1017. doi: 10.1093/gerona/58.11.m1012. [DOI] [PubMed] [Google Scholar]
- 23.Vandervoort AA. Aging of the human neuromuscular system. Muscle & nerve. 2002;25(1):17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 24.Chio A, Mora G, Calvo A, Mazzini L, Bottacchi E, Mutani R. Epidemiology of ALS in Italy A 10-year prospective population-based study. Neurology. 2009;72(8):725–731. doi: 10.1212/01.wnl.0000343008.26874.d1. [DOI] [PubMed] [Google Scholar]
- 25.Rutkove SB, Fogerson PM, Garmirian LP, Tarulli AW. Reference values for 50-kHZ electrical impedance myography. Muscle & Nerve. 2008;38(3):1128–1132. doi: 10.1002/mus.21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimnes S, Martinsen OG. Sources of error in tetrapolar impedance measurements on biomaterials and other ionic conductors. J Phys D: Appl Phys. 2007;40:9–14. [Google Scholar]
- 27.Tarulli AW, Chin AB, Partida RA, Rutkove SB. Electrical impedance in bovine skeletal muscle as a model for the study of neuromuscular disease. Physiological measurement. 2006;27(12):1269. doi: 10.1088/0967-3334/27/12/002. [DOI] [PubMed] [Google Scholar]
- 28.Robin DA, Somodi LB, Luschei ES. Measurement of tongue strength and endurance in normal and articulation disordered subjects. Dysarthria and apraxia of speech: Perspectives on management. 1991:173–184. [Google Scholar]
- 29.Robin DA, Goel A, Somodi LB, Luschei ES. Tongue strength and endurance: relation to highly skilled movements. Journal of Speech, Language and Hearing Research. 1992;35(6):1239. doi: 10.1044/jshr.3506.1239. [DOI] [PubMed] [Google Scholar]
- 30.Crow HC, Ship JA. Tongue strength and endurance in different aged individuals. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1996;51(5):M247–M250. doi: 10.1093/gerona/51a.5.m247. [DOI] [PubMed] [Google Scholar]
- 31.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. Journal of the neurological sciences. 1999;169(1):13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 32.Beukelman D, Yorkston K, Hakel M, Dorsey M. Speech Intelligibility Test. Computer software] Madonna Rehabilitation Hospital; Lincoln: 2007. 2007. [Google Scholar]
- 33.Ball LJ, Willis A, Beukelman DR, Pattee GL. A protocol for identification of early bulbar signs in amyotrophic lateral sclerosis. Journal of the neurological sciences. 2001;191(1):43–53. doi: 10.1016/s0022-510x(01)00623-2. [DOI] [PubMed] [Google Scholar]
- 34.Abdelnour-Mallet M, Tezenas Du Montcel S, Cazzolli PA, Assouline A, Pointon C, Leveque N, et al. Validation of robust tools to measure sialorrhea in amyotrophic lateral sclerosis: A study in a large French cohort. Amyotrophic Lateral Sclerosis. 2013;(0):1–6. doi: 10.3109/21678421.2012.735238. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Staats WL, Spieker A, Sung M, Rutkove SB. A technique for performing electrical impedance myography in the mouse hind limb: data in normal and ALS SOD1 G93A animals. PloS one. 2012;7(9):e45004. doi: 10.1371/journal.pone.0045004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarulli AW, Duggal N, Esper GJ, Garmirian LP, Fogerson PM, Lin CH, et al. Electrical impedance myography in the assessment of disuse atrophy. Archives of physical medicine and rehabilitation. 2009;90(10):1806–1810. doi: 10.1016/j.apmr.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narici MV, Maganaris CN, Reeves ND, Capodaglio P. Effect of aging on human muscle architecture. Journal of applied physiology. 2003;95(6):2229–2234. doi: 10.1152/japplphysiol.00433.2003. [DOI] [PubMed] [Google Scholar]
- 38.Clark HM, Solomon NP. Age and sex differences in orofacial strength. Dysphagia. 2012;27(1):2–9. doi: 10.1007/s00455-011-9328-2. [DOI] [PubMed] [Google Scholar]
- 39.Dworkin JP, Aronson AE, Mulder DW. Tongue force in normals and in dysarthric patients with amyotrophic lateral sclerosis. Journal of Speech, Language and Hearing Research. 1980;23(4):828. doi: 10.1044/jshr.2304.828. [DOI] [PubMed] [Google Scholar]
- 40.Robbins J, Bridges AD, Taylor A. Oral, pharyngeal and esophageal motor function in aging. GI Motility online. 2006 [Google Scholar]
- 41.Cha CH, Patten BM. Amyotrophic lateral sclerosis: abnormalities of the tongue on magnetic resonance imaging. Annals of neurology. 1989;25(5):468–472. doi: 10.1002/ana.410250508. [DOI] [PubMed] [Google Scholar]
- 42.Konagaya M, Konagaya Y, Konishi T, Mano Y. [MRI findings of the tongue in neurodegenerative diseases with bulbar sign]. Rinsho shinkeigaku. Clinical neurology. 1990;30(6):665–667. [PubMed] [Google Scholar]
