Abstract
We examined whether statins are associated with better cerebral white (WM) and gray matter (GM) indices in community-dwelling elders. In 295 older adults, we compared white matter hyperintensities (WMH) on brain MRI and, total WM fractional anisotropy (FA) and GM mean diffusivity (MD) on diffusion tensor imaging, of Alzheimer’s Disease (AD) relevant regions in statin-exposed and statin-unexposed participants stratified by Modified Mini-Mental Status Examination (3MS) score. There was no overall effect of statin exposure on cerebral structural indices. Interaction between statin exposure and 3MS was significant for total-WMH and WM-FA (both p<0.05) but not GM-MD. In the lowest 3MS tertile (mean: 86), statin-exposed individuals had lower total-WMH and higher WM-FA (p=0.005 and p=0.044) and FA of tracts linked to clinical AD (p-value range= 0.005 – 0.04) despite statistical adjustments. These differences were not significant in the two higher 3MS tertiles. Statins may benefit WM indices in elders vulnerable to dementia.
Keywords: statins, white matter hyperintensities, fractional anisotropy, mean diffusivity, cognition, older adults
1. Introduction
Alzheimer’s disease (AD) with cerebrovascular disease and vascular dementia, the commonest causes of dementia in population studies,1–3 share common vascular risk factors and cerebral vascular lesions potentiate the clinical symptomatology of AD pathology.1,4 Statins, important for management of cerebrovascular disease, are purported to benefit AD.5 However, early evidence of benefit of statins on cognition in elders with and without AD6,7 has not borne out in AD clinical trials.8,9 The discrepancy in these findings may relate to the neuroprotective effects of statins being possibly limited to the earliest stages of AD.10–13 Despite possible beneficial effects of statins on cognition,6,7 cerebrovascular disease,5 and even AD pathology 14 it is not known whether statins are associated with better microstructural brain integrity or lesser small-vessel disease severity in older adults vulnerable to dementia.
Cerebral small-vessel disease, quantified by assessing volume of white matter hyperintensities (WMH) on brain MRI, coexists with AD pathology,1 and is associated with decline in cognition in older adults.15 Furthermore, loss of structural integrity of gray (GM) and white matter (WM), quantified on diffusion tensor imaging (DTI) as an increase in cortical mean diffusivity (MD) and a decrease in fractional anisotropy (FA) respectively, is linked to AD risk.16 Specifically, increase in MD in dorsolateral prefrontal cortex (DLPFC), cingulate and medial temporal region (MTL)17,18 and decrease in FA in the superior longitudinal fasciculus (SLF), splenium (sCC) and genu (gCC) of the corpus callosum and anterior thalamic radiation (ATR) are linked to preclinical AD.19–21
The objective of this study was to compare statin-exposed to statin unexposed older adults on measures of cortical integrity and small-vessel disease in regions important for the clinical evolution of AD. In population-based samples, lower cognitive performance is suggestive of preclinical dementia,2,22–24 which in these populations is more likely of mixed etiology - an overlap of AD and cerebrovascular disease.25 Statins may particularly benefit cortical structure in these individuals in at least two ways - it may influence microvascular disease pathology through its direct effects on cholesterol metabolism and influence small-vessel disease burden; statins may influence AD pathology and GM and WM integrity in regions associated with the clinical evolution of AD.17–19 We, therefore, hypothesized that in older adults with lower cognitive performance, a sample likely to represent those with cognitive impairment of mixed etiology with greater small-vessel disease burden and poor white and gray matter integrity, statin-exposed individuals would have smaller small-vessel disease burden and better GM and WM integrity in regions relevant to the clinical evolution of AD.17–19
2. Methods
2.1. Subjects
Data analyzed was obtained from the ongoing Health Aging and Body Composition (Health ABC),26 which included 3075 well-functioning community-dwelling elders of whom 1501 were enrolled at the Pittsburgh site. In 2006, 819 surviving participants at the Pittsburgh site were screened for a brain-imaging ancillary study (no contraindications for a MRI, ability to walk 20 meters independently and an absence of dementia diagnosis) and 339 were enrolled. The current study sample included 295 of 339 (87.02%) eligible participants with complete MRI, DTI and medication information. This study was approved by the IRB at both clinical sites of Health ABC study (Pittsburgh and Memphis). All participants provided informed consent.
2.2. Cognitive status
Overall cognitive abilities were assessed in the Health ABC study in 2004/2005 on the Modified Mini-Mental Status Examination (3MS) that ranges from 0–100.27 The 3MS has excellent specificity and sensitivity in identifying dementia using standardized criteria.27 We included stratified analysis by 3MS tertiles to identify those with lower cognitive performance who were at a higher likelihood of having cognitive impairment/dementia.28
2.3. Neuroimaging
2.3.1 MRI Image acquisition
Brain imaging was performed in 2006–2008 on a 3T Siemens Tim Trio MR scanner with a Siemens 12-channel head coil at the MR Research Center of the University of Pittsburgh. All subjects were scanned using the same pulse sequences. Magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted images were acquired in the axial plane with the following parameters: repetition time (TR)=2300ms; echo time (TE)=3.43ms; TI=900 ms; flip angle=9; slice thickness=1 mm; field of vision (FOV)=256 × 224 mm; voxel size=1 mm × 1mm; matrix size=256 × 224; and number of slices=176. Fluid-attenuated inversion recovery (FLAIR) images were acquired in the axial plane with the following parameters: TR=9160ms; TE=89ms; TI=2500 ms; FA=150; FOVmm; slice thickness=3 mm; matrix size=256 × 240; number of slices=48 slices; and voxel size=1 mm × 1 mm. Diffusion weighted images were acquired using single-short pulsed-gradient spin-echo sequence with the following parameters: TR=5300 ms; TE=88 ms; TI=2500 ms; flip angle=90; FOV=256 × 256 mm; two diffusion values of b=0 and 1000 s/mm; 12 diffusion directions; four repeats; 40 slices; matrix size=128 × 128; voxel size=2 mm × 2 mm; slice thickness=3 mm; and GRAPPA=2. All MR images were reviewed by a radiologist to ascertain absence of unexpected clinical radiological findings.
2.3.2 Image processing and analysis
Cerebral WMH were obtained from T2-weighted FLAIR sequences using an Automated Labelling Pathway (ALP) method that involved fuzzy-connected algorithm with seed selection to classify WMH based on specific thresholds.29 Voxels classified as WMH were summed to obtain total WMH volume and were normalized to total brain volume (sum of voxels classified as GM, WM and cerebrospinal fluid obtained from skull-stripped T1 image) to adjust for age-related brain atrophy.30 All diffusion-weighted images were pre-processed using the FMRIB's Diffusion Toolbox31 to remove distortions due to motion artifacts and eddy currents.31 The tensor applied was diagonalized and eigenvalues were determined from which FA and MD maps were computed.32 The FA and MD maps were registered to the FMRIB58_FA template31 using the FMRIB's Non-linear Image Registration Tool.33 Then, using the segmentation of cerebral GM and WM and WMH that were obtained from the MPRAGE and T2-weighted FLAIR image, FA and MD of normal appearing cerebral GM and WM were obtained.34,35 Partial volume effects of MD were minimized by masking the aligned images with the GM segmented from the T1 images. Median FA was calculated for the normal appearing WM as well as in the WM tracts.
