Abstract
The retina drives various non-image-forming photoresponses including circadian photoentrainment and pupil constriction. Previous investigators showed that in humans, photic suppression of the clock-controlled hormone melatonin is most sensitive to 460-nm blue light, with a threshold of ~12 log photons cm−2 s−1. This threshold is surprising because non-image-forming vision is mediated by intrinsically photosensitive retinal ganglion cells (ipRGCs), which receive rod-driven synaptic input and can respond to light levels as low as ~7 log photons cm−2 s−1. Using a protocol that enhances data precision, we have found the threshold for human melatonin suppression to be ~10 log photons cm−2 s−1 at 460 nm. This finding has far-reaching implications since there is mounting evidence that nocturnal activation of the circadian system can be harmful.
Keywords: Pineal, melatonin, ipRGC, circadian photoentrainment, retinal ganglion cell, light, human, threshold
The visual system mediates not only pattern vision but also non-image-forming photoresponses including pupillary reflexes, entrainment of circadian rhythms to the light/dark cycle, and modulation of hormone secretion. Because excessive night-time photic stimulation of this system is harmful (Bedrosian and Nelson, 2013; Amaral et al., 2014), it is important to ascertain the intensity threshold of human non-image-forming vision. To this end, researchers have assessed the photosensitivity of the circadian pathway in which retinal neurons signal through the suprachiasmatic nucleus (SCN) to the pineal gland, which secretes melatonin during subjective night. Melatonin secretion can be suppressed acutely by light and earlier work found such suppression to be most sensitive to 460-nm light, with a threshold of ~12 log photons cm−2 s−1 (Brainard et al., 2001; Thapan et al., 2001). This threshold is surprisingly high because retinal input to the SCN is now known to be mediated by intrinsically photosensitive retinal ganglion cells (ipRGCs), which receive excitatory input from rod photoreceptors and can respond robustly to intensities as low as ~7 log photons cm−2 s−1 (Dacey et al., 2005). Mouse behavioral studies have likewise demonstrated a rod contribution to circadian photoentrainment (Altimus et al., 2010; Lall et al., 2010; Butler and Silver, 2011; Morin and Studholme, 2011). These new findings prompted us to re-examine the threshold for human melatonin suppression.
All procedures were approved by the Institutional Review Board at the University of Michigan and complied with the Declaration of Helsinki. Six authors of this paper (four Caucasians and two Asians, aged 19 – 37) served as subjects. All had normal color vision according to the Ishihara test. Each person served as subject for 2 – 13 months during which s/he adhered to the sleep/wake schedule in the 7-day protocol (Fig. 1A); proper photoentrainment was confirmed daily by actigraphy (Jawbone UP and UP24 activity trackers; San Francisco, CA). Throughout the protocol, each subject engaged in their normal daytime activities from 7:30 AM to 11 PM and slept from 11 PM to 7:30 AM, except on days 5 (the “control” session) and 7 (the “photostimulation” session) when s/he was in a completely dark room from 9 to 11 PM – the pair of sessions constituted a “trial”. In these sessions, the subject sat upright before a Ganzfeld dome with the head stabilized by a chin rest and a forehead band, and used salivettes (SciMart; Saint Louis, MO) to collect his/her own saliva every 20 min (Fig. 1A, asterisks). On the control night, the Ganzfeld dome remained dark, but on the photostimulation night, a 460-nm LED light with a half-peak width of ~25 nm (PAR20-B36, superbrightleds.com; Saint Louis, MO) was presented from 10 to 11 PM through a ceiling aperture of the Ganzfeld dome, with intensity adjusted using neutral density filters and calibrated using an S370 radiometer (Gamma Scientific, San Diego, CA). Each saliva sample was stored immediately at 4 °C for 12 – 16 hr and subsequently at −70 °C for up to 2 months, before it was subjected to a melatonin radioimmunoassay (Bühlmann Laboratories; Switzerland). Each subject generated all 12 samples in every trial. To reduce inter- and intra-assay variability, all samples from each trial were analyzed in triplicate using the same assay kit. Throughout the 7-day protocol, all subjects avoided caffeine, alcohol, bananas, beverages containing artificial colorants, over-the-counter medications, melatonin supplements, and strenuous exercise.
