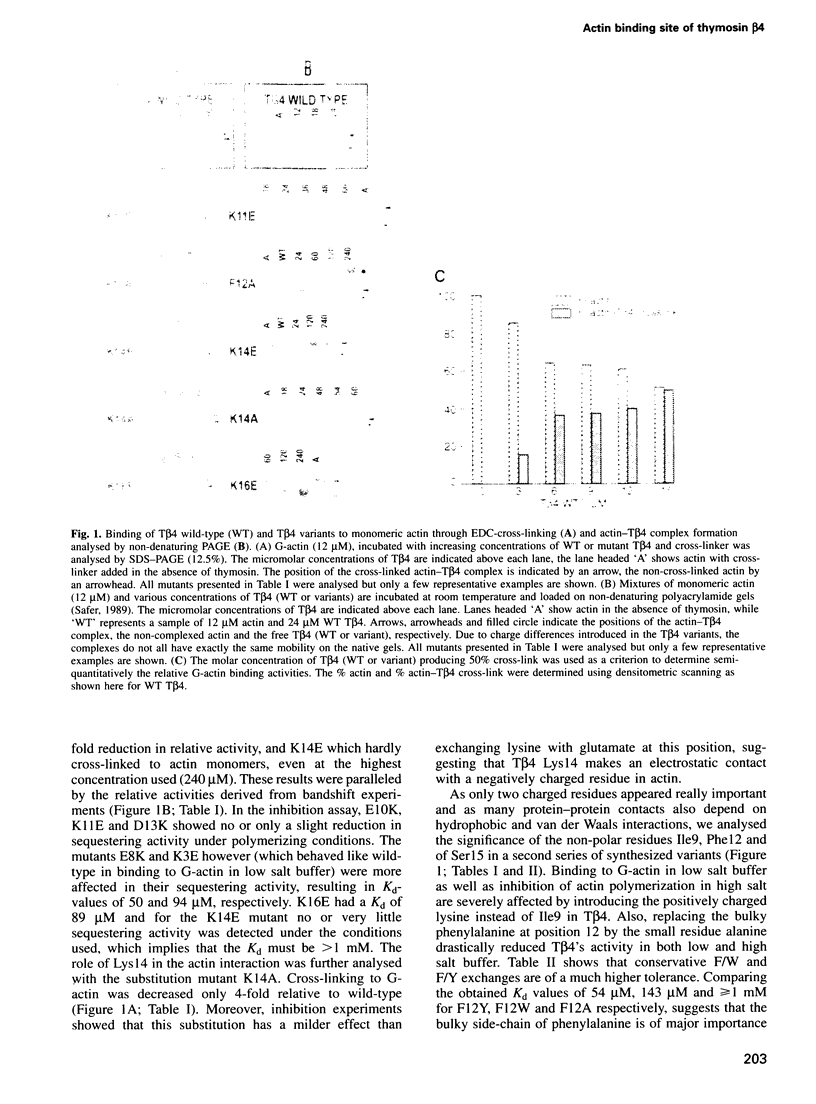
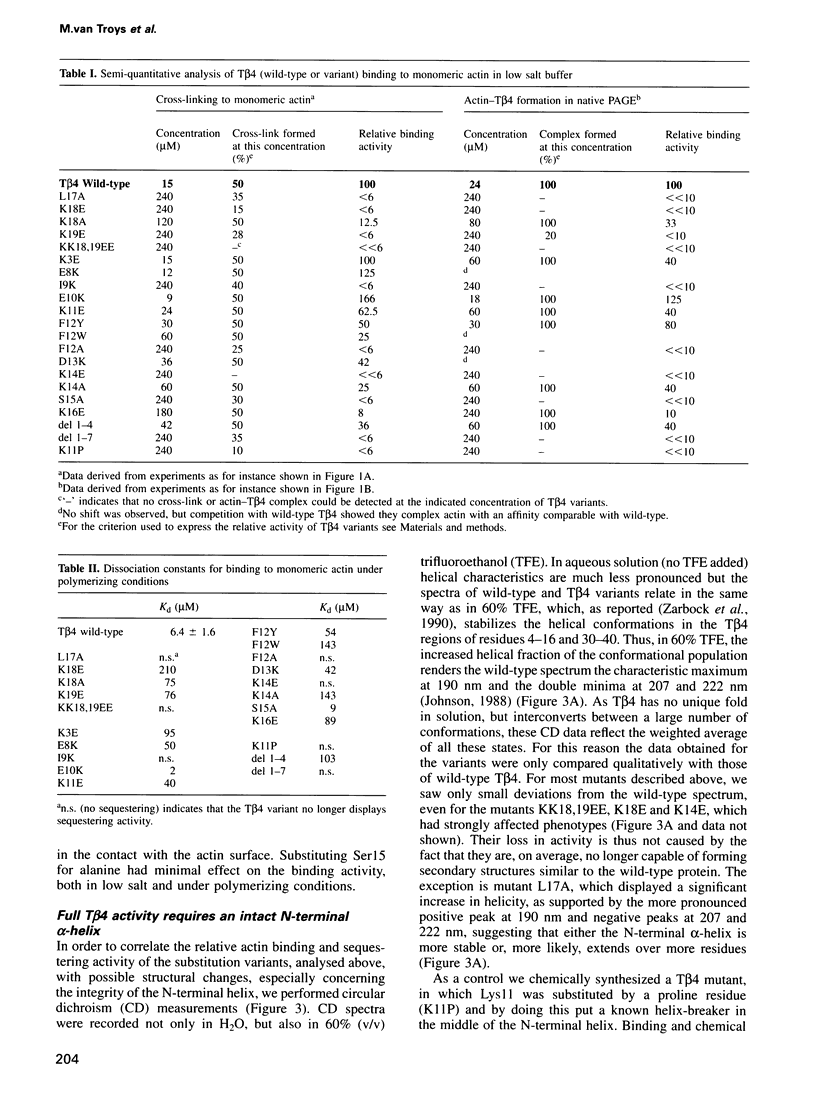
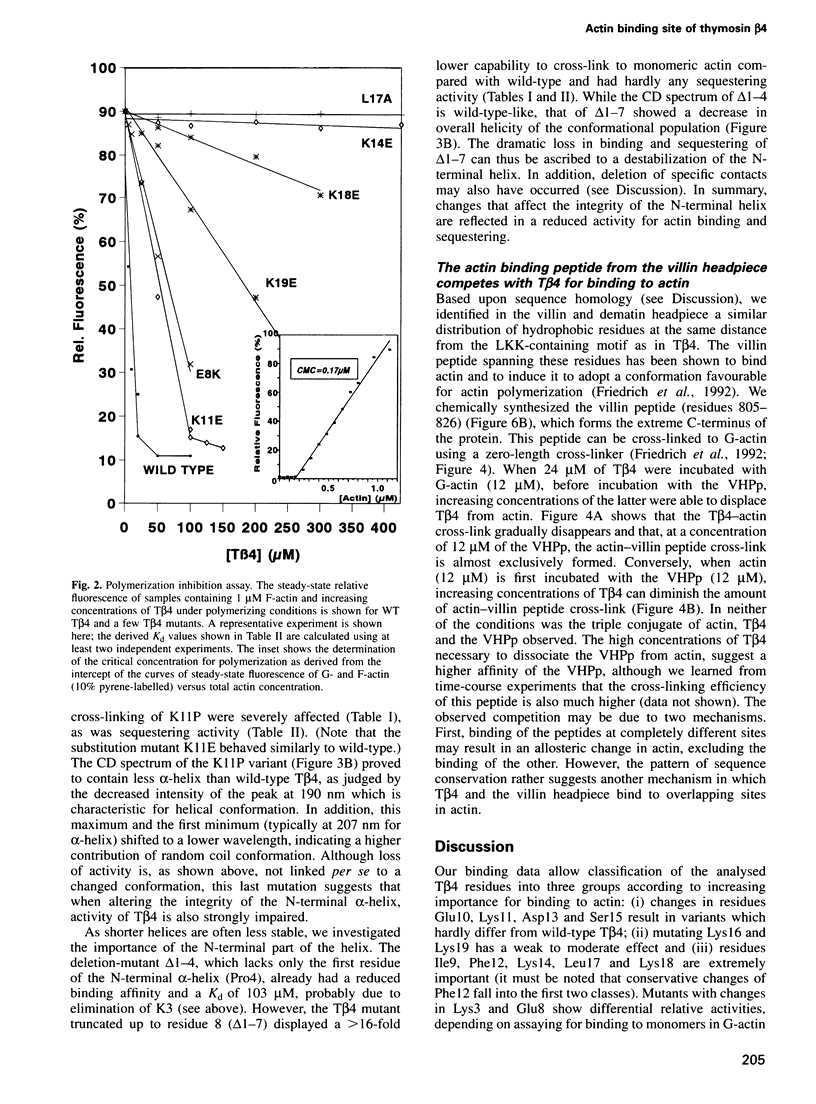
Abstract
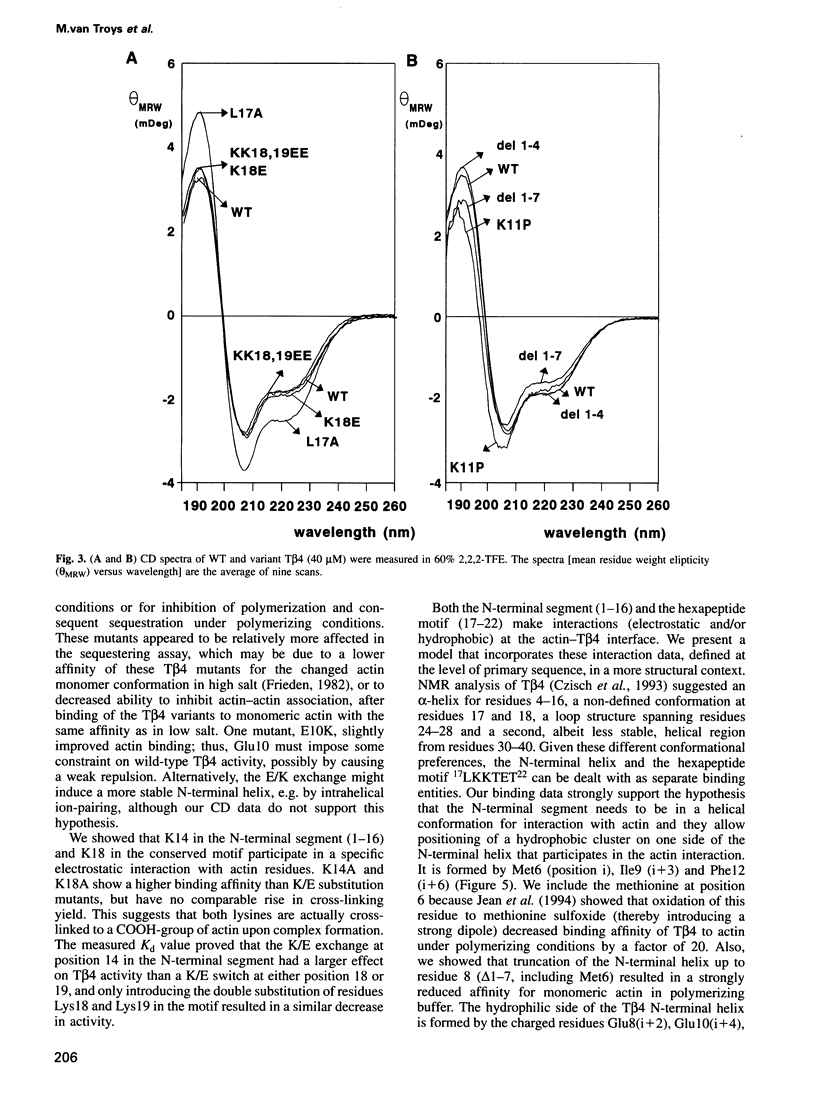
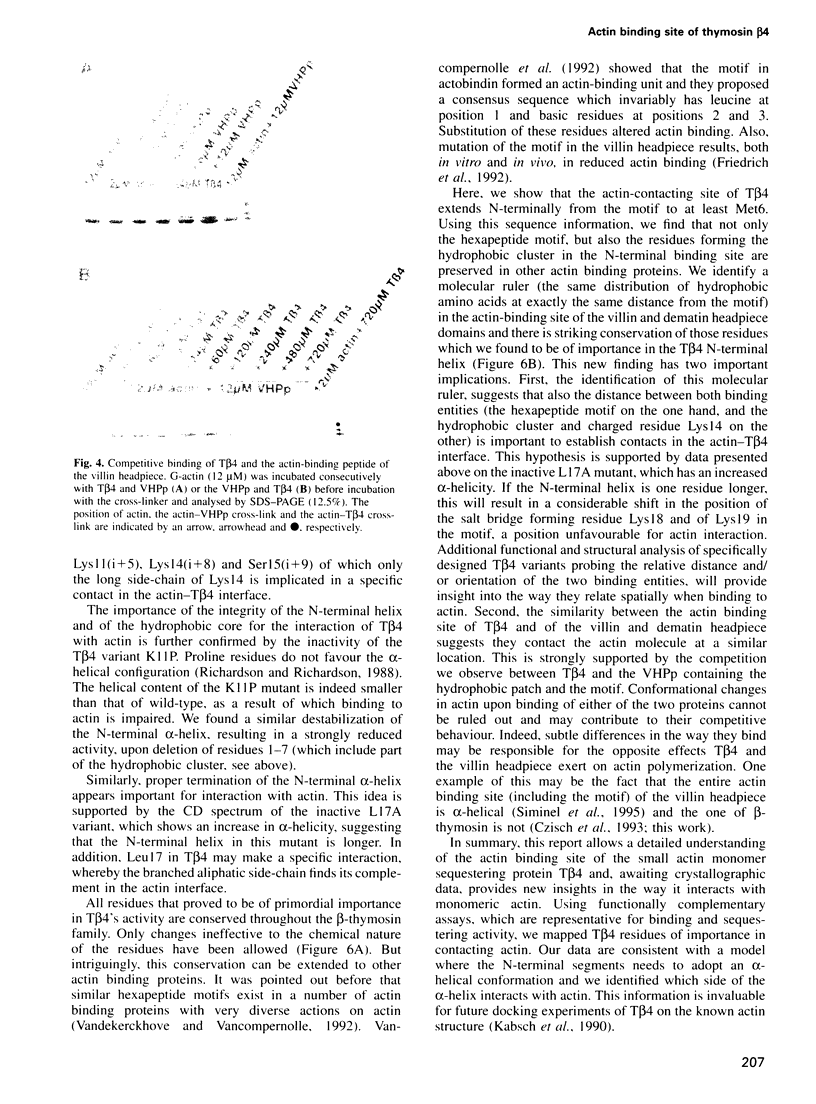
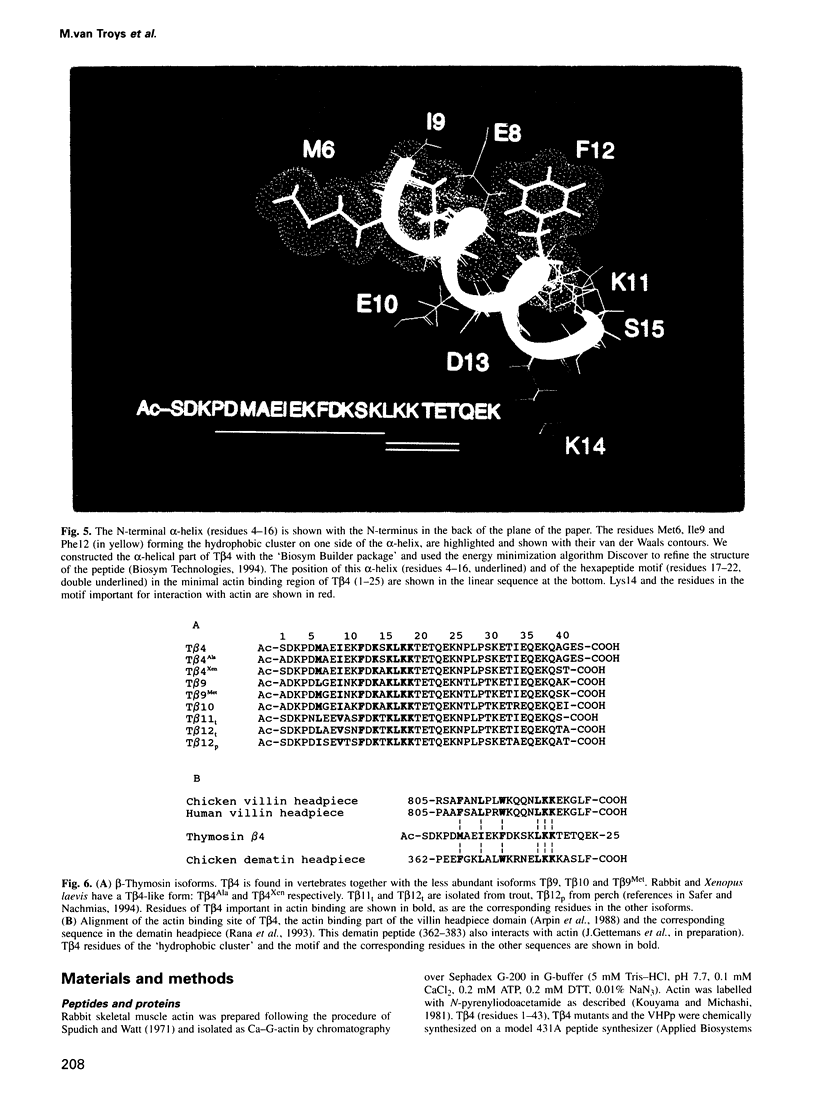
We characterized in detail the actin binding site of the small actin-sequestering protein thymosin beta 4 (T beta 4) using chemically synthesized full-length T beta 4 variants. The N-terminal part (residues 1-16) and a hexapeptide motif (residues 17-22) form separate structural entities. In both, we identified charged and hydrophobic residues that participate in the actin interaction using chemical cross-linking, complex formation in native gels and actin-sequestering experiments. Quantitative data on the activity of the variants and circular dichroism experiments allow to present a model in which the N-terminal part needs to adopt an alpha-helix for actin binding and interacts through a patch of hydrophobic residues (6M-I-F12) on one side of this helix. Also, electrostatic contacts between actin and lysine residues 18, in the motif, and 14, in the N-terminal alpha-helix, appear important for binding. The