Abstract
Objectives
Pain is the hallmark symptom of sickle cell disease (SCD), yet the types of pain that these patients experience, and the underlying mechanisms, have not been well characterized. The study purpose was to determine the safety and utility of a mechanical and thermal quantitative sensory testing (QST) protocol and the feasibility of utilizing neuropathic pain questionnaires among adults with SCD.
Methods
A convenience sample (N=25, 18 women, mean age 38.5 ± 12.5 [20–58 years]) completed self-report pain and quality-of-life tools. Subjects also underwent testing with the TSA-II NeuroSensory Analyzer and calibrated von Frey microfilaments.
Results
We found that the QST protocol was safe and did not stimulate a SCD pain crisis. There was evidence of central sensitization (n=15), peripheral sensitization (n=1), a mix of central and peripheral sensitization (n=8), or no sensitization (n=1). The neuropathic pain self-report tools were feasible with evidence of construct validity; 40% of the subjects reported S-LANSS scores that were indicative of neuropathic pain and had evidence of central, peripheral or mixed sensitization.
Discussion
The QST protocol can be safely conducted in adults with SCD and provides evidence of central or peripheral sensitization, which is consistent with a neuropathic component to SCD pain. These findings are novel, warrant a larger confirmatory study, and indicate the need for normative QST data from African American adults and older adults.
Keywords: sickle cell, pain, quantitative sensory testing, neuropathic pain, PAINReportIt
INTRODUCTION
For patients with sickle cell disease (SCD), pain is the chief complaint that leads to acute healthcare utilization,1 but the types of pain these patients experience, and the underlying mechanisms, have not been well characterized. Pain among patients with SCD is typically considered nociceptive and related to vaso-occlusion.2, 3 Recent evidence from adults with SCD,4 however, suggests that neuropathic pain mechanisms may contribute to the SCD pain experience. Also, evidence from animal studies5, 6,7, 8 implicates neuropathic pain mechanisms in SCD. Sensitivity to mechanical and thermal stimuli, hallmarks of neuropathic pain, were observed in Berkeley transgenic mice with SCD but not in their normal littermates.7 The use of quantitative sensory testing (QST) to detect the presence of sensitivity9, 10 among adult patients with SCD is needed to determine if neuropathic pain is part of the adult SCD pain experience. But, given the susceptibility of adults to pain crisis, it is important to demonstrate that the QST protocol does not stimulate a pain crisis. It would also be helpful to know how QST findings relate to the self-report neuropathic pain questionnaires, which have not been tested among adults with SCD. The purpose of our study was to determine the safety and utility of a mechanical and thermal QST protocol and the feasibility of neuropathic pain questionnaires among adults with SCD.
Pain of SCD is recurrent over a lifetime and nearly two-thirds of adults report that their pain is continuous with unpredictable acute episodes.4 The unpredictability of the acute vaso-occlusive pain episode (pain crisis) is problematic for patients’ work, school, and home life. Treatment for the acute vaso-occlusive pain episode often requires emergency care and prolonged hospitalizations at a high cost, estimated at $2.4 billion annually.11 Avoiding stimulation of an acute vaso-occlusive pain episode by the implementation of research protocols must be a high priority for ethical SCD pain research. Therefore, safety must be demonstrated for a QST protocol focused on the detection of mechanical and thermal sensations and pain thresholds. Specifically, it must be established that a QST protocol will not trigger a pain crisis before a larger scale study can be conducted to detect the prevalence of neuropathic pain among adults with SCD. Such knowledge is important because if neuropathic pain is found to be part of the SCD pain experience, it could shift the treatment paradigm for children and adults with SCD to include adjuvant drugs to treat the neuropathic component of their pain. Improved treatments are desperately needed to reduce SCD pain intensity, improve patients’ quality of life, lessen suffering, and reduce medical costs for people with SCD.11
QST is valuable for assessing large and small sensory fiber function, particularly to quantify mechanical and thermal sensitivity, the positive sensory phenomena that help to characterize neuropathic pain syndromes.10 There are only 2 recently published studies of children and adolescents with SCD that include QST results.12, 13 There are no published studies of QST results from adults, particularly older adults, with SCD or any published studies on the use of self-report neuropathic pain tools for children or adults with SCD.14–16 The Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS)15 is noted for its utility as a screening tool for neuropathic pain.10 The Neuropathic Pain Symptom Inventory (NPSI)17 is noted for its usefulness to monitor changes with therapy.10 Neither the S-LANSS nor the NSPI has been tested in adults with SCD.
The aim of this study was to determine the safety of a QST protocol in adults with SCD and if their responses on self-report questionnaires for neuropathic pain and quality of life were consistent with their QST findings. We hypothesized that a sensory detection and pain threshold protocol would be safe. We also hypothesized that the proportion of adults with SCD with positive neuropathic pain indicators from the QST and self-report tools would be as large or larger than proportions of people with neuropathic pain detected in community-based general population studies.18 These study findings were intended to inform a larger study that will be designed to determine the prevalence of neuropathic pain among adults with SCD.
METHODS
Design
For this cross-sectional study, we recruited subjects from the outpatient sickle cell clinic at the University of Illinois at Chicago, a large minority-serving urban hospital. The institutional review board at the University of Illinois at Chicago approved the protocol and procedures, and all subjects signed written informed consent documents.
Subjects
From sequential patients approached at an outpatient clinic, we recruited a convenience sample of subjects who met study eligibility criteria. The eligibility criteria included adults at least 18 years of age, diagnosed with SCD who spoke and read English, had care continuity at the recruitment site, and had a self-reported worst pain score of at least 3/10 related to SCD over the past 24 hours. Adults not eligible were those legally blind or cognitively unable to complete the study procedures.
We recruited 38 adult outpatients with SCD who were eligible and consented, but 13 did not participate because of scheduling difficulties. A total of 25 subjects started and completed the study (18 women, 7 men, mean age 38.5 years ± 12.5 [ranged from 20–58 years]. Seven of the subjects completed high school, and 18 completed some or graduated college. All subjects were African American with genotype SS (n=17), SC (n=5), Sβ+ (n=1), SSα thal (n=1), or Sβ0 thal (n=1). Four subjects reported they had diabetes. None reported mental health issues such as post-traumatic stress disorder or depression/anxiety conditions. Six subjects reported alcohol use, one drink per day. The mean body mass index for subjects was 25.0 ± 5.2 with 4% underweight, 54% normal weight, 25% overweight and 17% obese.
