Abstract
Noninvasive stimulation can alter the function of brain networks, although the duration of neuroplastic changes are uncertain and likely vary for different networks and stimulation parameters. We have previously shown that multiple-day repetitive transcranial magnetic stimulation can influence targeted hippocampal-cortical networks, producing increased functional MRI connectivity of these networks and concomitant improvements in memory that outlast stimulation by ~24 hours. Here we present new analyses showing that multiple-day targeted stimulation of hippocampal-cortical networks produces even longer-lasting enhancement. The ability to learn novel, arbitrary face-word pairings improved over five consecutive daily stimulation sessions, and this improvement remained robust at follow-up testing performed an average of 15 days later. Further, stimulation increased functional MRI connectivity of the targeted portion of the hippocampus with distributed regions of the posterior hippocampal-cortical network, and these changes in connectivity remained robust at follow-up testing. Neuroplastic changes of hippocampal-cortical networks caused by multiple-day noninvasive stimulation therefore persist for extended periods. These findings have implications for the design of multiple-day stimulation experiments and for the development of stimulation-based interventions for memory disorders.
Keywords: rTMS, relational memory, resting-state fMRI, hippocampus, hippocampal-cortical network
Large-scale brain networks critically support cognition (Mesulam, 1990). Considerable interest thus exists in modulating these networks using brain stimulation. Noninvasive methods such as repetitive transcranial magnetic stimulation (rTMS) can robustly change network functional connectivity for up to several hours (e.g., Fox, Halko, Eldaief, & Pascual-Leone, 2012; Gratton, Lee, Nomura, & D'Esposito, 2013; Zanto, Rubens, Thangavel, & Gazzaley, 2011). Multiple consecutive days of stimulation could produce longer-lasting effects (Dayan, Censor, Buch, Sandrini, & Cohen, 2013; Wassermann & Zimmermann, 2012). However, the duration and magnitude of brain-network changes caused by multiple-day stimulation are largely unknown.
We recently reported that multiple-day rTMS can be used to selectively enhance networks of the human hippocampus (Wang, et al., 2014). Hippocampal-cortical networks are critical for memory storage and retrieval (Ranganath & Ritchey, 2012). The hippocampus is a central hub that interacts with many anterior and posterior cortical regions, thereby supporting binding of information from distinct processing modules into coherent associative/relational memories (Aggleton, 2012; Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Battaglia, Benchenane, Sirota, Pennartz, & Wiener, 2011; Eichenbaum & Cohen, 2001; Eichenbaum, Yonelinas, & Ranganath, 2007; Ranganath & Ritchey, 2012; Staresina, Cooper, & Henson, 2013). In our recent experiment (Wang, et al., 2014), we selected a target within each subject’s left hippocampal body and used this as a seed region in order to define the resting-state fMRI connectivity network. From this network, we identified a region of superficial lateral parietal cortex that was accessible to rTMS. Lateral parietal cortex has high functional connectivity with the hippocampal target (Kahn, Andrews-Hanna, Vincent, Snyder, & Buckner, 2008) likely mediated by lateral-parietal projections to retrosplenial and parahippocampal cortex (Cavada & Goldman-Rakic, 1989; Mesulam, Van Hoesen, Pandya, & Geschwind, 1977). This subject-specific parietal location received high-frequency rTMS for five consecutive daily sessions.
Targeted multiple-day stimulation increased resting-state fMRI connectivity of the hippocampal target with its distributed cortical network, as measured during a Post-Tx assessment given ~24 hours after the final stimulation sessions relative to baseline. These fMRI connectivity increases occurred among the hippocampal target and regions of the posterior-medial hippocampal-cortical network (Ranganath & Ritchey, 2012), including parietal, retrosplenial, posterior cingulate, and parahippocampal cortex. Furthermore, increases were selective for the hippocampal target that was selected in each subject and did not occur for sham stimulation or stimulation of a distinct network (Wang, et al., 2014). Memory for novel, arbitrary face-word pairings also improved due to stimulation (Wang, et al., 2014). Notably, memory was assessed ~24 hours before (Baseline) and ~24 hours after (Post-Tx) multiple-day stimulation sessions and using novel stimuli for each assessment. We did not test whether stimulation influenced retention of specific pairings across days, but rather showed that stimulation produced lasting increases in the ability to learn/remember arbitrary relational information during each assessment.
