Abstract
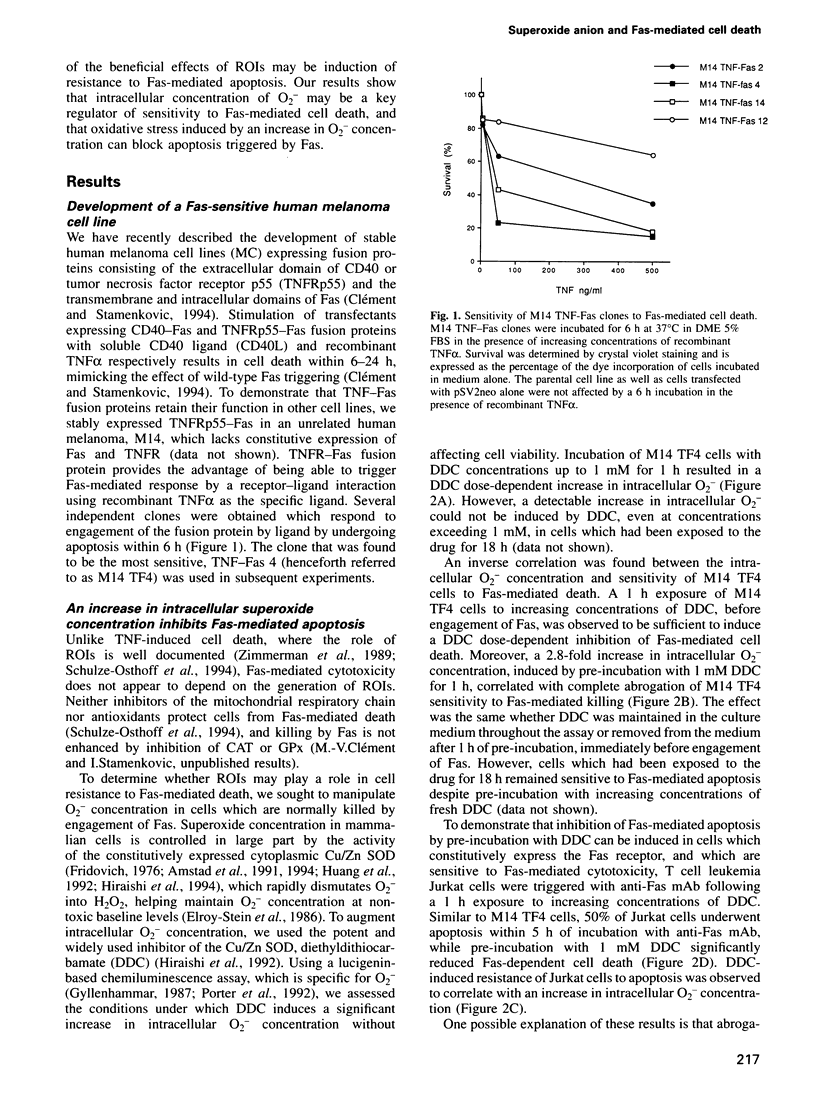
The cell surface receptor Fas is a major trigger of apoptosis. However, expression of the Fas receptor in many tumor cell types does not correlate with sensitivity to Fas-mediated cell death. Because a prooxidant state is a common feature of tumor cells, we examined the role of intracellular reactive oxygen intermediates in the regulation of Fas-mediated cytotoxicity. Our results show that an oxidative stress induced by increasing the intracellular superoxide anion (O2-) concentration can abrogate Fas-mediated apoptosis in cells which are constitutively sensitive to Fas. Conversely, an O2- concentration decrease is observed to sensitize cells which are naturally resistant to Fas signals. These observations suggest that intracellular O2- may play a key role in regulating cell sensitivity to a potentially lethal signal and provide tumor cells with a natural, inducible mechanism of resistance to Fas-mediated apoptosis.
