Abstract
Objectives
This study used proton magnetic resonance spectroscopy (1H MRS) to evaluate the in vivo effects of extended-release divalproex sodium on the glutamatergic system in adolescents with bipolar disorder and to identify baseline neurochemical predictors of clinical remission.
Method
Adolescents with bipolar disorder who were experiencing a manic or mixed episode (n=25) were treated with open-label, extended-release divalproex (serum levels 85–125 mcg/mL) and underwent 1H MRS scans at baseline (prior to treatment) and on days 7 and 28. Healthy comparison subjects (n=15) also underwent 1H MRS scans at the same time points. Glutamate (Glu) and Glutamate+glutamine (Glx) concentrations were measured in three voxels: anterior cingulate cortex (ACC) and left and right ventrolateral prefrontal cortex (LVLPFC and RVLPFC) and were compared between bipolar and healthy subjects. Within the bipolar subjects, Glu and Glx concentrations at baseline and each time point were also compared between remitters and non-remitters following divalproex treatment.
Results
At baseline, no differences in Glu or Glx concentrations between bipolar and healthy subjects were observed. Group (HC vs BP) by time effects revealed an interaction for Glu in the ACC and change over time effects for Glx were noted in the ACC in patients with bipolar disorder (increase from day 0 to day 7 and then a decrease from day 7 to day 28) but not in HC. Remitters had significantly lower baseline Glx concentrations in LVLPFC and in remitters, change in LVLPFC Glu correlated with the change in YMRS score.
Conclusions
Successful treatment of mania with divalproex may be predicted by lower baseline concentrations of Glx in the LVLPFC and, in remitters, the degree of symptomatic improvement is related to the change in Glu concentrations in this region, suggesting that divalproex may work via modulation of the prefrontal glutamatergic system in youth with bipolar disorder.
Keywords: bipolar disorder, mania, Glx, glutamate, glutamine
Divalproex is frequently used in the treatment of youth with bipolar disorder,1 though clinical trials of this agent in adolescents with acute mania have produced variable response rates or failed to show statistically-significant differences from placebo.2,3 Despite its use as a mood stabilizer in both pediatric and adult patients with bipolar disorder, the mechanism by which divalproex achieves mood stabilization remains unknown. At least some of the therapeutic effects of divalproex however, may be mediated through modulation of glutamate (Glu), the primary excitatory neurotransmitter in the central nervous system, and the inhibitory neurotransmitter γ-aminobutyric acid (GABA).4 Valproate has been reported to reduce the release of GABA and decreases neuronal excitation induced by NMDA-type Glu receptors.5 Also, divalproex stimulates glutamine removal in human kidneys through accentuating the activity of glutaminase.6 In addition, pre-clinical data suggest that divalproex attenuates transport of Glu into astrocytes within the rat cerebral cortex7 and decreases the expression of the vesicular Glu transporter in the central nervous system.8 Together, the extant data suggest that valproate at least partially blocks neuronal responses to Glu, and raise the possibility that antagonism of NMDA Glu receptor–mediated neuronal excitation may be a central mechanism of valproate efficacy.
Recently, the use of proton magnetic resonance spectroscopy (1H MRS) has facilitated in vivo assessments of the glutamatergic system in patients with bipolar disorder.9,10 The majority of these 1H MRS imaging studies have evaluated concentrations of a composite of Glu, glutamine and GABA (collectively termed Glx). Glx concentrations are elevated in the left dorsolateral prefrontal cortex (DLPFC) of adult manic patients10 as well as in the left cingulate cortices of adults with bipolar disorder experiencing depressive or mixed episodes.11 In addition, other studies have observed increased Glx concentrations in the medial prefrontal cortex, parieto-occiptal cortex, insula and hippocampus in patients with bipolar disorder (during depressive episodes, manic episodes and euthymic periods) (see reference 9 for recent review).
In youth with bipolar disorder however, Glx concentrations were found to be lower in the anterior cingulate cortex (ACC) compared with healthy comparison subjects.12 In contrast, Glx concentrations in this region were higher in youth with bipolar disorder who were “stably treated” with the second generation antipsychotic, risperidone.13 Untreated children, 6–12 years of age with bipolar disorder showed increased concentrations of Glu/glutamine in both the right and left prefrontal cortex compared to control subjects.14
Although divalproex is commonly used to treat adolescents with bipolar disorder,1 its effects on the central glutamatergic system in youth remain poorly understood. With these considerations in mind, we prospectively evaluated the effects of divalproex on Glx and Glu in a group of manic adolescents with bipolar disorder. Importantly, measurement of Glu and Glx may provide valuable information—by inference— regarding the relative concentrations of other components of Glx (e.g. glutamine + GABA) as Glu is the primary component within the Glx peak.15 We hypothesized that prefrontal Glx and Glu concentrations would normalize following successful treatment with divalproex. Additionally, we examined the effects of treatment with divalproex on prefrontal Glx and Glu concentrations to determine if pre-treatment (e.g. baseline) concentrations serve as a predictor of remission following treatment with divalproex.
Method
Subjects
Twenty-five adolescents with bipolar disorder experiencing a manic or mixed episode were recruited from community referrals during a two-year period. Patients were included in the study if they were 12 to 18 years old, met DSM-IV-TR criteria for a manic or mixed episode associated with bipolar I disorder, had a screening and baseline Young Mania Rating Scale (YMRS) score ≥16 and had no treatment with an anticonvulsant, antidepressant or antipsychotic within one week of study participation. Additionally, a group of demographically similar, unmedicated, healthy comparison subjects between the ages of 12–18 years (n=15) were recruited from the same community.