2.3.3 WM tracts of GM regions of interest
We identified boundaries of WM tracts regions using the John Hopkins University (JHU) White Matter Atlas 31,36 to measure FA and WMH volume in SLF, ATR, sCC and gCC. GM regions were demarcated using standard neuroanatomical atlas37 from which MD in the DLPFC, cingulate and MTL (comprised of the hippocampus, entorhinal cortex and parahippocampal gyrus) were obtained.38
2.4. Primary Independent Variable: Statin exposure
Prescription medication use data was collected by label-review of all medications that participants were taking during the previous 2 weeks of the scheduled annual visit. The medication data were coded using the Iowa Drug Information System codes.39 We defined statin exposure as those who in 2004–2005 were identified as using a statin based on codes belonging to statin class of drugs (IDIS codes: 24060201–24060208).39 Those without these medication codes were identified as statin unexposed. Based on studies that suggest that the time required for statins to attain favorable cardiovascular endpoints ranges from 4–6 months (through “direct” effects from improved endothelial function) to 1-year (through “indirect” effects through cholesterol lowering),40–43 we chose a two-year lag time between medication exposure and brain MRI corresponding to Health ABC data collection schedule to allow for sufficient time for potential pleotropic effects of statins to occur.
We also created a statin duration of use variable (number of years exposed) for an exploratory analyses. Lastly, because lipophilicity of statins can potentially influence statin uptake in the brain across the blood-brain and influence neuroprotective effects of statins,44 we analyzed the data after exclusion of predominantly hydrophilic statins (IDIS codes: 24060201 (rosuvastatin) and 24060207 (pravastatin)). No information regarding statin dosage was collected during the 2004/05 visit prohibiting an examination of potential dose-response relationships.
2.5. Covariates
We controlled for factors that could potentially confound any association between statin use and brain neuroimaging measures. These included demographics (age, race, gender, education, body-mass index), health behaviors (smoking, alcohol use), cardiovascular conditions (hypertension, diabetes mellitus, angina, myocardial infarction, coronary bypass surgery or angioplasty, stroke or transient ischemic attack and peripheral arterial disease), low-density lipoprotein (LDL) levels and apolipoprotein E-4 allele (APOE4) status.
2.6. Statistical analysis
This is a cross-sectional analysis, which also included a longitudinal component, of statin exposure (independent variable) on WMH, FA and MD measures (as separate dependent variables) stratified by cognitive performance tertiles on 3MS and adjusted for the covariates listed above. Chi-square and t-tests were used to compare those exposed to statins and those not exposed. We used independent samples t-tests stratified by cognitive performance tertiles to make unadjusted comparisons of brain imaging measures between the two statin groups. We analyzed main effects of statin and interaction between statin exposure and cognitive performance for each dependent variable. We then used analysis of covariance type models stratified by cognitive performance tertiles with each brain imaging measure as the dependent variable; statin exposure (yes/no) as the main independent factor of interest and demographic and other relevant confounders mentioned above as covariates. Additional multivariable exploratory analyses controlled for APOE4 status and serum LDL levels. Exploratory analyses also included a longitudinal component based on duration (years) of statin use as main predictor and comparisons between those ever exposed to statin and those never reporting statin use. SAS® version 9.3 (SAS Institute, Inc., Cary, North Carolina) was used for all statistical analyses.
3. Results
3.1. Characteristics of study population
Overall, 36.94% of the sample reported statin use. The mean duration of statin use in the exposed group was 3.7 ± 1.9 years and 85% of those on statins in 2004/2005 also reported statin use at time of MRI (2006/2008). Majority of the statin exposed participants in this sample were on lipophilic statins (simvastatin (42%), atorvastatin (40%), lovastatin (4%) and fluvastatin (<1.0%)) compared to hydrophilic statins (pravastatin (11%) and rosuvastatin (2%)).45
Table 1 compares the characteristics of the sample by statin exposure status. As expected, statin-exposed individuals had a higher proportion of cardiovascular disease. There was no difference in the average 3MS score in those with and without statin use. (Table 1).
Table 1.
Characteristics of sample and differences between statin exposed* vs unexposed.
| All | Statin exposed |
Statin unexposed |
P value | |
|---|---|---|---|---|
| Number | 295 | 109 | 186 | |
| Age (mean±SD), years | 80.1±2.8 | 79.8 | 80.3 | 0.300 |
| Women, N (%) | 168 (57%) | 55 (51%) | 113 (61%) | 0.089 |
| Black, N (%) | 118 (40%) | 75 (40%) | 43 (40%) | 0.902 |
| High school Education, N (%) | 256 (87%) | 97 (90%) | 159 (86%) | 0.367 |
| Hypertension, N (%) | 174 (60%) | 77 (71%) | 97 (52%) | 0.002 |
| Diabetes mellitus, N (%) | 48 (16%) | 25 (23%) | 23 (12%) | 0.022 |
| Coronary heart disease, N (%) | 64 (22%) | 37 (34%) | 27 (15%) | <0.001 |
| Stroke, N (%) | 23 (7%) | 9 (8%) | 14 (8%) | 0.825 |
| Alcohol consumption, N (%) | 172 (58%) | 66 (61%) | 106 (57%) | 0.624 |
| Peripheral arterial disease, N (%) | 10 (3%) | 7 (6%) | 3 (2%) | 0.042 |
| APOE4-positive status N (%) | 73 (26.1%) | 30 (28.0%) | 43 (24.9%) | 0.5557 |
| Serum LDL levels (mg/dl) | 115.1±31.7 | 96.0±24.8 | 125.9±30.2 | <0.0001 |
| 3MS | 92.77±6.17 | 93.1 | 92.3 | 0.30 |
Statin exposure defined as those identified as using a statin based on codes belonging to statin class of drugs.
3MS: Modified Mini-mental Status Examination
APOE4 positive stats: presence of apolipoprotein-epsilon 4 allele
LDL: low-density lipoprotein
The mean 3MS score for the lowest, middle and highest 3MS tertile were 86 (range: 59 to 92), 94 (range: 93 to 96) and 98 (97 to 100) respectively. Table 2 compares participants by statin exposure for those within the lowest tertile of 3MS. No significant differences were noted in demographic variables but as expected the statin-exposed group had a higher proportion of cardiovascular disease (p=0.02) and a lower LDL levels (p<0.0001).
Table 2.