Full text
PDF
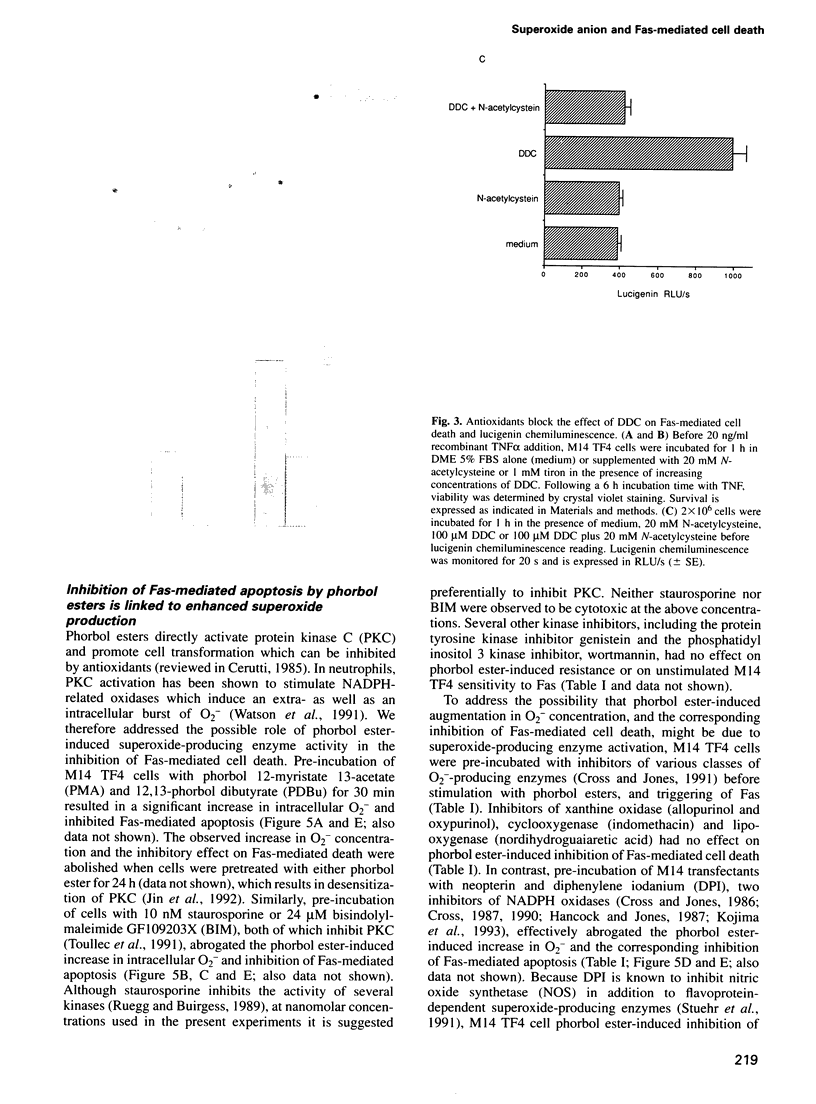
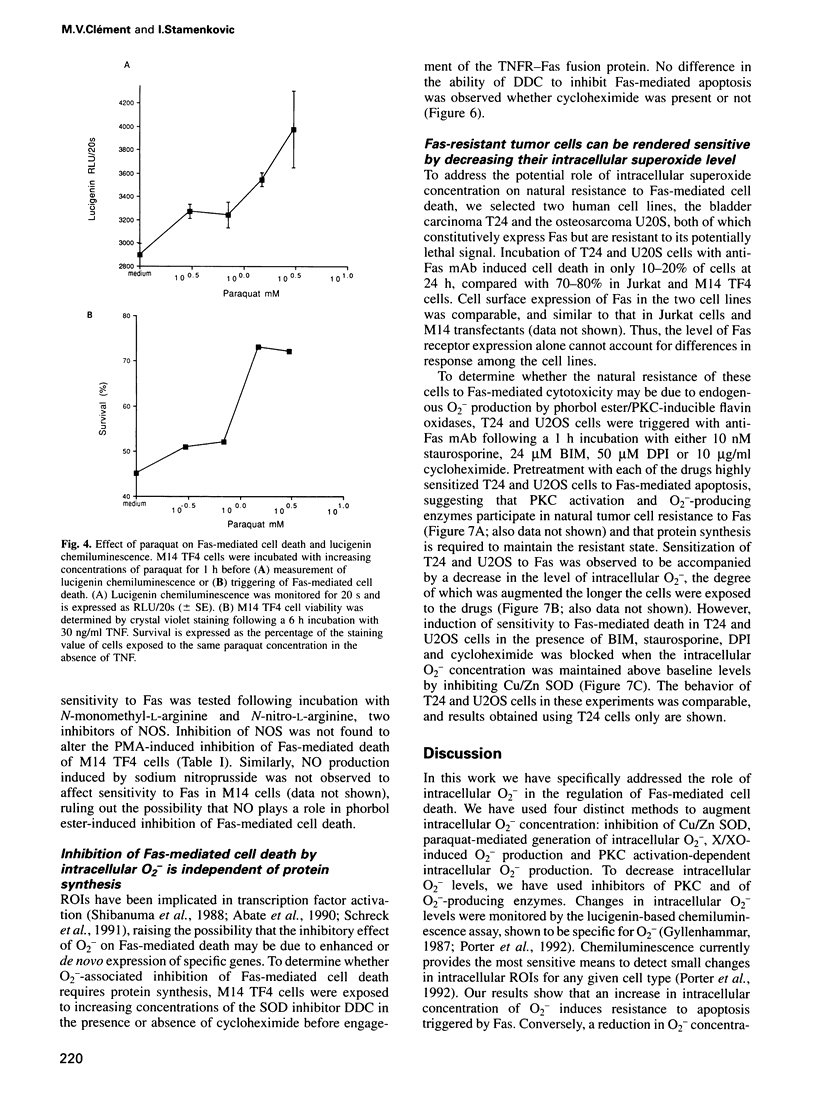
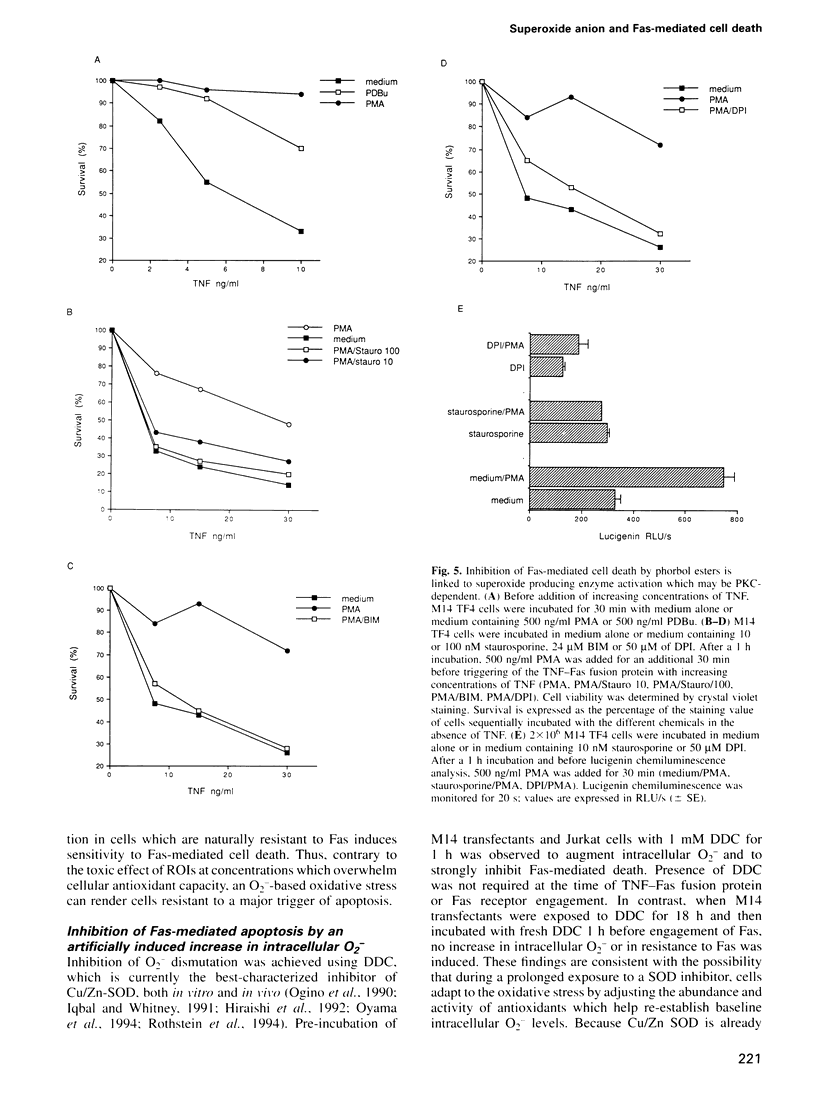
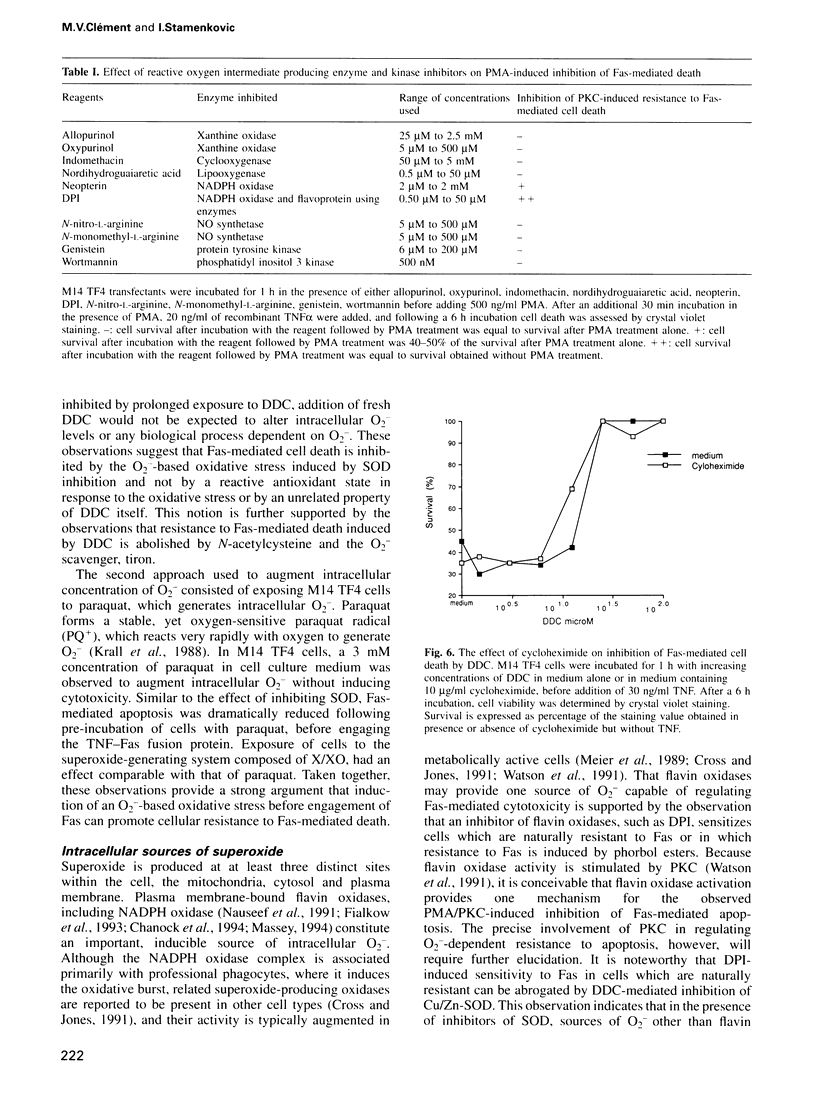


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Amstad P., Moret R., Cerutti P. Glutathione peroxidase compensates for the hypersensitivity of Cu,Zn-superoxide dismutase overproducers to oxidant stress. J Biol Chem. 1994 Jan 21;269(3):1606–1609. [PubMed] [Google Scholar]
- Amstad P., Peskin A., Shah G., Mirault M. E., Moret R., Zbinden I., Cerutti P. The balance between Cu,Zn-superoxide dismutase and catalase affects the sensitivity of mouse epidermal cells to oxidative stress. Biochemistry. 1991 Sep 24;30(38):9305–9313. doi: 10.1021/bi00102a024. [DOI] [PubMed] [Google Scholar]
- Behl C., Davis J. B., Lesley R., Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994 Jun 17;77(6):817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Brunner T., Mogil R. J., LaFace D., Yoo N. J., Mahboubi A., Echeverri F., Martin S. J., Force W. R., Lynch D. H., Ware C. F. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995 Feb 2;373(6513):441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- Buttke T. M., Sandstrom P. A. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994 Jan;15(1):7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Chanock S. J., el Benna J., Smith R. M., Babior B. M. The respiratory burst oxidase. J Biol Chem. 1994 Oct 7;269(40):24519–24522. [PubMed] [Google Scholar]