Adolescents provided written assent and their parents/legal guardians provided written informed consent after study procedures were fully explained. This study was approved by the University of Cincinnati Institutional Review Board. After providing informed consent and assent for participation, diagnoses were established using the Washington University at St. Louis Kiddie-Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS)16 by trained individuals with established diagnostic reliability (kappa = 0.94).17 All diagnoses were reviewed by a child and adolescent psychiatrist (MPD, JN). Subjects were excluded by an IQ <70 (determined by Weschsler Abbreviated Scale of Intelligence [WASI] which was administered by trained psychometrists), a diagnosis of substance abuse during the past 90 days or lifetime history of substance dependence, unstable medical or neurologic illness, pregnancy or nursing, a history of sensitivity or intolerance to divalproex, or a contraindication to having a 1H MRS scan. In addition, psychopathology and treatment in first degree relatives of the healthy comparison subjects, was assessed using the Family History Research Diagnostic Criteria;18 no healthy subject was included if there was a family history of mood, anxiety or psychotic disorder in any first degree relative.
Efficacy and tolerability measures
Symptoms were rated using the YMRS,19 the Children's Depression Rating Scale-Revised (CDRS-R),20 and the Clinical Global Impressions-Improvement (CGI-I) and Severity (CGI-S) scales. Efficacy, safety and tolerability ratings were obtained at baseline and days 7 and 28, or study termination. Serum valproic acid levels were obtained at days 7 and 28.
Divalproex Dosing
After meeting all study criteria, subjects with bipolar disorder received an initial dose of extended-release divalproex (15 mg/kg, maximum starting dose 750 mg). The dose of extended-release divalproex was adjusted by a child and adolescent psychiatrist to achieve a serum valproic acid level of 85–125 mcg/mL. No concomitant medications, including psychostimulants, were permitted at any point during the study.
Proton Magnetic Resonance Spectroscopy (1H MRS)
All subjects, including healthy subjects, underwent 1H MRS scans at baseline (prior to receiving medication for subjects with bipolar disorder) and days 7 and 28, or study termination. All MRI and MRS data were acquired on a Varian 4T whole-body scanner, for improved resolution and increased signal-to-noise ratio, in the Center for Imaging Research at the University of Cincinnati. A 1H TEM (Transverse ElectroMagnetic) volume head coil was used as a transmitter and receiver. A multi-slice axial image was initially acquired for patient positioning and MRS voxel positioning. The axial image was followed by the acquisition of a 3-D whole head MRI using MDEFT (Modified Driven Equilibrium Fourier Transform) pulse sequence.21 The MDEFT image was acquired in the axial orientation with repetition time (TR) 13.1 ms, echo time (TE) 6 ms, magnetization preparation time (TMD) 1.1 ms, data matrix = 256×192×192, field of view (FOV) 256×192×150 mm, slab thickness 150 mm and 32 segments. Three MRS voxels (8 cc in volume) were positioned, one in the ACC, predominately gray matter) and one each in the left and right ventral lateral prefrontal cortex (LVLPFC and RVLPFC, predominately white matter) (Figure 1A–C). Specifically, the ACC and VLPFC voxels were chosen because these regions are involved in executive function, regulation of affect and emotional processing—functions which are impaired during acute mania. A spectroscopist, blind to each subject's diagnosis, positioned the voxels to ensure consistent placement. Each of the three voxels was positioned at the same level to avoid signal artifacts from the orbits. The level was chosen such that the inferior portion of the lateral prefrontal white matter voxels would not include orbital gyri. The ACC voxel was placed in the midline and abutted posteriorly upon the anterior portion of the corpus callosum (Figure 1A); this voxel included the orbital frontal, middle frontal, and cingulate gyri, which corresponds to Brodmann areas 9, 10, 24, 32, and 47.22,23 Both left and right VLPFC voxels were placed at the same superior-inferior position as the ACC voxel, and all of these voxels were positioned lateral to Brodmann areas 44, 45, and 46 (Figure 1B and 1C). After MRS voxel positioning, the magnetic field homogeneity was optimized using FASTMAP (Fast Automatic Shimming Technique by Mapping Along Projections).24 A typical linewidth at half height of water signal in the MRS voxel was 10-12 Hz. Three single-voxel PRESS (Point RESolved Spectroscopy) spectra were collected in the ACC, LVLPFC and RVLPFC (Figure 1D). The spectra were acquired with repetition time (TR) 3000 ms, echo time (TE) 23 ms, voxel size 8cc and 128 averages with water suppression by VAPOR (VAriable Pulse powers and Optimizing Relaxation delays) method.25 For computations of Glx levels and eddy current correction, one reference spectrum without water suppression was collected at the same voxel positions with the same parameters except included four averages and reduced receiver gain.
Figure 1.