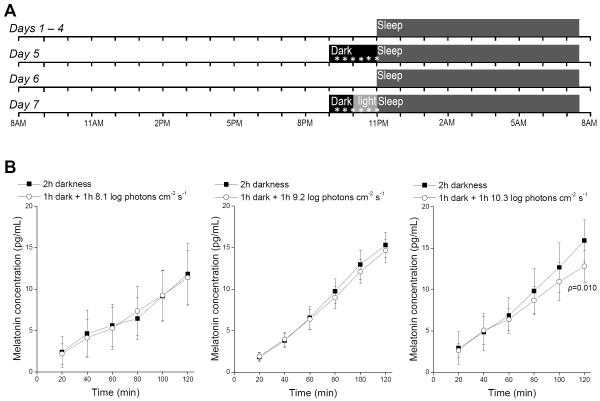
Figure 1.
Measuring the threshold for photic suppression of melatonin. A) The experimental protocol. Days 5 and 7 are the “control” and “photostimulation” sessions respectively, and together they constitute one “trial”. The asterisks represent saliva collection. B) In each plot, the black and white curves show data averaged from all control and photostimulation sessions, respectively. Each white curve’s last three data points were collected during light exposure. Left: Stimulus intensity was 8.1 log photons cm−2 s−1. N = 3 subjects, who contributed 1, 3 and 6 trials. Middle: 9.2 log photons cm−2 s−1 intensity. N = 5 subjects, who contributed 2, 2, 2, 3 and 5 trials. Right: 10.3 photons cm−2 s−1 intensity. N = 6 subjects, who contributed 1, 1, 1, 1, 3 and 4 trials. The p-value was calculated using the randomization test. Error bars represent S.E.M.
Three stimulus intensities were examined. Each intensity was tested on 3 – 6 subjects, with each subject contributing 1 – 6 trials per intensity (see Fig. 1 legend). The data were initially analyzed using the Wilcoxon signed-rank test, a widely used non-parametric, paired-difference test. For the lowest light intensity, 8.1 log photons cm−2 s−1, the data from the control and photostimulation sessions were statistically indistinguishable at all time points (Fig. 1B left), indicating it was too low to suppress melatonin. At 9.2 log photons cm−2 s−1, an apparent suppression was seen as all three data points during light treatment fell below control values (Fig. 1B center), although these data were not significantly different between the two nights. The two nights’ data deviated further when stimulus intensity increased to 10.3 log photons cm−2 s−1, with a significant difference at the fifth time point (p=0.034) and the final time point (p=0.003) (Fig. 1B right). However, the Wilcoxon signed-rank test assumes single testing of each subject whereas our subjects often contributed multiple trials per stimulus. Thus, we reanalyzed the 10.3 log photons cm−2 s−1 data using the randomization test (Ernst, 2004), a non-parametric test compatible with our repeated-measures design (supplemental material). The control-vs-photostimulation difference became insignificant at the fifth time point (p=0.143), but remained significant for the sixth time point (p=0.010).
In conclusion, we detected significant melatonin suppression at a light intensity about 2 log units lower than previously reported thresholds (Brainard et al., 2001; Thapan et al., 2001). This difference is likely due to the higher precision of our data: all our measurements were made during the first two hours of subjective night when melatonin level rises nearly monotonically, whereas the earlier studies were done at later time points when it fluctuates substantially. The number of subjects (6) we tested at 10.3 log photons cm−2 s−1 may seem small, but is comparable to the subject numbers (5 – 8) the earlier studies employed for each stimulus. There are, however, two plausible caveats. First, our data cannot be compared directly with the earlier studies since our photostimulation was done at early night but theirs around midnight, and the sensitivity of melatonin suppression is phase-dependent (McIntyre et al., 1989). Specifically, McIntyre et al. found a higher photosensitivity at midnight than at early night, suggesting the 2-log-unit threshold difference between our study and the earlier ones could be an underestimate. Second, our control session always preceded the photostimulation session, whereas some laboratories prefer to randomize the order of testing. We reasoned that, had the photostimulation been performed first, the light exposure could induce a circadian phase shift that would interfere with the control session conducted two days later. Indeed, for all three stimulus intensities, the control and photostimulation data were nearly identical at the first three time points, confirming that our protocol avoided phase shifts.