Here we seek to determine whether changes in hippocampal-cortical fMRI connectivity and memory persist beyond the ~24 h period that we previously tested. We thus report a new analysis of data from subjects in the Wang et al. (2014) study aimed at evaluating long-lasting effects of multiple-day rTMS targeted at hippocampal-cortical networks. Each subject (N=16) participated in an active stimulation week involving five consecutive daily sessions and in a sham week in which stimulation was delivered at sub-threshold intensity using the same parameters, with the ordering of weeks counterbalanced across subjects (see Detailed Methods). In the original study, a within-subjects design was used, with Baseline and Post-Tx assessments administered ~24 h before and after the first and last stimulation sessions of each week (Figure 1A). Here we focused on assessing long-lasting effects of stimulation by testing whether changes persisted after the Post-Tx assessment until the Baseline assessment of Week 2, using between-group analyses. We evaluated between-group differences in the Week 2 Baseline assessment for subjects who received stimulation during Week 1 (the W1-Stimulation group, n=8) versus subjects who received sham during Week 1 (the W1-Sham group; n=8; Figure 1A). The mean delay between the final stimulation/sham session during Week 1 and the Baseline Week 2 assessment was 15.3 days (range = 6–26 days; median = 16 days; no between-group delay difference, P=0.85).
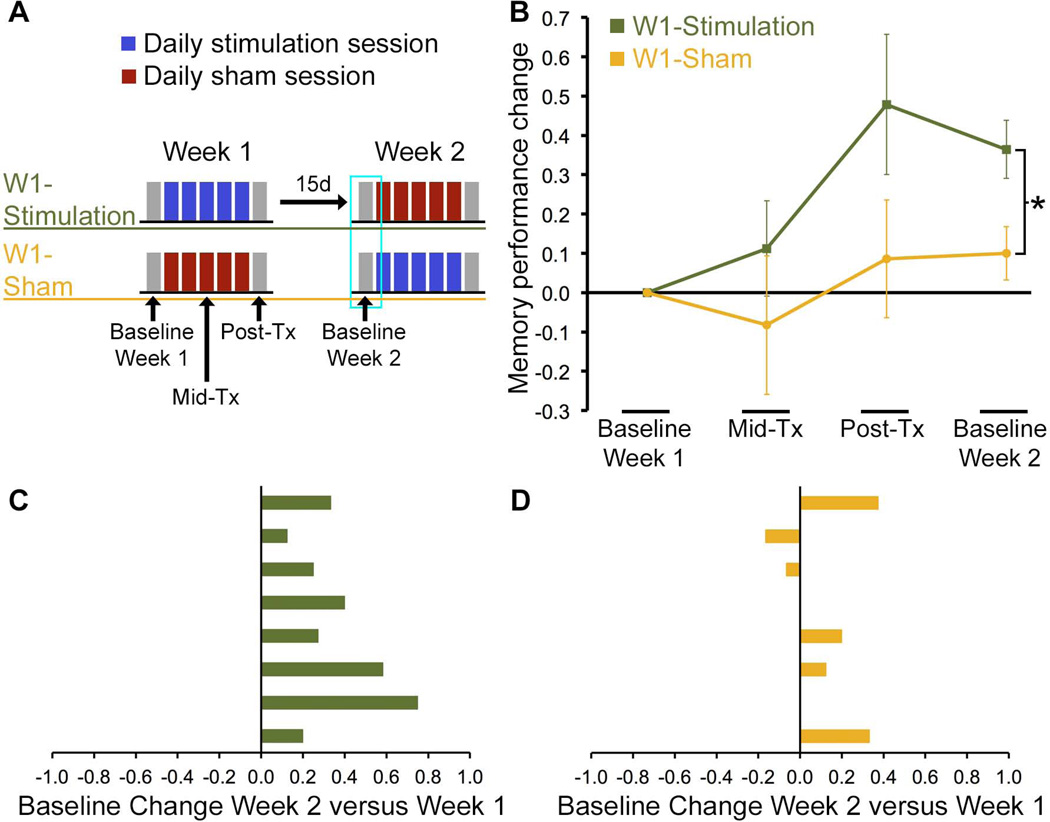
Figure 1. Lasting enhancement of memory for face-word pairings due to stimulation.