Sample characteristics of statin exposed* vs statin unexposed participants among those in the lowest 3MS tertile (score range: 59 to 92, mean score: 86.4).
| Statin exposed |
Statin unexposed |
P value | |
|---|---|---|---|
| Number | 45 | 61 | |
| Age (mean±SD), years | 80.02 ± 2.86 | 80.7 ± 2.83 | 0.30 |
| Women N (%) | 22 (49%) | 37 (61%) | 0.24 |
| Black N (%) | 22 (49%) | 26 (42%) | 0.56 |
| High school Education N (%) | 35 (80%) | 40 (66%) | 0.13 |
| Hypertension N (%) | 30 (67%) | 41 (67%) | 1.00 |
| Diabetes mellitus N (%) | 11 (24%) | 10 (16%) | 0.33 |
| Coronary heart disease N (%) | 17 (38%) | 11 (18%) | 0.03 |
| Stroke N (%) | 2 (4%) | 6 (10%) | 0.46 |
| Alcohol consumption N (%) | 21 (47%) | 34 (56%) | 0.43 |
| Peripheral arterial disease N (%) | 3 (67%) | 2 (3%) | 0.65 |
| APOE4 positive status N (%) | 10 (23%) | 20 (35%) | 0.22 |
| Serum LDL levels (mg/dl) | 93.8±26.2 | 125.0±30.7 | <0.0001 |
Statin exposure defined as those identified as using a statin based on codes belonging to statin class of drugs.
APOE4 positive stats: presence of apolipoprotein-epsilon 4 allele
LDL: low-density lipoprotein
3.2. Statin exposure and indices of WM and GM integrity
In the whole sample (N=295) there were no statistically significant main effects of statins on total WMH (estimate= −0.74 × 10−3; SE= 0.48 × 10−3, CI= −1.7 × 10−3 to 0.2 × 10−3; p=0.12) or total WM FA (estimate= 0.00123; SE= 0.00179; CI= −0.231 × 10−2 to 0.477 × 10−2; p=0.49). These differences remained non-significant after adjustments for covariates (total WMH: estimate= −0.84 × 10−3; SE= 0.49 × 10−3, CI= −1.79 × 10−3 to 0.12 × 10−3; p=0.09 and total WM FA: estimate= 0.059 × 10−2; SE= 0.18 × 10−2; CI= −0.3 × 10−2 to 0.48 × 10−2; p=0.74). There was a significant interaction between statin exposure and 3MS for WMH (p=0.02) and FA (p=0.01) of total WM (with and without adjustments for covariates) suggesting differential associations between statin use and brain imaging measures across 3MS tertiles. In contrast, the interaction between statin exposure and 3MS was not significant for GM MD (p=0.1) and therefore further examination of differential association between statin exposure and cortical regional MD across 3MS tertiles was not examined.
3.3. WMH and FA Differences in WM by Statin Exposure within 3MS Tertiles
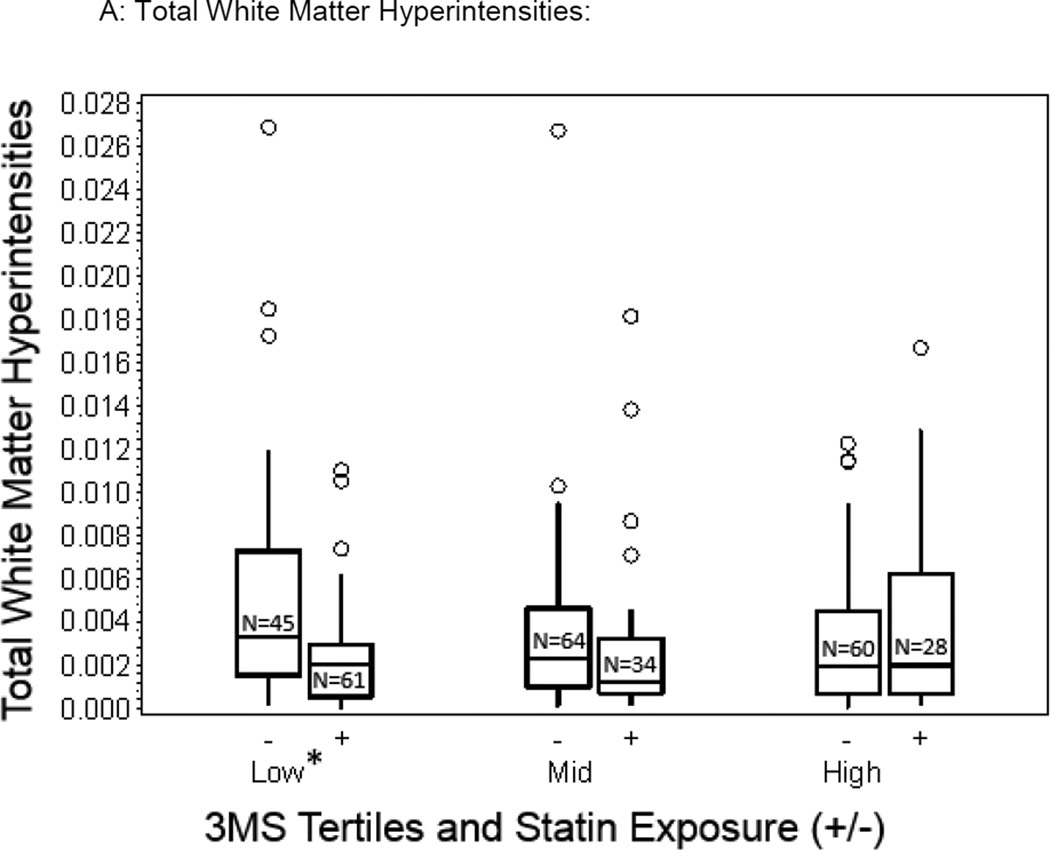
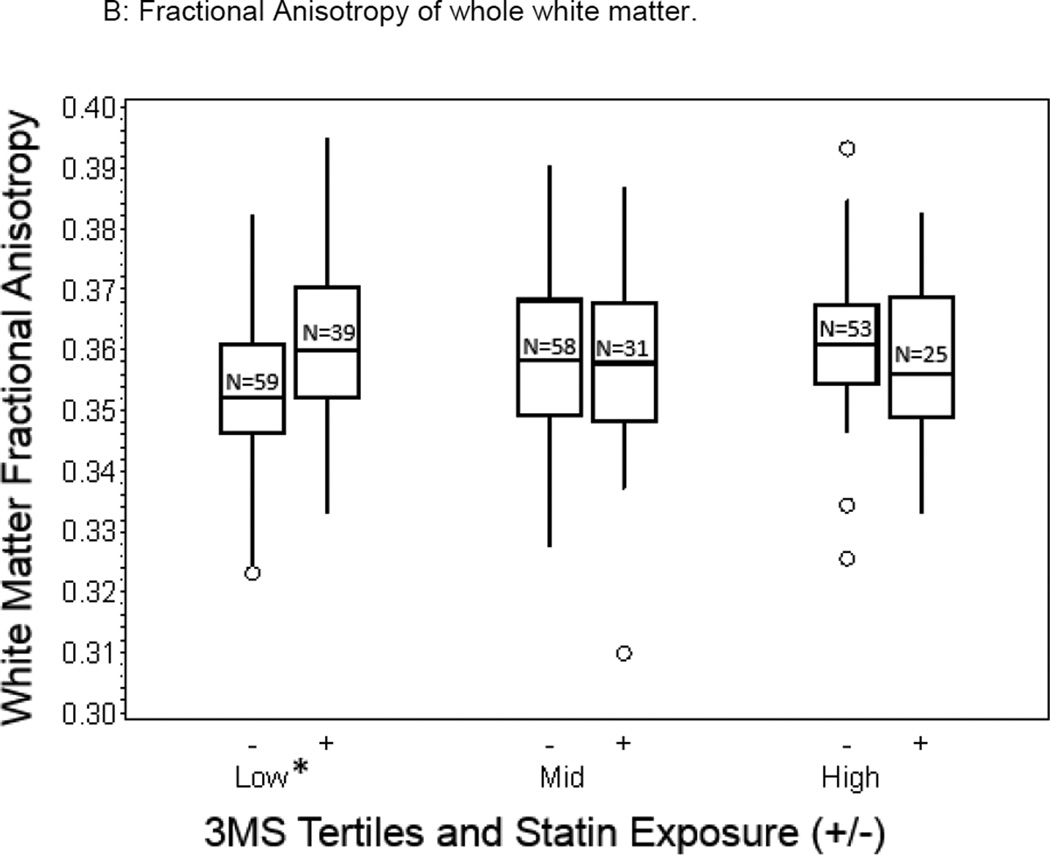
We compared differences in brain measures in statin exposed and unexposed within each 3MS tertile (Table 3). Differences in total WMH (Figure 1a) and total FA measures (Figure 1b) between the statin-exposed and statin unexposed groups were significant, both measures favoring the statin exposed group in the lowest 3MS tertile (p=0.005 and p=0.004 respectively), but not for those in the middle (p=0.408 and p=0.412) or highest 3MS tertiles (p=0.294 and p=0.240). Therefore, analyses of the spatial distribution of WMH and FA in relationship with statin exposure were examined for those in the lowest tertile. Specifically, there was a 0.23% lower total WMH volume favoring the statin exposed compared to the statin unexposed group for those in the lowest 3MS