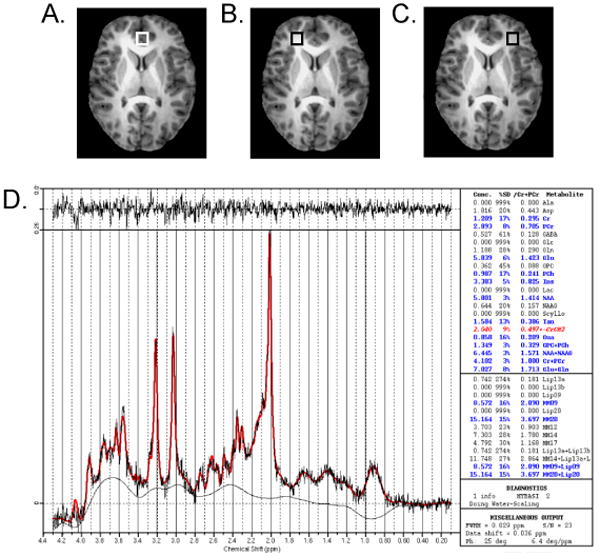
Placement of proton magnetic resonance spectroscopy (1H MRS) voxel for anterior cingulate (A) and left (B) and right (C) ventrolateral prefrontal cortex. Note: In addition, a representative spectrum (anterior cingulate cortex [ACC] in a healthy subject) and its Linear Combination (LC) Model fitting output are shown (D).
The tissue contents within MRS voxels were determined by MDEFT images using a contrast-driven algorithm in SPM (Statistical Parametrical Mapping; http://www.fil.ion.ucl.ac.uk/spm/) software. The tissue segmentation data were presented as percentage of gray matter (%GM), white matter (%WM) and cerebrospinal fluid (%CSF).
Glx levels were determined by analyzing spectra using software LCModel (Linear Combination of Model spectra) with the water reference in unsuppressed-water spectra.26 All metabolites levels except Glx and Glu were corrected based on tissue content in each voxel, and the T1 and T2 loss. In addition, the differences of water concentrations, T1 and T2 relaxation times in gray matter, white matter and CSF were taken into consideration for the computation and metabolite levels are presented in absolute concentrations (mM). For completeness, the concentrations of myo-inositol (mI, a sugar involved in cellular second messenger signaling and potential marker of the phosphoinositide cycle), choline (Cho, a marker for membrane phospholipid metabolism), N-acetyl aspartate (NAA, a marker of neuronal viability) and creatine (Cr) were also examined within each voxel. Cramer-Rao standard deviations for any neurometabolite level which exceeded 20% were excluded.
Statistical Analysis
Changes in symptom scores over time were examined in both groups using mixed-model repeated measures analysis of variance implemented in SAS Version 9.2 (SAS Institute, Inc.). For bipolar subjects, rates of response and remission to divalproex treatment were determined. Clinical response was defined as a decrease of 50% or more in baseline YMRS scores. Clinical remission was defined by an endpoint YMRS score of ≤12 and an endpoint CGI-I score ≤2.
Two-sided student's t-tests were used to compare baseline Glx, Glu and other metabolite concentrations between bipolar and healthy subjects. Group by time repeated measures ANOVAs were used to compare change in metabolite levels over time between bipolar vs. healthy comparison adolescents. Post-hoc repeated measures analyses of variance were used to examine within group changes over time in Glx, Glu and other metabolite concentrations in each of the three voxels of interest (ACC, RVLPFC, LVLPFC). Post-hoc t-tests were performed for each metabolite at each time period. Baseline concentrations in each region-of-interest were compared between remitters and non-remitters using analysis of covariance. Furthermore, changes in Glx, Glu and other metabolite concentrations over time were compared between remitters and non-remitters using a repeated measures analysis of variance. We conducted similar analyses for other metabolites (e.g. Cr, NAA, mI). For these exploratory analyses of other metabolite levels, we corrected for multiple comparisons with a significance threshold of 0.004 (4 metabolites × 3 regions = 12, p = 0.05/12 or 0.004).
Results
Characteristics of Bipolar and Healthy Comparison Subjects
The groups were well matched on demographic data (Table 1). Twenty-five (100%) bipolar subjects received a baseline scan. Twenty-two (88%) of the bipolar subjects received scans on days 7 and 28; one subject withdrew from the study after the baseline scan because of hospitalization for suicidal ideation and two subjects were lost to follow-up prior to day 7. Rates of co-morbid psychiatric disorders did not differ between patients who were classified as remitters and non-remitters for anxiety disorders (p=0.4), attention deficit hyperactivity disorder (ADHD) (p=1), disruptive behavior disorders (p=0.4) or substance use disorders (p=0.3) (Table 1).
Table 1.
Comparison of demographic and clinical variables among bipolar subjects who were divalproex remitters (n=7), non-remitters (n=18), and healthy comparison subjects (n=15).
