Abstract
Spinal cord injury (SCI) disrupts the long axonal tracts of the spinal cord leading to devastating loss of function. Cell transplantation in the injured spinal cord has the potential to lead to recovery after SCI via a variety of mechanisms. One such strategy is the formation of neuronal relays between injured long tract axons and denervated neurons. The idea of creating a neuronal relay was first proposed over 25 years ago when fetal tissue was first successfully transplanted into the injured rodent spinal cord. Advances in labeling of grafted cells and the development of neural stem cell culturing techniques have improved the ability to create and refine such relays. Several recent studies have examined the ability to create a novel neuronal circuit between injured axons and denervated targets. This approach is an alternative to long-distance regeneration of damaged axons that may provide a meaningful degree of recovery without direct recreation of lost pathways. This brief review will examine the contribution of fetal grafting to current advances in neuronal grafting. Of particular interest will be the ability of transplanted neurons derived from fetal grafts, neural precursor cells and neural stem cells to reconnect long distance motor and sensory pathways of the injured spinal cord.
Keywords: spinal cord injury, transplantation, neuronal relay, fetal graft, neural stem cells, neural progenitor cells
Introduction
Spinal cord injury (SCI) disrupts the neuronal connections between the brain and the periphery, resulting in a devastating loss of function. Endogenous regeneration fails after SCI due to a combination of factors, including extrinsic signaling molecules in the adult spinal cord that limit growth and the intrinsic growth state of injured neurons. Cell transplantation is an attractive strategy for SCI because transplanted cells have the potential to modulate the extrinsic environment, provide a growth substrate for injured axons and to replace cells lost after injury. Over the past several decades many cellular grafting strategies, with varied rationales have been studied in animal models of SCI (reviewed, Kwon et al., 2011). One specific strategy, neuronal relay formation, uses grafts capable of generating neurons within the injury site to function as novel interneurons between the injured axons and denervated neurons distal to the injury (Abematsu et al., 2010; Bonner et al., 2011; Fujimoto et al., 2012; Hou et al., 2013; Lee et al., 2014; Lu et al., 2012; Medalha et al., 2014; Sharp et al., 2014). The advantages of this approach are that injured axons do not need to regenerate long distances and the onus for axon growth is placed on the transplant-derived neurons, which can be selected for the ability to extend long axons in vivo. Attempts to create neuronal relays for SCI repair began with the grafting of whole pieces of fetal CNS tissue in the injured spinal cord (Bregman and Reier, 1986; Reier et al., 1986). Recent technical advances such as the development of neural stem cell (NSC) and neural progenitor cell (NPC) culturing as well as the availability of genetically labeled cells have made the results of transplantation studies easier to interpret and led to the improvement of neuronal relays. The goal of this brief review is to re-examine the seminal work that first proposed the ability for neuronal relay formation in the injured spinal cord with an eye towards the further development and refinement of neuronal relay therapies.
Spinal cord injury and endogenous neuronal relay formation
The idea that formation of new relays might restore function is based on processes that occur naturally in the injured nervous system. Despite the barriers to regeneration, some spontaneous recovery occurs after incomplete SCI as the result of sprouting and plasticity. In one well studied example of such plasticity, axons of the corticospinal tract (CST) can sprout rostral to an incomplete injury and synapse with long propriospinal axons that are spared around the injury site (Bareyre et al., 2004), making a new, relay circuit. This new circuit shows signs of activity dependent synaptic pruning and has a direct role in recovery of function (Courtine et al., 2008). Thus, the adult CNS – while unable to regenerate – remains capable of reorganizing existing neurons into novel circuits and using these connections in a meaningful way.
Recovery of function due to circuit reorganization suggests that transplant-derived neuronal relays might also contribute to recovery. Using these endogenous neuronal relays as a reference point, it is highly plausible that supraspinal centers would be able to adapt to novel neuronal connections and use those new connections functionally. Moreover, it seems likely that functional reorganization of transplant mediated neuronal relays would be further enhanced by training procedures commonly employed to improve plasticity mediated recovery of function, including task specific (Dai et al., 2011; Hurd et al., 2013), non-specific (Ward et al., 2014) and the training that is embedded in the testing procedures used to assess function (Girgis et al., 2007).
The Neuronal Relay Strategy of SCI Repair
This review will consider some of the conceptual issues underlying the strategy of using transplant derived neurons to create a novel relay circuit between injured axons in the spinal cord and distal target neurons. This repair strategy differs from traditional neuronal replacement in that a new circuit is established rather than the repair of a pre-existing but damaged circuit (Figure 1). Within the context of SCI, neuronal replacement would attempt to restore local interneuron populations or motor neurons, whereas a neuronal relay attempts to add new neurons at the site of the injury to restore communication along the long tracts of the spinal cord. The neuronal relay strategy differs from cellular/tissue bridges such as glial restricted progenitor (GRP) grafts and peripheral nerve grafts which can be used as a substratum that provides structural and trophic support through the injury site. Cellular bridges can effectively allow injured axons to grow into a permissive bridge but axons rarely re-enter the host spinal cord, with a few notable exceptions (Alto et al., 2009; Houle et al., 2006; Taylor et al., 2006; Tom et al., 2009). Neuronal relays have the advantage of utilizing NPC or NSC derived neurons that are already capable of extending long axons in the injured spinal cord rather than trying to induce long-distance growth capability in injured neurons.
Figure 1.