tertiles (2.52 × 10−3 vs 4.842 × 10−3, p=0.005, Figure 1a) and only a 0.06% lower and 0.09% higher volume of total WMH for those in the mid and highest 3MS tertiles respectively. Similarly, there was a 0.83% greater FA of total WM favoring the statin exposed compared to the statin unexposed group for those in the lowest 3MS tertile (0.36 vs 0.35, p=0.003, Figure 1b) whereas there was a 0.1% and 0.4% lower FA values in the two groups for those in the mid and highest 3MS tertiles respectively.
Table 3.
Mean Differences in WMH volumes and FA in statin exposed vs statin non-exposed groups in the lowest tertile of 3MS score (score range: 59 to 92, mean score: 86).
| Neuroimaging measures compared between exposed vs. unexposed |
Unadjusted comparison Estimate ± Standard Error (p value) |
Adjusted comparisonsa Estimate ± Standard Error (p value) |
Adjusted comparisons b Estimate ± Standard Error (p value) |
|---|---|---|---|
| White Matter Hyperintensities | |||
| Total white matter | −2.32 × 10−3 ± 0.81 × 10−3 (0.005) | −2.44 × 10−3 ± 0.8 × 10−3 (0.003) | −3.22 × 10−3 ± 0.9 × 10−3 (0.0009) |
| Anterior thalamic tract | −5.05 × 10−3 ± 0.262 × 10−3 (0.043) | −0.53 × 10−3 ± 0.27 × 10−3 (0.053) | −0.75 × 10−3 ± 0.30 × 10−3 (0.016) |
| Superior longitudinal fasciculus | −0.334 × 10−3 ± 0.17 × 10−3 (0.035) | −0.30 × 10−3 ± 0.17 × 10−3 (0.081) | −0.38 × 10−3 ± 0.19 × 10−3 (0.049) |
| Corpus callosum- genu | −0.304 × 10−3 ± 0.133 × 10−3 (0.002) | −0.32 × 10−3 ± 0.13 × 10−3 (0.018) | −0.34 × 10−3 ± 0.16 × 10−3 (0.035) |
| Corpus callosum-splenium | −0.487 × 10−3 ± 0.166 × 10−3 (0.016) | −0.49 × 10−3 ± 0.17 × 10−3 (0.005) | −0.57 × 10−3 ± 0.19 × 10−3 (0.0048) |
| Fractional Anisotropy values: | |||
| Total white matter | 8.35 × 10−3 ± 2.74 × 10 3 (0.004) | 7.94 × 10−3 ± 2.76 × 10 3 (0.005) | 7.23 × 10−3 ± 3.27 × 10 3 (0.030) |
| Anterior thalamic tract | 2.29 × 10−2 ± 0.817 × 10−2 (0.005) | 2.11 × 10−2 ± 0.84 × 102 (0.014) | 1.15 × 10−2 ± 0.87 × 102 (0.19) |
| Superior longitudinal fasciculus | 1.73 × 10−2 ± 0.781 × 10−2 (0.033) | 1.83 × 10−2 ± 0.78 × 10−2 (0.021) | 1.45 × 10−2 ± 0.98 × 10−2 (0.14) |
| Corpus callosum- genu | 0.80 × 10−2 ± 0.26 × 10−2 (0.002) | 0.79 × 10−2 ± 0.27 × 10−2 (0.005) | 0.89 × 10−2 ± 0.32 × 10−2 (0.007) |
| Corpus callosum-splenium | 0.14 × 10−2 ± 0.54 × 10−2 (0.009) | 1.38 × 10−2 ± 0.56 × 10−2 (0.015) | 1.04 × 10−2 ± 0.66 × 10−2 (0.122) |
adjusted for demographics (age, race, gender, education), health behaviors (smoking, alcohol use) and cardiovascular risk (angina, myocardial infarction, coronary artery bypass, angioplasty, diabetes mellitus, peripheral arterial disease, stroke).
adjusted for covariates in ‘a’ and, apolipoprotein epsilon-4 (ApoE4) and serum low-density lipoprotein levels (LDL).
Figure 1.
Box plots depicting unadjusted differences in total WMH and whole WM FA values in statin exposed and statin unexposed older adults across the 3MS tertiles (Lowest tertile: 3MS score of 59 to 92, mean=86; Middle tertile: 3MS score of 93 to 96, mean=95; Highest tertile: 3MS score of 97 to 100, mean=98; statistically significant adjusted differences are denoted by an asterix (p<0.05)).