Procedures
One of 2 trained researchers conducted all study-related procedures. We approached sequential patients to recruit eligible adults with SCD. After subjects consented and kept a scheduled appointment to participate in the study, the researchers asked them to complete a computerized version of the McGill Pain Questionnaire (MPQ) called PAINReportIt® and paper versions of the S-LANSS, NPSI, and Short Form-36 Health Survey (SF-36). Next, the subjects practiced at the non-painful site, then they underwent thermal and mechanical QST at 2 painful sites randomly selected by software attached to PAINReportIt,® and finally at the non-painful site. Subjects reported the intensity of pain perceived with each series of thermal and mechanical stimuli at each site. One day later, the researcher called the subjects to inquire whether they had any changes in pain intensity at the tested sites after the QST was completed.
Instruments
We administered PAINReportIt® on a pentablet computer with each item displayed on a separate screen. PAINReportIt® 19 contains 13 screens for McGill Pain Questionnaire (MPQ)20 items (version 1975), as well as 11 additional screens. The screens relevant to this report include those that allowed subjects to draw pain locations on a body outline and report their present pain intensity on a 0 to 10 scale. The validity and reliability of the MPQ,21, 22 as well as equivalence of the paper and pencil MPQ and PAINReportIt®23, have been reported. In addition, there is strong content validity of PAINReportIt® for adults with SCD.24
The Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) is a 7-item tool that is a screen for pain of neuropathic origin.15 The items in the S-LANSS ask subjects to draw the locations of their pain on a body outline, describe their worst pain level over the last week, and rate their worst pain level over the last week via a 0 to 10 scale.15 Possible total scores range from 0 to 24 and a score of 12 or higher is indicative of neuropathic pain.15 There is support for the validity and reliability of the S-LANSS.15
The Neuropathic Pain Symptom Inventory (NPSI) is a 12-item tool that examines neuropathic pain symptoms.25 The items ask the subject to rate, on a 0 to 10 scale, a total of 10 neuropathic pain descriptors. The other 2 items of the NPSI ask the subjects to identify temporal descriptors of their pain. The NPSI total score ranges from 0 to 100. Higher scores are indicative of neuropathic pain, but cut scores have not been published. Investigators have reported validity and reliability data regarding the NPSI.25
The Short Form-36 Health Survey (SF-36) is a 36-item self-report tool. The items represent 8 domains: physical functioning; social functioning; role limitations (physical problems); role limitations (emotional problems); mental health; vitality; pain; and general health perception.26 The SF-36 total score ranges from 0 to 100. The validity and reliability of the SF-36 are well documented.26, 27, 28
The TSA-II NeuroSensory Analyzer (Medoc, Ramat Yishai, Israel) is a precise, computer-controlled device capable of generating and documenting response to highly repeatable thermal stimuli, such as cool detection, warm detection, cold-induced pain, and heat-induced pain. The TSA-II evokes responses in small-diameter afferents (A-delta and C fiber). We used the thermal detection and threshold for pain protocol, known as the Limits Protocol. The subject is asked to indicate when the cool or warm sensation is detected and when the sensation is first perceived as painful (threshold) by clicking a mouse device. For each sensation type, cool or warm, we used a non-painful site to allow the subject to practice the detection tasks. After the practice session, we tested 2 randomly selected pain sites and then retested the non-painful site that had been used previously as the practice site about 5 to 10 minutes earlier. We asked the subject to rate the intensity of the pain at the end of each trial, at each site, and to indicate the sensation they felt (cool/cold, warm/hot). Because normative scores are not available for all sites tested in this study, Table 1 lists the literature-based norms that we used for thermal data analysis. Based on an author’s extensive QST expertise (**), we defined an abnormal thermal pain threshold (sensitivity) as (1) a value for cold pain threshold ½ SD above the cold pain norms for age and site, (2) a value for heat pain threshold ½ SD below the heat pain norms for age and site, or (3) the patient reported a pain intensity score for a thermal stimulus that was > 3/10.
Table 1.
Literature reported threshold temperatures (° C) for thermal pain detection and temperature (° C) means ± SD by age group for detection of cool and warm sensation and thresholds for cold and heat pain by non-painful and painful sites in upper body and lower body.
| Test Site | Age Group | Cool Sensation | Cold Pain | Warm Sensation | Heat Pain |
|---|---|---|---|---|---|
| Literature Reported Values | |||||
| Upper body | 18–39 yr | 14.2 [1.3,27.6]42 | 42.3±4.237 | ||
| Upper body | ≥ 40 yr | 14.2 [1.3,27.6]42 | 42.9 [37,47.4]42 | ||
| Lower body | 18–39 yr | 10.8±9.533 | 45±2.733 | ||
| Lower body | ≥ 40 yr | 8±8.833 | 47±2.133 | ||
| Findings from Current Study (n refers to number of subjects) | |||||
| Non-Painful Site (Upper Body) | 18–39 yr (n=12) | 29.04 ± 2.96 | 19.16 ± 9.86 | 35.89 ± 2.33 | 40.46 ± 4.60 |
| ≥ 40 yr (n=13) | 28.43 ± 3.84 | 20.58 ± 8.66 | 35.81 ± 2.01 | 41.62 ± 4.66 | |
| Painful Site (Upper Body) | 18–39 yr (n=8) | 29.14 ± 2.40 | 18.82 ± 10.19 | 35.38 ± 2.00 | 40.48 ± 3.97 |
| ≥ 40 yr (n=11) | 29.87 ± 1.34 | 20.75 ± 10.48 | 36.09 ± 1.43 | 42.71 ± 5.26 | |
| Painful Site (Lower Body) | 18–39 yr (n=7) | 29.54 ± 0.67 | 22.84 ± 10.83 | 36.58 ± 2.26 | 41.00 ± 5.95 |
| ≥ 40 yr (n=7) | 28.78 ± 0.90 | 23.23 ± 8.23 | 38.11 ± 3.48 | 42.87 ± 2.90 | |
We used standard, well-calibrated von Frey microfilaments to test for mechanical sensitivity. We used 7 of the filaments -- starting with 3.84 (=0.6 g) and ending with 5.88 (=60.0 g). Per standard technique, we applied each filament once to the skin, after which we asked the subject to indicate if he/she detected the pressure sensation and if it was painful. If the sensation was painful, we asked the subject to rate the amount of pain on a 0 to 10 scale. We tested each von Frey filament a total of 3 times at each test site with at least 3 seconds between testing at each site and between sites. If the subject reported pain with any filament, the testing was concluded and larger filaments were not tested at that site. We averaged the reported pain scores over the 3 measurements per site. We defined mechanical stimuli responses as abnormal (sensitivity) if a subject reported any pain to the 0.6g to 10g filaments, or if a subject reported average pain of 3 or greater to the 26g to 60g filaments. We classified the rest of the responses as normal.
