Abstract
An acute bout of exercise can exacerbate pain, hindering participation in regular exercise and daily activities. The mechanisms underlying pain in response to acute exercise are poorly understood. We hypothesized that proton accumulation during muscle fatigue activates ASIC3 on muscle nociceptors to produce hyperalgesia. We investigated the role of ASIC3 using genetic and pharmacological approaches in a model of fatigue-enhanced hyperalgesia. This model uses two injections of pH 5.0 saline into muscle in combination with an electrically-induced fatigue of the same muscle just prior to the second injection of acid to induce mechanical hyperalgesia. We show a significant decrease in muscle force and decrease in muscle pH after 6 minutes of electrical stimulation. Genetic deletion of ASIC3 using knockout mice and pharmacological blockade of ASIC3 with APETx2 in muscle prevents the fatigue-enhanced hyperalgesia. However, ASIC3−/− mice and APETx2 have no effect on the fatigue response. Genetic deletion of ASIC3 in primary afferents innervating muscle using an HSV-1 expressing miRNA to ASIC3 surprisingly had no effect on the development of the hyperalgesia. Muscle fatigue increased the number of macrophages in muscle, and removal of macrophages from muscle with clodronate liposomes prevented the development of fatigue-enhanced hyperalgesia. Thus, these data suggest that fatigue reduces pH in muscle that subsequently activates ASIC3 on macrophages to enhance hyperalgesia to muscle insult.
Introduction
Regular exercise remains one of the more effective treatments for chronic pain conditions such as fibromyalgia and low back pain [1, 2], but exercise can acutely exacerbate pain, hindering participation in regular exercise and daily activities [3–6]. Indeed, people with chronic pain show significant reduction in physical activity levels when compared to healthy controls and poor compliance with regular exercise [7, 8]. Therefore, reducing pain during exercise and activity is critical for promoting participation in regular daily activities and effective exercise programs.
The mechanisms that produce an acute exacerbation of muscle pain with exercise in people with chronic pain are not well understood. The enhanced pain response occurs with levels of activity that do not typically produce tissue damage or pain in healthy subjects [9, 10]. Animal studies similarly show an enhanced hyperalgesia when combining fatiguing, non-damaging exercise with a non-painful low-dose muscle insult [11–13] We previously show that whole-body fatiguing exercise enhances hyperalgesia to muscle insult and is associated with increased activity in brainstem neurons without significant changes in the muscle metabolites [11, 14,15]. On the other hand, enhanced hyperalgesia to muscle insult after fatiguing a single muscle was not associated with changes in brainstem neurons [13].
Muscle fatigue releases a wide range of by-products, including protons (decreasing pH) and lactate, which could subsequently activate nociceptors to produce pain. Decreasing pH in muscle produces pain and hyperalgesia in healthy human subjects [16, 17], produces hyperalgesia in animals [18], and activates acid sensing ion channels (ASICs) [19, 20]. During moderate fatiguing exercise, intramuscular pH decreases to approximately 6.6 [21, 22], values that would activate ASICs in muscle [23]. In fact, both protons and lactate produce ASIC-like currents and enhanced intracellular calcium when applied to dorsal root ganglia neurons including those innervating muscle [19, 20, 24–26]. ASIC3, in particular, has been implicated in the development of hyperalgesia in models of muscle pain, both inflammatory and non-inflammatory [27–29]. Muscle nociceptors express ASIC3 mRNA and protein and ASIC3 is found in higher quantities in afferents innervating muscle when compared to skin [26, 27, 30]. We therefore hypothesized that decreases in pH during muscle fatigue activates ASIC3 on nociceptors to enhance the hyperalgesia to a low-dose muscle insult.
Materials and Methods
Animals
All experiments were approved by the Institutional Animal Care and Use Committee and performed in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and IASP Ethical Guidelines for the Use of Animals in Research. Male and female mice (6–10 weeks old) were bred at the University of Iowa. Congenic mice on C57BL/6J background lacking the ASIC3 gene (ASIC3−/−, n=7 male, 8 female) and C57BL/6J mice (n=97 male, 22 female). ASIC3−/− mice have been previously characterized [31, 32], and show similar results when compared against wild-type littermates and C57/BL6 in study of muscle pain [27, 28].
Fatigue Paradigm
Muscle fatigue was induced using a modified Burke protocol that produces rapidly recovering single muscle fatigue as previously described [13, 33, 34]. Briefly, mice were deeply anesthetized using 2–4% isoflurane. Needle electrodes connected to a Grass S88 solid-state square waveform generator (Grass Technologies, West Warwick, RI) were inserted into the belly of the gastrocnemius. Baseline maximum force was established by applying three 100 Hz trains at 7 volts. To induce fatigue, mice were given six minutes of sub-maximal contractions using 7 volt stimulations at 40 Hz for 3.75 seconds with 4.25 seconds of rest between contractions. Three additional maximum force contractions were then elicited to determine the decline in force after fatiguing contractions. Force was measured by attaching the plantar surface of the foot to a force plate connected to an iWORX FT-302 force transducer (iWorx, Dover, NH). Data was collected using LabVIEW software and analyzed using Freemat and Python scripts. Force transducer data was converted to mN using a standard curve of 1g weights applied to the apparatus. Fatigue was operationally defined as a decline in force between baseline and final maximum force contractions.
ASIC3−/− (n=7 male, n=7 female) mice were compared to wild type mice (male n=6, n=6 female) for initial maximum contraction force, final maximum contraction force, and at each of the submaximal fatiguing contractions. Additionally, muscle fatigue was measured in mice pre-treated with the ASIC3 antagonist APETx2 at low (20 µM, n=8) and high (200µM, n=7) doses or saline control (n=9). Animals missing 20 or more data points (≥45%) in a fatigue recording were excluded from the fatigue analysis (males: n=2 ASIC3−/−, n=2 wild type, saline control n=1, low dose APETx2 n=2, high dose APETx2 n=2; females: none excluded).
Metabolite Recording
Muscle pH was measured in deeply anesthetized animals using a pH probe inserted into the belly of gastrocnemius before and after electrically stimulated muscle contractions. After shaving the overlying hair, an incision was made in the skin to open an 8 mm × 8 mm square over the center of the gastrocnemius muscle. Muscle fibers were gently teased apart and the tip of the probe was inserted into the muscle.
For the measurement of pH, a micro-pH probe (Lazar Research Laboratories, Los Angeles) connected to a JENCO 6230N (Jenco Instruments, San Diego) pH meter was calibrated at pH 7.0 and 4.0 before insertion into the muscle of male wild type mice. In the control group (n=4 male, n=4 female), the muscle was exposed and measurements were taken 6 m apart. For the experimental group (n=5 male, n=4 female), the muscle was exposed and then measurements were taken before and after the 6m fatigue treatment. The pH and force data were sampled continuously at 30 Hz using a Python script to record data from the JENCO 6230N pH meter. Though the sampling was continuous, the pH probe was applied to the muscle for only 30 s at a time to avoid loss of pH sensitivity. When not measuring muscle pH, the sensor was stored in a pH 6.8 phosphate buffer containing heparin, which served as both a reference to ensure sensitivity was maintained and as a cleaning solution. After each contact with the muscle, the tip was cleaned briefly with a cotton swab soaked in acetone.
In order to avoid electrical interference caused by unwanted circuits forming between the main power supply, electrical stimulator, and measurement probes, a wirelessly controlled battery powered electrical stimulator was developed. An Arduino Uno R3 microcontroller was programmed with the electrically stimulated muscle fatigue protocol. A digital output pin was used to control a transistor that gated a 7 volt current through needle electrodes. An RN-42 bluetooth antenna (Sparkfun Electronics, Boulder, CO) transmitted serial input to and from an Android device in order to receive and confirm instructions from the user. This device was validated against the Grass S88 solid-state square waveform generator and produced identical contractions at 40 and 100 Hz.