3.4. WMH volumetric differences
Unadjusted differences in WMH volume between statin exposed and unexposed groups in the lowest 3MS tertile were statistically significant in all tracts examined and favored the statin exposed group varying from 0.03% lower WMH in the SLF (p=0.035) 0.05% in the ATR (p=0.043), 0.05% in sCC (p=0.00019) and 0.03% in gCC (p=0.0163). Results were overall similar after controlling for demographic, health behaviors, cardiovascular conditions for total WMH volume (p=0.0036) and for WMH volume in ATR (p=0.05), sCC (p=0.005) and gCC (p=0.019, Table 3). Further adjustment for APOE4 status and serum LDL levels did not substantially alter the statistically significant differences in WMH measures.
3.5. Fractional anisotropy differences
Unadjusted differences in FA favored the statin exposed group compared to the statin unexposed group in the lowest 3MS tertile and varied from 2.3% greater FA in the SLF (p=.0.006), 1.7% in the ATR (p=0.029), 1.4% in sCC (p=0.009) and 0.8% in the gCC (p=0.002). After controlling for demographic, health behaviors and cardiovascular conditions, the differences in FA values in the two groups were similar for SLF (p=0.014), ATR (p=0.021), sCC (p=0.015) and gCC (p=0.005, unadjusted and adjusted values are shown in Table 3). Inclusion of intracranial volume in the adjustments did not substantially alter the statistically significant differences in the FA values. Further adjustment for APOE4 status and serum LDL levels did not substantially alter the statistically significant differences in total FA measures in the statin exposed and unexposed groups but individual differences in FA of the SLF, ATR and sCC in the two groups were no longer statistically significant.
In the lowest 3MS tertile further analysis exploratory longitudinal analysis with duration of statin exposure as the predictor variable revealed that with every year of statin use, WMH volume decreased by 0.05% per year in total WM (p=0.014), 0.01% in both the sCC and gCC (p=0.013 and 0.053 respectively) while the decrease of 0.01% in the ATR and SLF was not significant (p=0.1 for both). Correspondingly, with each additional year of statin use, the increase in FA was statistically significant for sCC (0.4% increase, p=0.009) and gCC (0.3% increase, p=0.033) but showed a trend in the expected direction for total WM (0.1% increase, p=0.1) and, ATR and SLF (both 0.2% increase, p=0.1). After adjustment the results remained essentially unchanged. The duration of statin exposure in the statin exposed older adults had no statistically significant effects on any of the measures in those in mid and high 3MS tertiles.
Exploratory analysis comparing those ever exposed to statin to those never exposed to statins in the lowest 3MS tertile revealed significant differences (unadjusted) in WMH and FA for total WM (p=0.005 and p=0.003), ATR (p=0.05 and p=0.02), SLF (p=0.04 and p=0.006), sCC (p=0.02 and p=0.009) and gCC (p=0.004 and p=0.002), favoring the statin exposed individuals. After adjusting for demographic, health behaviors, cardiovascular condition and APOE4, the differences in total WMH volumes and total FA remained significant (p=0.008 and p=0.06) while WMH and FA differences in the specific WM tracts were less significant (p value between 0.06 and 0.1). Again, no statistically significant differences were observed between the statin groups in any WMH or FA measures in the mid and high 3MS tertiles.
3.6. Mean diffusivity differences
We found no statistically significant differences in whole GM MD (p=0.4) or in the MD of DLPFC (p=0.3), cingulate (p=0.2) or MTL (p=0.9) in the lowest 3MS tertile.
3.7 Analysis based on statin lipophilicity
Thirteen percent of statin-exposed individuals were identified as exposed to predominantly hydrophilic statins (pravastatin and rosuvastatin). Exclusion of these individuals and reanalysis of the data revealed no appreciable change in main differences in the total and regional WMH or total WM FA that were noted above. However, the statistically significant differences in regional FA measures (SLF, ATR and sCC) that were no longer significant after adjusting for covariates in the whole sample, in fact were statistically significant when the hydrophilic statin users were eliminated from the analysis. Specifically, in the lowest 3MS tertile, after adjustment of covariates that included APOE4 and LDL, differences in WMH and FA for total WM (p=0.0005 and p=0.04), ATR (p=0.02 and p=0.02), SLF (p=0.04 and p=0.006), sCC (p=0.006 and p=0.009) and gCC (p=0.02 and p=0.01), favored the lipophilic statin exposed individuals. These significant differences in WMH and FA measures were not seen in the higher 3MS tertiles.
4. Discussion
In this cohort of older adults living in the community, there was no overall relationship between statin exposure and WMH or FA measures. However, statin exposed older adults in the lowest cognitive performance strata were more likely to have more favorable WM structural integrity measures - specifically lower small-vessel disease burden (smaller WMH volumes, Figure 1a) and better WM tract integrity (higher WM FA values, Figure 1b), independent of demographic and cardiovascular risk and comorbidities. These results suggest that statins may have a beneficial influence on WM integrity in older adults with early evidence of cognitive impairment which in population-based samples is typically of mixed etiology (AD and vascular) providing putative in-vivo evidence for the possible pathways involved in the benefit of statins on cognition in vulnerable older adults.
Our results support other studies suggesting that timing of statin exposure may be relevant. A secondary analysis of the Ginkgo Evaluation Memory Study reported that in older adults without dementia cognitive benefit of statins was not evident in those with diagnosed mild cognitive impairment at baseline.12 Other studies also suggest that benefit of statins may be stronger in older adults in more early stages of cognitive impairment.7,46,47 Statins showed no benefit on cognition in more severe stages of AD8,9 and it is debated whether statin exposure influences AD pathology or biomarkers of AD.14,48 The findings of our study suggest that statins may also influence WM regions rather than GM in the brain of older adults, particularly in those with early cognitive impairment. We did not look at the effects of statins on cognitive measures and cannot contrast out study findings to the null effects of statins on cognition demonstrated in trials in mild-moderate stage AD 9,49 and other epidemiological studies.10,11 However, the positive effects of statins in our subgroup with the lowest 3MS scores could likely relate to the timing of statin exposure and its protective effect being limited to the earliest stages of dementia that supports prior observations that statins may affect the earliest stages of AD pathology. 12,13 In addition, neuroimaging markers are more sensitive to change than general measures of cognition used in the clinical trials and epidemiological studies. While the results of our study must be interpreted with caution, it brings forward an important question whether statins can prevent progressive structural changes in older adults with early signs of cognitive impairment. Larger studies focusing on this subgroup would enable more conclusive evidence.
