Abstract
Systemic and topical glucocorticoids (GC) can cause significant adverse effects not only on the dermis, but also on epidermal structure and function. In epidermis, a striking GC-induced alteration in permeability barrier function occurs that can be attributed to an inhibition of epidermal mitogenesis, differentiation and lipid production. As prior studies in normal hairless mice demonstrated that topical applications of a flavonoid ingredient found in citrus, hesperidin, improve epidermal barrier function by stimulating epidermal proliferation and differentiation, we assessed here whether its topical applications could prevent GC-induced changes in epidermal function in murine skin and the basis for such effects. When hairless mice were co-treated topically with GC and 2% hesperidin twice-daily for 9 days, hesperidin co-applications prevented the expected GC-induced impairments of epidermal permeability barrier homoeostasis and stratum corneum (SC) acidification. These preventive effects could be attributed to a significant increase in filaggrin expression, enhanced epidermal β-glucocerebrosidase activity and accelerated lamellar bilayer maturation, the last two likely attributable to a hesperidin-induced reduction in stratum corneum pH. Furthermore, co-applications of hesperidin with GC largely prevented the expected GC-induced inhibition of epidermal proliferation. Finally, topical hesperidin increased epidermal glutathione reductase mRNA expression, which could counteract multiple functional negative effects of GC on epidermis. Together, these results show that topical hesperidin prevents GC-induced epidermal side effects by divergent mechanisms.
Keywords: epidermal permeability barrier, glucocorticoid, hesperidin, skin pH
Introduction
Systemic and topical glucocorticoids (GC) are widely used for treating inflammatory disorders, including a variety of kidney, respiratory, cardiovascular and skin diseases (1–5). While systemic or topical GC often demonstrate numerous short-term benefits, significant side effects, including generalised infections, diabetes, Cushing’s syndrome and osteoporosis, can limit their usage (6–13). Topical GC are among the most widely used form of anti-inflammatory therapy in dermatology, but physicians also often are reluctant to deploy them due to their atrophogenic side effects, including skin hypopigmentation, telangiectasia, delayed wound healing and impaired cutaneous innate immunity (14–19). GC also induce side effects on the epidermis, including defects in epidermal structure (thinning) and permeability barrier function, which can be attributed, in large part, to an inhibition of epidermal proliferation, differentiation and lipid production (20–23). Although some side effects of topical GC can be formidable, topical applications of stratum corneum (SC) physiological lipids improve epidermal permeability barrier homoeostasis and SC integrity/cohesion in GC-treated animal models (21,23,24). Moreover, topical applications of certain activators of peroxisome proliferator-activated receptor (PPAR) and liver X receptor prevent the GC-induced decrease in epidermal differentiation marker protein expression and/or proliferation (25). Furthermore, either topical ammonium lactate or all-trans-retinoic acid could prevent GC-induced skin atrophy (26,27).
Recent studies demonstrated that certain topical herbal ingredients, including the citrus-derived flavonoids, hesperidin at concentration of 2%, improve epidermal permeability barrier function, largely attributable to the upregulation of epidermal differentiation (28,29), but hesperidin also stimulates epidermal proliferation in normal mouse skin (29). It is known that epidermal proliferation is required for permeability barrier formation (reviewed in 30,31). Among epidermal differentiation-related proteins, filaggrin, which is selectively upregulated by topical hesperidin, is particularly interesting. Skins with filaggrin deficiency are known to exhibit defective permeability barrier function both in vivo and in vitro (32,33). Although it is well known that GC down regulates filaggrin expression and proliferation while hesperidin stimulates filaggrin expression and epidermal proliferation, whether topical herbal medicines, such as hesperidin, could prevent GC-induced abnormalities in epidermal function is unknown. Therefore, in this study, we tested here whether topical applications of hesperidin could prevent the emergence of divergent abnormalities in epidermal structure and function induced by topical GC and described the mechanisms responsible for this response.
Materials and methods
Materials
Six- to eight- week-old female hairless mice (h/h) and C57BL/6J were purchased from Charles River Laboratories (Wilmington, MA, USA) and fed mouse diet (Ralston-Purina Co., St Louis, MO, USA) and water ad libitum. Hesperidin powder was from P&G Corporation. Clobetasol propionate powder, 12-O-tetradecanoylphorbol-13-acetate (TPA), monoclonal anti-mouse β-actin antibody and TRI Reagent were from Sigma Chemical Co (St Louis, MO, USA). Affinity-purified, rabbit anti-mouse antibodies to loricrin, involucrin and filaggrin were purchased from Covance (Emeryville, CA, USA) for immunohistochemistry. Biotinylated monoclonal antibody against proliferating cell nuclear antigen (PCNA) was from CalTag Laboratories (Burlingame, CA, USA). MPA5 and pH900 were from C&K (Cologne, Germany). Bis-Tris gel was from Invitrogen (Carlsbad, CA, USA). Polyclonal antifilaggrin against the 37 kD (monomer), polyclonal anti-involucrin against the 56 kD and polyclonal antiloricrin against the 39 kD were from Covance, and enhanced chemiluminescence was from Thermo Fisher Sci. (Rockford, IL, USA). The iScript cDNA synthesis kit was purchased from Bio-Rad (Hercules, CA, USA).
Experimental protocols and functional studies
All animal procedures were approved by the Animal Studies Subcommittee of the San Francisco Veterans Affairs Medical Center and performed in accordance with their guidelines. As our prior studies have shown that topical 0.05% clobetasol propionate induced profound changes in epidermal structure and barrier function (21,23,25), we used this agent in the present study. Because clobetasol propionate is the most potent corticosteroid and because both propylene glycol and ethanol are penetration enhancers (34), we used propylene glycol/ethanol/water (6/2/2, v/v/v), instead of propylene glycol/ethanol (7/3, v/v) as the vehicle to reduce potential systemic adverse effects from topical clobetasol. Our prior study showed that topical applications of 2% hesperidin improve epidermal permeability barrier function in normal murine skin and that orally administrated hesperidin at dose of 100 mg per day for up to one year caused no changes in laboratory parameters such as haematology, liver and renal function, (35). Two percentage of hesperidin was used in this study. Both flanks of mice were treated topically with 60 µl of 0.05% clobetasol in propylene glycol/ethanol/water (6/2/2) twice-daily for 9 days. One hour after each clobetasol application, either 70% ethanol alone or 2% hesperidin in 70% ethanol was applied to clobetasol (GC)-treated area. The pH for 2% hesperidin solution was 7.5. On day 10, 18 h after the last hesperidin or 70% ethanol application, basal stratum corneum biophysical properties were assessed by measuring transepidermal water loss (TEWL) and stratum corneum hydration using respective probes, TM300 for TEWL and CM825 for hydration, connected to MPA5. pH900 was used to measure skin surface pH. For barrier recovery, TEWL was measured at 0, 2 and 4 h after tape stripping 10-fold increase in TEWL), and percentage barrier recovery was calculated as described earlier (36,37).