(A) Baseline and Post-Tx assessments were performed ~24 hours before and ~24 hours after five consecutive days of stimulation/sham sessions. Each subject underwent one week of stimulation and one week of sham, with Week 2 separated from the final stimulation session of Week 1 by a mean delay of 15 days. The focus of the current analyses was on long-lasting effects that were evident for the Week 2 Baseline, as highlighted with a cyan box, which were assessed separately for subjects receiving stimulation during Week 1 (W1-Stimulation group) versus subjects receiving sham during Week 1 (W1-Sham group). (B) Mean accuracy of face-cued word recall is shown calculated as a proportion-change score relative to the Baseline value from Week 1. (C) Proportion-change scores for the Week 2 Baseline relative to the Week 1 Baseline are shown for each W1-Stimulation subject (one subject per row), demonstrating lasting improvement in 100% of subjects. (D) In contrast, proportion-change scores for W1-Sham subjects clearly did not differ from zero, with only 50% of subjects showing improvement. *P=0.02 W1-Stimulation versus W1-Sham.
Robust improvement in face-cued word recall, calculated as proportion-change scores, was evident at Week 2 Baseline relative to Week 1 Baseline for W1-Stimulation subjects [t(7)=4.9, P=0.002] but not for W1-sham subjects (t(7)=1.5, P=0.185; Figure 1B). Group differences between W1-Stimulation and W1-Sham subjects for Week 2 Baseline relative to Week 1 Baseline were also significant [t(14)=2.6, P=0.020]. Additionally, these findings were evident when calculated using raw memory performance scores rather than proportion-change scores [Table 1; W1-Stimulation: t(7)=4.4, P=0.003; W1-Sham: t(7)=0.7; P=0.50; W1-Stimulation vs. W1-Sham: t(14)=3.0, P=0.009]. Improvement for the Week 2 Baseline relative to the Week 1 Baseline occurred for 100% of W1-Stimulation subjects (Figure 1C), but for only 50% of W1-Sham subjects (Figure 1D). The effects of stimulation on improved memory performance at the Week 2 Baseline assessment were thus highly consistent across subjects.
Table 1.
Face-cued word recall performance provided as mean raw scores (numbers of words correctly recalled to associated face cues) and as mean raw change scores (i.e., not as a proportion of baseline, as in the primary analyses presented in Figure 1) relative to each baseline assessment calculated for each subject. Parentheses indicate standard error of the mean.
| Raw score | Raw change from Week 1 Baseline | |
|---|---|---|
| W1-Stimulation | ||
| Baseline Week 1 | 8.5 (1.1) | N/A |
| Mid-Tx | 9.0 (1.1) | 0.5 (0.8) |
| Post-Tx | 11.4 (0.8) | 2.9 (0.6)** |
| Baseline Week 2 | 11.5 (1.6) | 3.0 (0.7)*† |
| W1- Sham | ||
| Baseline Week 1 | 8.9 (1.5) | N/A |
| Mid-Tx | 7.8 (1.3) | −1.1 (1.1) |
| Post-Tx | 9.0 (1.5) | 0.1 (1.3) |
| Baseline Week 2 | 9.3 (1.2) | 0.4 (0.5)† |
P=0.003 versus zero;
P=0.002 versus zero; P>0.344 for all other comparisons versus zero for raw change scores.
Raw changes from Baseline Week 1 were significantly greater for W1-Stimulation versus W1-Sham subjects for Baseline Week 2, with pairwise between-group differences P=0.009, and were trending for Post-Tx (P=0.07).
We next assessed corresponding long-lasting changes in fMRI connectivity of the hippocampal target with its cortical network. The primary question was whether changes in fMRI connectivity evident at the Post-Tx timepoint for the stimulation week (relative to the sham week) remained robust when measured at Week 2 Baseline. We therefore performed a targeted analysis within an inclusive mask of those regions found previously to show increased connectivity with the hippocampal target due to stimulation (Table 2A; within-subjects Post-Tx minus Baseline whole-brain comparison for stimulation versus sham, as reported in Wang, et al. 2014). Within these regions, we performed between-group analysis of the Week 2 Baseline minus the Week 1 Baseline for W1-Stimulation versus W1-Sham subjects.
Table 2.