The findings of our study that statins are associated with better WM indices in a sub-group of older adults are in contrast with others neuroimaging studies. The PROSPER trial of pravastatin showed a trend to lesser number of new infarcts in the treatment arm but failed to demonstrate an effect on progression of WMH volumes,50 which is likely because the PROSPER trial included a 1.5-T MRI, shorter duration of follow-up and their sample had a low prevalence of cardiovascular disease. The Cardiovascular Health Study revealed that statin use was not associated change in visually rated WMH severity over 5 years despite a reduced rate of cognitive decline in the statin-exposed group.46 These discrepancies in findings are also likely because the severity of small-vessel disease in these studies was visual rated and not based on more sensitive signal based rendering of WMH as applied in our neuroimaging protocol.
Several reasons could account for the putative protective effects of statins on WM in older adults with early cognitive impairment. Majority of brain cholesterol is located within the WM as cholesterol constitutes a large proportion of myelin sheath and is integral to myelin integrity and signal conduction within axons.51,52 Myelin degradation is hastened early in the course of small-vessel disease and AD leading to excess cerebral cholesterol metabolism and extracellular cholesterol deposition in the earliest stages of AD.53–55 Brain-specific cholesterol catabolites that are associated with development of incident cognitive impairment and severity of WMH54 are also shown to decline with 12-weeks of statin use without affecting AD biomarkers.53 Statins may also target isoprenoid regulation independent of its effects on cholesterol metabolism. 44,56 Isopreniods that are upstream in the cholesterol pathway are significantly elevated in the WM in AD. 56 Our results support these findings suggesting that statins may play a role in AD disease-specific targeting of cholesterol and isoprenoid regulation in the WM, which may reflect on better neuroimaging indices of myelin integrity and small-vessel disease. Moreover, WMH borne regions are particularly susceptible to hypoxic damage and statins have been shown to attenuate loss of WM integrity and hypomyelination and even increase axonal density in face of experimentally induced hypoxic injury in rodent models.57–59 Another potential mechanism could be related to statin effects on endothelium. Endothelial dysfunction is an integral step in the pathogenesis of small vessel disease.60,61 Subcortical WM where SLF, ATR and corpus callosum traverse, have a predilection to small-vessel disease.62 Statins may stabilize vascular endothelium and attenuate the progression of small-vessel disease and decrease WMH volume. 57,58,63Furthermore, blood-brain permeability increases in WMH rich regions including in the adjacent normal appearing WM.64 Lipophilic statins, which comprised the majority in our sample, readily cross the blood brain barrier and potentially exert further pleotropic effects that include anti-inflammation, amyloid clearance besides others.65,66 As lipophilicity of statins influences the pharmacokinetics of statin brain uptake and its potential neuroprotective effects 44 we performed an exploratory analysis that showed that after elimination of hydrophilic statins users, the group differences in total and regional WM and FA measures were statistically significant and independent of relevant covariates favoring the lipophilic statin exposed older adults. These reasons may explain why statin exposure in older adults with lower cognitive performance showed beneficial effect on small-vessel disease (lower WMH) and better WM microstructure (higher FA values).
APOE4 genetic status appears to influence statin effects on the brain. APOE4 is an established genetic risk factor for AD and is associated with both increased cerebral amyloid deposition and vascular risk factors.67 Furthermore, APOE4 status may differentially affect large association fibers (SLF and ATR) and posterior interhemispheric fibers (sCC) independent of WMH.20,68 Our analysis focused on these specific WM tracts. Furthermore, statins have a greater protective effect in APOE4 negative older adults without dementia than those who are APOE4 positive47 These reasons, in addition to the diminution in sample size by limiting to those with known APOE4 status, may point to why the inclusion of APOE4 status and LDL in the adjustment rendered the differences in regional WM microstructure (FA of the SLF, ATR and sCC) insignificant while the differences in total and regional WMH remained statistically significant. However, it is noteworthy that elimination of hydrophilic statin users retained the microstructural differences in the FA of SLF, ATR, gCC and sCC despite adjustment for APOE4 status and LDL. This suggests that lipophilicity of statins may play an important role in neuroprotective effects of statins on brain WM.
The implications of these findings is that in a general population of older adults, those in the presumably earliest stages of cognitive impairment/dementia on statins are likely to have beneficial WM structural indices. Lower WMH, indicative of lesser small-disease burden, and higher FA, indicative of better tract integrity, are linked to better cognitive and mobility measures in both older adults with and without AD. 21,69,70Taken together these findings suggest that better indices of WM in statin exposed older adults with poorer cognitive function may reflect better functional ability. We did not find an interaction between statin exposure and MD in gray matter regions which is supported by prior reports that statins do not affect cognitive performance,9,49 AD biomarkers,53 or AD pathology.48 Whether our findings of a 0.85% greater WM FA, 0.23% lower WMH volume and no significant difference on GM MD in older adults in the lowest cognitive performance strata on statins suggests that statins effects are localized to WM rather than GM or whether these effects translates to clinically identifiable functional outcomes needs to be addressed in future studies.
Several limitations of this analysis inherent to the observational nature of the data arise. However, the likelihood of selection bias or misclassification of neuroimaging brain measures is small. Despite adjustment for common indications of statins in this analysis we cannot entirely eliminate confounding by indication. As the indications for statins may also be related to risk of dementia one would expect that the two groups may not be entirely comparable. However, if statin-exposed individuals had a much higher burden of vascular diseases, then we would expect that this group would also have a worse WM integrity and greater prevalence of small-vessel disease, not better brain integrity and lesser small-vessel disease burden as we found. Another limitation is that the dosage of statins was not recorded and therefore not assessed. Lipophilic statins such as atorvastatin and simvastatin are more likely to cross the blood-brain barrier and penetrate the brain compared to hydrophilic statins such as pravastatin. 45 Finally, the generalizability of our findings to other samples is unknown.
Certain strengths of this study are worth noting. This analysis was based on a priori hypothesis using Health ABC data that was well characterized. Relying on the prospective nature of the data collected, this analysis also factored in longitudinal effects of statins by incorporating years of statin exposure in the ten year period prior to neuroimaging. The analytical methods were robust and accounted for important covariates associated with statin use and overall brain health. Lastly, the study incorporated sensitive neuroimaging measures to quantify small-vessel disease and GM and WM integrity.
In conclusion, this study suggests that in older adults with suboptimal cognition, statins may mitigate small-vessel disease burden and enable better WM integrity. Larger studies targeting vulnerable older adults are warranted to confirm these findings.
Acknowledgement
We would like to acknowledge Yihuang Kang, PhD and Wei Hsuan Lo Ciganic, PhD for their assistance with statistical analysis and computation of statin variables.
Study funding: This research was supported by the Intramural Research Program of the NIH, NIA (N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, NIA grants R01-AG028050, R01AG 027017, K07AG033174, NINR grant R01-NR012459), and the Pittsburgh Claude D. Pepper Older American's Independence Center (P30 AG024827).