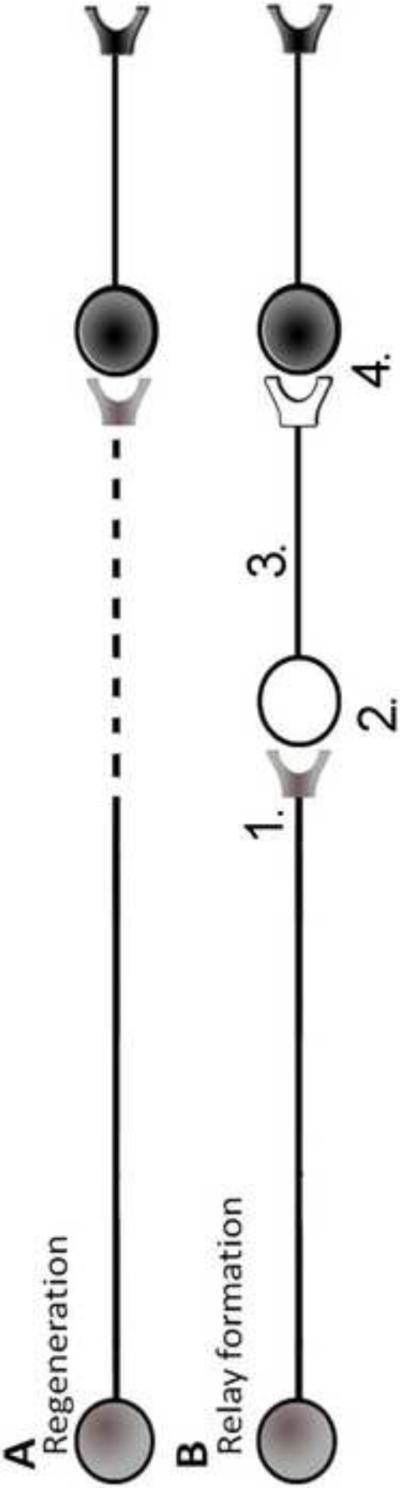
Regeneration and relay formation are 2 different models of spinal cord injury repair. (A) Regeneration, in the simplest form, involves in the regrowth of an injured axon back to the original target. While regeneration is a highly desirable outcome it has proved difficult to achieve. (B) Relay formation, again in the simplest form, involves the insertion of a new neuron between the injured axon and the target neuron. Relay formation has the advantage of utilizing developmentally young neurons that are intrinsically primed for growth in the injured spinal cord, while also having the disadvantage of requiring the creation of a novel CNS circuit.
Demonstrating a neuronal relay
The simplest neuronal relay is made up of 3 neurons: The injured neuron, the transplant derived neuron and the target neuron. Anatomical analysis of a neuronal relay requires the ability to identify the 3 component neurons. The development of a neuronal relay can be divided into 4 discrete steps: 1) synapse formation between injured axons and graft neurons, 2) generation of appropriate neuronal phenotypes (i.e. neurotransmitters) at the transplant site, 3) extension of axons from the graft to a target and 4) synapse formation between graft axons and a host target (Figure 1). Identifying grafted neurons can be achieved by isolating cells from transgenic rats or mice that express a marker such as green fluorescent protein (GFP) and Alkaline Phosphatase (AP) or by infecting cells with a vector that induces marker expression (Boldogkoi et al., 2002). Human cells can be identified with antibodies such as anti-human cytoplasmic antigen (SC121), which labels axons as well as cell bodies. Genetic labeling is preferable to cellular dyes, which can be diluted by proliferation or spread to other cell types in the host and give inconclusive results.
Injured and target host neurons can be identified by the lack of a transgenic marker, but the presence of an anterograde (injured) or retrograde (target) tracer will provide less ambiguous results. Retrograde tracers can also be applied to the transplant in order to label host axons projecting into the graft, but diffusion must be considered as a possible confound, particularly when looking at locally projecting neurons. Transsynaptic viral labeling such as pseudorabies virus (PRV) (Lee et al., 2014) and wheat germ agglutinin (WGA) expressing vectors (Fujimoto et al., 2012) can be used to trace the entire circuit, but alternate routes of viral transmission should be considered when interpreting data from incomplete injuries. Although the available data supports the hypothesis that both PRV and WGA are transferred at active synaptic connections, many of those studies have been conducted in vitro (Hogue et al., 2014) or intact CNS (Ohashi et al., 2011) rather than a relay model of SCI repair. The release of vesicles from growth cones (Sabo and McAllister, 2003; Tojima et al., 2007), or the establishment and retraction of functional synapses within a relay could lead to labeling of neurons without permanent synaptic connections. Transsynaptic labeling is most useful in the study of relay circuits when used in conjunction with other methods, such as behavioral outcome measures (Lee et al., 2014), to understand relay connections.
Fetal grafts: the first steps toward the creation of a neuronal relay
Reports of attempts to transplant CNS tissue date back to the late 19th century and over the years, many preparations of fetal grafts have been attempted, across different species and with different isolation techniques (for a historical review, see Das, 1990). While much of the early transplantation work focused on embryology and development, by the 1970s modern attempts at therapeutic transplantation of specific neuronal populations began. Bjorklund and colleagues demonstrated that fetal dopaminergic neurons could partially reinnervate the denervated hippocampus (Bjorklund et al., 1976) and that septal neurons could integrate into the hippocampus (Segal et al., 1985), rescuing spatial memory in aged rats (Gage et al., 1984). The success of these studies was driven in part by the ability to largely destroy host monoaminergic neurons with specific toxins (e.g. by using 6-OHDA to destroy host dopamine neurons) and to also visualize transplanted monoaminergic neurons using the Falck-Hillarp fluorescent method. These studies demonstrated that cells could be harvested from fetal tissue, transplanted into the diseased CNS and lead to functional recovery, while also highlighting the practical importance of being able to identify neurons and their axons after transplantation. Successful and reliable identification of transplanted cells and their processes allows for the fate analysis of neurons after transplantation, a key methodological consideration for relay formation.
Because long tracts are mostly glutamatergic, a guiding rationale for relay repair strategies for SCI is to use excitatory interneurons that respond to and releases glutamate. A source of glutamatergic neurons that can survive and integrate in the microenvironment of the injured spinal cord is a requirement for the development of such a therapeutic neuronal relay. Reier and colleagues established the ability of grafts of fetal spinal cord and fetal cortex to survive transplantation in the lateral ventricles (Reier et al., 1983), the injured brain (Jakeman et al., 1989) and the injured spinal cord (Bregman and Reier, 1986). These studies pre-dated the availability of genetically labeled cells, so identification of host-graft border, cell migration and axon growth was based on less reliable methods such as neuronal tracers (Jakeman and Reier, 1991) and autoradiography (Bregman and Reier, 1986). Despite these technical limitations, retrograde labeling demonstrated that E12-E14 fetal grafts generated neurons that spontaneously extended axons up to 5mm long after transplantation in the injured spinal cord (Reier et al., 1986). This finding was later confirmed with AP-labeled E14 fetal grafts (Lepore and Fischer, 2005; Medalha et al., 2014). Further studies demonstrated the complexity of the neuronal crosstalk between fetal grafts and the injured host (Jakeman and Reier, 1991), extended these findings to chronically injured spinal cord (Houle and Reier, 1988; Houle and Reier, 1989) and later demonstrated the potential of fetal graphs to modulate phrenic motor output and ventilation following SCI (Lee et al., 2014; White et al., 2010).