Fatigue Enhanced Pain Model
Muscle pain was induced by combining low-intensity muscle insult with six minutes of fatiguing contractions. On day 1, mice were anesthetized with 2–4% isoflurane and given an intramuscular (i.m.) injection of 20 µl normal saline adjusted to pH 5.0. On day 5, mice were anesthetized with 2–4% isoflurane and underwent six minutes of fatiguing contractions. Immediately after completing fatiguing muscle contractions, the mice were given a second i.m. injection of 20 µl pH 5.0 normal saline. The pH of the normal saline was adjusted with HCl to pH 5.0 ± 0.1. Control injections consisted of two injections of normal saline (pH 7.2 ± 0.1) 5 days apart without the six minutes of fatiguing contractions. The unbuffered pH 5 saline injections reduce muscle pH to approximately 6.9 [18], which is comparable to decreases seen after intense exercise [35, 36]. Previous studies show that the combination of pH 5.0 saline with muscle fatigue is critical for the development of mechanical hyperalgesia, as neither 2 injections of pH 5.0 alone nor 2 injections of pH 7.2 saline combined with fatigue produce hyperalgesia [11–13]. On the other hand two injections of pH 4.0 produce decreases in muscle pH to an average of pH 6.5 and results in long-lasting hyperalgesia [18].
Muscle withdrawal thresholds
Muscle withdrawal thresholds (MWT) were measured by applying force sensitive tweezers to the belly of the gastrocnemius muscle as previously described [11], where lower thresholds indicate greater sensitivity. Mice were acclimated to this behavioral paradigm in two 5 minute sessions over a two day period prior to the first injection. Briefly, mice were placed in a gardener’s glove, the hindlimb was held in extension, and the muscle was squeezed with force sensitive tweezers until the animal withdrew its hindlimb. An average of 3 trials per animal was taken at each time period. A decrease in withdrawal thresholds was interpreted as muscle hyperalgesia. Our prior work shows that anesthetizing the skin during this test does not change withdrawal thresholds but anesthetizing the deep tissue increases the threshold thus validating this as a measure of muscle hyperalgesia [37].
Genetic Deletion of ASIC3 in the Exercise Enhanced Pain Model
The role of ASIC3 was assessed using genetic and pharmacological approaches. ASIC3−/−(n=8 male, 4 female) were compared side-by-side with C57BL/6 mice (n=7 male, n=4 female} in the exercise enhanced pain model. Muscle withdrawal thresholds were measured at baseline and 24 h after the induction of the pain model, i.e. after the second injection of pH 5.0 saline.
Antagonism of ASIC3 in the Exercise Enhanced Pain Model
For the behavioral pharmacology studies, male and female C57BL/6 mice were pre-treated with i.m. injection of APETx2 (males: 20 µM, n=14; 70 µM, n=8; 200 µM, n=7, females: 200 µM, n=4) or vehicle (0.9% saline, males n=16, females n = 4) 5 minutes before muscle fatigue on day 5. 24 h after the second pH 5 saline injection, animals were tested for muscle withdrawal threshold. Animals were divided into multiple groups tested across several weeks. Each testing group consisted of vehicle control and multiple doses of both APETx2.
Neuron-Selective Knockdown of ASIC3 in the Fatigue-Enhanced Pain Model
To test the role of neuronal ASIC3 in fatigue-enhanced muscle pain, ASIC3 was selectively downregulated using Herpes Simplex Virus 1 (HSV-1) expressing an artificial miRNA against ASIC3 (HSV-miR844). Our laboratory developed and characterized this construct showing efficient and functional knockdown of ASIC3 in dorsal root ganglia cells after injection of HSV-miR844 into the gastrocnemius muscle [38]. Further, this strain of HSV selectively targets neuronal cells [39, 40]. In the present study, male C57/BL6 were assessed for muscle withdrawal threshold at three time points: prior to any manipulation (baseline 1), 4 weeks after HSV-1 treatment (baseline 2), and after exposure to the exercise enhanced pain model. After the first baseline, mice were injected with either HSV-1 expressing miR844 (active, n=7) or HSV-1 expressing eGFP (control, n=7). Specifically, an incision was made into the skin and the needle was passed into the belly of the gastrocnemius muscle through that opening. 20µL of solution was injected over the course of 1 minute. After injection, the wound covered by sterile gauze and the tissue was allowed to absorb the solution for 15 minutes before closure with a subcuticular suture. The mice were then allowed to recover for 4 weeks. Following incubation, a second baseline muscle withdrawal threshold was taken to assess for potential effects of viral infection on pain behavior. Then mice were exposed to the fatigue-enhanced pain model and muscle withdrawal thresholds were repeated for a final time.
The same animals used in the behavioral experiment were then sacrificed and L4-L6 dorsal root ganglia (DRG) were tested for ASIC3 mRNA using qPCR as previously described [29, 38]. Briefly, the DRGs were removed from the animal, and stored in RNALater (Qiagen, Valencia, CA). DRGs were transferred to Trizol (Life Technologies, Carlsbad, CA) and homogenized and RNA was then isolated using chloroform extraction, glycogen and isopropanol. First-strand cDNA was synthesized from 0.2 to 1µg RNA using the SuperScript VILO protocol (Life Technologies). qPCR using validated, pre-designed Taqman assays for ASIC3 (Mm00805460_m1) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was carried out using an ABI prism 7900HT sequence detector (Applied Biosystems, Foster City, CA). Each cDNA sample was run in triplicate and expression of ASIC3 was normalized to the expression of GAPDH based on quantification cycle (Cq). The results are expressed as means and SEM of the relative abundances (2−ΔCq) for the DRGs ipsilateral to virus injection.
Quantification of Macrophages in the Fatigue-Enhanced Pain Model
To determine if electrical stimulation with or without pH 5.0 saline injections increases the number of macrophages in gastrocnemius muscle tissue, male and female C57BL/6 mice (n=2 of each sex per condition) were left untreated, exposed to electrical stimulation alone (immediately after fatigue task), or exposed to electrical stimulation with pH 5.0 saline injections (24h after second acid injection). Mice were then anesthetized (sodium pentobarbital, 100 mg/kg, i.p.) and transcardially perfused with 4% paraformaldehyde. The gastrocnemius muscle was removed, stored in 30% sucrose and frozen. The muscle was cut with a cryostat onto slides at 20µm. Standard immunohistochemical techniques were used. Sections were incubated in the primary antibody, rat anti-mouse F4/80 (1:500, AbD Serotec, Raleigh, NC), overnight at room temperature. The next day sections were rinsed and inclubated in the secondary antibody, goat anti-rat Alexa 488 (Invitrogen, Grand Island, NY), for 1h at room temperature. Sections were coverslipped with Vectashield and imaged on an Olympus BX-51 light microscope. The total number of F4/80+ cells in 5 muscle sections were counted from each animal off line using Image-J software.
Depletion of Macrophages in the Fatigue-Enhanced Pain Model
To determine if macrophages contribute to the development of mechanical hyperalgesia in the fatigue-enhanced pain model, macrophages were depleted in the muscle using local injection of liposomes containing clodronate into the muscle of male C57/BL6 mice [41]. Muscle withdrawal thresholds were assessed prior to liposome injection and after exposure to the fatigue-enhanced pain model. Mice were injected with either active liposomes containing clodronate (n=4) or inactive liposomes containing PBS (n=4). 24h after liposome injection, mice were treated with the fatigue-enhanced pain model and withdrawal threshold measured. Depletion of macrophages was confirmed by F4/80 immunohistochemical staining of treated gastrocnemius muscle (n=4 clodronate liposomes; n=3 inactive liposomes) using the same approach detailed above.
Statistical Analysis
Data are reported as means +/− S.E.M. Muscle force, withdrawal thresholds, and pH measurements were analyzed with repeated measures ANOVA followed by post-hoc with a Tukey’s test. For pH measurements, differences between groups at baseline and after treatment were assessed by independent t-tests and differences within groups from baseline to post-treatment were assessed by paired t-tests. Macrophage quantification was assessed by one-way ANOVA followed by Tukey test. Significance for the repeated measures ANOVA and Tukey test was set at 0.05. To control for multiple comparisons, Bonferroni correction was applied to all of the t-tests for differences in pH, adjusting the level of significance to 0.0125.