- Chinnaiyan A. M., O'Rourke K., Tewari M., Dixit V. M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995 May 19;81(4):505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Clement M. V., Stamenkovic I. Fas and tumor necrosis factor receptor-mediated cell death: similarities and distinctions. J Exp Med. 1994 Aug 1;180(2):557–567. doi: 10.1084/jem.180.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R. Inhibitors of the leukocyte superoxide generating oxidase: mechanisms of action and methods for their elucidation. Free Radic Biol Med. 1990;8(1):71–93. doi: 10.1016/0891-5849(90)90147-b. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. Enzymic mechanisms of superoxide production. Biochim Biophys Acta. 1991 May 6;1057(3):281–298. doi: 10.1016/s0005-2728(05)80140-9. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem J. 1986 Jul 1;237(1):111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R. The inhibitory effects of some iodonium compounds on the superoxide generating system of neutrophils and their failure to inhibit diaphorase activity. Biochem Pharmacol. 1987 Feb 15;36(4):489–493. doi: 10.1016/0006-2952(87)90356-x. [DOI] [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
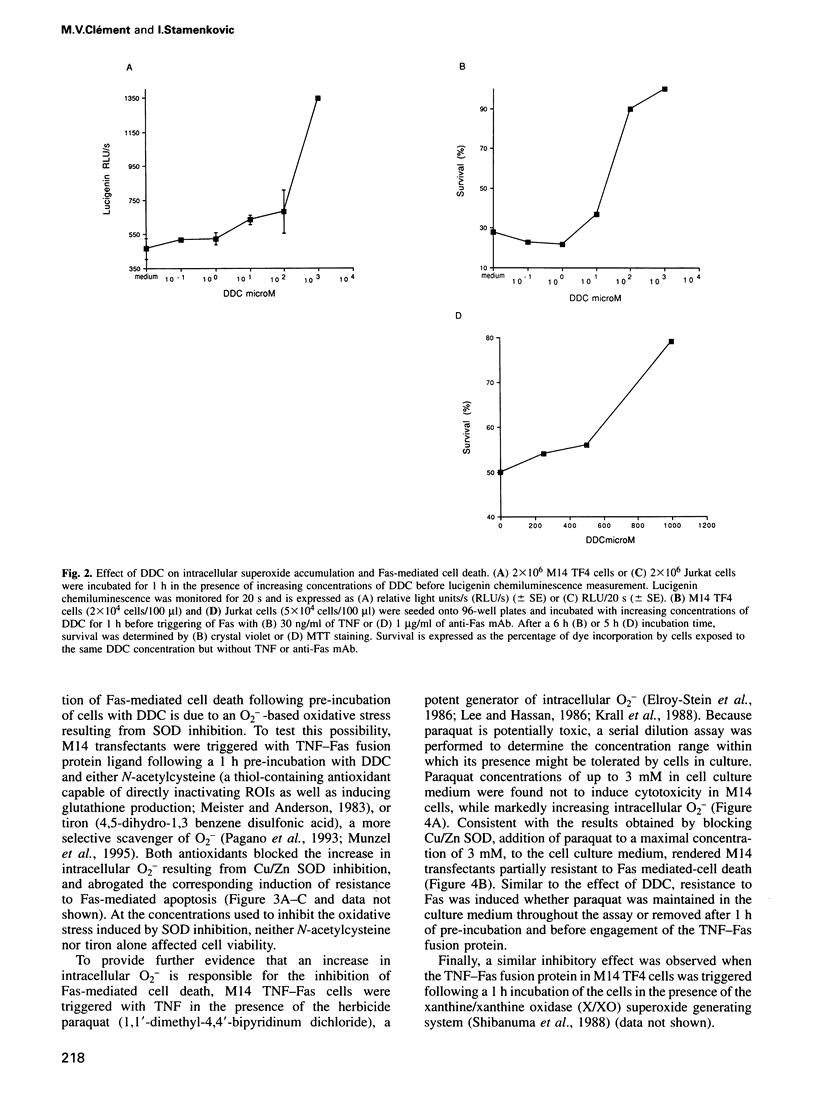
- Elroy-Stein O., Bernstein Y., Groner Y. Overproduction of human Cu/Zn-superoxide dismutase in transfected cells: extenuation of paraquat-mediated cytotoxicity and enhancement of lipid peroxidation. EMBO J. 1986 Mar;5(3):615–622. doi: 10.1002/j.1460-2075.1986.tb04255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enari M., Hug H., Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature. 