Analysis
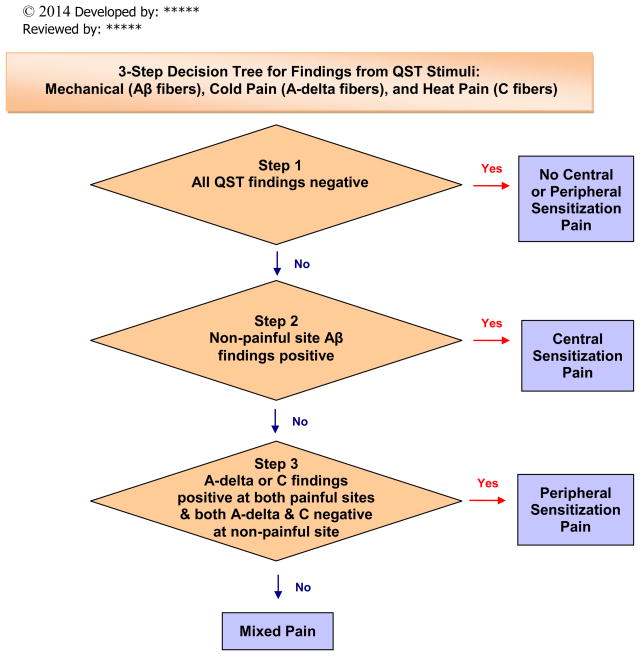
We exported the data for analysis using statistical software R29 and computed descriptive (mean, standard deviation) and inferential (correlation, t-test) statistics. There was a minimal amount of missing data (less than 1% each for NPSI and S-LANSS). For data analysis involving these 2 scales, we generated multiple imputed data sets and pooled analysis outputs for these imputed datasets to generate the final output. With less than 1% missing data, any potential violation of the missing at random assumption would have negligible impact.30 Based on well-known evidence of neural mechanisms of sensation and pain,9, 10 we created a decision tree (Figure 1) to classify each subject as normal or abnormal with consideration of the responses for each of the 3 test sites, the 2 thermal stimuli, and the mechanical stimuli. This decision tree has been reviewed for content validity by pain experts, including a national QST expert.
Figure 1.
Decision Tree for Synthesis of Quantitative Sensory Testing Findings. Copyright 2014 UIC Pain & Symptom Management Research Group, used with permission. Group Leaders: Diana J. Wilkie, PhD, RN, FAAN, Robert Molokie, MD, Z. Jim Wang, PhD. Developed by: Yingwei Yao, PhD, Diana J. Wilkie, PhD, RN, FAAN. Reviewed by: Robert Molokie, MD, Z. Jim Wang, PhD, Roger Fillingim, PhD.
RESULTS
Study participants reported that they believed their pain was related to their SCD. From medical record review, no subject had an acute care visit for a vaso-occlusive event within 2 weeks prior to the date of participating in our study; one subject had an acute care visit for a vaso-occlusive event within a 2-week window after the study was completed. Analgesics consumed within 24 hours before the study are listed in Table 2.
Table 2.
Analgesic drugs consumed within 24 hours before testing procedures (N=25)
| Fiber Function (number of subjects) [Mechanism] | Drug Class | Drug Name (subject code number)1 | Dose mg/24 hrs1 |
|---|---|---|---|
| Normal & abnormal mechanical and thermal (n=8) [mixed sensitization] | Non-opioid | Acetaminophen (12/6, 24) | 1300/0 |
| NSAID | Acetylsalicylic acid (1) | 325 | |
| Naproxen (13/16) | 500/0 | ||
| Ibuprophen (24, 2, 14) | 0 | ||
| Step 2 opioid | Tramadol (16/14) | 50/200 | |
| Hydrocodone (2/13/24) | 0/10/0 | ||
| Step 3 opioid | Morphine – Injectable (13) | 45 | |
| Morphine-immediate release (9) | 15 | ||
| Morphine-sustained release (9) | 30 | ||
| Methadone (14) | 40 | ||
| Hydromorphone (14) | 0 | ||
| Adjuvant | Amitriptyline (14) | 75 | |
| carbamazepine (14) | 200 | ||
| Normal mechanical; Abnormal thermal (n=1) [peripheral sensitization] | Step 3 opioid | Hydromorphone –injectable (22) | 0 |
| Abnormal mechanical and thermal (n=15) [central sensitization] | Non-opioid | Acetaminophen (5, 10, 17, 25/23/19) | 0/650/1,500 |
| Ibuprophen (4/17, 23/25) | 3200/0/800 | ||
| Acetylsalicylic acid (8) | 650 | ||
| Step 2 opioid | Hydrocodone (4/11/21) | 25/UK/0 | |
| Codeine (23) | 0 | ||
| Tylenol with codeine or hydrocodone (5, 6, 7, 8, 17, 21, 23) | 0 | ||
| Tramadol (10/25) | 200/0 | ||
| Tylenol with codeine (19) | 90 | ||
| Step 3 opioid | Morphine-immediate release (5) | 0 | |
| Fentanyl patch (10) | 375 | ||
| Morphine -immediate release (23) | 0 | ||
| Morphine -injectable (23) | 0 | ||
| Morphine -sustained release (8, 18, 23) | 0 | ||
| Oxycodone -controlled release (3) | 10 | ||
| Adjuvant | Amitriptyline (20) | 5 | |
| All normal (n=1) [No sensitization] | Non-opioid | Acetaminophen (15) | 650 |
| NSAID | Ibuprophen (15) | 800 | |
| Step 3 opioid | Morphine-immediate release (15) | 0 |
Key:
/ separates code numbers corresponding to doses in the last column
Safety
Three subjects reported having residual pain (worse and enduring pain) after completing the QST protocol. One subject reported residual pain 24 hours after the QST protocol. She considered an emergency department visit until her physician reminded her to take a pregabalin dose that he had previously prescribed. The subject reported that she had not been adherent to the pregabalin prescription and that she had been shopping after the study. Subsequent to that experience, to assure patient safety, we asked subjects to bring their prescribed analgesics to the study site in case the QST protocol increased their pain. One subject consumed an analgesic immediately after completing the QST protocol. None of the 3 subjects felt that their residual pain represented an acute pain crisis.