| Variable | BP remitters | BP non-remitters | Healthy comparison subjects |
|---|---|---|---|
| (n=7) | (n=18) | (n=15) | |
| Age, mean ± SD | 15.4±1 | 14.1±2.2 | 14.4±1.6 |
| Sex, males, N (%) | 4 (57) | 6 (33) | 6 (40) |
| Race, Caucasian, N (%) | 7 (100) | 13 (72) | 12 (80) |
| Tanner Stage | |||
| Breast | 4±0.9 | 3.7±1 | 3.7±0.7 |
| Pubic | 3.8±0.8 | 3.6±1 | 3.7±0.7 |
| YMRS, mean (SD) | N/A | ||
| Day 0 (baseline) | 25±8 | 28±8 | |
| Day 7 | 15±8 | 22±9 | |
| Day 28 (end point) | 6±8 | 21±9 | |
| CDRS-R, mean (SD) | N/A | ||
| Day 0 (baseline) | 31± 9 | 37.3±9 | |
| Day 7 | 31±9 | 32±9 | |
| Day 28 (end point) | 23±9 | 35±10 | |
| Co-morbid conditions | |||
| ADHD (current) | 4 | 11 | 0 |
| Anxiety disorders | 2 | 4 | 0 |
| Disruptive behavior disorders | 3 | 5 | 0 |
| Substance abuse | 1 (hypnotic) | 1 (hypnotic) | 0 |
| Prior treatment | |||
| SSRI | 2 (29%) | 2 (11%) | None |
| Stimulant | 0 (0%) | 6 (33%) | None |
| Benzodiazepine | 0 (0%) | 0 (0%) | None |
| SGA | 1 (14%) | 7 (39%) | None |
| Lithium | 0 (0%) | 2 (11%) | None |
| Segmentation (ACC) | |||
| Percent gray matter (%) | 70±7 | 69±8 | 75±8 |
| Percent white matter (%) | 9±4 | 12±11 | 7±5 |
| Percent CSF (%) | 22±6 | 19±9 | 18±7 |
| Segmentation (LVLPFC) | |||
| Percent gray matter (%) | 31±12 | 38±12 | 35±9 |
| Percent white matter (%) | 67±15 | 60±14 | 65±10 |
| Percent CSF (%) | 3±4.5 | 2±3 | 1±1 |
| Segmentation (RVLPFC) | |||
| Percent gray matter (%) | 34±12 | 34±10 | 37±12 |
| Percent white matter (%) | 63±15 | 64±11 | 62±13 |
| Percent CSF (%) | 3±4 | 2±1 | 1±1 |
Note: ACC = anterior cingulate cortex; ADHD = Attention Deficit-Hyperactivity Disorder; BP = bipolar; CDRS-R = Children's Depression Rating Scale-Revised; CSF = cerebrospinal fluid; LVLPFC = left ventrolateral prefrontal cortex; RVLPFC = right ventrolateral prefrontal cortex; SGA = second generation antipsychotic; SSRI = Selective Serotonin Reuptake Inhibitor; YMRS = Young Mania Rating Scale.
Symptom Rating Measures
YMRS and CGI-S scores significantly decreased over time (F(2,42.1)=12.6, p<0.001 and F(2,41.9)=12.2, p<0.001, respectively), while the CDRS-R score did not significantly change over time (F(2,40.7)=2.1, p=0.1) for the bipolar group. (Figure 2). Eleven (44%) subjects were responders, while seven (28%) were considered remitters (Table 1).
Figure 2.
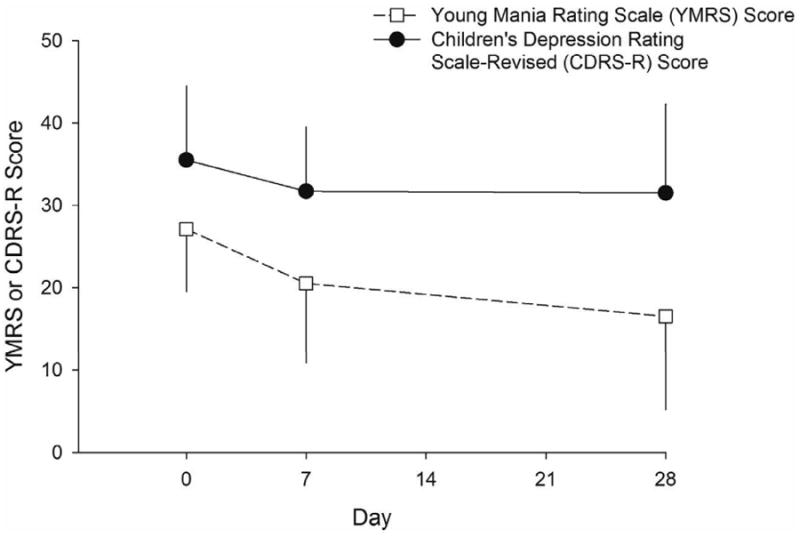
Mean Young Mania Rating Scale (YMRS) scores significantly improved from baseline (F(2,42.1)=12.6, p<0.001) over the course of 28 days of treatment with extended release divalproex in bipolar adolescents experiencing an acute manic or mixed episode. Note: However, in these patients, Children's Depression Rating Scale - Revised (CDRS-R) did not significantly change over the course of treatment (F(2,40.7)=2.1, p=0.1). Error bars represent standard deviations.
Tolerability
Adverse events that were classified as related to divalproex and which occurred at a rate >10% were: headaches (n=4, 15%), nausea (n=4, 15%), abdominal pain (n=4, 15%), and vomiting (n=3, 11%).
Glu and Glx Concentrations
At baseline, patients with bipolar disorder did not differ from healthy comparison subjects in Glu concentrations in the ACC (healthy comparison subjects: 5.0±1.2 ppm, bipolar patients: 5.6±1.1, p = 0.16), the LVLPFC (healthy comparison subjects: 5.7±1.1, bipolar patients: 5.7±0.8, p = 0.96) or the RVLPFC (healthy comparison subjects: 5.3±1.5, bipolar patients: 5.1±1.3, p = 0.56). Similarly, Glx concentrations did not differ between the two groups in the ACC (healthy comparison subjects: 7.5±2, bipolar patients: 7.8±2, p = 0.76), in the LVLPFC (healthy comparison subjects: 7.9±2, bipolar patients: 7.0±2, p = 0.27) or in the RVLPFC (healthy comparison subjects: 7.7±2.5, bipolar patients: 7.3±2, p = 0.5).
Group by time effects were observed for Glu concentrations in the ACC (p = 0.01), but were not observed in the LVLPFC (p=0.52), or in the RVLPFC (p = 0.89). No group by time effects were noted for Glx in any of the regions examined (ACC, p = 0.3; LVLPFC, p = 0.29; RVLPFC, p = 0.28).