Anti-inflammatory study
Animal treatment procedure and number of mice were showed in Figure S1. Ear inflammation on C57BL/6J mice was induced by topical application of 15 µl of 0.05% TPA in ethanol to both inner and outer surfaces of both ears (38). Forty-five minutes and 4 h following TPA treatment, either 20 µl of vehicle (propylene glycol/ethanol, 7:3, v/v) or 0.05% clobetasol was applied to both surfaces of the ear. One hour after each vehicle or clobetasol application, 20 µl of 70% ethanol was applied to both surfaces of vehicle and one group of clobetasol-treated ears, and 20 µl of 2% hesperidin to another group of clobetasol-treated ears. Ear thickness was measured with a digital caliper (Mitutoyo, Tokyo, Japan) before and 20 h after TPA application.
Western blot analysis of epidermal differentiation proteins
Epidermis from mice treated topically with clobetasol with/without 2% hesperidin for 9 days was obtained by EDTA separation (29). Epidermis was prepared in RIPA (radio-immunoprecipitation assay) buffer and was resolved by electrophoresis on 4–12% Bis-Tris Gel. Resultant bands were blotted onto polyvinylidene fluoride membranes and were subsequently probed with monoclonal anti-mouse β-actin antibody (1:30 000), polyclonal antifilaggrin (1:1000) against the 37 kD, monomer, polyclonal anti-involucrin (1:1000) against the 56 kD or polyclonal antiloricrin (1:1000) against the 39 kD, and the corresponding bands were detected by enhanced chemiluminescence and quantified by scanning densitometry. β-Actin was used to normalise changes in expression levels. Results were presented as percentage of vehicle-treated control, setting vehicle-treated as 100%.
Q-PCR for mRNA expression
Total epidermal RNA was isolated from mouse treated for 9 days and analysed as described previously (39). First-strand cDNA was synthesised from 1 µg of total RNA with the iScript cDNA Synthesis Kit (Bio-Rad). The real-time PCR contained 20 ng of reversed transcribed total RNA, 450 nm forward and reverse primers, and 10 µl of 2× LightCycler 480 SYBR Green I Master in a final volume of 20 µl in 96-well plates using Mx3000P™ Real-time PCR System (Stratagene, La Jolla, CA, USA). The primers for filaggrin, involucrin, loricrin and lipid synthetic enzymes, including 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCoA), serine–palmitoyl transferase 1 (SPT1), fatty acid synthase (FAS), glutathione reductase (GSR) and superoxide dismutase (SOD), are listed in Table S1. The PCR was performed at 50°C for 2 min, 95°C for 10 min and then 40 cycles of amplification of melting at 95°C for 30 s, annealing at 60°C for 30 s and extension at 72°C for 45 s, respectively. Gel electrophoresis and melting curve analyses were performed to confirm accurate PCR product sizes and absence of non-specific bands. The expression levels of each gene were normalised against 36B4 (is an invariant transcript) using the comparative Ct method. This method compared the Ct value of one target gene to the housekeeping gene (36B4) using the 2−ΔΔCT formula: 2−ΔΔCT = 2^−{[CT gene of interest (ABCA12) − CT internal control (36B4)] Treated Mice − [CT gene of interest (ABCA12) − CT internal control (36B4)] Untreated Mice]}. The fold changes were expressed as percentage of control, with the control as 100%.
Immunohistochemistry
Immunohistochemical staining for changes in epidermal proliferation was performed as described previously (29). Briefly, 5-µm paraffin sections were incubated with the primary anti-PCNA antibody at the dilutions of 1:500 overnight at 4°C. After washes ×3, sections were incubated with the secondary antibody for 30 min. Staining was detected with ABC-peroxidase kit from Vector Lab, and sections were then counterstained with haematoxylin. Sections were examined with a Zeiss fluorescence microscope (Jena, Germany), and digital images were captured with AxioVision software (Carl Zeiss Vision, Munich, Germany). All pictures were taken with the same exposure times.
In situ β-glucocerebrosidase activity
β-glucocerebrosidase is one of the key enzymes to process extracellular glucoceramides to ceramides (40–42). The latter is required for formation of permeability barrier (40). Therefore, β-glucocerebrosidase activity was evaluated by in situ zymography as described earlier (43,44). Briefly, after 9-day treatment, skin samples were taken for in situ zymography. Frozen sections (5 mm) were washed with the 1% Tween 20 washing solution and incubated at 37°C for 2 h with 250 µl of 1 mm resorufin α-D-glucopyranoside in deionised water The acidification-reversal experiments were performed in 10 mm MES buffer (pH 5.5), as above. All sections were then rinsed with the washing solution, cover-slipped and visualised under the confocal microscope at an excitation wavelength of 588 nm and an emission wavelength of 644 nm.
Electron microscopy
Skin biopsies from both vehicle and hesperidin-treated mice were taken for electron microscopy (45). Briefly, samples were minced to <0.5 mm3, fixed in a modified Karnovsky’s fixative overnight and postfixed in either 0.2% ruthenium tetroxide or 1% aqueous osmium tetroxide, containing 1.5% potassium ferrocyanide. After fixation, all samples were dehydrated in a graded ethanol series and embedded in an Epon–epoxy mixture. Ultrathin sections were examined, with or without further contrasting with lead citrate, in a Zeiss 10A electron microscope (Carl Zeiss, Thornwood, NJ, USA), operated at 60 kV.
Statistics
Data are expressed as the mean + SEM. GraphPad Prism 4 software (GraphPad Software, La Jolla, CA, USA) was used for all statistical analyses. Unpaired two-tailed Student’s t-test with Welch’s correction was used to determine the statistical significances when two groups were compared. One-way ANOVA with Tukey’s correction was used when three or more groups were compared.