Thermal QST
None of the subjects reached the limits for the cool and warm detection tests, which indicated that none of them had an insensate abnormality and were able to detect cool and warm at temperatures within normal ranges. Table 1 presents the temperatures at which the subjects detected cool and warm stimuli and reported cold and heat pain at non-painful and painful sites located in upper or lower body areas. Comparing values with published norms in Table 1, subjects with SCD reported sensitivity to cold at much higher cold pain threshold values than the norms even at non-painful sites. The difference in heat pain thresholds was not as dramatic, but still showed that subjects with SCD reported lower thresholds to heat pain than the published norms, some of which were from a relatively large sample of college-aged African Americans.
With thermal QST across the 2 painful sites, the average pain intensity reported for the cold pain stimuli was 4.15 ± 2.55 (ranged from 0 to10), and the average intensity for the heat pain stimuli was 4.84 ± 2.85 (ranged from 0 to 10). For the non-painful site, the average intensity for the cold pain stimuli was 4.54 ± 3.08 (ranged from 0 to 10) and for the heat pain stimuli was 4.96 ± 3.03 (ranged from 1 to 10). At 1 to 2 of the tested sites, 5 subjects (3 under age 30 and 2 over age 40) reported they perceived the cold pain stimulus as a warm sensation, but none reported the hot pain stimulus was perceived as either cool or cold.
Mechanical QST
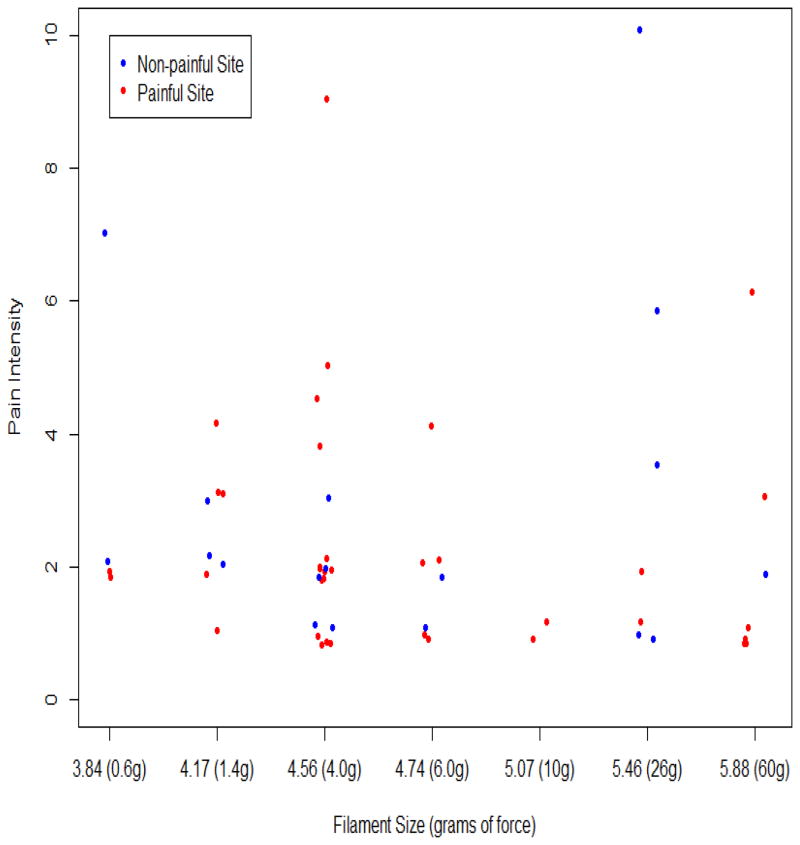
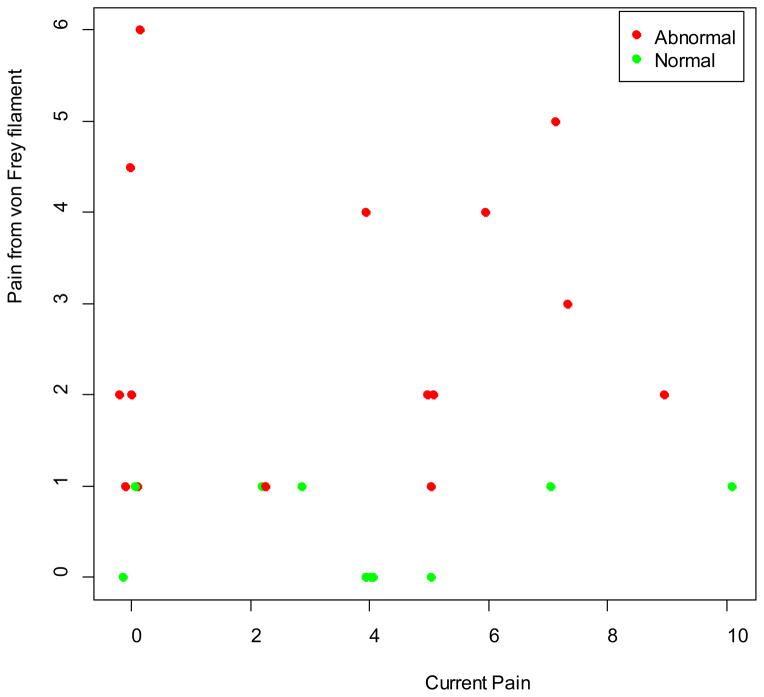
No subject failed to sense any of the tested von Frey filaments, which indicates that all were adequately sensate of mechanical pressure. Five subjects reported no pain for the 60g force at non-painful sites (all upper body). Eight subjects reported no mechanical pain in at least 1 upper body painful site, and 3 reported no mechanical pain in at least 1 lower body painful site. From the various grams of force tested across the sites, 20 (80%) subjects reported pain at the non-painful sites; 11 (58%) out of 19 with at least 1 upper body painful site reported pain in at least 1 painful site located in upper body areas; and, 12 (86%) of 14 with at least 1 lower body painful site reported pain in at least one painful site located in lower body areas. Figure 2 represents the intensity of pain that subjects reported for the various grams of force. Figure 3 shows the relationship between the subjects’ baseline current pain intensity before initiating any QST procedures and the average pain intensity reported upon stimulation with the von Frey filaments. Six subjects reported normal responses (−) to the mechanical stimuli at all tested sites, 11 subjects reported abnormal responses (+) at all tested sites, and 8 subjects reported a mix of normal and abnormal responses at the tested sites (Table 3).
Figure 2.
von Frey filament size when pain first reported by the reported pain intensity (Each dot represents one of the 55 test sites of the 21 subjects who reported pain with von Frey testing; 4 subjects reported no pain for any of the 7 filaments. We added jitters to separate identical measurement values.)
Figure 3.
Reported pain intensity at non-painful sites from von Frey filaments compared to current pain intensity prior to quantitative sensory testing
Table 3.