- 43.DePaul R, Waclawik AJ, Abbs JH, Brooks BR. Histopathological characteristics in lingual muscle tissue in ALS: Perspectives on the natural history of the disease. Neuromotor speech disorders: Nature, assessment, and management. 1998:69–84. [Google Scholar]
- 44.Urban PP, Vogt T, Hopf HC. Corticobulbar tract involvement in amyotrophic lateral sclerosis. A transcranial magnetic stimulation study. Brain. 1998;121(6):1099–1108. doi: 10.1093/brain/121.6.1099. [DOI] [PubMed] [Google Scholar]
- 45.Rutkove SB. Electrical impedance myography: background, current state, and future directions. Muscle & nerve. 2009;40(6):936–946. doi: 10.1002/mus.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green JR, Yunusova Y, Kuruvilla MS, Wang J, Pattee GL, Synhorst L, Berry JD. Bulbar and speech motor assessment in ALS: Challenges and future directions. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2013;14(7-8):494–500. doi: 10.3109/21678421.2013.817585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomik B, Guiloff RJ. Dysarthria in amyotrophic lateral sclerosis: a review. Amyotrophic Lateral Sclerosis. 2010;11(1-2):4–15. doi: 10.3109/17482960802379004. [DOI] [PubMed] [Google Scholar]
- 48.Maeda S. Speech production and speech modelling. Springer; Netherlands: 1990. Compensatory articulation during speech: Evidence from the analysis and synthesis of vocal-tract shapes using an articulatory model. pp. 131–149. [Google Scholar]
- 49.Kelso JS, Tuller B, Vatikiotis-Bateson E, Fowler CA. Functionally specific articulatory cooperation following jaw perturbations during speech: evidence for coordinative structures. Journal of Experimental Psychology: Human Perception and Performance. 1984;10(6):812. doi: 10.1037//0096-1523.10.6.812. [DOI] [PubMed] [Google Scholar]
- 50.Robin DA, Goel A, Somodi LB, Luschei ES. Tongue strength and endurance: relation to highly skilled movements. Journal of Speech and Hearing Research. 1992;35(6):1239. doi: 10.1044/jshr.3506.1239. [DOI] [PubMed] [Google Scholar]
- 51.Solomon NP, Robin DA, Luschei ES. Strength, endurance, and stability of the tongue and hand in Parkinson disease. Journal of Speech, Language & Hearing Research. 2000;43(1) doi: 10.1044/jslhr.4301.256. [DOI] [PubMed] [Google Scholar]
- 52.Robbins JA, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1995;50(5):M257–M262. doi: 10.1093/gerona/50a.5.m257. [DOI] [PubMed] [Google Scholar]
- 53.Dworkin JP, Aronson AE. Tongue strength and alternate motion rates in normal and dysarthric subjects. Journal of communication disorders. 1986;19(2):115–132. doi: 10.1016/0021-9924(86)90015-8. [DOI] [PubMed] [Google Scholar]
- 54.Solomon NP, Munson B. The effect of jaw position on measures of tongue strength and endurance. Journal of Speech, Language, and Hearing Research. 2004;47(3):584. doi: 10.1044/1092-4388(2004/045). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solomon NP, Lorell DM, Robin DA, Rodnitzky RL, Luschei ES. Tongue strength and endurance in mild to moderate Parkinson's disease. Journal of Medical Speech-Language Pathology. 1995;3(1):15–26. [Google Scholar]
- 56.Neel AT, Palmer PM. Is tongue strength an important influence on rate of articulation in diadochokinetic and reading tasks?. Journal of Speech, Language, and Hearing Research. 2012;55(1):235–246. doi: 10.1044/1092-4388(2011/10-0258). [DOI] [PubMed] [Google Scholar]
- 57.Nicosia MA, Hind JA, Roecker EB, Carnes M, Doyle J, Dengel GA, Robbins J. Age effects on the temporal evolution of isometric and swallowing pressure. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2000;55(11):M634–M640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- 58.Kays SA, Hind JA, Gangnon RE, Robbins J. Effects of dining on tongue endurance and swallowing-related outcomes. Journal of Speech, Language, and Hearing Research. 2010;53(4):898–907. doi: 10.1044/1092-4388(2009/09-0048). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stierwalt JA, Youmans SR. Tongue measures in individuals with normal and impaired swallowing. American journal of speech-language pathology. 2007;16(2):148–156. doi: 10.1044/1058-0360(2007/019). [DOI] [PubMed] [Google Scholar]
- 60.Devine MS, Woodhouse H, McCombe PA, Henderson RD. The relationship between limb dominance, disease lateralization and spread of weakness in amyotrophic lateral sclerosis (ALS). Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2013;14(2):150–151. doi: 10.3109/17482968.2012.725416. [DOI] [PubMed] [Google Scholar]