List of Abbreviations
- AD
Alzheimer’s Disease (AD)
- WMH
White matter hyperintensities
- DTI
Diffusion tensor imaging
- FA
Fractional anisotropy
- MD
Mean diffusivity
- SLF
Superior longitudinal fasciculus
- sCC
splenium of corpus callosum
- gCC
genu of corpus callosum
- ATR
anterior thalamic radiation
- Health ABC
Health Aging and Body Composition
- 3MS
Modified Mini-Mental Status Examination
- LDL
low-density lipoprotein
- APOE4
apolipoprotein E-4 allele
References
- 1.Gorelick PB, Scuteri A, Black SE, et al. Vascular Contributions to Cognitive Impairment and Dementia: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2011 Sep;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003 Jan-Feb;22(1):1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- 3.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009 Aug;66(2):200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. Jama. 1997 Mar 12;277(10):813–817. [PubMed] [Google Scholar]
- 5.Kuller LH. Statins and dementia. Curr Atheroscler Rep. 2007 Aug;9(2):154–161. doi: 10.1007/s11883-007-0012-9. [DOI] [PubMed] [Google Scholar]
- 6.Bernick C, Katz R, Smith NL, et al. Statins and cognitive function in the elderly - The Cardiovascular Health Study. Neurology. 2005 Nov;65(9):1388–1394. doi: 10.1212/01.wnl.0000182897.18229.ec. [DOI] [PubMed] [Google Scholar]
- 7.Yaffe K, Barrett-Connor E, Lin F, Grady D. Serum lipoprotein levels, statin use, and cognitive function in older women. Arch Neurol. 2002 Mar;59(3):378–384. doi: 10.1001/archneur.59.3.378. [DOI] [PubMed] [Google Scholar]
- 8.Feldman HH, Doody RS, Kivipelto M, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010 Mar 23;74(12):956–964. doi: 10.1212/WNL.0b013e3181d6476a. [DOI] [PubMed] [Google Scholar]
- 9.Sano M, Bell KL, Galasko D, et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology. 2011 Aug 9;77(6):556–563. doi: 10.1212/WNL.0b013e318228bf11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: II. Review of human trials and recommendations. Arch Neurol. 2011 Nov;68(11):1385–1392. doi: 10.1001/archneurol.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: I. Review of epidemiological and preclinical studies. Arch Neurol. 2011 Oct;68(10):1239–1244. doi: 10.1001/archneurol.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bettermann K, Arnold AM, Williamson J, et al. Statins, risk of dementia, and cognitive function: secondary analysis of the ginkgo evaluation of memory study. J Stroke Cerebrovasc Dis. 2012 Aug;21(6):436–444. doi: 10.1016/j.jstrokecerebrovasdis.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sparks DL, Sabbagh M, Connor D, et al. Statin therapy in Alzheimer's disease. Acta Neurol Scand Suppl. 2006;185:78–86. doi: 10.1111/j.1600-0404.2006.00689.x. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Larson EB, Sonnen JA, et al. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology. 2007 Aug 28;69(9):878–885. doi: 10.1212/01.wnl.0000277657.95487.1c. [DOI] [PubMed] [Google Scholar]
- 15.Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008 Jul 8;71(2):108–113. doi: 10.1212/01.wnl.0000316799.86917.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith CD, Chebrolu H, Andersen AH, et al. White matter diffusion alterations in normal women at risk of Alzheimer’s disease. Neurobiol Aging. 2010 Jul;31(7):1122–1131. doi: 10.1016/j.neurobiolaging.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bozzali M, Giulietti G, Basile B, et al. Damage to the cingulum contributes to Alzheimer’s disease pathophysiology by deafferentation mechanism. Hum Brain Mapp. 2012 Jun;33(6):1295–1308. doi: 10.1002/hbm.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sexton CE, Mackay CE, Lonie JA, et al. MRI correlates of episodic memory in Alzheimer’s disease, mild cognitive impairment, and healthy aging. Psychiatry Res. 2010 Oct 30;184(1):57–62. doi: 10.1016/j.pscychresns.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Radanovic M, Pereira FR, Stella F, et al. White matter abnormalities associated with Alzheimer’s disease and mild cognitive impairment: a critical review of MRI studies. Expert Rev Neurother. 2013 May;13(5):483–493. doi: 10.1586/ern.13.45. [DOI] [PubMed] [Google Scholar]
- 20.Lee DY, Fletcher E, Martinez O, et al. Vascular and degenerative processes differentially affect regional interhemispheric connections in normal aging, mild cognitive impairment, and Alzheimer disease. Stroke. 2010 Aug;41(8):1791–1797. doi: 10.1161/STROKEAHA.110.582163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kavcic V, Ni H, Zhu T, Zhong J, Duffy CJ. White matter integrity linked to functional impairments in aging and early Alzheimer's disease. Alzheimers Dement. 2008 Nov;4(6):381–389. doi: 10.1016/j.jalz.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Arch Neurol. 2003 Oct;60(10):1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 23.Barnes DE, Cauley JA, Lui LY, et al. Women who maintain optimal cognitive function into old age. J Am Geriatr Soc. 2007 Feb;55(2):259–264. doi: 10.1111/j.1532-5415.2007.01040.x. [DOI] [PubMed] [Google Scholar]
- 24.Yaffe K, Fiocco AJ, Lindquist K, et al. Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology. 2009 Jun 9;72(23):2029–2035. doi: 10.1212/WNL.0b013e3181a92c36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011 Sep;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001 Oct;56(10):M644–M649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 27.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987 Aug;48(8):314–318. [PubMed] [Google Scholar]
- 28.Ebly EM, Hogan DB, Parhad IM. Cognitive impairment in the nondemented elderly. Results from the Canadian Study of Health and Aging. Arch Neurol. 1995 Jun;52(6):612–619. doi: 10.1001/archneur.1995.00540300086018. [DOI] [PubMed] [Google Scholar]
- 29.Wu M, Rosano C, Butters M, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006 Dec 1;148(2–3):133–142. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001 Jan;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 31.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 32.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996 Dec;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 33.Andersson JLR, Jenkinson M. Non-linear registration, aka spatial normalisation. FMRIB Technical Report TR07JA2. 2007 www.fmrib.ox.ac.uk/analysis/techrep.