Tessler and colleagues transplanted E14.0 fetal tissue into a dorsal hemisection injury and re-implanted the avulsed dorsal root into the fetal graft to study the interaction between regenerating host dorsal root axons and grafted fetal tissue. Immunhistochemical-electron microscopy (immuno-EM) for calcitonin gene related peptide (CGRP) demonstrated the presence of host-derived presynaptic connections between regenerating CGRP+ dorsal root axons and graft derived neurons (Itoh and Tessler, 1990; Itoh et al., 1993a). They later studied the function of synapses by stimulating the dorsal root and recording within the fetal graft and showed that the grafted neurons received excitatory synaptic input from the regenerating dorsal root axons (Itoh et al., 1993b). This established the proof of principle that grafted tissue could receive synaptic input from the host and that the adult CNS axons could form novel synaptic connections with grafted tissue.
Fetal grafts contain neural stem cell and neural progenitor cells
The development of NSC culturing techniques, and particularly the ability to isolate single cells for clonal culture, led to the identification of the cell types present in E14.0 fetal grafts. The E10.5 rat spinal cord contains neuroepithelial cells (NEP), a multipotent NSC capable of producing neurons, oligodendrocytes and astrocytes (Kalyani et al., 1997) whereas neuronal restricted progenitors (NRP) (Mayer-Proschel et al., 1997) and glial restricted progenitors (GRP) (Rao et al., 1998) appear in the fetal spinal cord by E13.5. At E14.0, fetal grafts contain approximately 10% NEP, 80% NRP/GRP and 10% post-mitotic neural or non-neural cells (Cai et al., 2002). Since NEP and NRP/GRP require different culture substrates and media, culturing cells derived from fetal spinal cord allows for the selection of NEP and NRP/GRP.
Fetal grafts also contain non-cellular elements such as extracellular matrix molecules that may enhance the survival, differentiation and integration of cells in the fetal grafts. Fetal grafts undergo extensive cell death immediately after transplantation in the injured spinal cord and the surviving cells expand to fill the lesion cavity (Lepore and Fischer, 2005; Theele et al., 1996). Interestingly, isolated and cultured NRP/GRP survive and integrate in the lesioned spinal cord, while isolated and cultured NEP do not (Lepore et al., 2004), suggesting that NRP/GRP present in fetal grafts repopulate the lesion site. However, freshly isolated E14.0 fetal grafts survive in a full transection lesion (Medalha et al., 2014) whereas it has been reported (data not shown) that fetal spinal cord tissue treated with the protease trypsin (Lu et al., 2012) does not survive well unless treated with a growth factor cocktail and fibrin. Many conditions may account for the failure of grafted cells to survive, and it is especially difficult to draw conclusions from data that are not shown. Nevertheless, one possible conclusion is that protease treatment may degrade extracellular factors present in the fetal grafts that are important for survival and/or proliferation of NEP, NRP/GRP or both.
Axon growth of transplanted neuron in the adult CNS
To create relays that restore transmission along long tracts, transplant derived neurons must extend long axons and synapse with a target. Transplant derived axons must overcome the same inhibitory molecules that prevent endogenous axon growth but it appears that transplant derived neurons have the intrinsic state to overcome those barriers. While myelin associated molecules are inhibitory to axon growth in vitro, at least some adult neurons (Davies et al., 1997) and NRP derived neurons (Jin et al., 2012) are capable of extending long axons along the intact white matter of the adult CNS. Similarly, transplanted DRG axons grow along intact white matter in the injured spinal cord until reaching a chondroitin sulfate proteoglycan (CSPG) rich glial scar (Davies et al., 1999). Thus, transplanted neurons have been shown to extend axons along long white matter tracts if they can avoid or overcome CSPG boundaries.
Recent evidence has demonstrated that axons express at least 2 unique receptors that respond to CSPG (Fisher et al., 2011; Shen et al., 2009) and that the distribution of CSPGs, particularly in a molecular gradient (Kuboyama et al., 2013), play a role in the entrapment of growing axons within the glial scar (Cregg et al., 2014). In vitro evidence suggests two potential mechanisms for improved growth of NRP derived axons on CSPG: 1) neurons derived from NRP express low levels of CSPG receptors compared to other neuronal populations and 2) GRP secrete a factor that limits the inhibitory effect of CSPG on axon growth (Ketschek et al., 2012). After transplantation, GRP can physically disrupt the glial scar, reducing the physical and chemical inhibition of the scar (Haas et al., 2012). Thus, GRP present in a fetal graft or NRP/GRP graft may improve the ability of injured axons to make synaptic connections with graft derived neurons by limiting the impact of the glial scar.
Whole tissue rat fetal grafts readily extend axons several mm long in the partially injured spinal cord (Jakeman and Reier, 1991; Lepore and Fischer, 2005) and mechanically dissociated E14.0 rat fetal grafts extend axons of several mm in the fully transected rat spinal cord (Medalha et al., 2014). Enzymatically dissociated fetal grafts require supplementation with a cocktail of trophic factors to survive and extend axons in a full transection injury (Lu et al., 2012). The same cocktail produces similar results when applied to fetal brain stem derived monoaminergic precursors in the fully transected spinal cord (Hou et al., 2013). NRP/GRP grafts fail to spontaneously extend long axons when grafted acutely in the injured spinal cord (Lepore and Fischer, 2005), but respond to a single dose of exogenous BDNF by extending axons for several mm in both the rostral and caudal directions along white matter tracts. NRP/GRP respond to a BDNF gradient by preferentially extending axons towards the source of BDNF (Bonner et al., 2010), indicating that graft derived axons can be specifically guided towards a chosen target. Furthering this concept, Smith and colleagues demonstrated that adult dorsal root ganglion (DRG) neurons transplanted into the corpus callosum would extend axons along a preformed pathway of NGF for the full length of the corpus callosum, even crossing a midline injury site (Jin et al., 2008). Later studies demonstrated that by combining NGF with a repulsive cue, semaphorin 3A (Sema 3A) DRG axons could be induced to make a 90° turn from the corpus callosum into the striatum (Ziemba et al., 2008). A similar study demonstrated the same principles with dopaminergic neurons and gradients of GDNF and Netrin-1 (Zhang et al., 2013). These studies demonstrate that combining attractive and repulsive cues, as well as diffusible and matrix cues, may generate complex axon phenotypes that will be required to make specific connections between injured axons and denervated targets. Overall, the published data suggests that applying exogenous growth factors at time of transplantation may maximize the number of axons extending from a graft while viral vectors may be useful for directed growth and axon path finding.