Significant change over time effects were observed for Glu in the ACC in healthy comparison subjects in the ACC (day 0 = 5; day 7 = 4.7; day 28 = 5.9; p = 0.05) but were not observed in the LVLPFC (p=0.46) or RVLPFC (p=0.45). No significant change over time effects were observed for Glu in the patients with bipolar disorder in any region (ACC: p = 0.49; LVLPFC: p = 0.5; RVLPFC: p = 0.24). Change over time effects were not observed for Glx in any region in the healthy comparison subjects (ACC: p = 1; LVLPFC: p = 0.27; RVLPFC: p = 0.4); however, a change over time effect was noted for Glx in the ACC in patients with bipolar disorder (p = 0.04) (in whom Glx concentrations increase from day 0 (7.8) to day 7 (9.1) and then decreased by day 28 (7.8). However, similar effects were not observed in the LVLPFC (p = 0.5) or RVLPFC (p = 0.46).
Endpoint (day 28) valproic acid concentrations positively correlated with the endpoint Glu concentrations in the LVLPFC (r=0.47, p<0.05), but did not correlate with the Glu or glx concentrations in the ACC (Glu: r=-0.24, p=0.3; Glx: r=-0.2, p = 0.4) or RVLPFC (Glu: r=0.02, p=0.9; Glx: r=-0.05, p=0.8). Also, no relationship was observed between endpoint valproic acid concentration and LVLPFC concentrations of Glx (0.26, p=0.3). Finally, no statistically significant relationships were observed between endpoint valproic acid concentrations and the change in Glx or Glu from day 0 to day 28 in the ACC (Glu: r=-0.2, p=0.3; Glx: r=-0.06, p=0.8), LVLPFC (Glu: r=-0.4, p=0.2; Glx: r=-0.2, p=0.6) or RVLPFC (Glu: r=0.3, p=0.4; Glx: r=-0.2, p=0.6).
In patients with bipolar disorder change in Glu was not associated with change in YMRS score in any of the voxels of interest (ACC: r = 0.17, p = 0.4; LVLPFC: 0.14, p = 0.5; RVLPFC r = 0.26, p = 0.27). Similarly, change in Glx was not correlated with change in YMRS in the ACC (r = 0.19, p = 0.36) but trended towards significance in the LVLPFC (r = -0.39, p = 0.06)) and was significant in the RVLPFC (r = 0.55, p = 0.01).
Predictors of Remission: Glu and Glx
Bipolar subjects who experienced remission following treatment with divalproex did not differ in terms of any demographic variables from those who did not (Table 1). Baseline Glu concentrations in the LVLPFC trended towards lower values in the remitters compared to non-remitters (5.0+0.8 vs. 5.3+0.7; p = 0.09, Cohen's d = 0.57) but were similar between remitters and non-remitters in the ACC (p = 0.56) and RVLPFC (p=0.9). Lower baseline Glx concentrations in the LVLPFC were observed in non-remitters [5.5±1.9 vs. 7.7±1.8; p = 0.01, Cohen's d = 0.9] (Figure 3). Baseline Glx concentrations in the ACC (p = 0.53) and RVLPFC (p = 0.73) did not differ between remitters and non-remitters.
Figure 3.
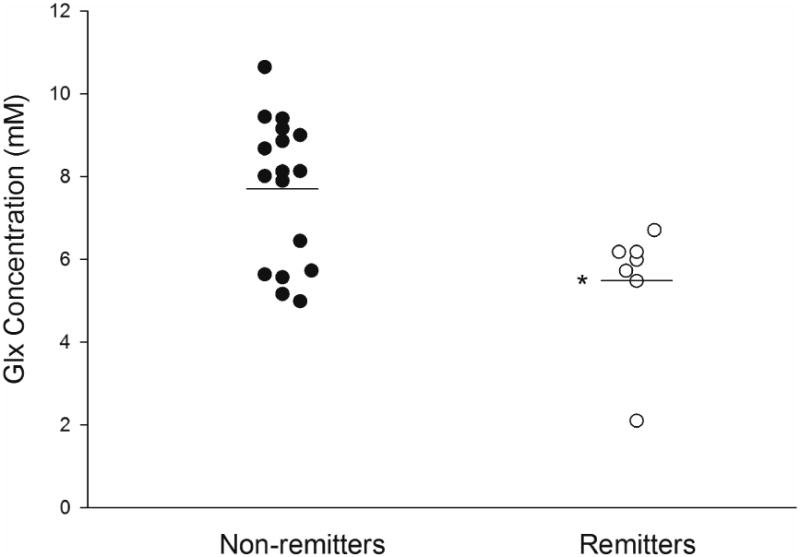
Left ventrolateral prefrontal cortex (LVLPFC) concentrations of glutamate/glutamine (Glx) were significantly lower in patients who experienced remission (p<0.005) and no patient with a LVLPFC Glx concentration >6.7 mM was classified as a remitter. Note: Horizontal bars represent the means for both groups.