Results
Muscle fatigue is not changed by genetic deletion or blockade of ASIC3
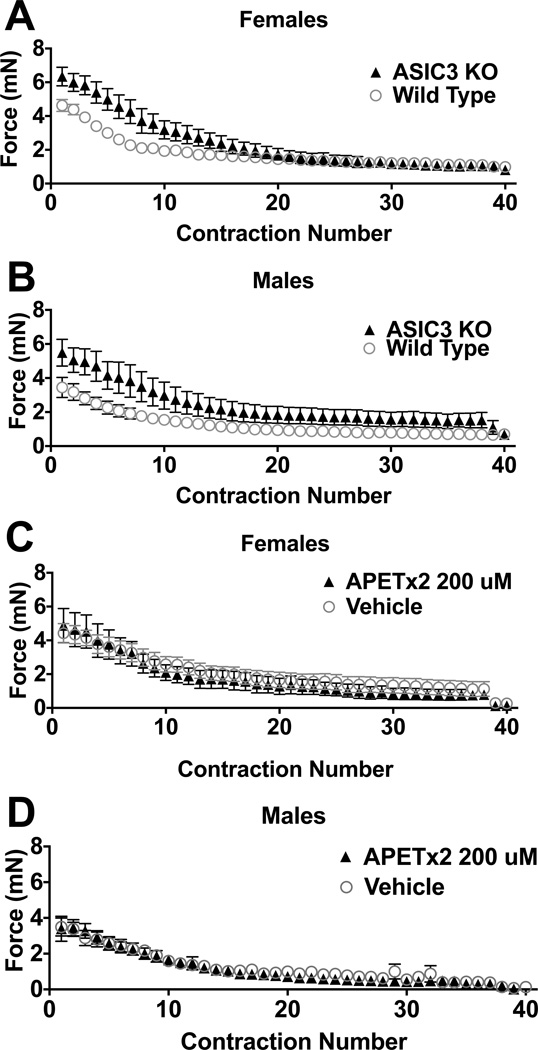
The force of muscle contractions across fatiguing stimuli were recorded for ASIC3−/− and compared to wildtype mice (Figure 1A,B). The initial force of contraction, i.e. the first fatiguing stimuli, was significantly greater in the ASIC3−/− mice (female 6.3 ± 0.59mN; male 5.5 ± 0.78mN) when compared to wildtype mice (female 4.6 ± 0.35mN; male 3.4 ± 0.59mN)(univariate ANOVA F1,25=22.8, p=0.005). There were no sex differences for the initial force of contraction. The force of contraction decreased significantly across time in all three groups. However, there were no significant differences between groups in the post-fatigue force when data were controlled with the initial force as a co-variate. There were also no differences in the time to peak fatigue, defined as peak decrease + 10% between wildtype and knockouts or between sexes (wildtype: female 269 ± 48s, male 268 ± 30s; ASIC3−/−: female 276 ± 26s, male 237 ± 75s). In the animals treated withAPETx2 there were no differences in the initial force, rate of fatigue, or post-fatigue force when compared to those treated with vehicle in either male or female mice (Fig. 1C,D).
Fig. 1.
ASIC3 does not contribute to the development of muscle fatigue. (A) Female and (B) male C57/BL6 mice develop muscle fatigue at similar rates. Initial force on the first contraction was significantly greater in ASIC3−/− mice compared to C57/BL6 mice (p<0.05), but not different between sexes. However, there was no significant differences between wild type (males n = 7, females n = 7) and ASIC3−/− (males n = 6, females n = 6) mice for force of contraction across the 6 minute period when normalized for baseline, and no difference in force between sexes. In (C) female and (D) male mice, pre-treatment with the ASIC3 antagonist APETx2 (200 µM, males n = 7, females n = 4) doses had no effect on the rate at which muscle fatigue developed as compared to controls (males n = 9, females n = 4).
Muscle fatigue decreases muscle pH in males but not females
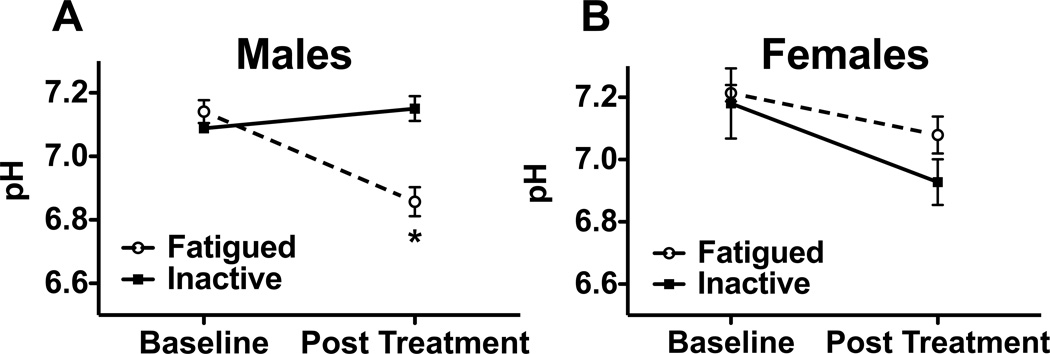
The effect of muscle fatigue on muscle pH was assessed by inserting a pH probe into the belly of the gastrocnemius muscle before and after either six minutes of fatiguing muscle contractions or six minutes of inactivity. For males, the initial muscle pH of both groups was comparable: 7.14 (+/− 0.04 SEM) and 7.09 (+/− 0.01 SEM) for the fatigued and inactive groups, respectively. After fatiguing muscle contractions, muscle pH dropped to 6.86 (+/− 0.05 SEM) while the inactive group remained relatively unchanged at 7.15 (+/− 0.04 SEM) (Fig 2A). Muscle fatigue had a significant effect on muscle pH (repeated measures ANOVA, F1,7 =26.505, p < 0.05). At baseline, there were no significant differences between groups (Student’s t-test, t7 = 1.230, p = 0.258). The pH of muscles exposed to fatiguing muscle contractions decreased significantly from baseline (Paired t-test, t4 = 5.778, p = 0.004) and was significantly lower than the inactive controls (Student’s t-test, t7 = −4.736, p = 0.002). The pH of inactive control muscles did not change from baseline (Fig. 2A, paired t-test, t3 = −1.452, p = 0.243). In contrast, in female mice electrically stimulated muscle contractions had no effect on muscle pH (fig. 2B, repeated measures ANOVA, F1,4 = 2.280, p = 0.206). In contrast to males, for females muscle fatigue did not result in a decrease in pH from baseline and there were no differences between inactive and fatigued muscles
Fig. 2.
Electrically stimulated muscle contractions produce significant decreases in interstitial muscle pH in male but not female mice. (A) Male mice treated with 6 minutes of fatiguing muscle contraction (n = 4) showed a significant decrease in interstitial muscle pH as compared to inactive controls (n = 5). * P < 0.05. (B) Female mice showed no significant difference between fatigued animals (n = 4) and inactive controls (n = 4).