Specificity and fidelity of information transfer in a non-specific network
The idea of relay formation is to bridge between injured axons and their normal targets to restore information transfer. In the normal brain and spinal cord, circuit function is based on highly specific connections that are formed early in development based on axonal guidance cues (Baier and Bonhoeffer, 1992; Kennedy et al., 2006), molecular specificity of synapses (Schuman et al., 2006), and activity-dependent remodeling (Martin et al., 1999). Relays formed by grafts are by definition novel, and the connections made by grafted neurons will not be as specific as the original connections of the damaged axons. Even if graft-derived neurons connect with the normal targets of damaged axons, it is likely that they will also connect with other neurons that are not normally targets of the damaged axons (Bonner et al., 2011). Refinements of relay circuit strategies may require approaches to enhance specificity of connections made by transplanted neurons and/or develop approaches (perhaps through re-training and activity dependent plasticity) to enable the nervous system to exert specific activation of components of a non-specifically organized circuit.
Another conceptual consideration in terms of relay circuits is whether relay neurons transmit information that is actually related to what is coming in via the host axon. For example, if relay neurons have an inhibitory phenotype when the original connection was excitatory then the relay will likely fail to restore function. Sources of transplants can be selected for their ability to generate particular phenotypes in vivo (Bonner et al., 2013; Braz et al., 2012; Hou et al., 2013; White et al., 2010), and protocols exist to promote specific neuronal phenotypes (Hu and Zhang, 2010; Kirkeby et al., 2012) in order to improve relay function. Alternatively, transplants containing multiple phenotypes may contribute to the repair of multiple spinal cord pathways and circuits. This strategy is likely to require training to organize a specific function after transplantation. Lee et al (2012) used hypoxia to improve task specific function after transplantation of fetal grafts in a C2 hemisection and the result suggests that specific training techniques may improve information transfer through graft derived neurons.
If the relay neuron matches the phenotype of the original circuit, the interposed neuron is likely to integrate inputs, so output will not perfectly reflect input (Figure 2). Spike trains from descending input are likely to carry important information within the timing and pattern of activity that may be decomposed in a relay circuit with an interposed neuron that integrates input into spiking output in a way that is determined by its phenotype. Decomposition of the pattern of spike trains may limit functional benefit of neuronal relays. Thus, on first principals, it may be overly-optimistic to hope that relays could restore skilled voluntary motor abilities mediated by the CST or discriminative touch mediated by the dorsal column-medial lemniscal system. On the other hand, the CNS has a remarkable capacity to learn and there are pathways in the spinal cord that seem to rely less on precise patterns than on the total amount of activity (i.e. the pathways from the brainstem that are responsible for driving locomotor circuits at segmental levels), so it is certainly conceivable that decomposed patterns may still provide function. The same may be true of central autonomic pathways and it is important to utilize outcome measures that can accurately measure these improvements (Hou et al., 2013; Lee et al., 2014).
Figure 2.
Potential relay circuits after cell transplantation in spinal cord injury. Transplant derived neurons are likely to integrate signals from multiple sources as the relay forms, and the ability of these connections to become physiologically useful is likely to depend on the ability of useful connections to be strengthened while other connections are pruned. In this schematic example, Neuron #1 receives input from two regenerating corticospinal tract neurons. These inputs while likely be integrated and any information encoded within the timing of the spike train will be lost. Neuron #2 receives excitatory input from both the rubrospinal and corticospinal tract in addition to inhibitory input from a local interneuron. Although rubrospinal and corticospinal tract serve complementary roles in the intact CNS, they relay neuron will now convey a single, integrated, input to motor centers below the injury. Additionally, relay neurons are likely to receive input from highly plastic local inhibitory neurons present in the adjacent spinal cord. Inhibitory input could interrupt information transfer through the relay. Neuron #3 receives input from rubrospinal and raphaespinal neurons. Information transfer through neuron #3 would depend largely on the 5-HT receptor types expressed by the relay neuron, adding another level of complexity to neuronal relay formation. There are many potential novel circuits that could form after transplantation of neurogenic cells or tissues into the site of spinal cord injury, for simplicity this figure only examines some of the potential variety of descending input, but variety of grafted neurons and ascending input should also be considered when interpreting the results of relay experiments.
Recent Studies of Neuronal Relay Formation
Studies of fetal spinal cord grafting in the injured spinal cord have led to the methods that allow for the reliable production of developmentally young neurons in the injured spinal cord. These studies also demonstrated that several host spinal cord tracts could extend axons into fetal grafts and make electrophysiologically active synapses. Furthermore, fetal grafts can spontaneously extend axons into the host spinal cord, potentially meaning that the recovery of function observed after fetal grafting is due to the neuronal relay formation and host plasticity similar to that observed in spontaneous recovery after SCI. Several recent publications have attempted to build on these earlier studies by using additional cues and new techniques to refine and add specificity to the formation of the neuronal relays in the injured spinal cord.
Abematsu et al (2010) examined neuronal relay formation in mice following relatively severe T9-10 contusion injuries (90kD, IH Device) using mouse fetal NPC isolated from E14.5 mouse forebrain. NPC transplants combined with systemic valproic acid treatment produced neurons in the injured spinal cord. Electron microscopy demonstrated that graft-derived neurons rostral to the graft received synapses from the host and that graft-derived axons extended caudally from the transplant to form synapses with host neurons below the injury site. It is important to note, however, that these data do not document a relay. This would require data showing that host axons synapse on individual neurons that also extend axons caudally to synapse on host neurons.