No group (remitters vs. non-remitters) by time effects were observed for Glu in the ACC (p = 0.56), LVLPFC (p = 0.88) or RVLPFC (p = 0.22). Similarly, no group by time effects were observed for Glx concentrations in the ACC (p = 0.39), LVLPFC (p = 0.57) or RVLPFC (p = 0.06). Change over time effects were not observed for Glu concentrations in the remitters or non-remitters in the ACC (remitters: p = 0.93; non-remitters: p = 0.26), LVLPFC (remitters: p = 0.98; non-remitters: p = 0.5). However, a significant change over time effect was observed for Glu in the RVLPFC (remitters: baseline = 5.2; day 7 = 6.1; day 28 = 4.4, p = 0.05; non-remitters: p = 0.7). Change over time effects were observed for Glx in the ACC in the non-remitters (baseline = 7.6; day 7 = 9.3; day 28 = 7.4, p = 0.03;), but not in the remitters (p = 0.94). In addition, change over time effects were not observed for Glx in the LVLPFC for either remitters or non-remitters (remitters: p = 0.32; non-remitters: p = 0.91), whereas there was a change over time effect for Glx in the RVLPFC in (remitters: p = 0.05; non-remitters: p = 0.8).
Finally, in the remitters, change in YMRS score was correlated with change in LVLPFC Glu concentrations (r = 0.82, p = 0.03), but no similar association was noted in the ACC or RVLPFC in either remitters or non-remitters. There were no significant associations between change in Glx and change in YMRS score in any of the pre-frontal voxels in either remitters or non-remitters.
Other Metabolites
No differences in baseline concentrations of choline were observed between the healthy comparison subjects and the bipolar patients in the ACC (p = 0.33), LVLPFC (p = 0.67) or RVLPFC (p = 0.52), nor did baseline creatine concentrations differ in the ACC (p = 0.18), LVLPFC (p = 0.65) or RVLPFC (p = 0.28). NAA concentrations did not differ between the patients with bipolar disorder compared to healthy subjects (ACC: p = 0.02; LVLPFC: p = 0.56; or RVLPFC: p = 0.33) nor did mI concentrations differ between patients and the healthy comparison subjects (ACC: p = 0.55; LVLPFC: p = 0.38; or RVLPFC: p = 0.87).
Group (patient vs. healthy subjects) by time effects were not observed for NAA, choline, creatine, or mI levels in the ACC, LVLPFC or RVLPFC. Remitters and non-remitters did not differ in their baseline concentrations of creatine, choline, NAA or mI in the ACC, LVLPFC or RVLPFC. No group by time or time effects with in each group were observed for remitters or non-remitters for any of the metabolites in any of the ROIs.
Discussion
This study is the first to prospectively examine the longitudinal effects of divalproex on the neurochemistry of bipolar disorder in adolescents with mania or mixed mania and raise the possibility that pre-treatment Glx concentrations in the LVLPFC could potentially predict remission following short-term treatment with divalproex. Moreover, these data suggest that adolescents experiencing a manic or mixed episode might not respond to divalproex if their baseline LVLPFC Glx is above a threshold concentration of 6.7 mM. Importantly, Glx concentrations reflect contributions of the excitatory neurotransmitters Glu, glutamine and the inhibitory neurotransmitter GABA; as such, a shift in any single component of Glx could be responsible for this predictive value of pre-treatment Glx concentrations. However, we did not note significant differences in pre-treatment Glu concentrations in any of the prefrontal voxels suggesting that the potential predictive value of pre-treatment Glx may relate to a disturbance in either glutamine or GABA or in the homeostatic equilibrium of Glu and glutamine. Of note, divalproex increases concentrations of GABA in lower animals and also attenuates neuronal excitation via NMDA-type Glu receptors in vitro.5 Therefore, it is possible divalproex may affect one or multiple components of Glx in youth with bipolar disorder. Last, neurochemical differences were observed between remitters and non-remitters in the LVLPFC, which is closely connected with the amygdala, a structure that has been reported to be hyperactivated in adolescents with bipolar disorder.27
Our findings also suggest that endpoint valproic acid concentrations are related to endpoint Glu concentrations in the LVLPFC (although similar relationships do not exist between endpoint valproic acid concentrations and Glu in the RVLPFC or ACC) and that, in remitters, the change in Glu concentrations within this region is related to the change in YMRS score. In addition, these findings suggest that the absolute concentrations of Glx in these prefrontal regions may not be altered by acute treatment with divalproex. This finding contrasts with studies of the neurochemical effects of second generation antipsychotics (e.g. risperidone) in these brain regions in bipolar youth.12 Accumulating evidence suggests that mood stabilizers/antiepileptic medications may be less effective than second generation antipsychotics in the treatment of bipolar disorder. Specifically, Correll and colleagues,28 in a recent meta-analysis of mood stabilizers/antiepileptic drugs for mania, found that the effect sizes (a surrogate of efficacy) for mood stabilizers/antiepileptic drugs were notably lower (Cohen's d effect size: 0.2) than second generation antipsychotics (Cohen's d effect size: 0.65). Whether the differential effect of the two classes of medications on brain neurochemistry relates to differences in efficacy remains to be determined.