Summary of fMRI connectivity findings used, including MNI-305 coordinates of the centroid (mm), volume (mm3), and Brodmann Area(s) for the cluster (BA). Baseline Week 2 versus Baseline Week 1 differences are referred to as “long-lasting changes.”
| Coordinates | |||||
|---|---|---|---|---|---|
| X | Y | Z | Volume (mm3) |
BA | |
| A. Inclusive mask used in the targeted analysis (975-mm3 cluster-size threshold) | |||||
| Retrosplenial/precuneus/cuneus/posterior cingulate (bilateral) | −2 | −76 | +20 | 18,039 | 7/19/31 |
| Fusiform and parahippocampal cortex (bilateral) | +13 | −66 | −23 | 2,933 | 18/19 |
| Superior parietal lobule (left) | −30 | −51 | +57 | 1,283 | 7/40 |
| Lateral parietal cortex (left) | −52 | −54 | +33 | 975 | 40 |
| B. Targeted analysis of long-lasting changes for W1-Stimulation versus W1-Sham (169-mm3 cluster-size threshold) | |||||
| Precuneus/cuneus/posterior cingulate (bilateral, predominantly right) | +5 | −79 | +21 | 3,594 | 7/19/31 |
| Fusiform (left) | −8 | −66 | −5 | 392 | 19 |
| Fusiform (right) | +28 | −67 | −23 | 321 | 19/30 |
| Retrosplenial/parahippocampal (right) | +11 | −59 | −7 | 294 | 19/30 |
| Precuneus/cuneus (left) | −20 | −78 | +32 | 240 | 19/31 |
| Precuneus/cuneus (left) | −19 | −91 | +22 | 182 | 18/19 |
| C. Targeted analysis of long-lasting changes for W1-Stimulation (169-mm3 cluster-size threshold) | |||||
| Precuneus/cuneus/retrosplenial/parahippocampal/posterior cingulate (bilateral) | +0 | −78 | +17 | 1,721 | 7/18/19/30/31 |
| Fusiform (right) | +33 | −66 | −23 | 182 | 19/30 |
| D. Whole-brain analysis of long-lasting changes (975-mm3 cluster-size thresholds) | |||||
| Precuneus/cuneus/retrosplenial/parahippocampal/fusiform/posterior cingulate (bilateral) | +4 | −77 | +18 | 5,981 | 7/18/19/30/31 |
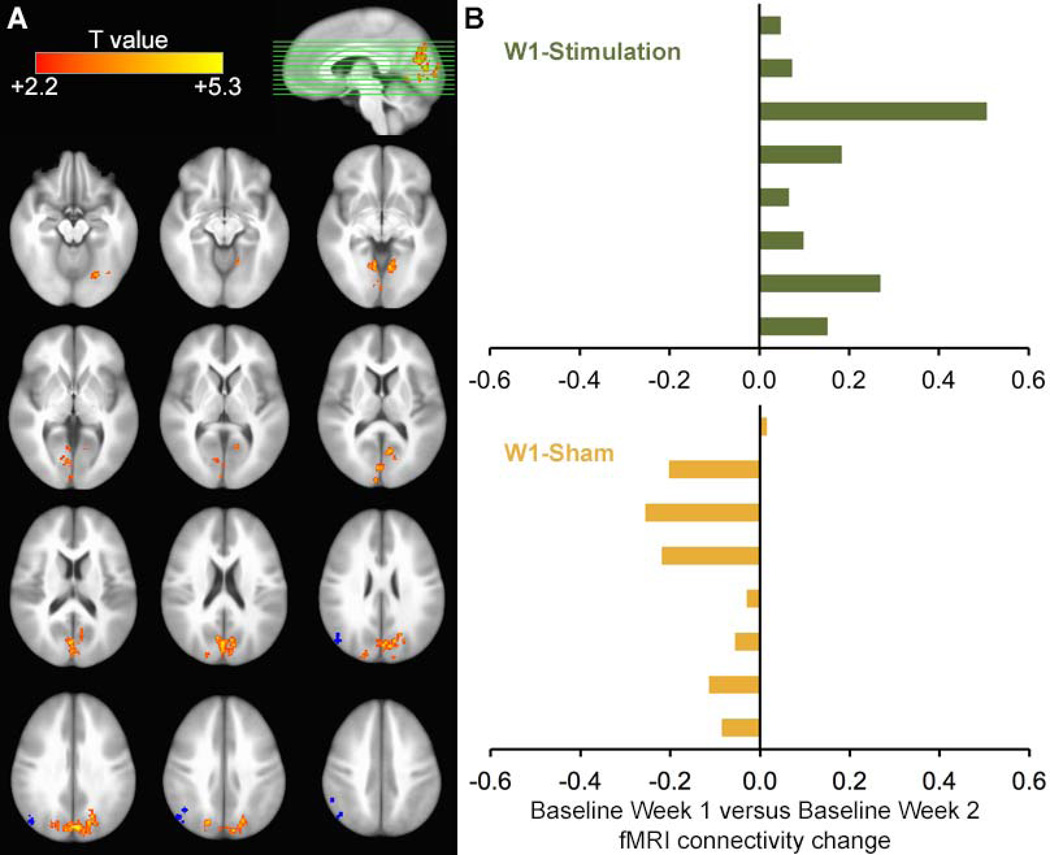
This analysis identified significant long-lasting effects of stimulation (versus sham) on fMRI connectivity with the hippocampal target for regions including bilateral parieto-occipital, fusiform, retrosplenial, and parahippocampal cortex (Figure 2A; Table 2B). Thus, portions of the posterior-medial hippocampal-cortical network (Ranganath & Ritchey, 2012) that demonstrated significant stimulation-induced changes in fMRI connectivity with the hippocampal target at Post-Tx continued to show significantly elevated fMRI connectivity at Week 2 Baseline. Notably, increases in fMRI connectivity for all supra-threshold voxels were evident in 100% of W1-Stimulation subjects (Figure 2B). Although corresponding decreases were evident in most W1-Sham subjects, voxels were selected based on the between-group difference and were therefore subject to selection bias for negative changes. We therefore also compared the Week 2 to Week 1 Baselines for W1-Stimulation subjects (i.e., without comparison to W1-Sham subjects). This analysis identified fMRI connectivity increases for many of the same regions that showed W1-Stimulation versus W1-Sham differences (Table 2C), indicating that significant increases for W-1 Stimulation subjects did not require identification based on comparison against W1-Sham subjects.
