Abstract
Hematopoietic cell transplantation (HCT) is a potential cure for certain hematologic malignancies. However, because of risks of complications and mortality, this treatment option is limited to patients with minimal comorbidities. We performed a retrospective cohort study evaluating the impact of pre-HCT systolic dysfunction on outcomes. We identified 49 subjects with systolic dysfunction, defined as left ventricular ejection fraction (LVEF) < 50% and 49 controls (matched by age, gender, conditioning regimen, and HCT donor number; all with LVEF ≥ 50%) undergoing HCT at the University of Minnesota between 2002 and 2012. Treatment complications, use of beta-blockers and angiotensin-converting enzyme inhibitors, and overall survival (OS) after HCT out to 24 months were analyzed. The median LVEF was 45% (range, 27.5% to 49%) for the study group and 60% (range, 50% to 69%) for controls. The majority of patients in both groups (81.6%) received reduced-intensity conditioning (RIC). Treatment-related mortality (TRM) at day 100 was identical, with a cumulative incidence of 14% in the study (95% confidence interval [CI], 5% to 24%) versus 14% in controls (95% CI, 5% to 24%) (P = .89). Two-year OS was similar in the study group (53%; 95% CI, 38% to 66%) versus controls (61%; 95% CI, 46% to 73%) (P = .34). LVEF ≥ 43% was associated with improved OS at 1 year (hazard ratio [HR], .36; 95% CI, .15 to .87; P = .02). There was no significant difference in the incidence of non–life-threatening cardiac complications (12.2% in cases versus 8.2% in controls, P = .50) or serious (life-threatening or fatal) cardiac complications (4.1% in cases versus 2.0% in controls, P .56). Pre-existing coronary artery disease was associated with increased TRM at 100 days (HR, 4.35; 95% CI,=1.24 to 15.32; P = .02). Cardiac medication use had no effect on TRM. Our study demonstrates that patients with asymptomatic borderline systolic dysfunction can safely undergo HCT with RIC. Coronary artery disease remains a risk factor for increased TRM. Patients with borderline systolic dysfunction can safely undergo HCT, but may need particular vigilance for potential hemodynamic or ischemic cardiac complications.
Keywords: Hematopoietic cell, transplantation, Systolic dysfunction, Left ventricular ejection, fraction, Survival
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (HCT) is a potential cure for various hematologic malignancies. Given the risk of treatment-related complications and mortality, this treatment is only offered to patients considered able to tolerate treatment toxicity. Reduced-intensity conditioning (RIC) regimens have expanded the patient population being offered HCT to include older patients [1-3]. This older population often presents with added comorbidities, which may require specific attention and management. The HCT-specific comorbidity index is a validated tool to help predict transplantation risk based on the presence of various comorbidities [4]. According to this model, a cardiac comorbidity (coronary artery disease [CAD], congestive heart failure [CHF], myocardial infarction [MI], or ejection fraction ≤ 50%) is considered low risk. However, with few studies supporting this data, there remains concern about transplantation outcomes in patients with cardiomyopathy. Additionally, the significance of these individual cardiac comorbidities is uncertain. The relative infrequency of major cardiac complications attributable to HCT, occurring in < 1% of transplantations at our institution, suggests that patients with impaired cardiac function are not predisposed to worse transplantation outcomes [5]. Qazilbash et al. showed that patients with reduced left ventricular ejection fraction (LVEF) can safely undergo allogeneic HCT [6]. We investigated whether patients with systolic dysfunction are able to tolerate transplantation. We report the outcomes of consecutive patients undergoing HCT with a reduced LVEF.
MATERIALS AND METHODS
Patient Selection
We performed a retrospective cohort study comparing the HCT outcomes of subjects with systolic dysfunction compared with controls who had normal systolic function. The University of Minnesota HCT database contains prospectively collected data on all patients who underwent transplantation at our institution. All adult patients (18 years or older) undergoing allogeneic HCT from January 1, 2002 to December 31, 2012 with a history of cardiovascular disease were identified. The initial screen yielded 117 subjects. Pretransplantation cardiac evaluation by either echocardio-gram or multiple gated acquisition scan identified 49 of these 117 subjects as having an LVEF < 50% before transplantation.
The control group comprised 49 randomly selected subjects who received transplants between 2002 and 2012. Control subjects were identified using HCT database at University of Minnesota, matching the study group by age (±5 years), gender, diagnosis, conditioning regimen (myeloablative versus reduced intensity), and number of stem cell units transplanted (single versus double).