Summary of Abnormal (+) and Normal (−) Findings by Subject (identified by code, age, and sex) for Thermal and Mechanical Quantitative Sensory Testing (QST) at the Two Painful Sites and One Non-painful Site, the Subjective Measures, and QST-based Decision Tree Outcome (N=25)
| Code | Age | Sex | Cold Pain Threshold | Cold Pain Intensity | Heat Pain Threshold | Heat Pain Intensity | Mechanical | NPSI | S-LANSS | Decision tree^ |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | >40 | M | −++ | (1,2,2) | ++− | (4,3,3) | −+− | 10 | 7 | 1 |
| 2 | >55 | F | +−− | (2,3,3) | +++ | (3,4,3) | ++− | 26 | 19 | 1 |
| 3 | >20 | M | +−+ | (4,3,4) | +++ | (6,5,5) | +−+ | 22 | 6 | 3 |
| 4 | >50 | F | +++ | (8,9,10) | +++ | (10,9,10) | +++ | 34* | 19 | 3 |
| 5 | >40 | F | +++ | (2,10,6) | +++ | (2,10,6) | +++ | 35 | 10 | 3 |
| 6 | >50 | F | +++ | (8,7,8) | +++ | (6,5,6) | +++ | 0 | 5 | 3 |
| 7 | >50 | F | +++ | (4,3,3) | +++ | (4,3,3) | +−+ | 28 | 14 | 3 |
| 8 | >40 | F | +++ | (6,6,4) | −++ | (0,9,9) | −−+ | 28 | 3 | 3 |
| 9 | >40 | F | +++ | (2,2,2) | +++ | (2,2,2) | 40 | 9 | 1 | |
| 10 | >20 | F | +++ | (7,6,10) | +++ | (10,8,10) | +++ | 58 | 24 | 3 |
| 11 | >40 | F | +++ | (6,7,5) | +++ | (9,7,8) | +++ | 80 | 17 | 3 |
| 12 | >30 | M | +++ | (2,2,3) | −++ | (3,2,2) | 33 | 8 | 1 | |
| 13 | >30 | F | +++ | (6,5.5,9.5) | +++ | (7,9,9) | −+− | 31 | 22 | 1 |
| 14 | >20 | F | +++ | (3,2,2) | +++ | (3,3,3) | 8 | 3 | 1 | |
| 15 | >20 | M | (0,0,0) | (3,2,2) | 6 | 0 | 0 | |||
| 16 | >50 | F | +++ | (1,1,1) | +++ | (1,1,1) | 20 | 11 | 1 | |
| 17 | >30 | F | ++− | (3,4,3) | +++ | (4,5,4) | +++ | 30 | 10 | 3 |
| 18 | >40 | F | +++ | (5,8,8) | +++ | (8,7,8) | +++ | 25 | 18* | 3 |
| 19 | >18 | F | +++ | (6,5,5) | +++ | (5,3,3) | +++ | 47 | 19 | 3 |
| 20 | >20 | M | +++ | (5,7,6) | +++ | (7,8,7) | +++ | 27 | 10 | 3 |
| 21 | >20 | F | +++ | (2,2,2) | +++ | (2,2,2) | +++ | 10 | 8 | 3 |
| 22 | >46 | M | ++− | (2,3,2) | (3,3,2) | −+− | 5 | 5 | 2 | |
| 23 | >20 | M | +++ | (3,3,3) | +++ | (3,3,3) | +−+ | 6 | 0 | 3 |
| 24 | >20 | F | +−− | (3,1,2) | (3,3,3) | 50 | 24 | 1 | ||
| 25 | >20 | F | +++ | (8,7,10) | +++ | (10,8,10) | +++ | 16 | 19 | 3 |
−normal finding; +abnormal finding;
Decision Outcome: 0. No Central or Peripheral Sensitization (normal), 1. Mixed Sensitization, 2. Peripheral Sensitization, 3. Central Sensitization;
Average of multiple imputed values.
Synthesis across QST Measures
To classify each subject as having normal or abnormal responses, we used the decision tree (Figure 1) to consider findings for each subject across the thermal and mechanical QST results for all test sites (Table 3). Only 1 subject reported normal responses to both the mechanical and thermal stimuli at all test sites, a clear indication of normal large and small fiber function. There were abnormalities across all 3 test sites from the mechanical stimuli for 11 (44%) subjects, from the cold pain stimuli for 18 (72%) subjects, and from the heat pain stimuli for 19 (76%) of the subjects. Based on our decision tree, 24 (96%) of the 25 subjects met criteria for neuropathic pain with an indication that 15 (60%) had widespread sensitivity (abnormalities at all 3 sites), which suggests central pain mechanism, and 1 (4%) had localized sensitivity, which suggests peripheral pain mechanisms. For 8 (32%) of the subjects, the cross-sectional data were mixed, suggesting either a combination of central and peripheral mechanisms across the testing sites and modalities, or the need for a repeat measurement to clarify the relative contributions of central or peripheral mechanisms. Table 3 presents the summary data across the QST and self-report measures and the decision tree conclusion for each subject.
Based on Brandow et al.’s recent results from children and adolescents with SCD and matched controls,12 we replicated the decision tree analysis using these newly published norms. Only 1 subject would have been classified differently. We classified this subject as mixed based upon our Table 1 norms but would have classified the subject (a 27-year-old female) as normal based upon these new norms from the matched controls.12 These findings indicate the robustness of our original decision tree classification (Figure 2).
Self-report Findings
The mean current pain intensity score was 3.57 ± 3.09 and the average (current, least, worst in previous 24 hours) pain intensity score was 3.76 ± 2.66. The subjects easily used the S-LANSS and NPSI tools and there was minimal missing data. The mean S-LANSS score was 11.58 ± 7.37, and the mean NPSI score was 26.99 ± 18.60. Table 4 presents additional information about S-LANSS and NPSI scores. Mean SF-36 scores appear in Table 5.
Table 4.
S-LANSS and NPSI Scores by QST Findings Group
| Neuropathic Pain Measures | QST Findings Group | Mean ± SD (min-max) |
|---|---|---|
| S-LANSS (ranges 0–24) 40% ≥ 12 | Mixed sensitization (n=8) | 12.9 ± 7.7 (3–24) |
| Peripheral sensitization (n=1) | 5 | |
| Central sensitizationl (n=15) | 12.1 ± 7.0 (0–24) | |
| No Sensitization (n=1) | 0 | |
| NPSI (ranges 0–100) | Mixed sensitization (n=8) | 27.3 ± 14.4 (8–50) |
| Peripheral sensitization (n=1) | 5 | |
| Central sensitizationl (n=15) | 29.9 ± 20.4 (0–80) | |
| No Sensitization (n=1) | 6 |
Table 5.