There are several limitations to this investigation. First, the present study included only adolescents with YMRS scores >16 and was conducted with only outpatients, thus generalizability to youth with more severe manic symptoms and perhaps more functional impairment requiring hospitalization might be limited. Second, neurochemical effects may have occurred in regions that were not examined in this study, such as other components of the anterior limbic network and cerebellar vermis.23 Nonetheless, the ACC and VLPFC voxels examined in this study were chosen because they are involved in executive function, regulation of affect and emotional processing—functions which are impaired during acute mania. Third, in this study, we did not resolve all of the individual components of Glx (except Glu) and therefore, we were unable to determine what single component of Glx (e.g. Gln or GABA) accounted for the potential predictive value of pre-treatment Glx concentrations. Nonetheless, we found baseline Glx levels in the LVLPFC are significantly different between the two groups (with Glu levels in this region trending towards lower values). Accordingly, as Glx is a summed term of Glu and glutamine with trace amounts of GABA,15 it is reasonable to suggest that the differences in Glx concentrations are dominated by the contribution of glutamine or GABA. However, to verify this, the accurate measurement of glutamine and GABA levels using different MRS pulse sequences in future studies is necessary. It is likely that this may be accomplished with several emerging MRS methods such as constant-time PRESS (CT-PRESS), echo-time-averaged PRESS (TE-averaged-PRESS) and STEAM (stimulated echo acquisition mode), which may resolve the Glu, Gln, and GABA contribution to Glx signals.29-31 Last, although there were too few subjects in our sample to examine the impact of prior depressive episodes and co-occurring anxiety, future studies assessing the effects of these factors, as well as family history of mood disorders on treatment response are needed.
To date, several clinical and historical factors have been suggested as predictors of poor response to divalproex in youth with bipolar disorder, including high baseline CGI-Severity scores, comorbid conduct disorder, concurrent psychotic symptoms;32 however, we are aware of no previously reported neurochemical markers of treatment response or remission. Thus, the potential existence of biochemical predictors of treatment remission in pediatric bipolar disorder, described herein, is encouraging and highlights the need for additional studies, especially those which can better resolve the individual components of Glx. Most studies have examined the neurochemical effects of lithium, rather than antiepileptic drugs and second generation antipsychotics.33 It is likely that as 1H MRS techniques advance and greater field strengths become increasingly available with associated improvement the spectral resolution of metabolites, additional neurochemical predictors of treatment response will become available for these commonly used medications. Certainly future studies will need to consider potential differences in the pathophysiology of pediatric and adult bipolar disorder, developmental differences which affect the course of the condition in adolescents and and the mood state of the patient.
Acknowledgments
This work was supported by a grant from the Thrasher Research Fund.
Disclosure: Dr. Strawn has received research support from The American Academy of Child and Adolescent Psychiatry, Eli Lilly and Co., and Shire. Dr. Adler served as a speaker for Johnson and Johnson, and Merck. Dr. Strakowski has served as a consultant to Pfizer and Consensus Medical Communications, and has served on the speakers' bureau for Adamed and CME Outfitters. Dr. DelBello served as a consultant, adviser, or on the speakers' bureau for Eli Lilly and Co., GlaxoSmithKline, Bristol-Myers Squibb, Merck, Alexza, and Consensus Medical Communications. Additionally, The Division of Bipolar Disorders Research (SS, MD, CA, and JS) has received research support from AstraZeneca, Eli Lilly and Co., Johnson and Johnson, Shire, Janssen, Johnson and Johnson, Pfizer, Bristol-Myers Squibb, Repligen, Martek, Somerset, Sumitomo, Nutrition 21, and GlaxoSmithKline.
Footnotes
Drs. Chu, Kim, Lee, Patel, Nandagopal, and Welge, and Ms. Bryan, Mr. Alfieri, and Mr. Blom report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhangoo RK, Lowe CH, Myers FS, Treland J, Curran J, Towbin KE, Leibenluft E. Medication use in children and adolescents treated in the community for bipolar disorder. J Child Adolesc Psychopharmacol. 2003;13:515–522. doi: 10.1089/104454603322724904. [DOI] [PubMed] [Google Scholar]
- 2.Kowatch RA, Suppes T, Carmody TJ, Bucci JP, Hume JH, Kromelis M, Emslie GJ, Weinberg WA, Rush AJ. Effect size of lithium, divalproex sodium, and carbamazepine in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2000;39:713–720. doi: 10.1097/00004583-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Wagner KD, Redden L, Kowatch RA, Wilens TE, Segal S, Chang K, Wozniak P, Vigna NV, Abi-Saab W, Saltarelli M. A double-blind, randomized, placebo-controlled trial of divalproex extended-release in the treatment of bipolar disorder in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:519–532. doi: 10.1097/CHI.0b013e31819c55ec. [DOI] [PubMed] [Google Scholar]
- 4.Kowatch RA, DelBello MP. The use of mood stabilizers and atypical antipsychotics in children and adolescents with bipolar disorders. CNS Spectr. 2003;8:273–280. doi: 10.1017/s1092852900018484. [DOI] [PubMed] [Google Scholar]
- 5.Löscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol. 1999;58:31–59. doi: 10.1016/s0301-0082(98)00075-6. [DOI] [PubMed] [Google Scholar]
- 6.Martin G, Durozard D, Besson J, Baverel G. Effect of the antiepileptic drug sodium valproate on glutamine and glutamate metabolism in isolated human kidney tubules. Biochim Biophys Acta. 1990;1033:261–6. doi: 10.1016/0304-4165(90)90130-o. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson M, Hansson E, Rönnbäck L. Interactions between valproate, glutamate, aspartate, and GABA with respect to uptake in astroglial primary cultures. Neurochem Res. 1992;17:327–332. doi: 10.1007/BF00974573. [DOI] [PubMed] [Google Scholar]