residues critical for contacting actin are conserved throughout the beta-thymosin family and in addition to this we identify a similar pattern in the C-terminal headpiece of villin and dematin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arpin M., Pringault E., Finidori J., Garcia A., Jeltsch J. M., Vandekerckhove J., Louvard D. Sequence of human villin: a large duplicated domain homologous with other actin-severing proteins and a unique small carboxy-terminal domain related to villin specificity. J Cell Biol. 1988 Nov;107(5):1759–1766. doi: 10.1083/jcb.107.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M. F., Pantaloni D. Actin assembly in response to extracellular signals: role of capping proteins, thymosin beta 4 and profilin. Semin Cell Biol. 1994 Jun;5(3):183–191. doi: 10.1006/scel.1994.1023. [DOI] [PubMed] [Google Scholar]
- Czisch M., Schleicher M., Hörger S., Voelter W., Holak T. A. Conformation of thymosin beta 4 in water determined by NMR spectroscopy. Eur J Biochem. 1993 Dec 1;218(2):335–344. doi: 10.1111/j.1432-1033.1993.tb18382.x. [DOI] [PubMed] [Google Scholar]
- Frieden C. The Mg2+-induced conformational change in rabbit skeletal muscle G-actin. J Biol Chem. 1982 Mar 25;257(6):2882–2886. [PubMed] [Google Scholar]
- Friederich E., Vancompernolle K., Huet C., Goethals M., Finidori J., Vandekerckhove J., Louvard D. An actin-binding site containing a conserved motif of charged amino acid residues is essential for the morphogenic effect of villin. Cell. 1992 Jul 10;70(1):81–92. doi: 10.1016/0092-8674(92)90535-k. [DOI] [PubMed] [Google Scholar]
- Jean C., Rieger K., Blanchoin L., Carlier M. F., Lenfant M., Pantaloni D. Interaction of G-actin with thymosin beta 4 and its variants thymosin beta 9 and thymosin beta met9. J Muscle Res Cell Motil. 1994 Jun;15(3):278–286. doi: 10.1007/BF00123480. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Jr Secondary structure of proteins through circular dichroism spectroscopy. Annu Rev Biophys Biophys Chem. 1988;17:145–166. doi: 10.1146/annurev.bb.17.060188.001045. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kouyama T., Mihashi K. Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur J Biochem. 1981;114(1):33–38. [PubMed] [Google Scholar]
- Nachmias V. T., Cassimeris L., Golla R., Safer D. Thymosin beta 4 (T beta 4) in activated platelets. Eur J Cell Biol. 1993 Aug;61(2):314–320. [PubMed] [Google Scholar]