Figure 2. Lasting enhancement of stimulation-induced fMRI connectivity increases.
(A) Resting-state fMRI connectivity of the hippocampal target for the Week 2 Baseline minus Week 1 Baseline was compared for the W1-Stimulation versus W1-Sham groups. Results are displayed on a template brain (ICBM-452). Blue markings indicate the subject-specific stimulation locations (one mark per subject). (B) Change in fMRI connectivity was averaged for all supra-threshold voxels and displayed for each subject, showing Week 2 Baseline versus Week 1 Baseline increases in 100% of W1-Stimulation subjects but only 13% of W1-Sham subjects.
To further evaluate these effects, we conducted two additional analyses. First, a whole-brain analysis of long-lasting changes in fMRI connectivity (i.e., the same analysis as that shown in Table 2B but without inclusive masking of regions reported in Table 2A). This analysis yielded one large cluster that encompassed most of the same regions that were identified by the targeted analysis, including parieto-occipital, fusiform, retrosplenial, and parahippocampal cortex (Table 2D). Second, we conducted a targeted analysis of functional connectivity changes between the hippocampal targets and subject-specific stimulation locations in parietal cortex. For W1-Sham subjects, we found a significant decrease in connectivity from W1 Baseline to W2 Baseline [t(7)=−.5, P=0.040], but no significant changes were found for W1 Baseline to W1 Post-Tx or for W1-Stimulation subjects at either timepoint. There were no significant group differences for these timepoints for W1-Stimulation versus W1-Sham subjects. These findings are in contrast to the robust increases in functional connectivity evident for midline aspects of the posterior hippocampal-cortical network (Figure 2A). It is possible that more robust changes for midline areas could result if the hippocampus were the critical locus of neuroplastic changes induced by the rTMS regimen, as these areas are more robustly/directly connected with hippocampus than the lateral parietal locations that were stimulated. The decrease in connectivity we observe for the W1-Sham group might be caused in part by habituation to resting-state scanning throughout the experiment, which is interesting as we are aware of no previous studies examining changes in functional connectivity for repeat scans at the close intervals used in our study (i.e., previous studies have examined test-retest reliability at longer intervals). That the W1-Stimulation group displays no such decrease could be evidence of ameliorating factors, possibly due to the rTMS regimen, and warrants further study.