SF-36 Mean Scores and SD by Subscale
| SF-36 scale | Mean | SD |
|---|---|---|
| Physical functioning | 44.6 | 24.8 |
| Role limitation due to physical health | 41.7 | 42.3 |
| Role limitation due to emotional problems | 65.3 | 41.4 |
| Energy/fatigue | 42.4 | 21.0 |
| Emotional well-being | 66.5 | 20.8 |
| Social functioning | 53.0 | 27.5 |
| Pain | 57.6 | 24.1 |
| General health | 43.1 | 19.3 |
There was a strong, statistically significant relationship between the S-LANSS and NPSI scores (r =.66, p<.001). There was moderate and statistically significant correlation between the S-LANSS score and the average pain intensity (r=.49, p<.02) and between the NPSI score and the average pain intensity (r=.59, p<.003), but the correlation between the S-LANSS score and SF-36 pain subscale (r=−.11, p=.61) and between the NPSI score and SF-36 pain subscale (r=−.31, p=.15) were not significant.
We found that 10 (40%) subjects met the S-LANSS criteria for neuropathic pain, meaning that their S-LANSS score was greater than or equal to 12 (High S-LANSS score group). For these subjects, the mean pain intensity was 5.0 ± 2.9. For subjects with S-LANSS score below 12 (Low S-LANSS score group), the mean pain intensity was 2.9 ± 2.2. The difference in mean pain intensity by S-LANSS High and Low groups approached statistical significance (p=.07). For the high S-LANSS score group, the SF-36 pain score was 50.3 ± 20.1 and for the low S-LANSS score group, the SF-36 pain score was 62.5 ± 25.9; this difference was not significant (p=.20).
We could not determine the percentage of subjects that met the criteria for neuropathic pain with the NPSI because a cut-off point for neuropathic pain has not been established. Subjects reported NPSI scores that ranged from 0 to 80, with a mean score 27 ± 18.6. Among subjects in the High S-LANSS score group, the NPSI scores ranged from 16 to 80, with mean 39.5 ± 25.0. For subjects in the Low S-LANSS score group, the NPSI scores ranged from 0 to 40, with mean 18.7 ± 20.3. The difference in mean NPSI scores by S-LANSS High and Low groups was significant (p=.01).
DISCUSSION
We are the first to report the safety of a QST protocol, results for detection of thermal and mechanical stimuli among adults with SCD, and self-report findings about their pain. We found that adults with SCD tolerated the TSA II Limits Protocol and von Frey filaments as stimuli for detecting sensation and pain thresholds; the QST protocol was safe and did not stimulate a SCD pain crisis. Previously, we found that adults with SCD select neuropathic pain descriptors to describe their pain.4 We also are the first to report the likelihood of neuropathic pain in this small sample of adults with SCD using thermal and mechanical QST and other self-report measures of neuropathic pain. With thermal and mechanical QST, we found evidence of widespread sensitivity in 15 (60%) of the 25 subjects and identified 1 (4%) subject with possible findings of small sensory fiber abnormality, 8 subjects with mixed normal and abnormal findings, and 1 subject with normal findings. The adults with SCD were able to use two self-report tools for neuropathic pain (S-LANSS, NPSI) with preliminary evidence of construct validity for both based on average pain intensity; 10 (40%) of the subjects reported S-LANSS scores indicative of neuropathic pain. Although encouraging as indicators of neuropathic pain in adults with SCD, all of the findings require replication in a larger sample before definitive conclusions can be made about neuropathic pain in adults with SCD.
Pain of adults with SCD includes chronic ongoing pain for as many as two-thirds of patients31, 4 but also includes unpredictable, recurrent acute pain that often results in hospitalization.32 The unpredictable nature of this acute pain must be considered as research protocols are designed. Although we demonstrated safety of our QST protocol, a few subjects reported residual pain after the testing protocol; for one subject, the pain continued for 24 hours, and another subject narrowly averted an ED visit after the testing, but neither considered the pain to be an acute pain crisis. Because of these issues, although we allowed subjects to remain on their routine analgesics, all but 1 of the subjects reported sensitivity to the mechanical and thermal stimuli. Additional studies are needed to confirm these observations and to focus on analgesics needed for at least 24 hours after the testing protocol. Because of the pain exacerbation issue, another research group (J.A. Haythornthwaite, personal communication, Sept 2011) also observed the need to modify testing protocols when studying pain mechanisms among adults with SCD. Investigators are encouraged to pilot test a standard pain testing protocol when studying people with SCD to assure its safety in this vulnerable population.
We used thermal and mechanical stimulation of sensory fibers to determine if adults with SCD report normal sensation or sensitivity; all subjects were sensate to the thermal and mechanical stimuli at all sites tested. The detection of warm sensation is usually at 1° to 2° C above 32° C (the TSA adaptation temperature) and is a sensation mediated by C fibers. Cold sensation usually occurs at a similar range below adaptation and is a sensation mediated by A-delta fibers. In our sample, detection of warm and cold sensations was 3° to 4° C higher or lower than the 32° C adaptation temperature, respectively, suggesting thermal hypoesthesia. The heat pain threshold is usually about 45° C12, 33 and mostly mediated by C fibers with some A-delta fiber involvement, but in our sample, the heat pain threshold was 2° to 4° C lower. The cold pain threshold is the most variable and difficult to assess of all these modalities, but it is usually sensed at about 10° C34 as mediated by a combination of both C and A-delta fibers. In our sample, cold pain threshold was 8° to 13° C higher than the typical 10° C temperature, which if confirmed in a larger sample, could help to explain the increase in acute care utilization for SCD pain at cooler temperatures that are usually well tolerated by others. Also, in our sample, the cold and heat pain thresholds were only 2° to 3° C higher for cold or lower for heat than norms from children and adolescents matched for age and race to those with SCD.12 Interestingly, a small number of both older and younger subjects reported they perceived the cold pain stimuli as warm rather than cold, a finding that others have reported in other populations.35, 36
In our sample, age and the tested body site may account for some of the differences in temperature detection or pain threshold temperatures. Also, our use of ½ SD from the mean to define abnormality may account for the low frequency of subjects with normal responses, but normative data from matched controls at all the tested sites was not available at the time of our study and we had to rely on published norms from African American college students or the best available evidence. There remains a need for normative data from subjects matched for age, race, and gender in all the sites to be tested to better inform future studies. Our conclusions might be altered if normative data are significantly different from those currently available for interpretation of our values, especially for the older adults.