Results
Topical hesperidin prevents the emergence of GC-induced, epidermal functional abnormalities
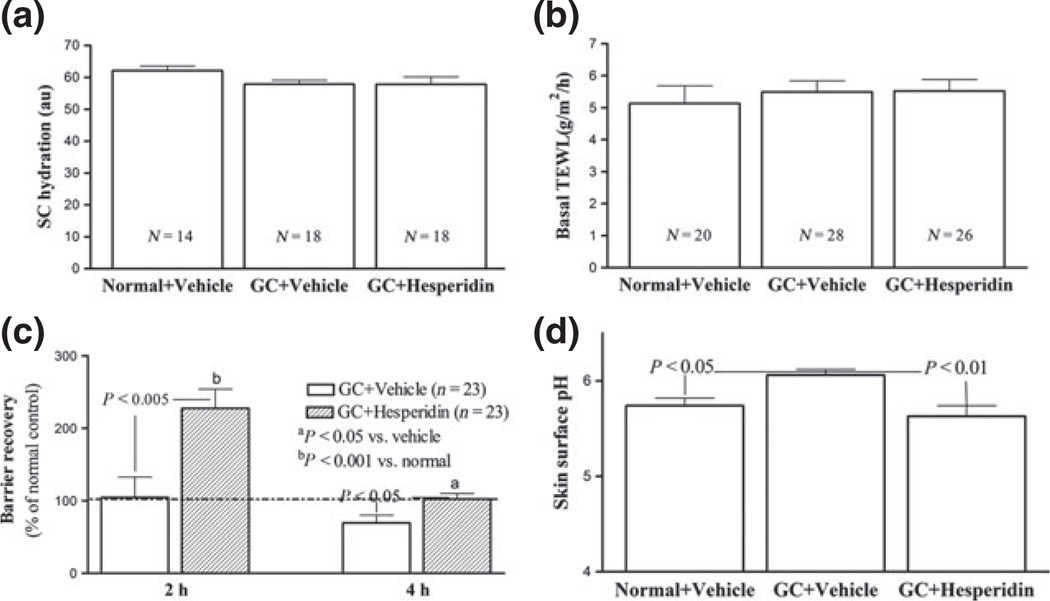
After 9 days of treatments with topical clobetasol (GC), treated skin sites on hairless mice appeared erythematous and atrophic as compared with vehicle-treated skin sites (Figure S2). Co-applications of hesperidin with GC largely prevented the emergence of these changes in skin appearance (Figure S2). The body sizes remained similar among normal, GC-treated and GC plus hesperidin-treated mice. Although baseline stratum corneum hydration and transepidermal water loss rates did not differ significantly in GC plus hesperidin versus vehicle-treated mice (Fig. 1a,b), the significant delay in barrier recovery, observed 4 h after acute barrier disruption in topical GC-treated skin was prevented by co-applications of topical hesperidin (Fig. 1c). Moreover, while topical GC treatments significantly raised skin surface pH, co-applications of hesperidin during GC treatments completely prevented the alteration in skin surface pH (Fig. 1d). These results indicate that topical hesperidin can prevent emergence of GC-induced alterations in epidermal permeability barrier homoeostasis and acidification.
Figure 1.
Topical hesperidin prevents the GC-induced abnormalities in SC pH and permeability homoeostasis in murine skin: Both flanks of mice were treated topically with 60 µl of 0.05% clobetasol in propylene glycol/ethanol/water (6/2/2) twice-daily for 9 days. One hour after each clobetasol application, either 70% ethanol alone or 2% hesperidin in 70% ethanol was applied to clobetasol-treated area. On day 10, 18 h after last 70% ethanol or hesperidin application, basal stratum corneum biophysical properties were assessed as described in Materials and Methods. (a) displays stratum corneum capacitance; (b) shows basal transepidermal water loss; (c) indicates the barrier recovery rates; and (d) exhibits skin surface pH. Numbers and significances are indicated in the figures.
Topical hesperidin increases filaggrin expression in GC-treated skin
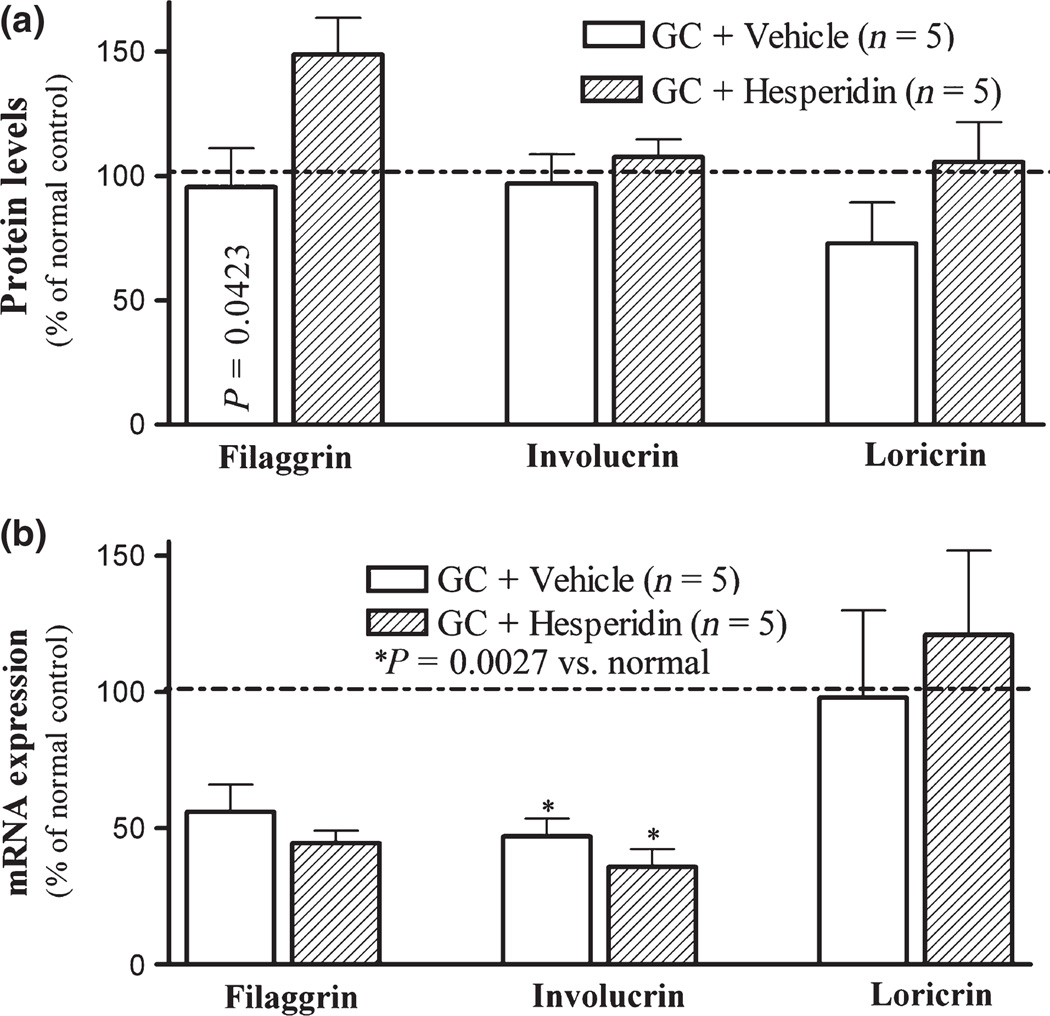
We next assessed the potential basis for the preventive benefits of hesperidin in GC-treated skin. As previous studies showed that topical GC impair epidermal permeability barrier homoeostasis in part by reducing expression of epidermal differentiation-related proteins (20) and that topical hesperidin stimulates epidermal differentiation and proliferation in normal mouse skin (29), we first assessed whether topical hesperidin stimulates the expression of epidermal differentiation-related proteins in GC-treated murine skin, providing one potential mechanism whereby hesperidin could improve barrier function. As seen in Fig. 2a, topical GC also moderately decreased epidermal loricrin expression, while topical co-treatments with hesperidin increased the expression of both loricrin and filaggrin (Fig. 2a. P = 0.0423 for filaggrin between vehicle and hesperidin). We next assessed whether the changes of epidermal differentiation marker protein expression induced by topical hesperidin could be attributed to upregulation of their gene expression. Specifically, we assessed changes in the levels of mRNA for epidermal differentiation marker-related proteins in GC ± hesperidin-treated skin sites. As shown in Fig. 2b, topical GC treatment dramatically reduced epidermal mRNA levels for filaggrin and involucrin, while mRNA levels for loricrin did not significantly change. These results indicate that while topical GC and hesperidin regulate epidermal filaggrin protein levels, the hesperidin-induced upregulation of filaggrin expression likely does not occur at a transcription level.