- 8.Kang TC, Kim DS, Kwak SE, Kim JE, Kim DW, Kang JH, Won MH, Kwon OS, Choi SY. Valproic acid reduces enhanced vesicular glutamate transporter immunoreactivities in the dentate gyrus of the seizure prone gerbil. Neuropharmacology. 2005;49:912–21. doi: 10.1016/j.neuropharm.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Yüksel C, Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michael N, Erfurth A, Ohrmann P, Gössling M, Arolt V, Heindel W, Pfleiderer B. Acute mania is accompanied by elevated glutamate/glutamine levels within the left dorsolateral prefrontal cortex. Psychopharmacology (Berl) 2003;168:344–346. doi: 10.1007/s00213-003-1440-z. [DOI] [PubMed] [Google Scholar]
- 11.Dager SR, Friedman SD, Parow A, Demopulos C, Stoll AL, Lyoo IK, Dunner DL, Renshaw PF. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry. 2004;61:450–458. doi: 10.1001/archpsyc.61.5.450. [DOI] [PubMed] [Google Scholar]
- 12.Moore CM, Frazier JA, Glod CA, Breeze JL, Dieterich M, Finn CT, Frederick B, Renshaw PF. Glutamine and glutamate levels in children and adolescents with bipolar disorder: a 4.0-T proton magnetic resonance spectroscopy study of the anterior cingulate cortex. J Am Acad Child Adolesc Psychiatry. 2007a;46:524–534. doi: 10.1097/chi.0b013e31802f5f2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore CM, Biederman J, Wozniak J, Mick E, Aleardi M, Wardrop M, Dougherty M, Harpold T, Hammerness P, Randall E, Lyoo IK, Renshaw PF. Mania, glutamate/glutamine and risperidone in pediatric bipolar disorder: a proton magnetic resonance spectroscopy study of the anterior cingulate cortex. J Affect Disord. 2007b;99:19–25. doi: 10.1016/j.jad.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castillo M, Kwock L, Courvoisie H, Hooper SR. Proton MR spectroscopy in children with bipolar affective disorder: preliminary observations. Am J Neuroradiol. 2000;21:832–838. [PMC free article] [PubMed] [Google Scholar]
- 15.Mangia S, Tkaac I, Gruetter R, Van De Moortele PF, Giove F, Maraviglia B, Urgaurbil K. Sensitivity of single-voxel 1H-MRS in investigating the metabolism of the activated human visual cortex at 7 T. Magnetic Resonance Imaging. 2006;24:343–348. doi: 10.1016/j.mri.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, DelBello MP, Soutullo C. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 17.DelBello MP, Schwiers ML, Rosenberg HL, Strakowski SM. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2002;41:1216–1223. doi: 10.1097/00004583-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria: reliability and validity. Arch Gen Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- 19.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 20.Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children's depression rating scale. J Am Acad Child Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, Ugurbil K. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med. 1995;34:308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- 22.Cecil KM, DelBello MP, Morey R, Strakowski SM. Frontal lobe differences in bipolar disorder as determined by proton MR spectroscopy. Bipolar Disord. 2002;4:357–365. doi: 10.1034/j.1399-5618.2002.02235.x. [DOI] [PubMed] [Google Scholar]
- 23.Cecil KM, DelBello MP, Sellars MC, Strakowski SM. Proton magnetic resonance spectroscopy of the frontal lobe and cerebellar vermis in children with a mood disorder and a familial risk for bipolar disorders. J Child Adolesc Psychopharmacol. 2003;13:545–555. doi: 10.1089/104454603322724931. [DOI] [PubMed] [Google Scholar]
- 24.Gruetter R, Boesch C. Fast, noniterative shimming of spatially localized signals. In vivo analysis of the magnetic field along axes. J Magn Reson. 1992;96:323–334. [Google Scholar]
- 25.Tkac I, Staruck Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1ms echo time. Magn Reson Med. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Provincher SW. Estimation of metabolite concentration from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 27.Kalmar JH, Wang F, Chepenik LG, Womer FY, Jones MM, Pittman B, Shah MP, Martin A, Constable RT, Blumberg HP. Relation between amygdala structure and function in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:636–642. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Correll CU, Sheridan EM, DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 2010;12:116–141. doi: 10.1111/j.1399-5618.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- 29.Mayer D, Spielman DM. Detection of Glutamate in the Human Brain at 3T Using Optimizing Constant Time Point Resolved Spectroscopy. Magnetic Resonance in Medicine. 2005;54:439–442. doi: 10.1002/mrm.20571. [DOI] [PubMed] [Google Scholar]
- 30.Hu J, Yang S, Xuan Y, Jian Q, Yang Y, Haacke EM. Simultaneous detection of resolved glutamate, glutamine, and γ-aminobutyric acid at 4T. Journal of Magnetic Resonance. 2007;185:204–213. doi: 10.1016/j.jmr.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang S, Hu J, Kou Z, Yang Y. Spectral simplification for resolved glutamate and glutamine measurement using a standard STEAM sequence with optimized timing parameters at 3, 4, 4.7, 7, and 9.4T. Magnetic Resonance in Medicine. 2008;59:236–244. doi: 10.1002/mrm.21463. [DOI] [PubMed] [Google Scholar]
- 32.Masi G, Perugi G, Millepiedi S, Mucci M, Pfanner C, Berloffa S, Pari C, Gagliano A, D'Amico F, Akiskal HS. Pharmacological response in juvenile bipolar disorder subtypes: A naturalistic retrospective examination. Psychiatry Res. 2010;177:192–198. doi: 10.1016/j.psychres.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 33.DelBello MP, Strakowski SM. Neurochemical predictors of response to pharmacologic treatments for bipolar disorder. Curr Psychiatry Rep. 2004;6:466–472. doi: 10.1007/s11920-004-0012-1. [DOI] [PubMed] [Google Scholar]