Genetic deletion of ASIC3 prevents development of fatigue-enhanced hyperalgesia
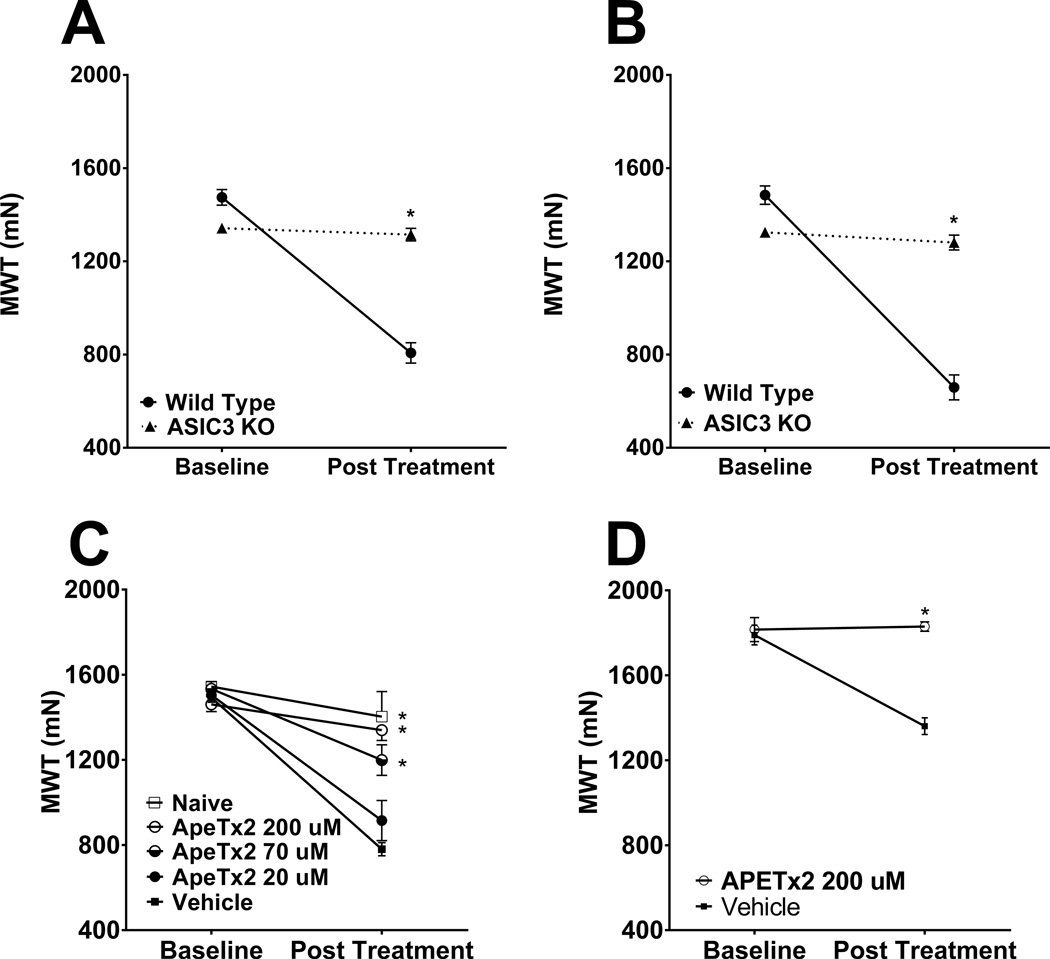
The role of ASIC3 in the development of exercise enhanced muscle pain was tested by comparing muscle withdrawal thresholds in ASIC3−/− animals after exposure to acidic saline and fatiguing muscle contractions and compared to wild-type controls. As previously shown [13], wild type mice showed a significant decrease in the muscle withdrawal threshold. ASIC3−/− mice showed no decrease and were significantly different from both wildtype mice (Fig. 3A & 3B, repeated measures ANOVA with post-hoc Tukey test, F2,18=20.614, p < 0.001). No sex differences were observed.
Fig. 3.
(A & B) ASIC3−/− mice do not develop mechanical hyperalgesia in the fatigue-enhanced pain model. (A) Male wild-type mice (n = 7) show significant decreases in muscle withdrawal threshold, while male ASIC3−/− mice (n =8) were significantly different, showing no change in muscle withdrawal threshold from baseline. * P < 0.05. (B) Female wild-type mice (n = 4) show significant decreases in muscle withdrawal threshold, while female ASIC3−/− mice (n = 4) were also significantly different, showing no change in muscle withdrawal from baseline * P < 0.05. (C & D) Antagonism of ASIC3 during the fatigue task prevents the development of mechanical hyperalgesia in the fatigue-enhanced pain model. (C) Male C57BL/6 mice were pretreated with 20µM (n = 14), 70µM (n = 8), or 200µM (n = 7) of the ASIC3 antagonist APETx2 or vehicle (n = 16) during the fatigue task. As a negative control, one group received no treatment (n = 4). Mice treated with 70 µM or 200 µM ApeTx2 had significantly higher post-treatment muscle withdrawal thresholds as compared to the vehicle control. (D) Female C57BL/6 mice were pretreated with either 200 µM APETx2 (n = 4) or vehicle (n = 4) during the fatigue task. Mice treated with APETx2 had significantly higher post-treatment muscle withdrawal thresholds as compared to vehicle control. * P < 0.05.
Local blockade of ASIC3 prevents development of fatigue-enhanced hyperalgesia
To confirm the results from ASIC3−/− mice and to test if ASIC3 activation at the site of fatigue was necessary, we injected the ASIC3-antagonist ApeTx2 directly into the muscle prior to the fatigue task. As previously shown, mice treated with vehicle (saline) showed a significant decrease in muscle withdrawal threshold. In contrast, APETx2 injected into muscle prevented the decrease in muscle withdrawal thresholds in both male and female mice. In males, the effect of APETx2 occurred in a dose-dependent manner - muscle withdrawal thresholds from mice injected with 70 and 200 µM doses were significantly higher than vehicle. (Fig. 3C, repeated measures ANOVA with post-hoc Tukey test, APETx2: F4,39=10.643, p <0.001). In females, only the highest dose of APETx2 was tested since no sex differences were observed. Females treated with 200 µM APETx2 had significantly higher muscle withdrawal thresholds than those given vehicle control (Fig. 3D, repeated measures ANOVA, F1,6=258.492, p <0.001).
Downregulation of ASIC3 in muscle afferents has no effect on fatigue-enhanced hyperalgesia
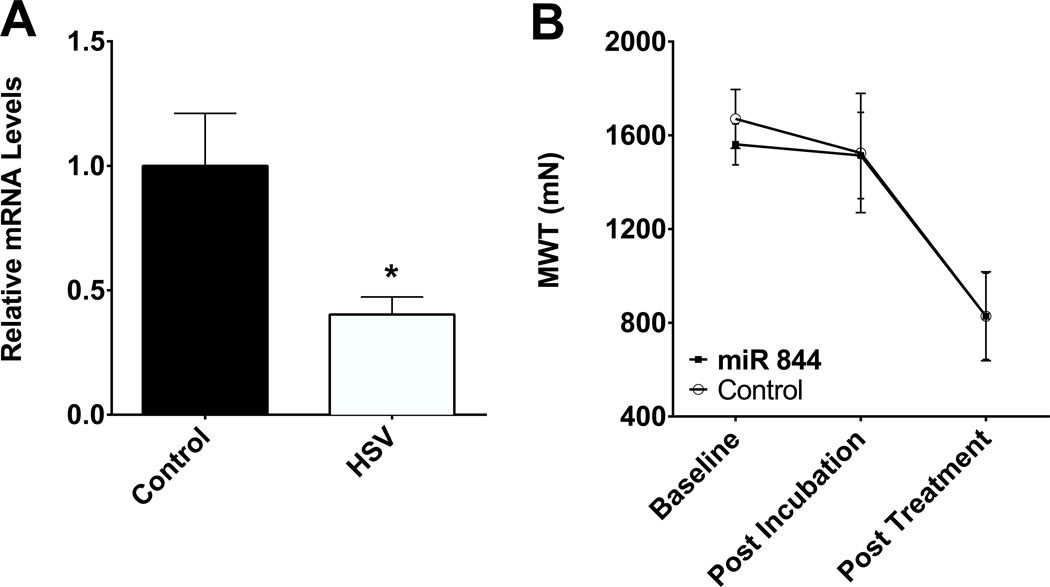
To test if ASIC3 in neurons innervating the fatigued muscle is required for the development of hyperalgesia in the fatigue-enhanced hyperalgesia, we downregulated ASIC3 by infecting DRGs with HSV-1 expressing a miRNA against ASIC3 [38]. DRGs from mice injected with the HSV-miR844 showed a significant decrease in ASIC3when compared to DRGs from mice injected with HSV-GFP as a control (Fig. 4A, student’s t-test, p=0.0139). Despite a significant decrease in ASIC3 in DRG innervating muscle, muscle withdrawal thresholds decreased significantly and to the same extent in animals injected with HSV-miR844 and HSV-GFP (Fig. 4B, repeated measures ANOVA, F1,14=0.325, p=0.578).
Fig. 4.
Neuron-selective knockdown of ASIC3 does not prevent the development of mechanical hyperalgesia in the fatigue-enhanced pain model. (A) Intramuscular injection of herpes simplex virus-1 (HSV-1) expressing miRNA against ASIC3 (n = 8) into the gastrocnemius muscle produces a 2-fold decrease in ASIC3 expression in the DRG innervating the gastrocnemius muscle as compared to injection of a control HSV-1 expressing GFP (n = 8). * P < 0.05. (B) Mice treated with HSV-1 expressing miRNA against ASIC3 (n =8) 4 weeks prior to exposure to the fatigue-enhanced pain model were similar to those treated with control HSV-1 (n = 8) at the pre-treatment baseline and after 4 weeks of incubation with HSV-1. After treatment with the fatigue-enhanced pain model, mice treated with HSV-1 expressing miRNA against ASIC3 developed hyperalgesia similar to those mice treated with control HSV-1 (n = 8).