- Pantaloni D., Carlier M. F. How profilin promotes actin filament assembly in the presence of thymosin beta 4. Cell. 1993 Dec 3;75(5):1007–1014. doi: 10.1016/0092-8674(93)90544-z. [DOI] [PubMed] [Google Scholar]
- Rana A. P., Ruff P., Maalouf G. J., Speicher D. W., Chishti A. H. Cloning of human erythroid dematin reveals another member of the villin family. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6651–6655. doi: 10.1073/pnas.90.14.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. S., Richardson D. C. Amino acid preferences for specific locations at the ends of alpha helices. Science. 1988 Jun 17;240(4859):1648–1652. doi: 10.1126/science.3381086. [DOI] [PubMed] [Google Scholar]
- Safer D. An electrophoretic procedure for detecting proteins that bind actin monomers. Anal Biochem. 1989 Apr;178(1):32–37. doi: 10.1016/0003-2697(89)90351-5. [DOI] [PubMed] [Google Scholar]
- Safer D., Nachmias V. T. Beta thymosins as actin binding peptides. Bioessays. 1994 Jul;16(7):473–479. doi: 10.1002/bies.950160706. [DOI] [PubMed] [Google Scholar]
- Sanders M. C., Goldstein A. L., Wang Y. L. Thymosin beta 4 (Fx peptide) is a potent regulator of actin polymerization in living cells. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4678–4682. doi: 10.1073/pnas.89.10.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simenel C., Rose T., Goethals M., Vandekerckhove J., Friederich E., Louvard D., Delepierre M. Conformational behaviour of a synthetic peptide of the C-terminus of villin that interacts with actin: an NMR, CD and stimulated annealing study. Int J Pept Protein Res. 1995 Jun;45(6):574–586. doi: 10.1111/j.1399-3011.1995.tb01322.x. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Staros J. V., Wright R. W., Swingle D. M. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal Biochem. 1986 Jul;156(1):220–222. doi: 10.1016/0003-2697(86)90176-4. [DOI] [PubMed] [Google Scholar]
- Vancompernolle K., Goethals M., Huet C., Louvard D., Vandekerckhove J. G- to F-actin modulation by a single amino acid substitution in the actin binding site of actobindin and thymosin beta 4. EMBO J. 1992 Dec;11(13):4739–4746. doi: 10.1002/j.1460-2075.1992.tb05579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancompernolle K., Vandekerckhove J., Bubb M. R., Korn E. D. The interfaces of actin and Acanthamoeba actobindin. Identification of a new actin-binding motif. J Biol Chem. 1991 Aug 15;266(23):15427–15431. [PubMed] [Google Scholar]