1995 May 4;375(6526):78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- Fialkow L., Chan C. K., Grinstein S., Downey G. P. Regulation of tyrosine phosphorylation in neutrophils by the NADPH oxidase. Role of reactive oxygen intermediates. J Biol Chem. 1993 Aug 15;268(23):17131–17137. [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Hancock J. T., Jones O. T. The inhibition by diphenyleneiodonium and its analogues of superoxide generation by macrophages. Biochem J. 1987 Feb 15;242(1):103–107. doi: 10.1042/bj2420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M. Biosynthesis and regulation of superoxide dismutases. Free Radic Biol Med. 1988;5(5-6):377–385. doi: 10.1016/0891-5849(88)90111-6. [DOI] [PubMed] [Google Scholar]
- Hiraishi H., Terano A., Razandi M., Sugimoto T., Harada T., Ivey K. J. Role of cellular superoxide dismutase against reactive oxygen metabolite injury in cultured bovine aortic endothelial cells. J Biol Chem. 1992 Jul 25;267(21):14812–14817. [PubMed] [Google Scholar]
- Hiraishi H., Terano A., Sugimoto T., Harada T., Razandi M., Ivey K. J. Protective role of intracellular superoxide dismutase against extracellular oxidants in cultured rat gastric cells. J Clin Invest. 1994 Jan;93(1):331–338. doi: 10.1172/JCI116964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J., Whitney P. Use of cyanide and diethyldithiocarbamate in the assay of superoxide dismutases. Free Radic Biol Med. 1991;10(1):69–77. doi: 10.1016/0891-5849(91)90023-v. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Jin L. W., Inaba K., Saitoh T. The involvement of protein kinase C in activation-induced cell death in T-cell hybridoma. Cell Immunol. 1992 Oct 1;144(1):217–227. doi: 10.1016/0008-8749(92)90238-k. [DOI] [PubMed] [Google Scholar]