The authors also present evidence for relay formation based on presumed trans-synaptic transport of WGA following injections of WGA-expressing adeno-associated virus into the motor cortex. In un-injured controls, WGA granules were found in neurons in the ventral horn throughout the spinal cord. The interpretation is trans-synaptic labeling from CST-tomotoneuron. In mice with contusion injuries, neurons above the injury contained WGA granules whereas neurons below the injury did not. In animals with NPC grafts treated with valproic acid, WGA granules were present in neurons above and below the injury. Although the interpretation is relay formation, the evidence is indirect and there could be other explanations for the results. For example, even the “positive control” of WGA granules in motoneurons is puzzling because there are relatively few direct CST-to-motoneuron connections in rodents (reviewed, Miri et al., 2013). Thus WGA granules in motoneurons would presumably have to come via a multi-synaptic relay. One would expect that the spinal neurons presynaptic to the motoneurons should be more heavily labeled than the motoneurons, but this was not reported. In any case, the exact structure of the proposed neuronal relay is unclear.
Despite these caveats, assessments of hindlimb locomotion with the BBB scale revealed dramatically higher BBB scores in transplanted+ valproic acid treated mice, which was eliminated when grafted cells were killed with diphtheria toxin. This does not document relay formation by the grafts, but does support the interpretation that grafts are contributing to improved locomotor function in some way. The same group showed similar results with human induced pluripotent stem cell derived NEP (Fujimoto et al., 2012). The structure of any human-NEP derived relay remains unclear since the transplant-derived neurons do not appear to extend axons beyond the lesion boundary.
Bonner et al (2011) took an approach addressing the other side of the issue—whether transplants could form a relay between ascending sensory axons and their targets in the dorsal column nuclei (DCN). The dorsal column was transected at C1, E13.5 fetal spinal cord derived NRP/GRP from human AP-expressing rats were transplanted into the C1 lesion site, and a BDNF expressing lentivirus was injected into the DCN. Injured axons were anterogradely labeled with cholera toxin subunit β, and target DCN cells were labeled retrogradely by fluorogold injections into the thalamus. Injured axons spontaneously regenerated into, but not beyond, the NRP/GRP graft and graft-derived neurons extended axons into the target. Electron microscopy demonstrated that host axons made synaptic connections with graft neurons and that graft derived neurons made synaptic connections in the DCN. Stimulation of the sciatic nerve produced evoked responses in the DCN with an approximately 1.2ms delay, consistent with monosynaptic transmission through a neuronal relay. No recordings were made in the graft itself, but c-fos expression was used to demonstrate that activation of regenerated dorsal column axons induced c-fos in graft neurons. In one animal, responses could be triggered in the DCN by brushing the hindpaw of an anesthetized animal with a cotton-tipped applicator, demonstrating that physiologically relevant sensory stimuli could be transmitted through an NRP/GRP derived neuronal relay. It would be fascinating to know how this activation was perceived by the animal. This work demonstrated that neuronal relays could be successfully applied to the sensory system. Further work will need to determine methods to improve fidelity of the neuronal relay and to combine a sensory relay with a motor relay in a larger injury model.
Another study reporting results relevant to relay formation (Lu et al., 2012) involved grafts of mechanically and enzymatically dissociated E14.0 fetal cells from GFP-expressing rats into complete thoracic lesion cavities. The cells were treated with a growth factor cocktail and transplanted in a fibrin matrix. The grafted cells survived and expanded to completely fill the lesion cavity. There was robust axon outgrowth for many mm into the host. Some axons were in bundles similar to those reported with fetal grafts that are not enzymatically dissociated (Jakeman and Reier, 1991; Lepore and Fischer, 2005). GFP-positive axons formed elaborate arbors in the host gray matter with varicosities suggestive of synaptic contacts.
This was not an explicit attempt to construct a relay and there was no pre-defined descending or ascending input pathway or pre-determined target for graft-derived axons. Nevertheless, the authors demonstrated ingrowth of host raphespinal and reticulospinal tract axons into the grafts. This work demonstrated connectivity between injured tracts and grafted cells as well as grafted cells and host neurons below the level of the injury. The effects on ascending tracts were not reported. Importantly, Lu et al (2012) also reported similar results with human fetal derived neural stem cells as well as with human embryonic stem cell derived neural stem cells. Although fetal grafts are a useful experimental model, a well characterized stem cell population is a more clinically relevant option. The authors reported axons of great length in this publication, and the presented data clearly show a great number of axons extending 10mm from the graft site.
As part of the NINDS-funded FORE-SCI replication project (Facilities of Research Excellence, Spinal Cord Injury), our group (Sharp et al., 2014) carried out a replication of the rodent fetal graft experiments of Lu et al. We confirmed that grafted cells could expand to completely fill the lesion site, depending on the method of transplant and that there was robust outgrowth of axons from the graft. Ingrowth of host axons was limited, however, and improved hindlimb locomotor function was not confirmed. One possible explanation for the lack of recovery is the presence of partitions in most of the grafts, which interrupted the continuity of the graft, preventing transmission of any signals across the lesion. The replication study clarified some methodological issues regarding transplantation techniques and demonstrated the need for follow-up studies to improve formation of continuous tissue bridges between rostral and caudal segments. Interestingly, during the replication study, we also discovered ectopic colonies of graft-derived cells at long distances from the transplant in the central canal, 4th ventricle and the subdural surface of the spinal cord and brain stem (Steward et al., 2014a; Steward et al., 2014b). It remains to be seen whether these colonies reflect the unique conditions of the experiment, for example the treatment of cells with growth factor cocktail, or whether fetal cells that are still capable of self-renewal (i.e. proliferation) have a previously unrecognized capacity for dispersal and colony formation. Continuing research from Paul Lu and Mark Tuszynski has now shown that human induced pluripotent stem cell derived NSC can also generate neurons with long axons when grafted with a growth factor matrix; however, further research is needed to promote lesion filling and functional recovery by these cells (Lu et al., 2014).