Figure 2.
Topical applications of hesperidin stimulates epidermal differentiation in GC-damaged murine skin: After 9-day treatment (detailed in materials and methods), skin samples were taken for immunohistochemical staining, Western blot and mRNA expression as described in Materials and Methods section. (a) depicts differentiation marker protein expression assessed by Western blot; (b) is differentiation marker mRNA expression assessed by Q-PCR. Numbers and significances are indicated in figures.
Hesperidin does not regulate the expression of lipid synthetic enzymes in GC-treated epidermis
As epidermal lipid synthesis is required for permeability barrier function, we next quantified the changes in mRNA levels of the rate-limiting enzymes for the synthesis of each of these key lipids, that is, HMGCoA, SPT1 and FAS, in murine epidermis after 9 days of treatments. The results showed that GC treatment alone slightly lowered the mRNA levels of HMGCoA, SPT1 and FAS, consistent with previous reports (20) (Figure S3), but a further reduction in mRNA levels of the lipid synthetic enzymes was observed following hesperidin co-applications to the GC-treated skin although the differences were not significant between these two groups. Thus, the hesperidin-induced improvement in epidermal permeability barrier function likely cannot be attributed to stimulation of epidermal lipid synthetic enzymes.
Topical hesperidin prevents GC-induced inhibition of epidermal proliferation
As epidermal proliferation is required for permeability barrier formation (reviewed in 30,31), and topical GC inhibits epidermal proliferation (16), we next assessed whether topical hesperidin could prevent the GC-induced reduction in epidermal proliferation. As expected, GC-treated epidermis became much thinner, with flattened keratinocytes, and a decrease in mitotic figures (Figure S4a vs b), and marked reduction of PCNA-positive cells in GC-treated epidermis (Fig. 4d vs e). Yet, co-applications of hesperidin largely prevented epidermal thinning by GC treatments and increased epidermal thickness and the number of PCNA-positive cells (Fig S4c,f). These results indicate that topical hesperidin prevents the GC-induced inhibition of epidermal proliferation.
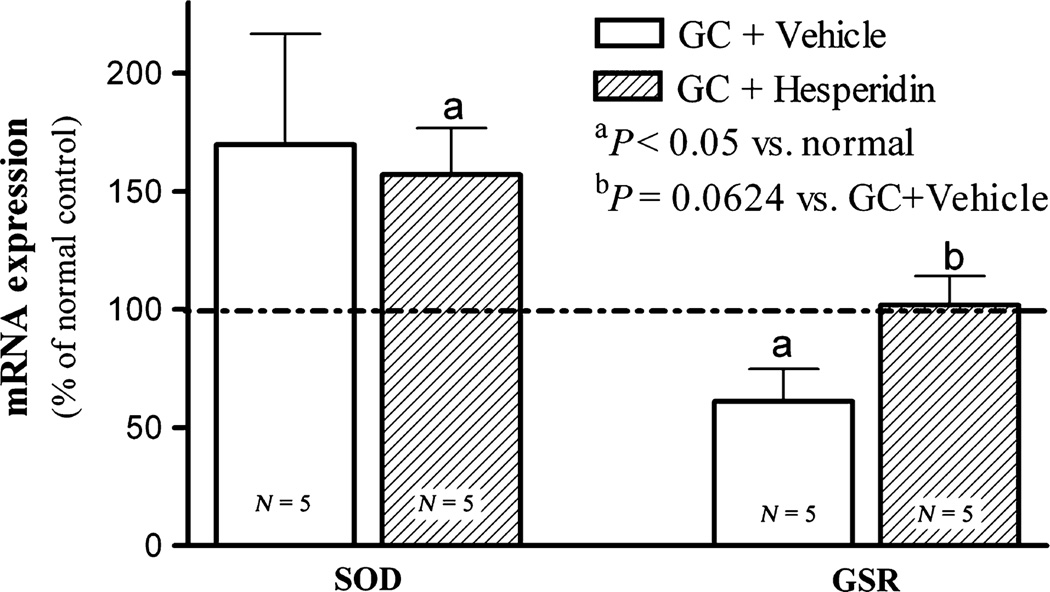
Figure 4.
Topical hesperidin increases glutathione reductase mRNA expression in GC-damaged murine skin: After 9-day treatment with GC plus vehicle or GC plus hesperidin, skin samples were taken for assessing mRNA expression as described in Materials and Methods section. Data are expressed as % of normal untreated epidermis. Numbers and significances are indicated in the figures.
Hesperidin prevents GC-induced abnormalities in extracellular lipid processing
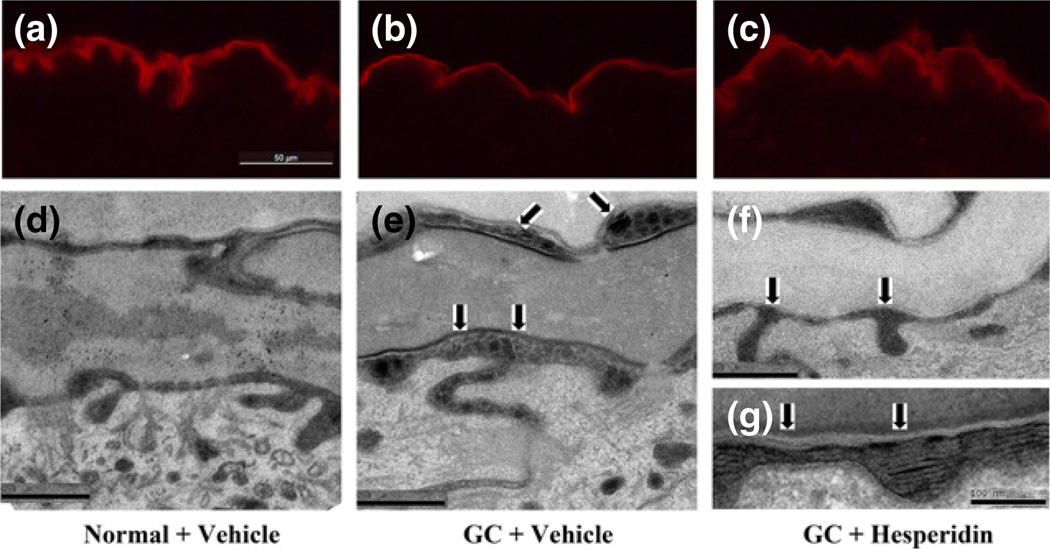
As noted above (Fig. 1b), GC treatment results in an increase in SC pH. Extracellular lipid processing is a critical, pH-dependent step leading to the formation of the lamellar bilayers that modulate epidermal permeability barrier function (40,46–50). Previous studies have shown that β-glucocerebrosidase, which optimal pH is ≈5–5.5, is a key enzyme for generating ceramides necessary for barrier formation (41,42,48). As co-applications of hesperidin counteract the GC-induced increase in SC pH (Fig. 1b), we next assessed whether this reduction in SC pH results in an elevation of β-glucocerebrosidase activity, using the in situ zymography technique (43,44). As predicted, a reduction of β-glucocerebrosidase activity was evident in GC-treated as compared to normal epidermis (Fig. 3a vs b). Yet, β-glucocerebrosidase activity largely normalised following co-applications of hesperidin with GC to normal skin (Fig. 3b vs c).