We conclude that multiple-day rTMS targeted to resting-state fMRI connectivity networks of the left hippocampal body can produce surprisingly long-lasting enhancement of memory and fMRI connectivity. These long-term neuroplastic changes could result from induction of LTP-like effects throughout hippocampal-cortical networks (Alvarez-Salvado, Pallares, Moreno, & Canals, 2014; Canals, Beyerlein, Merkle, & Logothetis, 2009). Indeed, recent experiments using voltage-sensitive dye imaging show that high-frequency rTMS increases excitation and reduces inhibition for stimulated regions (Kozyrev, Eysel, & Jancke, 2014), suggesting induction of an LTP-favorable state. However, additional research will be necessary to evaluate this hypothesized mechanism.
These findings have implications for multiple-day rTMS experiments. Because multiple-day stimulation can produce surprisingly long-lasting changes (15 days in the current experiment, and perhaps months for multiple-week stimulation of fronto-limbic networks performed to treat major depression; see Fox, Buckner, White, Greicius, & Pascual-Leone, 2012; Wassermann & Zimmermann, 2012), within-subjects paradigms should be designed with appropriately long washout periods between conditions. In terms of the within-subjects analyses of the current data (Wang, et al., 2014), Baseline values for the second week were partially influenced by carry-over effects of stimulation during the first week (in W1-Stimulation subjects). Thus, at the group level, significant differences between stimulation and sham were only evident when memory performance was calculated as a proportion-change score relative to the baseline value for each week (Figure 4B of Wang et al., 2014). When raw scores were used (Table S2 of Wang et al., 2014), the carry-over effect of Week 1 stimulation on the Week 2 Baseline obscured effects of stimulation. By assessing treatment effects separately for W1-Stimulation and W1-Sham subjects in the current report, changes were evident in both proportion-change scores and raw scores because carryover effects were eliminated. Notably, we did not assess longer-lasting changes, and so it is possible that changes could outlast the 15-day period tested here. Furthermore, stimulation for periods of longer than 5 days could potentially produce more robust and/or longer-lasting effects. These possibilities should be tested in future research.
Although long-lasting effects complicate multiple-day stimulation experiments, they motivate development of stimulation-based treatment of brain-network abnormalities. For instance, pathophysiology of hippocampus and reduced fMRI connectivity of hippocampal-cortical networks have been implicated in a variety of memory disorders (Budson, 2009; Dickerson & Eichenbaum, 2010; La Joie, et al., 2014; Small, Schobel, Buxton, Witter, & Barnes, 2011). Multiple-day rTMS of hippocampal-cortical networks using methods described here thus presents an attractive model for treatment of these disorders. Indeed, rTMS for treatment of various disorders is particularly effective when applied to the resting-state fMRI connectivity networks of regions for which deep-brain stimulation is effective (Fox, et al., 2014). Although it is uncertain whether deep-brain stimulation of hippocampus will be effective for treatment of memory disorders, the fact that rTMS of hippocampal-cortical networks as described here produces ~15-day enhancements suggests that noninvasive stimulation could provide an attractive alternative to neurosurgical approaches. Future research will be needed to determine whether yet longer stimulation regimens could produce more robust and longer-lasting effects on hippocampal brain networks and memory, in healthy as well as neurologically impaired individuals.
Detailed Methods
Healthy adult subjects (N=16; 9 female; ages 20–32 years) were randomly assigned to the W1-Stimulation and W1-Sham groups (Figure 1A; n=8 each). Baseline, Mid-Tx, and Post-Tx assessments for each week included resting-state fMRI and behavioral memory testing.
MRI data were collected using a Siemens 3T TIM Trio whole-body scanner with a 32-channel head coil. T1-weighted structural images provided anatomical localization. Eyes-open resting-state fMRI used whole-brain BOLD EPI. Resting-state fMRI analyses used standard preprocessing and analysis steps implemented in AFNI (Cox, 1996; see Wang, et al., 2014 for scanning parameters and preprocessing details). fMRI analyses used seed-based resting-state functional connectivity using the hippocampal target defined in each subject as the seed (see Wang, et al., 2014 for analysis details). All analyses used P<0.05 corrected thresholds, using combined voxel-wise (P<0.05) and cluster-size thresholds determined via Monte Carlo simulation by AFNI 3dAlphaSim. Spatial-extent thresholds are listed in Table 2. Comparisons were not directional (i.e., both increases and decreases were tested).