Relay-mediated repair is not limited to the repair of motor or sensory pathways and, indeed, many of the highest priority functional benefits would be to the restored control of autonomic function (Anderson, 2004). Early studies of transplantation in the injured spinal cord demonstrated that fetal grafts rich in monoaminergic neurons could replace input lost after spinal cord injury (Nygren et al., 1977; Privat et al., 1989). Repair of autonomic pathways has the added advantage of being a potentially close target to the injury as restoring function only a few segments within the cervical or thoracic cord could lead to meaningful restoration of function. Hou et al (2013), using a transplantation protocol similar to Lu et al., (2012), demonstrated the possibility of creating a relay between brain stem neurons that contribute to cardiovascular function and neurons in the intermediolateral nucleus of the spinal cord. The authors compared transplants of enzymatically dissociated fetal spinal cord or fetal brain stem, with the idea being that the latter would be rich in monoaminergic neurons, particularly serotonergic and noradrenergic neurons. This study found functional improvement in autonomic dysreflexia (after fetal spinal cord and fetal brain stem transplants) and improvement in hemodynamic measurements (after fetal brain stem transplants).
These studies demonstrate that neuronal relay approaches can be applied to a variety of injury models towards restoring sensory, motor and autonomic function. Although each study tends to focus on a single modality, researchers should consider the effects of multiple descending and ascending projections that integrate at the cell transplant site (Figure 2). While training is likely to promote some improvement of relay function, other methods to convey specificity of neuronal connections should also be explored.
Conclusion
Neuronal relays are a promising strategy for the repair of spinal cord injuries, but continued refinement of techniques will be required to improve specificity of connectivity, and enhance functional outcome and reproducibility. Early studies demonstrated that fetal grafts could improve the injury site and produce neurons capable of synaptic integration in the injured spinal cord, first raising the possibility of neuronal relays. Thanks to technical advancements recent studies of neuronal relay formation have demonstrated that neuronal relays can be formed with a variety of cells and applied to a range of injury models. Continuing studies can focus on three areas for the improvement of neuronal relays. Application of the neuronal relay strategy to a variety of contusion/compression injuries – particularly chronic injuries – will increase the clinical relevance of animal studies. Further experiments with well defined populations of human NPC or NSC derived from embryonic stem cells and/or induced pluripotent stem cells will improve the transition to the clinic. Finally, guidance of relay axons to specific motor, sensory and autonomic targets should improve the success and reproducibility of neuronal relays and hopefully lead to a successful treatment of SCI.
Highlights.
Neuronal relays are a potential repair strategy for spinal cord injury.
Fetal grafting studies laid the groundwork for neuronal relay research.
Recent neuronal relay studies are reviewed.
Acknowledgements
J.B. has been supported by a fellowship from the Craig H. Neilsen Foundation and is currently the recipient of a postdoctoral fellowship from the California Institute of Regenerative Medicine. O.S. is supported by NIH R01 NS073857 and generous private donations from Cure Medical, Research For Cure, Unite-2-Fight Paralysis, and other private donors. The authors would like to thank Dr. Arthi Amin for her critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abematsu M, Tsujimura K, Yamano M, Saito M, Kohno K, Kohyama J, Namihira M, Komiya S, Nakashima K. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin invest. 2010;120:3255–66. doi: 10.1172/JCI42957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto LT, Havton LA, Conner JM, Hollis li ER, Blesch A, Tuszynski MH. Chemotropic guidance faciiitates axonal regeneration and synapse formation after spinal cord injury. Nat Neurosci. 2009;12:1106–13. doi: 10.1038/nn.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–83. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Baier H, Bonhoeffer F. Axon guidance by gradients of a target-derived component. Science. 1992;255:472–5. doi: 10.1126/science.1734526. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–77. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Stenevi U, Svendgaard N. Growth of transplanted monoaminergic neurones into the adult hippocampus alongthe perforant path. Nature. 1976;262:787–90. doi: 10.1038/262787a0. [DOI] [PubMed] [Google Scholar]
- Boldogkoi Z, Szabo A, Vrbova G, Nogradi A. Pseudorabies virus-based gene delivery to rat embryonic spinal cord grafts. Hum Gene Ther. 2002;13:719–29. doi: 10.1089/104303402317322285. [DOI] [PubMed] [Google Scholar]
- Bonner JF, Blesch A, Neuhuber B, Fischer I. Promoting directional axon growth from neural progenitors grafted into the injured spinal cord. J Neurosci Res. 2010;88:1182–92. doi: 10.1002/jnr.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011;31:4675–86. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JF, Haas CJ, Fischer I. Preparation of neural stem cells and progenitors: neuronal production and grafting applications. Methods Mol Biol. 2013;1078:65–88. doi: 10.1007/978-1-62703-640-5_7. [DOI] [PubMed] [Google Scholar]
- Braz JM, Sharif-Naeini R, Vogt D, Kriegstein A, Alvarez-Buylla A, Rubenstein JL, Basbaum AI. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury- induced neuropathic pain. Neuron. 2012;74:663–75. doi: 10.1016/j.neuron.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman BS, Reier PJ. Neural tissue transplants rescue axotomized rubrospinal cells from retrograde death. J Comp Neurol. 1986;244:86–95. doi: 10.1002/cne.902440107. [DOI] [PubMed] [Google Scholar]
- Cai J, Wu Y, Mirua T, Pierce JL, Lucero MT, Albertine KH, Spangrude GJ, Rao MS. Properties of a fetal multipotent neural stem cell (NEP cell). Dev Biol. 2002;251:221–40. doi: 10.1006/dbio.2002.0828. [DOI] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Macarthur L, McAtee M, Hockenbury N, Das P, Bregman BS. Delayed rehabilitation with task-specific therapies improves forelimb function after a cervical spinal cord injury. Restor Neurol Neurosci. 2011;29:91–103. doi: 10.3233/RNN-2011-0583. [DOI] [PubMed] [Google Scholar]