Treatment with electrical stimulation with or without pH 5.0 saline increases the number of macrophages in muscle tissue
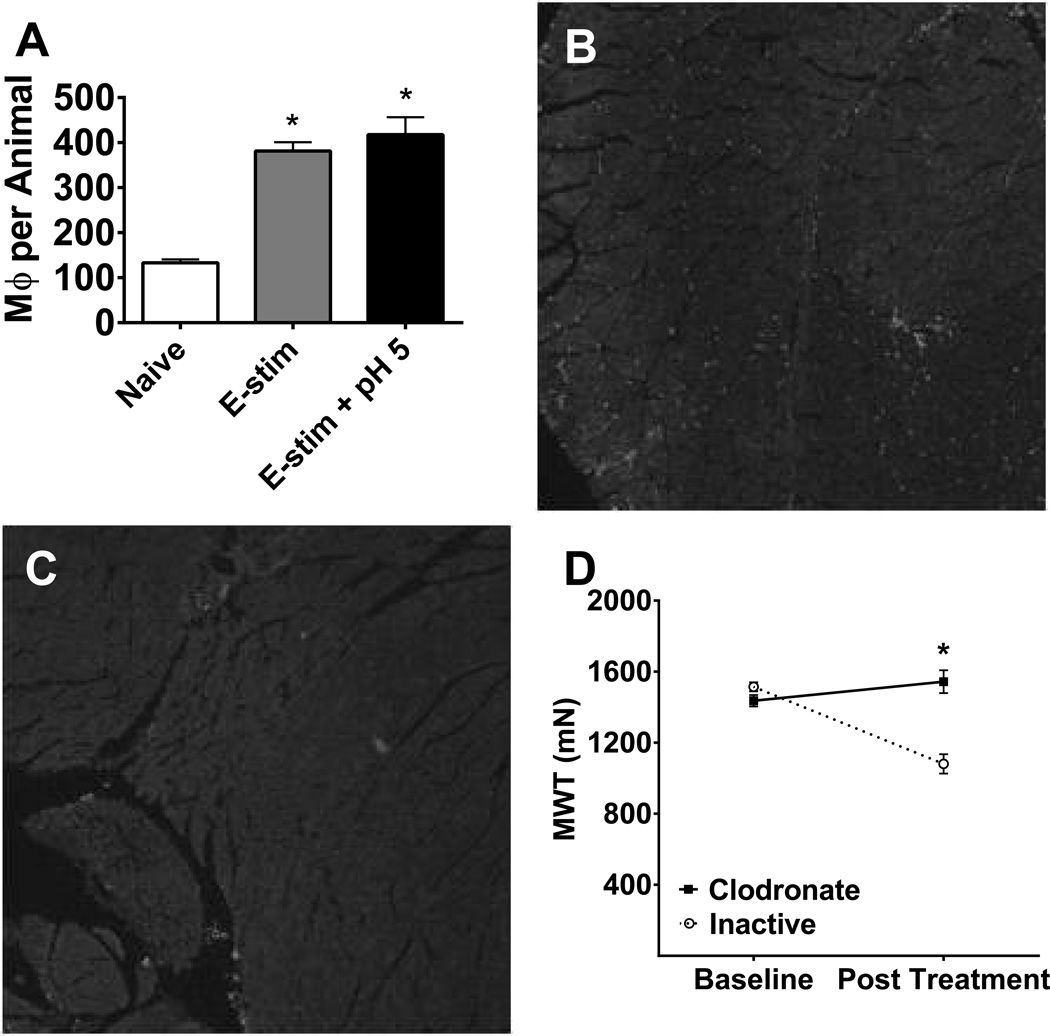
While previous studies have shown neutrophils are not changed by electrical stimulation or pH 5.0 saline injection [13], it is unknown if the number of macrophages in muscle tissue changes under these conditions. To test this, C57BL/6 mice were exposed to either electrical stimulation, electrical stimulation with pH 5.0 saline, or left untreated. Immunohistochemical staining with the macrophage marker F4/80 showed a significantly greater number of cells in the electrically stimulated (mean 381 ± 20, Tukey test, p < 0.001) or electrically stimulated and pH 5.0 saline treated animals (mean 418 ± 39, Tukey test, p < 0.001) when compared to naïve animals (mean 133 ± 7)(Fig 5A, one-way ANOVA F2,9 = 36.97, p < 0.001)
Fig. 5.
Fatigue increases macrophages in muscle and depletion of muscle macrophages prevents fatigue-enhanced hyperalgesia. A. The total number of F4/80+ macrophages from 5 sections of muscle were increased immediately after the fatigue task and 24h after the second pH 5.0 injection in combination with the fatigue task when compared to controls (male n = 2, female n = 2/group). *, P < 0.05 (B) F4/80 immunostain from a gastrocnemius muscle section from a mouse injected with inactive liposomes. (C) F4/80 immunostain from a gastrocnemius muscle section from a mouse injected with inactive liposomes. (D) Male C57BL/6 mice treated with intramuscular clodronate (n = 4) showed significantly higher post-treatment muscle withdrawal thresholds as compared to mice treated with inactive liposomes (n = 4) prior to exposure to the fatigue-enhanced pain model. * P < 0.05.
Depletion of macrophages prevents mechanical hyperalgesia in the fatigue-enhanced pain mode
Since immune cells, including macrophages, respond to decreases in pH and express ASIC3 [42, 43], we tested if resident macrophages in muscle were necessary for the development of mechanical hyperalgesia in the fatigue-enhanced pain model. Macrophages were depleted locally by intramuscular clodronate prior to induction of the model. Immunohistochemical staining for the macrophage marker F4/80 shows staining of macrophages in muscle from animals that received control liposomes (Fig 5B) and significantly less from animals that received clodronate liposomes (Fig 5C). Counting of positively stained macrophages, from 5 sections per animal, showed a significant decrease in the number of macrophages from those that received clodronate liposomes (18 ± 6.7, n=4) when compared to those that received control liposomes (66 ± 16, n=3) (p=0.03, one-way ANOVA). Mice treated with control liposomes showed significant decreases in muscle withdrawal threshold, while those treated with clodronate liposomes showed no change in muscle withdrawal threshold and were different from controls (Fig. 5D, repeated measures ANOVA, F1,6=117.16, p < 0.001).
Discussion
The current study shows that six minutes of electrically stimulated muscle contractions not only results in substantial fatigue, but also physiologically relevant accumulation of protons, in the detectable range for ASICs. While a number of channels can detect protons, heteromeric channels expressing ASIC3 are necessary for fatigue-enhanced hyperalgesia since genetic deletion and local blockade of ASIC3 prevents development of hyperalgesia. However, our data indicate that ASIC3 on primary afferent fibers innervating muscle is not required for development of hyperalgesia, suggesting ASIC3 on non-neuronal cells mediates the development of hyperalgesia in this particular model. ASIC3 is expressed in macrophages [43] and we show muscle fatigue increases the number of macrophages in muscle and removal of macrophages from muscle prevents the development of fatigue-induced hyperalgesia. Therefore, we suggest that decreases in pH from fatiguing muscle activate ASIC3 on resident macrophages that subsequently release algesic chemicals to produce hyperalgesia
ASIC3 is critical for development of hyperalgesia after muscle insult. ASIC3−/− mice do not develop hyperalgesia after repeated acid injections into muscle, inflammation of muscle, or inflammation of joint [27–29, 44–47]. Further, prior studies also show that blockade of ASICs locally in muscle both prevents and reverses muscle hyperalgesia associated with either repeated acid injections into muscle or muscle inflammation [29, 47, 48]. The current study is consistent with these prior studies and shows that fatigue-enhanced hyperalgesia does not occur in ASIC3−/− mice or after pharmacological blockade of ASIC3 in muscle. Muscle afferents express ASIC3, form heteromeric channels, and respond to pH within the ranges produced by fatiguing muscle contractions [25–27, 30, 45]. Muscle afferents from ASIC3−/− mice show no change in pH sensitivity, but have a slower rate of desensitization and slower recovery from desensitization [26]. However, the current study shows depletion of ASIC3 in muscle afferents, with HSV-miR844, has no effect on the development of hyperalgesia. This is in direct contrast to prior studies using the same HSV-miR844 in animals with carrageenan-induced muscle inflammation; hyperalgesia was prevented in these mice [38]. Further, re-expression of ASIC3 in muscle afferents from ASIC3−/− mice rescued the hyperalgesia to muscle inflammation [28]. Together these data suggest that the fatigue-induced hyperalgesia model is uniquely different from the muscle inflammation model. Indeed, the fatigue-induced hyperalgesia model is not associated with an acute neutrophil response in the muscle [13], while carrageenan muscle inflammation produces a robust neutrophilic inflammation [49]. Further, inflammatory models routinely show upregulation of ASICs and change in sensitivity to pH in afferent fibers innervating the inflamed tissue and in DRG [38, 44, 50], while repeated acid injections show no such changes in DRGs [25].