Face-cued word recall testing involved 20 unfamiliar faces and 20 common words, with different stimuli used for each assessment. Arbitrarily matched face-word pairs were studied for ~3 s each (visual face and auditory word). At test, subjects viewed faces individually and recalled associated words. Production of the same word paired with the face at study was scored as correct; all other responses were scored as incorrect.
rTMS was delivered using a Nexstim eXimia NBS 4.3 with a 70-mm figure-eight coil (Nexstim Ltd., Finland). A hippocampal target was defined as the location of hippocampal body nearest to MNI coordinate −24, −18, −18 in each subject. In each subject, the location of lateral parietal cortex with highest fMRI connectivity values with the hippocampal target was stimulated (as described in Wang, et al., 2014; see also Fox, Liu, & Pascual-Leone, 2013). rTMS was applied at 20 Hz in alternating 2-s/28-s on/off trains for 20 minutes per day at 100% of resting motor threshold in the stimulation condition and at effectively zero intensity in the sham condition.
Acknowledgments
Research was supported by award numbers P50-MH094263 from the National Institute of Mental Health and F32-NS083340 from the National Institute of Neurological Disorders and Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Neuroimaging was performed at the Northwestern University Center for Translational Imaging facility supported by the Northwestern University Department of Radiology
Footnotes
The authors declare no conflict of interest.
References
- Aggleton JP. Multiple anatomical systems embedded within the primate medial temporal lobe: implications for hippocampal function. Neurosci Biobehav Rev. 2012;36:1579–1596. doi: 10.1016/j.neubiorev.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Alvarez-Salvado E, Pallares V, Moreno A, Canals S. Functional MRI of long-term potentiation: imaging network plasticity. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130152. doi: 10.1098/rstb.2013.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia FP, Benchenane K, Sirota A, Pennartz CM, Wiener SI. The hippocampus: hub of brain network communication for memory. Trends Cogn Sci. 2011;15:310–318. doi: 10.1016/j.tics.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Budson AE. Understanding memory dysfunction. Neurologist. 2009;15:71–79. doi: 10.1097/NRL.0b013e318188040d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals S, Beyerlein M, Merkle H, Logothetis NK. Functional MRI evidence for LTP-induced neural network reorganization. Curr Biol. 2009;19:398–403. doi: 10.1016/j.cub.2009.01.037. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dayan E, Censor N, Buch ER, Sandrini M, Cohen LG. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat Neurosci. 2013;16:838–844. doi: 10.1038/nn.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. New York City, NY: Oxford University Press; 2001. [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci U S A. 2014;111:E4367–E4375. doi: 10.1073/pnas.1405003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Halko MA, Eldaief MC, Pascual-Leone A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS) Neuroimage. 2012;62:2232–2243. doi: 10.1016/j.neuroimage.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage. 2013;66:151–160. doi: 10.1016/j.neuroimage.2012.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Lee TG, Nomura EM, D'Esposito M. The effect of theta-burst TMS on cognitive control networks measured with resting state fMRI. Front Syst Neurosci. 2013;7:124. doi: 10.3389/fnsys.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozyrev V, Eysel UT, Jancke D. Voltage-sensitive dye imaging of transcranial magnetic stimulation-induced intracortical dynamics. Proc Natl Acad Sci U S A. 2014;111:13553–13558. doi: 10.1073/pnas.1405508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Joie R, Landeau B, Perrotin A, Bejanin A, Egret S, Pelerin A, Mezenge F, Belliard S, de La Sayette V, Eustache F, Desgranges B, Chetelat G. Intrinsic connectivity identifies the hippocampus as a main crossroad between Alzheimer's and semantic dementia-targeted networks. Neuron. 2014;81:1417–1428. doi: 10.1016/j.neuron.2014.01.026. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Van Hoesen GW, Pandya DN, Geschwind N. Limbic and sensory connections of the inferior parietal lobule (area PG) in the rhesus monkey: a study with a new method for horseradish peroxidase histochemistry. Brain Res. 1977;136:393–414. doi: 10.1016/0006-8993(77)90066-x. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Cooper E, Henson RN. Reversible information flow across the medial temporal lobe: the hippocampus links cortical modules during memory retrieval. J Neurosci. 2013;33:14184–14192. doi: 10.1523/JNEUROSCI.1987-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JX, Rogers LM, Gross EZ, Ryals AJ, Dokucu ME, Brandstatt KL, Hermiller MS, Voss JL. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345:1054–1057. doi: 10.1126/science.1252900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, Zimmermann T. Transcranial magnetic brain stimulation: therapeutic promises and scientific gaps. Pharmacol Ther. 2012;133:98–107. doi: 10.1016/j.pharmthera.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci. 2011;14:656–661. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]