Figure 3.
Topical hesperidin accelerates lipid processing in GC-damaged murine skin: After 9-day treatment with GC plus vehicle or GC plus hesperidin, skin samples were taken for both in situ zymography and ultrastructural study as in Materials and Methods. (a, b, c) are in situ zymography for beta-glucocerabrosidase. (a) is normal skin; (b) is GC + vehicle-treated skin; and (c) is GC + hesperidin-treated skin. Scale Bar = 50 µm. (d–g) are electron microscopy pictures. (d) depicts normal lipid processing (arrows) between stratum granulosum and stratum corneum intercellular space in normal epidermis. (e) displays retardation of lipid processing (arrows) in GC + vehicle-treated epidermis. (e) exhibits normal lipid processing (arrows) in GC + hesperidin-treated skin, and (f) shows normal lipid processing (arrows) at higher magnification. Scale bars for all electron microscopy pictures are 0.5 µm except for (f), which scale bars = 100 nm.
To further confirm that hesperidin-induced reduction in SC pH results in an accelerated extracellular lipid processing, changes in lamellar bilayer structure were evaluated by ruthenium tetroxide postfixation and electron microscopy. While GC treatment alone provoked an accumulation of unprocessed lamellar material in the intercellular space (arrows in Fig. 3e), a sign of retarded lipid processing, hesperidin co-treatment completely prevented the expected GC-induced delay in lipid processing (arrows in Fig. 3f, g). Yet, the hesperidin co-treatment did not alter either lamellar body contents or density (data not shown). Taken together, these results indicate that a GC-induced retardation of lipid processing is another potential, unrelated mechanism whereby GC cause a defective permeability barrier and by which hesperidin co-treatment could in part improve barrier function.
Hesperidin increases glutathione reductase expression in GC-treated skin
Oxidative stress is linked with epidermal permeability barrier function (51,52), and GC treatment has been shown to increase oxidative stress through reduction in glutathione reductase expression and activity (53,54). As both SOD and GSR are among the key enzymatic anti-oxidants and widely expressed in tissues, especially in epidermis (55), we next determined whether GC treatment alters the expression levels of epidermal GSR, as well as another anti-oxidant enzyme, superoxide dismutase (SOD), and whether hesperidin co-treated could prevent these changes. As shown in Fig. 4, GC treatment alone significantly reduced the epidermal levels of GSR mRNA levels, while hesperidin co-treatments prevented this expected decline in GSR expression. In contrast, SOD mRNA levels increased moderately with GC treatment and did not change with hesperidin co-treatment. These data indicate that topical GC reduces epidermal GSR expression, a change that could be prevented by co-applications of hesperidin.
Hesperidin does not attenuate anti-inflammatory effect of GC
We next determined whether topical hesperidin attenuated the anti-inflammatory efficacy of GC in a TPA-induced irritant contact dermatitis model. Our results showed that 20 h after TPA application, the percentage increase in ear thickness was similar in GC-treated ears with or without hesperidin applications (10.00 ± 4.17 in GC + Vehicle vs 13.39 ± 3.33 in GC + hesperidin). Moreover, hesperidin alone did not inhibit inflammation (23.83 ± 5.64 in TPA + Vehicle vs 23.03 ± 8.68 in GC + hesperidin). These results demonstrate that topical hesperidin neither interferes with the anti-inflammatory effect of GC, nor inhibits inflammation in TPA-induced dermatitis model.
Discussion
Well-recognised GC-induced cutaneous adverse effects include compromised epidermal permeability barrier function and skin atrophy. To date, approaches to overcome these GC-induced side effects are still limited. In agreement with our previous findings (20,22,25), the present study also showed that topical GC delay permeability barrier recovery. GC-induced epidermal structural and functional changes can be attributed to the inhibition of epidermal proliferation, differentiation and lipid production (20). Although topical applications of either stratum corneum lipid mixtures (22–24) or PPAR and LXR ligands (25) can improve some epidermal side effects of GC such as barrier abnormality in murine skin, their expense and limited availability can constrain their widespread clinical usage, particularly in developing countries. In the present study, we demonstrated that topical applications of an inexpensive and widely available natural ingredient, hesperidin, prevent topical GC-induced changes in epidermal structure and function. The underlying mechanisms accounting for hesperidin-induced improvement of epidermal permeability barrier function in GC-treated skin likely include stimulation of a) epidermal proliferation, b) filaggrin expression and/or c) stratum corneum acidification and processing that all are required for normal epidermal permeability barrier homoeostasis (46, reviewed in 56). Among many differentiation-associated proteins, filaggrin is particularly interesting, because filaggrin mutations compromise barrier function in humans (57), filaggrin deficient mouse models (32), as well as in vitro human skin model (33), while in contrast, selective upregulation of filaggrin expression accelerates barrier recovery (58). Therefore, the GC-induced permeability barrier abnormality could result, at least in part, from an inhibition of epidermal filaggrin expression (25). The present studies show that topical hesperidin markedly increases epidermal filaggrin expression in GC-treated skin, which could contribute to the prevention of GC-induced alterations in epidermal permeability barrier function by hesperidin. It is worth noting that previous study showed that GC delayed barrier recovery 3 h after barrier disruption (22), while in the present study, impaired barrier recovery occurred only at 4 h after barrier disruption in GC-treated skin. These discrepancies could result from the differences in vehicle. In previous study, propylene glycol/ethanol (7/3, v/v) was used as vehicle (20,22,25). It is known that both propylene glycol and ethanol are penetration enhancers (34), which would allow more GC penetrate into skin compared with the vehicle containing less propylene glycol and ethanol used in the present study. Thus, impairment of barrier recovery was less in the present study. Nevertheless, the present studies demonstrated that topical hesperidin prevents GC-induced barrier abnormality, possibly resulting from stimulation of filaggrin expression and epidermal proliferation.
In addition to epidermal differentiation, epidermal proliferation is required for permeability barrier function (reviewed in 30,31), and we demonstrated here that topical hesperidin prevents the expected GC-induced inhibition of epidermal proliferation. How hesperidin prevents the GC-induced decline in proliferation is less clear. Although several studies showed that hesperidin stimulates proliferation of osteoblasts, epithelial cells as well as keratinocytes (29,59,60), the responsible mechanisms in these cell types remain unclear. However, prior studies showed that hesperidin upregulates nuclear factor (erythroid-derived 2)-like 2 (Nrf2) expression and translocation (61,62), while Nrf2 in turn regulates both anti-oxidant enzyme expression (61–63) and cell proliferation (64). Our recent studies showed that topical applications of hesperidin increases epidermal Nrf2 expression in murine skin (MQ Man & PM Elias, unpublished observation). Therefore, the preventive effect of hesperidin on GC-induced hypoproliferation could be ascribed to an upregulation of Nrf2 expression. Thus, improvement of epidermal proliferation could represent another mechanism by which hesperidin prevents emergence of a barrier abnormality in GC-treated skin.