- Das GD. Neural transplantation: an historical perspective. Neurosci Biobehav Rev. 1990;14:389–401. doi: 10.1016/s0149-7634(05)80061-2. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–3. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19:5810–22. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, Yang XL, Bachoo R, Cannon S, Longo FM, Sheng M, Silver J, Li S. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci. 2011;31:14051–66. doi: 10.1523/JNEUROSCI.1737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto Y, Abematsu M, Falk A, Tsujimura K, Sanosaka T, Juliandi B, Semi K, Namihira M, Komiya S, Smith A, Nakashima K. Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell-derived long-term self-renewing neuroepithelial-like stem cells. Stem Cells. 2012;30:1163–73. doi: 10.1002/stem.1083. [DOI] [PubMed] [Google Scholar]
- Gage FH, Bjorklund A, Stenevi U, Dunnett SB, Kelly PA. Intrahippocampal septal grafts ameliorate learning impairments in aged rats. Science. 1984;225:533–6. doi: 10.1126/science.6539949. [DOI] [PubMed] [Google Scholar]
- Girgis J, Merrett D, Kirkland S, Metz GA, Verge V, Fouad K. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain. 2007;130:2993–3003. doi: 10.1093/brain/awm245. [DOI] [PubMed] [Google Scholar]
- Haas C, Neuhuber B, Yamagami T, Rao M, Fischer I. Phenotypic analysis of astrocytes derived from glial restricted precursors and their impact on axon regeneration. Exp Neurol. 2012;233:717–32. doi: 10.1016/j.expneurol.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogue IB, Bosse JB, Hu JR, Thiberge SY, Enquist LW. Cellular mechanisms of alpha herpesvirus egress: live cell fluorescence microscopy of pseudorabies virus exocytosis. PLoS Pathog. 2014;10:e1004535. doi: 10.1371/journal.ppat.1004535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Tom VJ, Graham L, Lu P, Blesch A. Partial restoration of cardiovascular function by embryonic neural stem cell grafts after complete spinal cord transection. J Neurosci. 2013;33:17138–49. doi: 10.1523/JNEUROSCI.2851-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle JD, Reier PJ. Transplantation of fetal spinal cord tissue into the chronically injured adult rat spinal cord. J Comp Neurol. 1988;269:535–47. doi: 10.1002/cne.902690406. [DOI] [PubMed] [Google Scholar]
- Houle JD, Reier PJ. Regrowth of calcitonin gene-related peptide (CGRP) immunoreactive axons from the chronically injured rat spinal cord into fetal spinal cord tissue transplants. Neurosci Lett. 1989;103:253–8. doi: 10.1016/0304-3940(89)90108-0. [DOI] [PubMed] [Google Scholar]
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–15. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BY, Zhang SC. Directed differentiation of neural-stem cells and subtype-specific neurons from hESCs. Methods Mol Biol. 2010;636:123–37. doi: 10.1007/978-1-60761-691-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd C, Weishaupt N, Fouad K. Anatomical correlates of recovery in single pellet reaching in spinal cord injured rats. Exp Neurol. 2013;247:605–14. doi: 10.1016/j.expneurol.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Tessler A. Ultrastructural organization of regenerated adult dorsal root axons within transplants of fetal spinal cord. J Comp Neurol. 1990;292:396–411. doi: 10.1002/cne.902920306. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Sugawara T, Kowada M, Tessler A. Time course of dorsal root axon regeneration into transplants of fetal spinal cord: an electron microscopic study. Exp Neurol. 1993a;123:133–46. doi: 10.1006/exnr.1993.1146. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Tessler A, Kowada M, Pinter M. Electrophysiological responses in foetal spinal cord transplants evoked by regenerated dorsal root axons. Acta Neurochir Suppl (Wien) 1993b;58:24–6. doi: 10.1007/978-3-7091-9297-9_5. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Reier PJ, Bregman BS, Wade EB, Dailey M, Kastner RJ, Himes BT, Tessler A. Differentiation of substantia gelatinosa-like regions in intraspinal and intracerebral transplants of embryonic spinal cord tissue in the rat. Exp Neurol. 1989;103:17–33. doi: 10.1016/0014-4886(89)90181-7. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Reier PJ. Axonal projections between fetal spinal cord transplants and the adult rat spinal cord: a neuroanatomical tracing study of local interactions. J Comp Neurol. 1991;307:311–334. doi: 10.1002/cne.903070211. [DOI] [PubMed] [Google Scholar]
- Jin Y, Ziemba KS, Smith GM. Axon growth across a lesion site along a preformed guidance pathway in the brain. Exp Neurol. 2008;210:521–30. doi: 10.1016/j.expneurol.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Sura K, Fischer I. Differential effects of distinct central nervous system regions on cell migration and axonal extension of neural precursor transplants. J Neurosci Res. 2012;90:2065–73. doi: 10.1002/jnr.23099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyani A, Hobson K, Rao MS. Neuroepithelial stem cells from the embryonic spinal cord: isolation, characterization, and clonal analysis. Dev Biol. 1997;186:202–223. doi: 10.1006/dbio.1997.8592. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Wang H, Marshall W, Tessier-Lavigne M. Axon guidance by diffusible chemoattractants: a gradient of netrin protein in the developing spinal cord. J Neurosci. 2006;26:8866–74. doi: 10.1523/JNEUROSCI.5191-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketschek AR, Haas C, Gallo G, Fischer I. The roles of neuronal and glial precursors in overcoming chondroitin sulfate proteoglycan inhibition. Exp Neurol. 2012;235:627–37. doi: 10.1016/j.expneurol.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkeby A, Nelander J, Parmar M. Generating regionalized neuronal cells from pluripotency, a step-by-step protocol. Front Cell Neurosci. 2012;6:64. doi: 10.3389/fncel.2012.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboyama T, Luo X, Park K, Blackmore MG, Tojima T, Tohda C, Bixby JL, Lemmon VP, Kamiguchi H. Paxillin phosphorylation counteracts proteoglycan-mediated inhibition of axon regeneration. Exp Neurol. 2013;248:157–69. doi: 10.1016/j.expneurol.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BK, Okon EB, Plunet W, Baptiste D, Fouad K, Hillyer J, Weaver LC, Fehlings MG, Tetzlaff W. A systematic review of directly applied biologic therapies for acute spinal cord injury. J Neurotrauma. 