Non-neuronal cells express ASIC3 and can mediate the effects of decreases in pH. Prior studies show expression of ASIC3 on synoviocytes, osteocytes, and immune cells [42, 43, 46, 51–53]. Macrophages are distributed throughout the body in nearly every tissue type, including skeletal muscle. They play a role in innate immunity, as well as tissue remodeling, endocrine function, and tumor biology [54]. Macrophages can secrete inflammatory cytokines like interleukin-6 (IL-6), IL-1β, or TNF which are all known to sensitize nociceptors and produce pain [54–56]. Furthermore, macrophages express a number of receptors and ion channels, including ASIC3, which are capable of monitoring the external environment. Acidic pH (pH 6.5) improves antigen internalization, enhances phagocytosis and modulates production of cytokines in macrophages [43, 57–59]. Lactate, a known activator of ASIC3, can also activate macrophages [60], and enhances the LPS-induced release of IL-6 from macrophages [61]. This suggests macrophages may be well suited to detecting and releasing pro-algesic factors in response to decreases in extracellular pH, these pro-algesic factors (i.e. cytokines, ATP) can subsequently activate receptors located on nociceptors. The current study is consistent with function of ASIC3 on macrophages since macrophages are elevated under conditions which produce fatigue-induced hyperalgesia and depletion of macrophages from muscle prevents the development of fatigue-induced hyperalgesia. Thus, we propose that decreases in pH that occur during fatigue activate ASIC3 on macrophages to release algesic chemical that can subsequently activate nociceptors to produce pain.
Surprisingly, we show sex differences in muscle pH after 6 minutes of fatiguing isometric contractions, without sex differences in the degree of fatigue. In male mice there is a decrease in the intramuscular pH to 6.8 with fatiguing contractions of the gastrocnemius muscle; there is no change in females. While the basis for this sex difference in muscle pH is not explored here, a previous study of human subjects performing 8 minutes of fatiguing isometric exercise showed a similarly greater decrease in muscle pH in males subjects as compare to females [62]. The reason for this may be related to sex differences in muscle metabolism [63]. Males have a greater proportion of the type II fibers that rapidly generate force [64, 65]. The predominant fibers in female muscle have lower Ca2+-ATPase activity and subsequently slower rates of contraction [66, 67]. Further, muscle in males rely more heavily on glycolysis for energy [68] while females make use of oxidative phosphorylation and lipid metabolism [69–71]. Sex differences in muscle metabolism have a significant effect on the production of lactic acid—after a short sprinting task, males develop significantly higher concentrations of lactic acid than females [72]. This greater accumulation of lactic acid in males may explain the difference in muscle pH between sexes.
The magnitude of pH decrease for males in the present study is consistent with prior studies that show pH values 6.4–6.85 with muscle fatigue [21, 22, 73, 74]. Venous effluent from muscle decreases to a similar degree with exercise (0.3 pH units to pH 7.1) but starts at a higher resting pH (7.4) [75, 76]. Sustained exposure to pH in the 7.0–7.1 range could lead to steady state desensitization of ASIC channels [77]. Since muscle nociceptors are located in the adventitia of arteries [30], they are in close proximity to both blood and intramuscular fluid. While the milieu surrounding these nerve terminals is not well understood, it may be the case that the terminals are typically surrounded by pH 7.4 serum and only transiently exposed to the more acidic intramuscular fluid, such as during muscle contractions. In this way, decrease in intramuscular pH may contribute to the fatigue-enhanced pain effect, at least in males. However, the role of pH in the fatigue-enhanced pain effect in females is less clear. It is possible that other metabolites released during muscle fatigue, such as ATP, may play a more significant role in females.
The role of ASIC3 in muscle fatigue is mixed. While the current study showed no difference in fatigue response to electrical stimulation in ASIC3−/− mice or after blockade of ASIC3, we previously show a role for ASIC3 in muscle fatigue. Specifically, male ASIC3+/+ mice muscle showed less fatigue than male ASIC3−/− mice and female ASIC3+/+ mice (1h-Rota-Rod task); this difference did not occur in female mice [78]. The discrepancy between studies may be explained by several factors, including the longer duration of exercise, the task-specific nature of muscle fatigue, the role of voluntary effort in task failure, or the muscle fatigued. For example, the current study used a short fatigue task (6m) resulting in fatigue lasting 10 m in a single muscle [13], as compared to a much longer (3 h) and widespread protocol that produced fatigue lasting (2 h) in our prior study [78]. Notably, when the duration of fatigue task was reduced in our prior study (1.5h), there was no difference in fatigue between ASIC3−/− and WT mice in males or females [78].
Worsening of pain after exercise remains a significant barrier to adherence to an exercise regimen that is crucial for treating diseases like myofascial pain syndrome, fibromyalgia, chronic fatigue syndrome, and low back pain [5, 79, 80]. Many mechanisms likely contribute to the development of pain after exercise, including tissue damage and inflammation, but for fibromyalgia patients whose symptoms acutely worsen with even light or moderate exercise that does not damage muscle, targeting ASICs may provide symptomatic relief [81, 82]. Studies show consistent exercise improves the symptoms of in people with fibromyalgia [83] and prevents the development of chronic and exercise-induced pain in animals [84]. Further, patients with chronic fatigue syndrome and fibromyalgia report worsening of their fatigue symptoms with exercise, which is correlated with significant upregulation of ASIC3 mRNA in the blood, suggesting ASIC3 may contribute to the subjective feeling of fatigue [6]. By inhibiting ASICs prior to exercise in the early period of an exercise regimen, it may be possible to address the acute exacerbation of pain and fatigue with exercise, leading to greater adherence to a regular exercise program.
In conclusion, these data implicate ASIC3 in the development of exercise enhanced pain through a decrease in pH from the fatigued muscle. Muscle fatigue reduces intramuscular pH which is in the range for activating ASICs. Studies in knockout mice indicate ASIC3 is the essential subunit within the wild type channel on muscle DRG; but that ASIC3 effects are not due to its location on muscle primary afferent fibers. We further show that depletion of macrophages prevents the fatigue-induced hyperalgesia, and suggest ASIC3 on macrophages mediated the fatigue-enhanced pain response.
Acknowledgements
Funded by National Institute of Health grants AR061371 and AR053509. The authors wish to thank Dr. Roxanne Walder for advice on qPCR and Lynn Rasmussen and Jing Danielson for technical assistance.
Footnotes
Conflict of interest. The authors declare no competing financial interests.