A notable finding that emerged during the present studies was that topical hesperidin prevented the topical GC-induced changes in skin surface pH. Not only does an acidic pH accelerate epidermal permeability barrier recovery in adult and neonatal barrier maturation (48,49,65), but it also prevents the development of atopic dermatitis in a murine model (66). To date, hesperidin is the only known agent shown to prevent the GC-induced abnormality in skin surface pH. Although the underlying mechanism remains unknown, the resultant improvement in SC pH could significantly impact epidermal function by acidification-induced increase in β-glucocerbrosidase activity, as shown here, which also correlated with accelerated maturation of SC extracellular lamellar bilayers. Thus, a reduced SC pH likely represents another mechanism by which hesperidin prevents the emergence of GC-induced permeability barrier abnormalities.
Another potential mechanism that could contribute to the hesperidin-induced improvement in epidermal permeability barrier function in GC-treated skin could be its stimulation of anti-oxidant defense. Previous studies have shown that exogenous GC provokes oxidative stress. For example, dexamethasone increases NADPH oxidase gene expression and the production of reactive oxygen species in hippocampal cultures (67). Similarly, both glutathione reductase content and activity declined following addition of dexamethasone to alveolar epithelial type II-like cell cultures (53,54). The present studies show that topical GC treatment reduces epidermal glutathione reductase mRNA expression, potentially compromising epidermal barrier function, because oxidative stress is closely linked with epidermal permeability barrier function (51,52,68). Studies have demonstrated that oxidative stress can damage stratum corneum lipids and proteins (69,70), both of which are key determinants for epidermal permeability barrier (31,56). Moreover, oxidative stress inhibits the formation of stratum corneum cornified envelope, a structural protein required for permeability barrier (71,72). Furthermore, either topical or systemic applications of anti-oxidants improve epidermal permeability barrier function (72–74). Hesperidin is a well-known anti-oxidant (75,76), and the present studies show that it increases epidermal glutathione reductase expression, which would result in increased anti-oxidant capacity. Hence, the ability of hesperidin to prevent GC-induced changes in epidermal permeability function could be also ascribed to its anti-oxidant properties.
Previous studies have demonstrated that inhibition of epidermal lipid synthesis delays barrier recovery (77). Conversely, topical applications accelerate barrier recovery (78). Although it has been reported that hesperidin increases lipid production in human preadipocytes (79) and PPAR α/γ mRNA expression in rat liver (80), data from the present study do not support the involvement of either lipid synthesis or PPARα or γ in the observed hesperidin-induced changes in epidermal function. While PPARα or γ activation increases epidermal lipid production and epidermal differentiation, stimulates filaggrin, involucrin and loricrin expression, epidermal proliferation is not altered (81–83). Moreover, in the present study, only filaggrin and loricrin expression increased. This discrepancy could be due to protocol differences. While we use GC-treated murine skin, others have deployed cultured human preadipocytes (79,80). Thus, the impact of hesperidin on epidermal proliferation and differentiation likely is independent of PPARs.
Although prior studies have shown preventive benefit of hesperidin in allergic (84,85) and irritant dermatitis model (86), in the present study, we did not observe any therapeutic effect of hesperidin in irritant dermatitis model. Moreover, our results from anti-inflammatory study suggest that topical hesperidin does not attenuate anti-inflammatory benefit of GC. Furthermore, topical hesperidin unlikely interferes with the penetration of GC into skin because hesperidin was applied 1 h after GC application. It is well known that GC could induce dermatitis. Whether co-application of GC with hesperidin or hesperidin alone could prevent or treat GC-induced dermatitis is unknown.
In conclusions, the present study demonstrates that topical hesperidin prevents GC-induced alterations of epidermal permeability barrier homoeostasis and acidification, likely due to an enhancement in epidermal differentiation and proliferation. Hesperidin alone could therefore be useful for preventing GC-induced changes of epidermal function. The combination of topical hesperidin and GC could be useful in treating inflammatory dermatoses, characterised by a barrier abnormality.
Supplementary Material
Acknowledgements
Authors are grateful to Ms. Joan Wakefield for her secretary assistance. This work was supported by grants (AR019089, PME; AR051930, TM) from the National Institutes of Health and Veterans Affairs Merit Review (BX000608, PEM).
Abbreviations
- GC
glucocorticoids
- TPA
12-O-tetradecanoylphorbol-13-acetate
- SC
stratum corneum
- TEWL
transepidermal water loss
- PPAR
peroxisome proliferator-activated receptor
- HMGCoA
3-hydroxy-3-methyl-glutaryl-CoA reductase
- SPT1
serine–palmitoyl transferase 1
- FAS
fatty acid synthase
- GSR
glutathione reductase
- SOD
superoxide dismutase
- RIPA
radio-immunoprecipitation assay
Footnotes
Author contribution
GM and CC: Performed functional studies; prepared samples for morphology. TMM: Designed experiment and interpreted data; approved final version. PLK: qPCR. MH: H&E and immunohistochemical staining. YZ and RS: Western blotting and qPCR. DC: Ultrastructural studies and analysis. ANG: Data analyses. PME: Designed experiment; critically reviewed and interpreted data; approved final version. MQM: Designed experiment and wrote manuscript; made figures; approved final version.
Conflict of interest
The authors have declared no conflicting interests.
Supporting Information
Additional supporting data may be found in the supplementary information of this article:
Figure S1. Treatment procedure for anti-inflammatory study.
Figure S2. Clinical appearance of mice.
Figure S3. Topical hesperidin does not affect lipid synthetic enzyme mRNA expression in GC-damaged murine skin.
Figure S4. Topical hesperidin prevents GC-induced inhibition of epidermal proliferation in murine skin.
Table S1. Primer sequences.