- Vandekerckhove J., Vancompernolle K. Structural relationships of actin-binding proteins. Curr Opin Cell Biol. 1992 Feb;4(1):36–42. doi: 10.1016/0955-0674(92)90056-i. [DOI] [PubMed] [Google Scholar]
- Weber A., Nachmias V. T., Pennise C. R., Pring M., Safer D. Interaction of thymosin beta 4 with muscle and platelet actin: implications for actin sequestration in resting platelets. Biochemistry. 1992 Jul 14;31(27):6179–6185. doi: 10.1021/bi00142a002. [DOI] [PubMed] [Google Scholar]
- Yu F. X., Lin S. C., Morrison-Bogorad M., Yin H. L. Effects of thymosin beta 4 and thymosin beta 10 on actin structures in living cells. Cell Motil Cytoskeleton. 1994;27(1):13–25. doi: 10.1002/cm.970270103. [DOI] [PubMed] [Google Scholar]
- Zarbock J., Oschkinat H., Hannappel E., Kalbacher H., Voelter W., Holak T. A. Solution conformation of thymosin beta 4: a nuclear magnetic resonance and simulated annealing study. Biochemistry. 1990 Aug 28;29(34):7814–7821. doi: 10.1021/bi00486a006. [DOI] [PubMed] [Google Scholar]