References
- 1.Macedo LG, Bostick GP, Maher CG. Exercise for prevention of recurrences of nonspecific low back pain. Phys Ther. 2013;93:1587–1591. doi: 10.2522/ptj.20120464. [DOI] [PubMed] [Google Scholar]
- 2.Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014:1547–1555. doi: 10.1001/jama.2014.3266. [DOI] [PubMed] [Google Scholar]
- 3.Kosek E, Kosek E, Ekholm J, et al. Modulation of pressure pain thresholds during and following isometric contraction in patients with fibromyalgia and in healthy controls. Pain. 1996;64:415–423. doi: 10.1016/0304-3959(95)00112-3. [DOI] [PubMed] [Google Scholar]
- 4.Vlaeyen JWS, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 5.Staud R. Treatment of fibromyalgia and its symptoms. Expert Opin Pharmacother. 2007;8:1629–1642. doi: 10.1517/14656566.8.11.1629. [DOI] [PubMed] [Google Scholar]
- 6.Light AR, Light AR, White AT, et al. Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. J Pain. 2009;10:1099–1112. doi: 10.1016/j.jpain.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin C-WC, Haas M, Maher CG, et al. Cost-effectiveness of guideline-endorsed treatments for low back pain: a systematic review. Eur Spine J. 2011;20:1024–1038. doi: 10.1007/s00586-010-1676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLoughlin MJ, Stegner AJ, Cook DB. The Relationship Between Physical Activity and Brain Responses to Pain in Fibromyalgia. The Journal of Pain. 2011;12:640–651. doi: 10.1016/j.jpain.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain. 2005;118:176–184. doi: 10.1016/j.pain.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Ge H-Y, Ge H-Y, Arendt-Nielsen L, Arendt-Nielsen L. Latent Myofascial Trigger Points. Curr Pain Headache Rep. 2011 doi: 10.1007/s11916-011-0210-6. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama T, Yokoyama T, Lisi TL, et al. Muscle fatigue increases the probability of developing hyperalgesia in mice. The Journal of Pain. 2007;8:692–699. doi: 10.1016/j.jpain.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sluka KA, Sluka KA, Rasmussen LA, Rasmussen LA. Fatiguing exercise enhances hyperalgesia to muscle inflammation. Pain. 2010;148:188–197. doi: 10.1016/j.pain.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregory NS, Gibson-Corley K, Frey-Law L, Sluka KA. Fatigue-enhanced hyperalgesia in response to muscle insult: Induction and development occur in a sex-dependent manner. Pain. 2013;154:2668–2676. doi: 10.1016/j.pain.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Da Silva LF, Da Silva LF, DeSantana JM, et al. Activation of NMDA receptors in the brainstem, rostral ventromedial medulla, and nucleus reticularis gigantocellularis mediates mechanical hyperalgesia produced by repeated intramuscular injections of acidic saline in rats. J Pain. 2010;11:378–387. doi: 10.1016/j.jpain.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sluka KA, Sluka KA, Danielson J, et al. Exercise-induced pain requires NMDA receptor activation in the medullary raphe nuclei. Med Sci Sports Exerc. 2012;44:420–427. doi: 10.1249/MSS.0b013e31822f490e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steen K, Steen K, Issbemer U, et al. Pain due to experimental acidosis in human skin: evidence for non-adapting nociceptor excitation. Neurosci Lett. 2003:1–4. doi: 10.1016/0304-3940(95)12002-l. [DOI] [PubMed] [Google Scholar]
- 17.Frey Law LA, Frey Law LA, Sluka KA, et al. Acidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humans. Pain. 2008;140:254–264. doi: 10.1016/j.pain.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sluka KA, Sluka KA, Kalra A, et al. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 20.Light AR, Light AR, Hughen RW, et al. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahlin K, Harris RC, Nylind B, Hultman E. Lactate content and pH in muscle obtained after dynamic exercise. Pflugers Arch. 1976;367:143–149. doi: 10.1007/BF00585150. [DOI] [PubMed] [Google Scholar]
- 22.Spriet LL, Söderlund K, Thomson JA, Hultman E. pH measurement in human skeletal muscle samples: effect of phosphagen hydrolysis. J Appl Physiol. 1986;61:1949–1954. doi: 10.1152/jappl.1986.61.5.1949. [DOI] [PubMed] [Google Scholar]
- 23.Waldmann R, Waldmann R, Champigny G, et al. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 24.Birdsong WT, Birdsong WT, Fierro L, et al. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron. 2010;68:739–749. doi: 10.1016/j.neuron.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gautam M, Benson CJ, Ranier JD, et al. ASICs Do Not Play a Role in Maintaining Hyperalgesia Induced by Repeated Intramuscular Acid Injections. Pain Res Treat. 2012;2012:817347. doi: 10.1155/2012/817347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gautam M, Benson CJ. Acid-sensing ion channels (ASICs) in mouse skeletal muscle afferents are heteromers composed of ASIC1a, ASIC2, and ASIC3 subunits. FASEB J. 2013;27:793–802. doi: 10.1096/fj.12-220400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sluka KA, Sluka KA, Price MP, et al. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 28.Sluka KA, Sluka KA, Radhakrishnan R, et al. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–112. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walder RY, Walder RY, Rasmussen LA, et al. ASIC1 and ASIC3 play different roles in the development of Hyperalgesia after inflammatory muscle injury. J Pain. 2010;11:210–218. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molliver DC, Molliver DC, Immke DC, et al. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Molecular pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price MP, McIlwrath SL, Xie J, et al. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 32.Wemmie JA, Chen J, Askwith CC, et al. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 33.Burke RE, Brennan TJ, Levine DN, et al. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol (Lond) 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enoka RM, Enoka RM, Rankin LL, et al. Fatigability of rat hindlimb muscle: associations between electromyogram and force during a fatigue test. J Physiol (Lond) 1989;408:251–270. doi: 10.1113/jphysiol.1989.sp017458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taguchi T, Taguchi T, Sato J, et al. Augmented mechanical response of muscle thin-fiber sensory receptors recorded from rat muscle-nerve preparations in vitro after eccentric contraction. J Neurophysiol. 2005;94:2822–2831. doi: 10.1152/jn.00470.2005. [DOI] [PubMed] [Google Scholar]
- 36.Bennett RM. Myofascial pain syndromes and their evaluation. Best Pract Res Clin Rheumatol. 2007;21:427–445. doi: 10.1016/j.berh.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Skyba DA, Radhakrishnan R, Sluka KA. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. The Journal of Pain. 2005;6:41–47. doi: 10.1016/j.jpain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Walder RY, Walder RY, Gautam M, et al. Selective targeting of ASIC3 using artificial miRNAs inhibits primary and secondary hyperalgesia after muscle inflammation. Pain. 2011;152:2348–2356. doi: 10.1016/j.pain.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson RL, Cook ML, Devi-Rao GB, et al. Functional and molecular analyses of the avirulent wild-type herpes simplex virus type 1 strain KOS. J Virol. 1986;58:203–211. doi: 10.1128/jvi.58.1.203-211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun N, Cassell MD, Perlman S. Anterograde, transneuronal transport of herpes simplex virus type 1 strain H129 in the murine visual system. J Virol. 1996;70:5405–5413. doi: 10.1128/jvi.70.8.5405-5413.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Rooijen N. Liposome mediated modulation of macrophage functions. Adv Exp Med Biol. 1994;355:69–74. doi: 10.1007/978-1-4615-2492-2_12. [DOI] [PubMed] [Google Scholar]
- 42.Tong J, Wu W-N, Kong X, et al. Acid-sensing ion channels contribute to the effect of acidosis on the function of dendritic cells. J Immunol. 2011;186:3686–3692. doi: 10.4049/jimmunol.1001346. [DOI] [PubMed] [Google Scholar]