Results from the mechanical stimuli indicate that only 6 (24%) of our 25 adult subjects reported responses consistent with normal responses to mechanical stimuli, an indication of Aβ fiber function. For the other subjects, the abnormalities from a theoretical perspective suggest widespread large fiber dysfunction for 11 (44%) and mixed responses for 8 (31%). These findings are different than those recently reported for children and adolescents with SCD whose responses to mechanical stimuli were not different from their matched controls.12
Our sample reported abnormal sensitivity to thermal and mechanical stimuli with 9 (28%) subjects reporting severe pain, 7 (36%) reporting moderate pain, and the other reporting mostly mild pain, with the stimuli at one or more of the test sites. Generally, subjects rated the thermal pain more intense than the mechanical stimuli. The scatter plot of pain intensity reported for the mechanical stimuli and the baseline current pain intensity (Figure 3) clearly shows variability in responses and an indication that the patient was responding to the QST stimuli rather than the baseline pain. Other researchers typically have not reported these pain report data, which makes interpretation of findings from this small sample difficult.
Although our sample is small, the findings provide the first support of the construct validity of the S-LANSS and NPSI as a pain measure for adults and older adults with SCD. Clearly a larger study is needed to confirm the validity of the neuropathic self-report questionnaires in the SCD population and to establish appropriate cut scores for the pain of SCD. It is evident that the subjects were able to use the tools and there is some evidence of their validity in adults with SCD.
Despite the importance and novelty of our findings, some limitations detract from them. We did not use random selection procedures to obtain our sample; therefore, it is not clear how representative our sample is to all adults with SCD and pain. Although this study focused on protocol safety, we did not exclude 4 subjects with diabetes; diabetes should be an exclusion criterion in future studies among adults with SCD. Adults with SCD who have moderate to severe intense pain are more likely to participate in pain studies because they are desperate for better pain control. The prevalence of abnormal thermal and mechanical sensitivity, which may be an indication of neuropathic pain, is likely to be lower in the general population with SCD. Lack of norms for all the sites we tested among African Americans of similar age to our sample is also a limitation. Additional research is needed to obtain more complete normative data, especially for older African Americans not represented adequately in previous studies.12, 37 Another limitation of our study is lack of data regarding the nervous system injury that is needed to support the narrow definition of neuropathic pain that others support.10, 38, 39 It may support the broader definition of neuropathic pain, since inflammation was not clearly evident at any of the sites we tested, as an alternate etiology of the thermal and mechanical sensitivity we observed.39 It is unknown if the strong correlation we found between the self-report measures of neuropathic pain, the S-LANSS and the NPSI, will be replicated in another sample of adults with SCD. Finally, the lack of correlation between the SF-36 pain subscale and either the S-LANSS or the NPSI may or may not be replicated in a larger sample.
In light of these limitations, the most important implication of our findings is the need for additional research to determine the contribution of neuropathic pain mechanisms to the SCD pain experience. Larger studies of adults with SCD with repeated QST measures are needed to determine the stability and prevalence of neuropathic pain as well as the mechanisms and treatments for this component of SCD pain. It is likely that adult patients with SCD experience both nociceptive and neuropathic pain, but at this time it is not clear which mechanisms contribute to their pain experiences at different time points. Given the evidence that animal studies document neuropathic pain in the transgenic mouse with SCD,5, 6, 7 historical evidence related to SCD,40 and the evidence we present here, it is highly likely that some adults with SCD have neuropathic pain as part of their pain experience. Determining the therapies most likely to relieve the neuropathic component of SCD pain could provide a paradigm shift for its treatment, which now focuses mostly on use of opioids but as our group showed, other therapies show promise.41
In conclusion, we present evidence supportive of a thermal and mechanical QST protocol that can be safely conducted in adults with SCD. All subjects were sensate in all sites tested. Using available norms for thermal QST, we documented that 60% of our sample reported widespread sensitivity, 36% of our small sample reported localized or mixed sensitivity, and only one subject reported no indication of sensitivity. Using published cut scores for self-report tools for neuropathic pain, evidence also supports the presence of neuropathic pain for 40% of the sample of adults with SCD. Although novel and intriguing, additional research studies are needed to confirm these findings.
Acknowledgments
This publication was made possible by Grant Numbers 1R01 HL078536 (subject recruitment) and 1U54 HL090513 (Scholars program, data collection) from the National Institutes of Health (NIH), National Heart Lung and Blood Institute (NHLBI) and Dr. Wilkie’s Harriet H. Werley Endowed Chair for Nursing Research fund. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI. The final peer-reviewed manuscript is subject to the NIH Public Access Policy. The authors thank the patients with sickle cell disease for participating in this study, the staff at the Comprehensive Sickle Cell Center for their continuous support of the study, and Harriett Wittert, BSN, RN for her assistance with subject recruitment. The authors declare no conflicts of interest related to this study. Drs. Molokie and Wilkie are co-investigators on an unrelated study funded by Pfizer.