- 34.Barrick TR, Charlton RA, Clark CA, Markus HS. White matter structural decline in normal ageing: a prospective longitudinal study using tract-based spatial statistics. Neuroimage. 2010 Jun;51(2):565–577. doi: 10.1016/j.neuroimage.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 35.Spilt A, Geeraedts T, de Craen AJ, Westendorp RG, Blauw GJ, van Buchem MA. Age-related changes in normal-appearing brain tissue and white matter hyperintensities: more of the same or something else? AJNR Am J Neuroradiol. 2005 Apr;26(4):725–729. [PMC free article] [PubMed] [Google Scholar]
- 36.Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007 Jul 1;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002 Jan;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 38.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004 Jan;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 39.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994 Aug;10(4):405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001 Apr 4;285(13):1711–1718. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 41.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008 Nov 20;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 42.Heart Protection Study Collaborative G. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002 Jul 6;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 43.Sharp Collaborative G. Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J. 2010 Nov;160(5):785–794. doi: 10.1016/j.ahj.2010.08.012. e710. [DOI] [PubMed] [Google Scholar]
- 44.Wood WG, Eckert GP, Igbavboa U, Muller WE. Statins and neuroprotection: a prescription to move the field forward. Ann N Y Acad Sci. 2010 Jun;1199:69–76. doi: 10.1111/j.1749-6632.2009.05359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005 Feb;19(1):117–125. doi: 10.1111/j.1472-8206.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 46.Bernick C, Katz R, Smith NL, et al. Statins and cognitive function in the elderly: the Cardiovascular Health Study. Neurology. 2005 Nov 8;65(9):1388–1394. doi: 10.1212/01.wnl.0000182897.18229.ec. [DOI] [PubMed] [Google Scholar]
- 47.Steenland K, Zhao L, Goldstein FC, Levey AI. Statins and cognitive decline in older adults with normal cognition or mild cognitive impairment. J Am Geriatr Soc. 2013 Sep;61(9):1449–1455. doi: 10.1111/jgs.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arvanitakis Z, Schneider JA, Wilson RS, et al. Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology. 2008 May 6;70(19 Pt 2):1795–1802. doi: 10.1212/01.wnl.0000288181.00826.63. [DOI] [PubMed] [Google Scholar]
- 49.Feldman HH, Doody RS, Kivipelto M, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010 Mar 23;74(12):956–964. doi: 10.1212/WNL.0b013e3181d6476a. [DOI] [PubMed] [Google Scholar]
- 50.ten Dam VH, van den Heuvel DM, van Buchem MA, et al. Effect of pravastatin on cerebral infarcts and white matter lesions. Neurology. 2005 May 24;64(10):1807–1809. doi: 10.1212/01.WNL.0000161844.00797.73. [DOI] [PubMed] [Google Scholar]
- 51.Bjorkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol. 2004 May;24(5):806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 52.Saher G, Simons M. Cholesterol and myelin biogenesis. Sub-cellular biochemistry. 2010;51:489–508. doi: 10.1007/978-90-481-8622-8_18. [DOI] [PubMed] [Google Scholar]
- 53.Serrano-Pozo A, Vega GL, Lutjohann D, et al. Effects of simvastatin on cholesterol metabolism and Alzheimer disease biomarkers. Alzheimer Dis Assoc Disord. 2010 Jul-Sep;24(3):220–226. doi: 10.1097/WAD.0b013e3181d61fea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hughes TM, Kuller LH, Lopez OL, et al. Markers of cholesterol metabolism in the brain show stronger associations with cerebrovascular disease than Alzheimer's disease. J Alzheimers Dis. 2012;30(1):53–61. doi: 10.3233/JAD-2012-111460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hughes TM, Lopez OL, Evans RW, et al. Markers of cholesterol transport are associated with amyloid deposition in the brain. Neurobiol Aging. 2014 Apr;35(4):802–807. doi: 10.1016/j.neurobiolaging.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eckert GP, Hooff GP, Strandjord DM, et al. Regulation of the brain isoprenoids farnesyl- and geranylgeranylpyrophosphate is altered in male Alzheimer patients. Neurobiol Dis. 2009 Aug;35(2):251–257. doi: 10.1016/j.nbd.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shehadah A, Chen J, Cui X, Roberts C, Lu M, Chopp M. Combination treatment of experimental stroke with Niaspan and Simvastatin, reduces axonal damage and improves functional outcome. J Neurol Sci. 2010 Jul 15;294(1–2):107–111. doi: 10.1016/j.jns.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li A, Lv S, Yu Z, et al. Simvastatin attenuates hypomyelination induced by hypoxia-ischemia in neonatal rats. Neurol Res. 2010 Nov;32(9):945–952. doi: 10.1179/016164110X12670144737774. [DOI] [PubMed] [Google Scholar]
- 59.Li Q, Zhuang QK, Yang JN, Zhang YY. Statins excert neuroprotection on cerebral ischemia independent of their lipid-lowering action: the potential molecular mechanisms. European review for medical and pharmacological sciences. 2014;18(8):1113–1126. [PubMed] [Google Scholar]
- 60.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013 May;12(5):483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thompson CS, Hakim AM. Living beyond our physiological means: small vessel disease of the brain is an expression of a systemic failure in arteriolar function: a unifying hypothesis. Stroke. 2009 May;40(5):e322–e330. doi: 10.1161/STROKEAHA.108.542266. [DOI] [PubMed] [Google Scholar]
- 62.Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009 Mar;40(3 Suppl):S48–S52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- 63.Sterzer P, Meintzschel F, Rosler A, Lanfermann H, Steinmetz H, Sitzer M. Pravastatin improves cerebral vasomotor reactivity in patients with subcortical small-vessel disease. Stroke. 2001 Dec 1;32(12):2817–2820. doi: 10.1161/hs1201.099663. [DOI] [PubMed] [Google Scholar]
- 64.Topakian R, Barrick TR, Howe FA, Markus HS. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry. 2010 Feb;81(2):192–197. doi: 10.1136/jnnp.2009.172072. [DOI] [PubMed] [Google Scholar]
- 65.Quist-Paulsen P. Statins and inflammation: an update. Current opinion in cardiology. 2010 Jul;25(4):399–405. doi: 10.1097/HCO.0b013e3283398e53. [DOI] [PubMed] [Google Scholar]
- 66.Fassbender K, Simons M, Bergmann C, et al. Simvastatin strongly reduces levels of Alzheimer’s disease beta-amyloid peptides A beta 42 and A beta 40 in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 2001 May;98(10):5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reed BR, Marchant NL, Jagust WJ, DeCarli CC, Mack W, Chui HC. Coronary risk correlates with cerebral amyloid deposition. Neurobiol Aging. 2012 Sep;33(9):1979–1987. doi: 10.1016/j.neurobiolaging.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heise V, Filippini N, Ebmeier KP, Mackay CE. The APOE varepsilon4 allele modulates brain white matter integrity in healthy adults. Mol Psychiatry. 2011 Sep;16(9):908–916. doi: 10.1038/mp.2010.90. [DOI] [PubMed] [Google Scholar]
- 69.Nadkarni NK, McIlroy WE, Mawji E, Black SE. Gait and subcortical hyperintensities in mild Alzheimer's disease and aging. Dement Geriatr Cogn Disord. 2009;28(4):295–301. doi: 10.1159/000245158. [DOI] [PubMed] [Google Scholar]
- 70.Venkatraman VK, Aizenstein HJ, Newman AB, et al. Lower Digit Symbol Substitution Score in the Oldest Old is Related to Magnetization Transfer and Diffusion Tensor Imaging of the White Matter. Front Aging Neurosci. 2011;3:11. doi: 10.3389/fnagi.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]