- 43.Kong X, Tang X, Du W, et al. Extracellular acidosis modulates the endocytosis and maturation of macrophages. Cell Immunol. 2013;281:44–50. doi: 10.1016/j.cellimm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Ikeuchi M, Ikeuchi M, Kolker SJ, et al. Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain. 2008;137:662–669. doi: 10.1016/j.pain.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yen Y-T, Tu P-H, Chen C-J, et al. Role of acid-sensing ion channel 3 in sub-acute-phase inflammation. Molecular pain. 2009;5:1. doi: 10.1186/1744-8069-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sluka KA, Rasmussen LA, Edgar MM, et al. Acid-sensing ion channel 3 deficiency increases inflammation but decreases pain behavior in murine arthritis. Arthritis Rheum. 2013;65:1194–1202. doi: 10.1002/art.37862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen W-N, Lee C-H, Lin S-H, et al. Roles of ASIC3, TRPV1, and NaV1.8 in the transition from acute to chronic pain in a mouse model of fibromyalgia. Molecular pain. 2014;10:40. doi: 10.1186/1744-8069-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karczewski J, Karczewski J, Spencer RH, et al. Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br J Pharmacol. 2010;161:950–960. doi: 10.1111/j.1476-5381.2010.00918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radhakrishnan R, Radhakrishnan R, Moore SA, et al. Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain. 2003;104:567–577. doi: 10.1016/s0304-3959(03)00114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deval E, Deval E, Noel J, et al. Acid-Sensing Ion Channels in Postoperative Pain. J Neurosci. 2011;31:6059–6066. doi: 10.1523/JNEUROSCI.5266-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jahr H, Jahr H, van Driel M, et al. Identification of acid-sensing ion channels in bone. Biochemical and Biophysical Research Communications. 2005;337:349–354. doi: 10.1016/j.bbrc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 52.Kolker SJ, Kolker SJ, Walder RY, et al. Acid-sensing ion channel 3 expressed in type B synoviocytes and chondrocytes modulates hyaluronan expression and release. Ann Rheum Dis. 2010;69:903–909. doi: 10.1136/ard.2009.117168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gong W, Kolker SJ, Usachev Y, et al. Acid-sensing ion channel 3 decreases phosphorylation of extracellular signal-regulated kinases and induces synoviocyte cell death by increasing intracellular calcium. Arthritis Res Ther. 2014;16:R121. doi: 10.1186/ar4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dina OA, Dina OA, Levine JD, et al. Enhanced cytokine-induced mechanical hyperalgesia in skeletal muscle produced by a novel mechanism in rats exposed to unpredictable sound stress. Eur J Pain. 2011;15:796–800. doi: 10.1016/j.ejpain.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Binshtok AM, Wang H, Zimmermann K, et al. Nociceptors are interleukin-1beta sensors. Journal of Neuroscience. 2008;28:14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mogi C, Tobo M, Tomura H, et al. Involvement of proton-sensing TDAG8 in extracellular acidification-induced inhibition of proinflammatory cytokine production in peritoneal macrophages. J Immunol. 2009;182:3243–3251. doi: 10.4049/jimmunol.0803466. [DOI] [PubMed] [Google Scholar]
- 58.Wang N, Gates KL, Trejo H, et al. Elevated CO2 selectively inhibits interleukin-6 and tumor necrosis factor expression and decreases phagocytosis in the macrophage. The FASEB Journal. 2010;24:2178–2190. doi: 10.1096/fj.09-136895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park S-Y, Bae D-J, Kim M-J, et al. Extracellular low pH modulates phosphatidylserine-dependent phagocytosis in macrophages by increasing stabilin-1 expression. Journal of Biological Chemistry. 2012;287:11261–11271. doi: 10.1074/jbc.M111.310953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zabel DD, Feng JJ, Scheuenstuhl H, et al. Lactate stimulation of macrophage-derived angiogenic activity is associated with inhibition of Poly(ADP-ribose) synthesis. Lab Invest. 1996;74:644–649. [PubMed] [Google Scholar]
- 61.Nareika A, He L, Game BA, et al. Sodium lactate increases LPS-stimulated MMP and cytokine expression in U937 histiocytes by enhancing AP-1 and NF-kappaB transcriptional activities. Am J Physiol Endocrinol Metab. 2005;289:E534–E542. doi: 10.1152/ajpendo.00462.2004. [DOI] [PubMed] [Google Scholar]
- 62.Kent-Braun JA, Kent-Braun JA, Ng AV, et al. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol. 2002;93:1813–1823. doi: 10.1152/japplphysiol.00091.2002. [DOI] [PubMed] [Google Scholar]
- 63.Hunter SK. Sex differences in human fatigability: mechanisms and insight to physiological responses. Acta Physiol (Oxf) 2014;210:768–789. doi: 10.1111/apha.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simoneau JA, Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol. 1989;257:E567–E572. doi: 10.1152/ajpendo.1989.257.4.E567. [DOI] [PubMed] [Google Scholar]
- 65.Staron RS, Hagerman FC, Hikida RS, et al. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem. 2000;48:623–629. doi: 10.1177/002215540004800506. [DOI] [PubMed] [Google Scholar]
- 66.Gollnick PD, Körge P, Karpakka J, Saltin B. Elongation of skeletal muscle relaxation during exercise is linked to reduced calcium uptake by the sarcoplasmic reticulum in man. Acta Physiol Scand. 1991;142:135–136. doi: 10.1111/j.1748-1716.1991.tb09139.x. [DOI] [PubMed] [Google Scholar]
- 67.Hunter SK, Thompson MW, Ruell PA, et al. Human skeletal sarcoplasmic reticulum Ca2+ uptake and muscle function with aging and strength training. J Appl Physiol. 1999;86:1858–1865. doi: 10.1152/jappl.1999.86.6.1858. [DOI] [PubMed] [Google Scholar]
- 68.Russ DW, Russ DW, Lanza IR, et al. Sex differences in glycolysis during brief, intense isometric contractions. Muscle Nerve. 2005;32:647–655. doi: 10.1002/mus.20396. [DOI] [PubMed] [Google Scholar]
- 69.Tarnopolsky LJ, MacDougall JD, Atkinson SA, et al. Gender differences in substrate for endurance exercise. J Appl Physiol. 1990;68:302–308. doi: 10.1152/jappl.1990.68.1.302. [DOI] [PubMed] [Google Scholar]
- 70.Roepstorff C, Steffensen CH, Madsen M, et al. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am J Physiol Endocrinol Metab. 2002;282:E435–E447. doi: 10.1152/ajpendo.00266.2001. [DOI] [PubMed] [Google Scholar]
- 71.Roepstorff C, Thiele M, Hillig T, et al. Higher skeletal muscle alpha2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise. J Physiol (Lond) 2006;574:125–138. doi: 10.1113/jphysiol.2006.108720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Esbjörnsson-Liljedahl M, Sundberg CJ, Norman B, Jansson E. Metabolic response in type I and type II muscle fibers during a 30-s cycle sprint in men and women. J Appl Physiol. 1999;87:1326–1332. doi: 10.1152/jappl.1999.87.4.1326. [DOI] [PubMed] [Google Scholar]
- 73.Costill DL, Sharp RL, Fink WJ, Katz A. Determination of human muscle pH in needle-biopsy specimens. J Appl Physiol Respir Environ Exerc Physiol. 1982;53:1310–1313. doi: 10.1152/jappl.1982.53.5.1310. [DOI] [PubMed] [Google Scholar]
- 74.Broch-Lips M, Overgaard K, Praetorius HA, Nielsen OB. Effects of extracellular HCO3(−) on fatigue pHi, and K+ efflux in rat skeletal muscles. J Appl Physiol. 2007;103:494–503. doi: 10.1152/japplphysiol.00049.2007. [DOI] [PubMed] [Google Scholar]
- 75.Hermansen L, Osnes JB. Blood and muscle pH after maximal exercise in man. J Appl Physiol. 1972;32:304–308. doi: 10.1152/jappl.1972.32.3.304. [DOI] [PubMed] [Google Scholar]
- 76.Juel C, Klarskov C, Nielsen JJ, et al. Effect of high-intensity intermittent training on lactate and H+ release from human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E245–E251. doi: 10.1152/ajpendo.00303.2003. [DOI] [PubMed] [Google Scholar]
- 77.Sherwood TW, Sherwood TW, Frey EN, et al. Structure and activity of the acid-sensing ion channels. Am J Physiol, Cell Physiol. 2012;303:C699–C710. doi: 10.1152/ajpcell.00188.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burnes LA, Burnes LA, Kolker SJ, et al. Enhanced muscle fatigue occurs in male but not female ASIC3−/− mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1347–R1355. doi: 10.1152/ajpregu.00687.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Tulder MW, Malmivaara A, Esmail R, Koes BW. Exercise therapy for low back pain. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD000335. CD000335. [DOI] [PubMed] [Google Scholar]
- 80.Thompson JM. Exercise in muscle pain disorders. PM R. 2012;4:889–893. doi: 10.1016/j.pmrj.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 81.Valkeinen H, Häkkinen A, Alen M, et al. Physical Fitness in Postmenopausal Women with Fibromyalgia. Int J Sports Med. 2008;29:408–413. doi: 10.1055/s-2007-965818. [DOI] [PubMed] [Google Scholar]
- 82.Verbunt JA, Verbunt JA, Pernot DHFM, et al. Disability and quality of life in patients with fibromyalgia. Health Qual Life Outcomes. 2008;6:8. doi: 10.1186/1477-7525-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Busch AJ, Barber KAR, Overend TJ, et al. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD003786.pub2. CD003786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sluka KA, Sluka KA, O'Donnell JM, et al. Regular physical activity prevents development of chronic pain and activation of central neurons. Journal of Applied Physiology. 2013;114:725–733. doi: 10.1152/japplphysiol.01317.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]