- Kojima S., Nomura T., Icho T., Kajiwara Y., Kitabatake K., Kubota K. Inhibitory effect of neopterin on NADPH-dependent superoxide-generating oxidase of rat peritoneal macrophages. FEBS Lett. 1993 Aug 23;329(1-2):125–128. doi: 10.1016/0014-5793(93)80207-b. [DOI] [PubMed] [Google Scholar]
- Krall J., Bagley A. C., Mullenbach G. T., Hallewell R. A., Lynch R. E. Superoxide mediates the toxicity of paraquat for cultured mammalian cells. J Biol Chem. 1988 Feb 5;263(4):1910–1914. [PubMed] [Google Scholar]
- Lee F. J., Hassan H. M. Biosynthesis of superoxide dismutase in Saccharomyces cerevisiae: effects of paraquat and copper. J Free Radic Biol Med. 1985;1(4):319–325. doi: 10.1016/0748-5514(85)90138-2. [DOI] [PubMed] [Google Scholar]
- Meier B., Radeke H. H., Selle S., Younes M., Sies H., Resch K., Habermehl G. G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-alpha. Biochem J. 1989 Oct 15;263(2):539–545. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Miura M., Zhu H., Rotello R., Hartwieg E. A., Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993 Nov 19;75(4):653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Münzel T., Sayegh H., Freeman B. A., Tarpey M. M., Harrison D. G. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J Clin Invest. 1995 Jan;95(1):187–194. doi: 10.1172/JCI117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S., Golstein P. The Fas death factor. Science. 1995 Mar 10;267(5203):1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Nauseef W. M., Volpp B. D., McCormick S., Leidal K. G., Clark R. A. Assembly of the neutrophil respiratory burst oxidase. Protein kinase C promotes cytoskeletal and membrane association of cytosolic oxidase components. J Biol Chem. 1991 Mar 25;266(9):5911–5917. [PubMed] [Google Scholar]