2011;28:1589–610. doi: 10.1089/neu.2009.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Lane MA, Dougherty BJ, Mercier LM, Sandhu MS, Sanchez JC, Reier PJ, Fuller DD. Intraspinal transplantation and modulation of donor neuron electrophysiological activity. Exp Neurol. 2014;251:47–57. doi: 10.1016/j.expneurol.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, Han SS, Tyler-Polsz CJ, Cai J, Rao MS, Fischer I. Differential fate of multipotent and lineage-restricted neural precursors following transplantation into the adult CNS. Neuron Glia Biol. 2004;1:113–126. doi: 10.1017/s1740925x04000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, Fischer I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp Neurol. 2005;194:230–42. doi: 10.1016/j.expneurol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–73. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Woodruff G, Wang Y, Graham L, Hunt M, Wu D, Boehle E, Ahmad R, Poplawski G, Brock J, Goldstein LS, Tuszynski MH. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014;83:789–96. doi: 10.1016/j.neuron.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH, Kably B, Hacking A. Activity-dependent development of cortical axon terminations in the spinal cord and brain stem. Exp Brain Res. 1999;125:184–99. doi: 10.1007/s002210050673. [DOI] [PubMed] [Google Scholar]
- Mayer-Proschel M, Kalyani AJ, Mujtaba T, Rao MS. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron. 1997;19:773–85. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- Medalha CC, Jin Y, Yamagami T, Haas C, Fischer I. Transplanting neural progenitors into a complete transection model of spinal cord injury. J Neurosci Res. 2014 doi: 10.1002/jnr.23340. [DOI] [PubMed] [Google Scholar]
- Miri A, Azim E, Jessell TM. Edging toward entelechy in motor control. Neuron. 2013;80:827–34. doi: 10.1016/j.neuron.2013.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren LG, Olson L, Seiger A. Monaminergic reinnervation of the transected spinal cord by homologous fetal brain grafts. Brain Res. 1977;129:227–35. doi: 10.1016/0006-8993(77)90003-8. [DOI] [PubMed] [Google Scholar]
- Ohashi Y, Tsubota T, Sato A, Koyano KW, Tamura K, Miyashita Y. A bicistronic lentiviral vector-based method for differential transsynaptic tracing of neural circuits. Mol Cell Neurosci. 2011;46:136–47. doi: 10.1016/j.mcn.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Privat A, Mansour H, Rajaofetra N, Geffard M. Intraspinal transplants of serotonergic neurons in the adult rat. Brain Res Bull. 1989;22:123–9. doi: 10.1016/0361-9230(89)90136-6. [DOI] [PubMed] [Google Scholar]
- Rao MS, Noble M, Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci USA. 1998;95:3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reier PJ, Perlow MJ, Guth L. Development of embryonic spinal cord transplants in the rat. Brain Res. 1983;312:201–219. doi: 10.1016/0165-3806(83)90137-2. [DOI] [PubMed] [Google Scholar]
- Reier PJ, Bregman BS, Wujek JR. Intraspinal transplantation of embryonic spinal cord tissue in neonatal and adult rats. J Comp Neurol. 1986;247:275–296. doi: 10.1002/cne.902470302. [DOI] [PubMed] [Google Scholar]
- Sabo SL, McAllister AK. Mobility and cycling of synaptic protein-containing vesicles in axonal growth cone filopodia. Nat Neurosci. 2003;6:1264–9. doi: 10.1038/nn1149. [DOI] [PubMed] [Google Scholar]
- Schuman EM, Dynes JL, Steward O. Synaptic regulation of translation of dendritic mRNAs. J Neurosci. 2006;26:7143–6. doi: 10.1523/JNEUROSCI.1796-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M, Bjorklund A, Gage FH. Transplanted septal neurons make viable cholinergic synapses with a host hippocampus. Brain Res. 1985;336:302–7. doi: 10.1016/0006-8993(85)90656-0. [DOI] [PubMed] [Google Scholar]
- Sharp KG, Yee KM, Steward O. A re-assessment of long distance growth and connectivity of neural stem cells after severe spinal cord injury. Exp Neurol. 2014 doi: 10.1016/j.expneurol.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–6. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Sharp KG, Matsudaira Yee K. Long-distance migration and colonization of transplanted neural stem cells. Cell. 2014a;156:385–7. doi: 10.1016/j.cell.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Steward O, Sharp KG, Yee KM, Hatch MN, Bonner JF. Characterization of ectopic colonies that form in widespread areas of the nervous system with neural stem cell transplants into the site of a severe spinal cord injury. J Neurosci. 2014b;34:14013–21. doi: 10.1523/JNEUROSCI.3066-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26:9713–21. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theele DP, Schrimsher GW, Reier PJ. Comparison of the growth and fate of fetal spinal iso- and allografts in the adult rat injured spinal cord. Exp Neurol. 1996;142:128–143. doi: 10.1006/exnr.1996.0184. [DOI] [PubMed] [Google Scholar]
- Tojima T, Akiyama H, Itofusa R, Li Y, Katayama H, Miyawaki A, Katmigufhi H. Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone. Nat Neurosci. 2007;10:58–66. doi: 10.1038/nn1814. [DOI] [PubMed] [Google Scholar]
- Tom VJ, Sandrow-Feinberg HR, Miller K, Santi L, Connors T, Lemay MA, Houle JD. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. J Neurosci. 2009;29:14881–90. doi: 10.1523/JNEUROSCI.3641-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PJ, Herrity AN, Smith RR, Willhite A, Harrison BJ, Petruska JC, Harkema SJ, Hubscher CH. Novel multi-system functional gains via task specific training in spinal cord injured male rats. J Neurotrauma. 2014;31:819–33. doi: 10.1089/neu.2013.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TE, Lane MA, Sandhu MS, O'Steen BE, Fuller DD, Reier PJ. Neuronal progenitor transplantation and respiratory outcomes following upper cervical spinal cord injury in adult rats. Exp Neurol. 2010 doi: 10.1016/j.expneurol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Jin Y, Ziemba KS, Fletcher AM, Ghosh B, Truit E, Yurek DM, Smith GM. Long distance directional growth of dopaminergic axons along pathways of netrin-1 and GDNF. Exp Neurol. 2013;250:156–64. doi: 10.1016/j.expneurol.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemba KS, Chaudhry N, Rabchevsky AG, Jin Y, Smith GM. Targeting axon growth from neuronal transplants along preformed guidance pathways in the adult CNS. J Neurosci. 2008;28:340–8. doi: 10.1523/JNEUROSCI.3819-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]