References
- 1.Yusuf HR, Atrash HK, Grosse SD, Parker CS, Grant AM. Emergency department visits made by patients with sickle cell disease: a descriptive study, 1999–2007. Am J Prev Med. 2010;38:S536–541. doi: 10.1016/j.amepre.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballas S. Sickle cell pain. Seattle: IASP Press; 1998. [Google Scholar]
- 3.Benjamin LJ, Dampier CD, Jacox AK, Odesina V, Phoenix D, Shapiro B, et al. Guidelines for the management of acute and chronic pain in sickle-cell disease. Glenview, IL: American Pain Society; 1999. [Google Scholar]
- 4.Wilkie DJ, Molokie R, Boyd-Seal D, Suarez ML, Kim YO, Zong S, et al. Patient-Reported Outcomes: Nociceptive and Neuropathic Pain and Pain Barriers in Adult Outpatients with Sickle Cell Disease. JNMA. 2010;102:18–27. doi: 10.1016/s0027-9684(15)30471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohli DR, Li Y, Khasabov SG, Gupta P, Kehl LJ, Ericson ME, et al. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood. 2010;116:456–465. doi: 10.1182/blood-2010-01-260372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hillery CA, Kerstein PC, Vilceanu D, Barabas ME, Retherford D, Brandow AM, et al. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood. 2011;118:3376–3383. doi: 10.1182/blood-2010-12-327429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang ZJ, Wilkie DJ, Molokie R. Neurobiological mechanisms of pain in sickle cell disease. Hematology Am Soc Hematol Educ. 2010;2010:403–408. doi: 10.1182/asheducation-2010.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zappia KJ, Garrison SR, Hillery CA, Stucky CL. Cold hypersensitivity increases with age in mice with sickle cell disease. Pain. 2014 doi: 10.1016/j.pain.2014.05.030. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Backonja MM, Walk D, Edwards RR, Sehgal N, Moeller-Bertram T, Wasan A, et al. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain. 2009;25:641–647. doi: 10.1097/AJP.0b013e3181a68c7e. [DOI] [PubMed] [Google Scholar]
- 10.Cruccu G, Sommer C, Anand P, Attal N, Baron R, Garcia-Larrea L, et al. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol. 2010;17:1010–1018. doi: 10.1111/j.1468-1331.2010.02969.x. [DOI] [PubMed] [Google Scholar]
- 11.Lanzkron S, Carroll CP, Haywood C., Jr The burden of emergency department use for sickle-cell disease: an analysis of the national emergency department sample database. Am J Hematol. 2010;85:797–799. doi: 10.1002/ajh.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandow AM, Stucky CL, Hillery CA, Hoffmann RG, Panepinto JA. Patients with sickle cell disease have increased sensitivity to cold and heat. Am J Hematol. 2012;88:37–43. doi: 10.1002/ajh.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Leary JD, Crawford MW, Odame I, Shorten GD, McGrath PA. Thermal pain and sensory processing in children with sickle cell disease. Clin J Pain. 2014;30:244–250. doi: 10.1097/AJP.0b013e318292a38e. [DOI] [PubMed] [Google Scholar]
- 14.Bennett MI, Attal N, Backonja MM, Baron R, Bouhassira D, Freynhagen R, et al. Using screening tools to identify neuropathic pain. Pain. 2007;127:199–203. doi: 10.1016/j.pain.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 15.Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain. 2005;6:149–158. doi: 10.1016/j.jpain.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Gray P. Acute neuropathic pain: diagnosis and treatment. Curr Opin Anaesthesiol. 2008;21:590–595. doi: 10.1097/ACO.0b013e32830c900c. [DOI] [PubMed] [Google Scholar]
- 17.Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108:248–257. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Yawn BP, Wollan PC, Weingarten TN, Watson JC, Hooten WM, Melton LJ., 3rd The prevalence of neuropathic pain: clinical evaluation compared with screening tools in a community population. Pain Med. 2009;10:586–593. doi: 10.1111/j.1526-4637.2009.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkie DJ, Judge MK, Berry DL, Dell J, Zong S, Gilespie R. Usability of a computerized PAINReportIt in the general public with pain and people with cancer pain. J Pain Symptom Manage. 2003;25:213–224. doi: 10.1016/s0885-3924(02)00638-3. [DOI] [PubMed] [Google Scholar]
- 20.Melzack R. The McGill pain questionnaire: Major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 21.Holroyd KA, Holm JE, Keefe FJ, Turner JA, Bradley LA, Murphy WD, et al. A multi-center evaluation of the McGill Pain Questionnaire: results from more than 1700 chronic pain patients. Pain. 1992;48:301–311. doi: 10.1016/0304-3959(92)90077-O. [DOI] [PubMed] [Google Scholar]
- 22.Pearce J, Morley S. An experimental investigation of the construct validity of the McGill Pain Questionnaire. Pain. 1989;39:115–121. doi: 10.1016/0304-3959(89)90182-6. [DOI] [PubMed] [Google Scholar]
- 23.Huang HY, Wilkie DJ, Zong SP, Berry D, Hairabedian D, Judge MK, et al. Developing a computerized data collection and decision support system for cancer pain management. Comput Inform Nurs. 2003;21:206–217. doi: 10.1097/00024665-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Jha A, Suarez ML, Ferrans CE, Molokie R, Kim YO, Wilkie DJ. Cognitive testing of PAINReportIt in adult African Americans with sickle cell disease. Comput Inform Nurs. 2010;28:141–150. doi: 10.1097/NCN.0b013e3181d7820b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouhassira D. Neuropathic pain: the clinical syndrome revisited. Acta Neurol Belg. 2001;101:47–52. [PubMed] [Google Scholar]
- 26.Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkinson C, Wright L, Coulter A. Criterion validity and reliability of the SF-36 in a population sample. Qual Life Res. 1994;3:7–12. doi: 10.1007/BF00647843. [DOI] [PubMed] [Google Scholar]
- 28.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 30.Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods. 2001;6:330–351. [PubMed] [Google Scholar]
- 31.Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148:94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- 32.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120:3647–3656. doi: 10.1182/blood-2012-04-383430. [DOI] [PubMed] [Google Scholar]
- 33.Smith V. Reference Values for Quantitative Sensory Testing (QST) in Healthy Individuals [Honor's College] Chicago, IL: Biobehavioral Health Science, University of Illinois at Chicago; 2011. [Google Scholar]
- 34.McKemy DD. The molecular and cellular basis of cold sensation. ACS Chem Neurosci. 2013;4:238–247. doi: 10.1021/cn300193h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Defrin R, Ohry A, Blumen N, Urca G. Sensory determinants of thermal pain. Brain. 2002;125:501–510. doi: 10.1093/brain/awf055. [DOI] [PubMed] [Google Scholar]
- 36.Hiura A. Is thermal nociception only sensed by the capsaicin receptor, TRPV1? Anat Sci Int. 2009;84:122–128. doi: 10.1007/s12565-009-0048-8. [DOI] [PubMed] [Google Scholar]
- 37.Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;113:20–26. doi: 10.1016/j.pain.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Cruccu G, Anand P, Attal N, Garcia-Larrea L, Haanpaa M, Jorum E, et al. EFNS guidelines on neuropathic pain assessment. Eur J Neurol. 2004;11:153–162. doi: 10.1111/j.1468-1331.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- 39.Haanpaa M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152:14–27. doi: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 40.Molokie RE, Wang ZJ, Wilkie DJ. Presence of neuropathic pain as an underlying mechanism for pain associated with cold weather in patients with sickle cell disease. Med Hypotheses. 2011;77:491–493. doi: 10.1016/j.mehy.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molokie RE, Wilkie DJ, Wittert H, Suarez ML, Yao Y, Zhao Z, et al. Mechanism-driven phase I translational study of trifluoperazine in adults with sickle cell disease. Eur J Pharmacol. 2014;723:419–424. doi: 10.1016/j.ejphar.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagander LG, Midani HA, Kuskowski MA, Parry GJ. Quantitative sensory testing: effect of site and skin temperature on thermal thresholds. Clin Neurophysiol. 2000;111:17–22. doi: 10.1016/s1388-2457(99)00192-3. [DOI] [PubMed] [Google Scholar]