References
- 1.Mentzelopoulos SD, Zakynthinos SG, Tzoufi M, et al. Arch Intern Med. 2009;169:15–24. doi: 10.1001/archinternmed.2008.509. [DOI] [PubMed] [Google Scholar]
- 2.Naldi L, Yawalkar N, Kaszuba A, et al. Am J Clin Dermatol. 2011;12:191–201. doi: 10.2165/11539780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Boscia JA, Pudi KK, Zvarich MT, et al. Clin Ther. 2012;34:1655–1666. e5. doi: 10.1016/j.clinthera.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Kim TY, Kim SB, Park SK. Clin Nephrol. 2012;78:100–105. doi: 10.5414/cn107418. [DOI] [PubMed] [Google Scholar]
- 5.Montecucco C, Todoerti M, Sakellariou G, et al. Arthritis Res Ther. 2012;14:R112. doi: 10.1186/ar3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basílio FM, Hammerschmidt M, Mukai MM, et al. An Bras Dermatol. 2012;87:767–771. doi: 10.1590/s0365-05962012000500017. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein RS. Endocrinol Metab Clin North Am. 2012;41:595–611. doi: 10.1016/j.ecl.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uçmak D, Harman M, Uçmak F, et al. Indian J Dermatol Venereol Leprol. 2013;79:211–215. doi: 10.4103/0378-6323.107638. [DOI] [PubMed] [Google Scholar]
- 9.Durmazlar SP, Oktay B, Eren C, et al. Eur J Dermatol. 2009;19:169–170. doi: 10.1684/ejd.2008.0584. [DOI] [PubMed] [Google Scholar]
- 10.Lansang MC, Hustak LK. Cleve Clin J Med. 2011;78:748–756. doi: 10.3949/ccjm.78a.10180. [DOI] [PubMed] [Google Scholar]
- 11.Liu MF, Yencha M. Otolaryngol Head Neck Surg. 2006;135:960–961. doi: 10.1016/j.otohns.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Pramick M, Whitmore SE. Int J Dermatol. 2009;48:100–101. doi: 10.1111/j.1365-4632.2009.03811.x. [DOI] [PubMed] [Google Scholar]
- 13.Tempark T, Phatarakijnirund V, Chatproedprai S, et al. Endocrine. 2010;38:328–334. doi: 10.1007/s12020-010-9393-6. [DOI] [PubMed] [Google Scholar]
- 14.Furue M, Terao H, Rikihisa W, et al. Br J Dermatol. 2003;148:128–133. doi: 10.1046/j.1365-2133.2003.04934.x. [DOI] [PubMed] [Google Scholar]
- 15.Gans EH, Sadiq I, Stoudemayer T, et al. J Drugs Dermatol. 2008;7:28–32. [PubMed] [Google Scholar]
- 16.Johnson E, Groben P, Eanes A, et al. J Midwifery Womens Health. 2012;57:296–299. doi: 10.1111/j.1542-2011.2012.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salava A, Alanko K, Hyry H. Contact Dermatitis. 2012;67:244–246. doi: 10.1111/j.1600-0536.2012.02115.x. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz C, Javvaji S, Feinberg JS. Dermatol Online J. 2012;18:11. [PubMed] [Google Scholar]
- 19.Aberg KM, Radek KA, Choi EH, et al. J Clin Invest. 2007;117:3339–3349. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi EH, Brown BE, Crumrine D, et al. J Invest Dermatol. 2005;124:587–595. doi: 10.1111/j.0022-202X.2005.23589.x. [DOI] [PubMed] [Google Scholar]
- 21.Kolbe L, Kligman AM, Schreiner V, et al. Skin Res Technol. 2001;7:73–77. doi: 10.1034/j.1600-0846.2001.70203.x. [DOI] [PubMed] [Google Scholar]
- 22.Kao JS, Fluhr JW, Man MQ, et al. J Invest Dermatol. 2003;120:456–464. doi: 10.1046/j.1523-1747.2003.12053.x. [DOI] [PubMed] [Google Scholar]
- 23.Ahn SK, Bak HN, Park BD, et al. J Dermatol. 2006;33:80–90. doi: 10.1111/j.1346-8138.2006.00018.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim HJ, Park HJ, Yun JN, et al. Immunol Res. 2011;3:96–102. doi: 10.4168/aair.2011.3.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demerjian M, Choi EH, Man MQ, et al. Exp Dermatol. 2009;18:643–649. doi: 10.1111/j.1600-0625.2009.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavker RM, Kaidbey K, Leyden JJ. J Am Acad Dermatol. 1992;26:535–544. doi: 10.1016/0190-9622(92)70076-r. [DOI] [PubMed] [Google Scholar]
- 27.Lesnik RH, Mezick JA, Capetola R, et al. J Am Acad Dermatol. 1989;21:186–190. doi: 10.1016/s0190-9622(89)70159-6. [DOI] [PubMed] [Google Scholar]
- 28.Man M, Hupe M, Mackenzie D, et al. Exp Dermatol. 2011;20:285–288. doi: 10.1111/j.1600-0625.2010.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou M, Man M, Man W, et al. Exp Dermatol. 2012;21:337–340. doi: 10.1111/j.1600-0625.2012.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proksch E, Fölster-Holst R, Jensen JM. J Dermatol Sci. 2006;43:159–169. doi: 10.1016/j.jdermsci.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Elias PM, Feingold KR. Skin Pharmacol Appl Skin Physiol. 2001;14:28–34. doi: 10.1159/000056387. [DOI] [PubMed] [Google Scholar]
- 32.Scharschmidt TC, Man MQ, Hatano Y, et al. J Allergy Clin Immunol. 2009;124:496–506. 506.e1–506.e6. doi: 10.1016/j.jaci.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mildner M, Jin J, Eckhart L, et al. J Invest Dermatol. 2010;130:2286–2294. doi: 10.1038/jid.2010.115. [DOI] [PubMed] [Google Scholar]
- 34.Williams AC, Barry BW. Adv Drug Deliv Rev. 2004;56:603–618. doi: 10.1016/j.addr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 35.Meyer OC. Angiology. 1994;45:579–584. doi: 10.1177/000331979404500614. [DOI] [PubMed] [Google Scholar]
- 36.Mao-Qiang M, Elias PM, Feingold KR. J Clin Invest. 1993;92:791–798. doi: 10.1172/JCI116652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Man MQ, Hupe M, Sun R, et al. Evid Based Complement Alternat Med. 2012;2012:912028. doi: 10.1155/2012/912028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Man M, Elias PM, Man W, et al. Exp Dermatol. 2009;18:962–968. doi: 10.1111/j.1600-0625.2009.00882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang YJ, Uchida Y, Lu B, et al. J Biol Chem. 2009;284:18942–18952. doi: 10.1074/jbc.M109.006973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holleran WM, Takagi Y, Menon GK, et al. J Clin Invest. 1993;91:1656–1664. doi: 10.1172/JCI116374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holleran WM, Takagi Y, Imokawa G, et al. J Lipid Res. 1992;33:1201–1209. [PubMed] [Google Scholar]