Though lower than previously published values, our threshold for melatonin suppression is still at least 3 log units above the threshold for primate ipRGCs’ rod-driven photoresponses (Dacey et al., 2005). While this fits the hypothesis that the human circadian system receives no excitatory rod input (Rea et al., 2005), it does not rule out such input. For example, our threshold could have been lower had the subjects’ pupils been dilated by mydiatrics (Gaddy et al., 1993). Furthermore, the threshold for light pulse-induced melatonin suppression appears higher than that for circadian entrainment to light-dark cycles (Zeitzer et al., 2000; Butler and Silver, 2011), suggesting that stimulus durations longer than ours could conceivably suppress melatonin at lower intensities.
Nevertheless, rods could indeed have little impact on the human circadian system. For example, nonlinearities downstream of ipRGCs could dictate the threshold for melanopsin suppression, in effect blocking low-amplitude rod-driven signals. Furthermore, retinal input to the primate SCN could be mediated by previously uncharacterized ipRGCs that receive weak rod input. Two types of primate ipRGCs have been recorded and both exhibited robust rod-driven light responses (Dacey et al., 2005), but five ipRGC types have since been discovered in rodents, of which only the M1 type innervates the SCN (Ecker et al., 2010). We learned recently that while mouse M1 cells display rod-driven photoresponses as robust as those of primate ipRGCs (Zhao et al., 2014), rat M1 cells’ rod/cone-mediated responses are far weaker (Reifler et al., 2015). The SCN-projecting ipRGCs in primates could resemble those in rats.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Josh Errickson and Prof. Kerby Shedden at the University of Michigan Center for Statistical Consultation & Research for help with statistics, and Teera Parr for performing the radioimmunoassay.
FUNDING
This work was funded by Research to Prevent Blindness [Career Development Award]; the NIH National Eye Institute [P30 EY007003]; and the Academy of Finland [253314].
Footnotes
CONFLICT OF INTERESTS
None.
REFERENCES
- Altimus CM, Guler AD, Alam NM, Arman AC, Prusky GT, Sampath AP, Hattar S. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci. 2010;13:1107–1112. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral FG, Castrucci AM, Cipolla-Neto J, Poletini MO, Mendez N, Richter HG, Sellix MT. Environmental Control of Biological Rhythms: Effects on Development, Fertility and Metabolism. J Neuroendocrinol. 2014 doi: 10.1111/jne.12144. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Nelson RJ. Influence of the modern light environment on mood. Mol Psychiatry. 2013;18:751–757. doi: 10.1038/mp.2013.70. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MP, Silver R. Divergent photic thresholds in the non-image-forming visual system: entrainment, masking and pupillary light reflex. Proc Biol Sci. 2011;278:745–750. doi: 10.1098/rspb.2010.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MD. Permutation methods: a basis for exact inference. Statistical Science. 2004;19:676–685. [Google Scholar]
- Gaddy JR, Rollag MD, Brainard GC. Pupil size regulation of threshold of light-induced melatonin suppression. J Clin Endocrinol Metab. 1993;77:1398–1401. doi: 10.1210/jcem.77.5.8077340. [DOI] [PubMed] [Google Scholar]
- Lall GS, Revell VL, Momiji H, Al Enezi J, Altimus CM, Guler AD, Aguilar C, Cameron MA, Allender S, Hankins MW, Lucas RJ. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre IM, Norman TR, Burrows GD, Armstrong SM. Human melatonin response to light at different times of the night. Psychoneuroendocrinology. 1989;14:187–193. doi: 10.1016/0306-4530(89)90016-4. [DOI] [PubMed] [Google Scholar]
- Morin LP, Studholme KM. Separation of function for classical and ganglion cell photoreceptors with respect to circadian rhythm entrainment and induction of photosomnolence. Neuroscience. 2011;199:213–224. doi: 10.1016/j.neuroscience.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Res Brain Res Rev. 2005;50:213–228. doi: 10.1016/j.brainresrev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Reifler AN, Chervenak AP, Dolikian ME, Benenati BA, Meyers BS, Demertzis ZD, Lynch AM, Li BY, Wachter RD, Abufarha FS, Dulka EA, Pack W, Zhao X, Wong KY. The rat retina has five types of ganglion-cell photoreceptors. Exp Eye Res. 2015;130:17–28. doi: 10.1016/j.exer.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(Pt 3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Stafford BK, Godin AL, King WM, Wong KY. Photoresponse diversity among the five types of intrinsically photosensitive retinal ganglion cells. J Physiol. 2014;592:1619–1636. doi: 10.1113/jphysiol.2013.262782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.