- Ogino K., Hobara T., Kawamoto T., Kobayashi H., Iwamoto S., Oka S., Okazaki Y. Mechanism of diethyldithiocarbamate-induced gastric ulcer formation in the rat. Pharmacol Toxicol. 1990 Feb;66(2):133–137. doi: 10.1111/j.1600-0773.1990.tb00719.x. [DOI] [PubMed] [Google Scholar]
- Owen-Schaub L. B., Radinsky R., Kruzel E., Berry K., Yonehara S. Anti-Fas on nonhematopoietic tumors: levels of Fas/APO-1 and bcl-2 are not predictive of biological responsiveness. Cancer Res. 1994 Mar 15;54(6):1580–1586. [PubMed] [Google Scholar]
- Oyama Y., Furukawa K., Chikahisa L., Hatakeyama Y. Effect of N,N-diethyldithiocarbamate on ionomycin-induced increase in oxidation of cellular 2',7'-dichlorofluorescin in dissociated cerebellar neurons. Brain Res. 1994 Oct 10;660(1):158–161. doi: 10.1016/0006-8993(94)90850-8. [DOI] [PubMed] [Google Scholar]
- Pagano P. J., Tornheim K., Cohen R. A. Superoxide anion production by rabbit thoracic aorta: effect of endothelium-derived nitric oxide. Am J Physiol. 1993 Aug;265(2 Pt 2):H707–H712. doi: 10.1152/ajpheart.1993.265.2.H707. [DOI] [PubMed] [Google Scholar]
- Porter C. D., Parkar M. H., Collins M. K., Levinsky R. J., Kinnon C. Superoxide production by normal and chronic granulomatous disease (CGD) patient-derived EBV-transformed B cell lines measured by chemiluminescence-based assays. J Immunol Methods. 1992 Nov 5;155(2):151–157. doi: 10.1016/0022-1759(92)90281-w. [DOI] [PubMed] [Google Scholar]