- 42.Holleran WM, Ginns EI, Menon GK, et al. J Clin Invest. 1994;93:1756–1764. doi: 10.1172/JCI117160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hachem JP, Crumrine D, Fluhr J, et al. J Invest Dermatol. 2003;121:345–353. doi: 10.1046/j.1523-1747.2003.12365.x. [DOI] [PubMed] [Google Scholar]
- 44.Hachem JP, Man MQ, Crumrine D, et al. J Invest Dermatol. 2005;125:510–520. doi: 10.1111/j.0022-202X.2005.23838.x. [DOI] [PubMed] [Google Scholar]
- 45.Man MQ, Hatano Y, Lee SH, et al. J Invest Dermatol. 2008;128:79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao-Qiang M, Feingold KR, Jain M, et al. J Lipid Res. 1995;36:1925–1935. [PubMed] [Google Scholar]
- 47.Mao-Qiang M, Jain M, Feingold KR, et al. J Invest Dermatol. 1996;106:57–63. doi: 10.1111/1523-1747.ep12327246. [DOI] [PubMed] [Google Scholar]
- 48.Schmuth M, Man MQ, Weber F, et al. J Invest Dermatol. 2000;115:459–466. doi: 10.1046/j.1523-1747.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 49.Fluhr JW, Mao-Qiang M, Brown BE, et al. J Invest Dermatol. 2004;123:140–151. doi: 10.1111/j.0022-202X.2004.22726.x. [DOI] [PubMed] [Google Scholar]
- 50.Mauro T, Holleran WM, Grayson S, et al. Arch Dermatol Res. 1998;290:215–222. doi: 10.1007/s004030050293. [DOI] [PubMed] [Google Scholar]
- 51.Man MQ, Elias PM. Chin J Dermatovenereol. 2013;27:407–409. [Google Scholar]
- 52.Man MQ, Elias PM. Chin J Dermatovenereol. 2013;27:722–724. [Google Scholar]
- 53.Walther UI. Arch Toxicol. 2004;78:402–409. doi: 10.1007/s00204-004-0557-0. [DOI] [PubMed] [Google Scholar]
- 54.Walther UI, Stets R. Toxicology. 2009;256:48–52. doi: 10.1016/j.tox.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Shindo Y, Witt E, Han D, et al. J Invest Dermatol. 1994;102:122–124. doi: 10.1111/1523-1747.ep12371744. [DOI] [PubMed] [Google Scholar]
- 56.Feingold KR. J Lipid Res. 2007;48:2531–2546. doi: 10.1194/jlr.R700013-JLR200. [DOI] [PubMed] [Google Scholar]
- 57.Gruber R, Elias PM, Crumrine D, et al. Am J Pathol. 2011;178:2252–2263. doi: 10.1016/j.ajpath.2011.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hou M, Sun R, Hupe M, et al. Exp Dermatol. 2013;22:210–215. doi: 10.1111/exd.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ko SY, Bae MS, Kim SW. Int J Oral Biol. 2006;31:119–125. [Google Scholar]
- 60.Acipayam C, Bayram I, Daglioglu K, et al. Med Princ Pract. 2013;00:45–52. doi: 10.1159/000355900. PMID: 24247210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elavarasan J, Velusamy P, Ganesan T, et al. J Pharm Pharmacol. 2012;64:1472–1482. doi: 10.1111/j.2042-7158.2012.01512.x. [DOI] [PubMed] [Google Scholar]
- 62.Chen MC, Ye YY, Ji G, et al. J Agric Food Chem. 2010;58:3330–3335. doi: 10.1021/jf904549s. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen T, Nioi P, Pickett CB. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Homma S, Ishii Y, Morishima Y, et al. Clin Cancer Res. 2009;15:3423–3432. doi: 10.1158/1078-0432.CCR-08-2822. [DOI] [PubMed] [Google Scholar]
- 65.Hachem JP, Roelandt T, Schürer N, et al. J Invest Dermatol. 2010;130:500–510. doi: 10.1038/jid.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hatano Y, Man MQ, Uchida Y, et al. J Invest Dermatol. 2009;129:1824–1835. doi: 10.1038/jid.2008.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.You JM, Yun SJ, Nam KN, et al. Can J Physiol Pharmacol. 2009;87:440–447. doi: 10.1139/y09-027. [DOI] [PubMed] [Google Scholar]
- 68.Vermeij WP, Alia A, Backendorf C. J Invest Dermatol. 2011;131:1435–1441. doi: 10.1038/jid.2010.433. [DOI] [PubMed] [Google Scholar]
- 69.Thiele JJ. Skin Pharmacol Appl Skin Physiol. 2001;14:87–91. doi: 10.1159/000056395. [DOI] [PubMed] [Google Scholar]
- 70.Hirao T, Takahashi M. FEBS Lett. 2005;579:6870–6874. doi: 10.1016/j.febslet.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 71.Rossi A, Catani MV, Candi E, et al. J Invest Dermatol. 2000;115:731–739. doi: 10.1046/j.1523-1747.2000.00116.x. [DOI] [PubMed] [Google Scholar]
- 72.Puch F, Samson-Villeger S, Guyonnet D, et al. Exp Dermatol. 2008;17:668–674. doi: 10.1111/j.1600-0625.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 73.Jeon HY, Kim JK, Kim WG, et al. Skin Pharmacol Physiol. 2009;22:137–141. doi: 10.1159/000201562. [DOI] [PubMed] [Google Scholar]
- 74.Gianeti MD, Mercurio DG, Maia Campos PM. Dermatol Ther. 2013;26:267–271. doi: 10.1111/j.1529-8019.2013.01552.x. [DOI] [PubMed] [Google Scholar]
- 75.de Oliveira DM, Dourado GK, Cesar TB. J Int Soc Sports Nutr. 2013;10:27. doi: 10.1186/1550-2783-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Henneberg R, Otuki MF, Furman AE, et al. Rev Bras Hematol Hemoter. 2013;35:52–55. doi: 10.5581/1516-8484.20130015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elias PM, Feingold KR. Semin Dermatol. 1992;11:176–182. [PubMed] [Google Scholar]
- 78.Man MQ, Elias P, Thornfeldt CR, et al. J Invest Dermatol. 1996;106:1096–1101. doi: 10.1111/1523-1747.ep12340135. [DOI] [PubMed] [Google Scholar]
- 79.Morikawa K, Nonaka M, Mochizuki H, et al. J Agric Food Chem. 2008;56:11030–11037. doi: 10.1021/jf801965n. [DOI] [PubMed] [Google Scholar]
- 80.Akiyama S, Katsumata S, Suzuki K, et al. Biosci Biotechnol Biochem. 2009;73:2779–2782. doi: 10.1271/bbb.90576. [DOI] [PubMed] [Google Scholar]
- 81.Kömüves LG, Hanley K, Man MQ, et al. J Invest Dermatol. 2000;115:361–367. doi: 10.1046/j.1523-1747.2000.00076.x. [DOI] [PubMed] [Google Scholar]
- 82.Mao-Qiang M, Fowler AJ, Schmuth M, et al. J Invest Dermatol. 2004;123:305–312. doi: 10.1111/j.0022-202X.2004.23235.x. [DOI] [PubMed] [Google Scholar]
- 83.Man MQ, Choi EH, Schmuth M, et al. J Invest Dermatol. 2006;126:386–392. doi: 10.1038/sj.jid.5700046. [DOI] [PubMed] [Google Scholar]
- 84.Fujita T, Shiura T, Masuda M, et al. J Nat Med. 2008;62:202–206. doi: 10.1007/s11418-007-0208-x. [DOI] [PubMed] [Google Scholar]
- 85.Nagashio Y, Matsuura Y, Miyamoto J, et al. J Funct Foods. 2013;5:1633–1641. [Google Scholar]
- 86.Koyuncu H, Berkarda B, Baykut F, et al. Anticancer Res. 1999;19:3237–3241. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.