- Rothstein J. D., Bristol L. A., Hosler B., Brown R. H., Jr, Kuncl R. W. Chronic inhibition of superoxide dismutase produces apoptotic death of spinal neurons. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4155–4159. doi: 10.1073/pnas.91.10.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Krammer P. H., Dröge W. Divergent signalling via APO-1/Fas and the TNF receptor, two homologous molecules involved in physiological cell death. EMBO J. 1994 Oct 3;13(19):4587–4596. doi: 10.1002/j.1460-2075.1994.tb06780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger B. Z., Leder P., Lee T. H., Kim E., Seed B. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995 May 19;81(4):513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995 Mar 10;267(5203):1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- Tewari M., Quan L. T., O'Rourke K., Desnoyers S., Zeng Z., Beidler D. R., Poirier G. G., Salvesen G. S., Dixit V. M. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995 Jun 2;81(5):801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- Thompson C. B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995 Mar 10;267(5203):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991 Aug 25;266(24):15771–15781. [PubMed] [Google Scholar]
- Watson F., Robinson J., Edwards S. W. Protein kinase C-dependent and -independent activation of the NADPH oxidase of human neutrophils. J Biol Chem. 1991 Apr 25;266(12):7432–7439. [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yuan J., Shaham S., Ledoux S., Ellis H. M., Horvitz H. R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993 Nov 19;75(4):641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Zimmerman R. J., Chan A., Leadon S. A. Oxidative damage in murine tumor cells treated in vitro by recombinant human tumor necrosis factor. Cancer Res. 1989 Apr 1;49(7):1644–1648. [PubMed] [Google Scholar]