Patient Characteristics
We reviewed available records for cardiovascular risk factors before transplantation, including tobacco use, CAD, cardiac arrhythmia, CHF, MI, hypertension, dyslipidemia, diabetes mellitus, chronic obstructive pulmonary disease, and chronic kidney disease (glomerular filtration rate < 60 mL/minute). Additionally, we documented use of the following cardiac medications before HCT: angiotensin-converting enzyme inhibitors (ACE inhibitors), angiotensin receptor blockers, beta blockers, aspirin, clopidogrel, statins, and loop diuretics. We collected data on the subjects’ anthracycline exposure before transplantation or estimated it based on cycles of treatment with an anthracycline. A doxorubicin equivalent dose for other antitumor antibiotics (daunorubicin, idarubicin, and mitoxantrone) was calculated (1 mg doxorubicin = 3 mg idarubicin = 2.8 mg mitoxantrone = .67 mg daunorubicin) based on previously reported maximum cumulative doses associated with cardiotoxicity [7-10].
Endpoints
The primary endpoint was 100 day transplantation-related morality (TRM) in the study and control groups. Secondary endpoints included TRM at 1 year and 2 years, overall survival (OS) at 1 year out to 2 years, and occurrence of cardiac complications (minor and serious) at any time after HCT. Cardiac events were identified by database and medical record review and were categorized by the Common Terminology Criteria for Adverse Events version 4.0 [11]. We subdivided events into minor (grades 1 to 3) and serious events (grades 4 and 5). In an attempt to accurately identify cardiac complications attributable to HCT, we excluded 7 cardiac events as review of the record identified a noncardiac cause as the underlying etiology (sepsis, n = 5; veno-occlusive disease, n = 1; and multiorgan failure, n = 1).
Statistical Analysis
Data on pretransplantation patient characteristics, transplantation complications, and outcomes were prospectively collected by the biostatistical support group at the University of Minnesota, using standardized collection procedures.
Comparison of patient and transplantation characteristics between study and control group were performed using chi-square or Wilcoxon's rank sum test, as appropriate. TRM and OS were the outcome variables studied. The Kaplan-Meier method was used to estimate OS, whereas cumulative incidence was estimated for the risk of TRM, with malignant relapse as the competing hazard. Cox regression models were conducted for OS and competing risk regression was employed for TRM [12,13]. We also investigated a threshold LVEF associated with different transplantation outcomes. We tested distribution of pre-HCT LVEF versus day 100 OS and found that the lowest 10th percentile of LVEF (corresponding to LVEF of 43%) was the optimal cut-off point. LVEF < 43% and ≥ 43% was the primary factor considered for each endpoint. Other covariates, including group (case versus control), medication, and comorbidity risk factors (listed in Table 1), were also compared for each endpoint. Multivariate analysis was not done because the factors showing significance in univariate analysis were highly correlated to each other.
Table 1.
Patient Comorbidities and Medication Use
| Case (n = 49) | Control (n = 49) | P Value | |
|---|---|---|---|
| Median LVEF | 45.0% (27.5%-49.0%) | 60.0% (50.0%-69.0%) | <.01 |
| Anthracycline dose, median (mg/m2) | 220.0 (.0-800.0) | 216.0 (.0-580.0) | .24 |
| CAD | 6 (12.2%) | 1 (2.0%) | .05 |
| Arrhythmia | 3 (6.1%) | 0 | .08 |
| CHF | 5 (10.2%) | 1 (2.0%) | .09 |
| Diabetes mellitus | 8 (16.3%) | 3 (6.1%) | .11 |
| COPD | 1 (2.0%) | 0 | .31 |
| CKD | 7 (14.3%) | 3 (6.1%) | .19 |
| Hypertension | 9 (18.4%) | 7 (14.3%) | .58 |
| Dyslipidemia | 8 (16.3%) | 5 (10.2%) | .37 |
| Prior MI | 3 (6.1%) | 0 | .08 |
| ACE inhibitor | 15 (30.6%) | 3 (6.1%) | <.01 |
| ARB | 3 (6.1%) | 1 (2.0%) | .31 |
| Beta blocker | 16 (32.7%) | 2 (4.1%) | <.01 |
| Aspirin | 6 (12.2%) | 0 | .01 |
| Clopidogrel | 2 (4.1%) | 0 | .15 |
| Statin | 9 (18.4%) | 2 (4.1%) | .03 |
| Loop diuretic | 4 (8.2%) | 1 (2.0%) | .17 |
COPD indicates chronic obstructive pulmonary disease; CKD, chronic kidney disease; ARB, angiotensin receptor blocker.
Data presented are n (%) unless otherwise indicated.
All P values were 2 sided. Analyses were performed using SAS 9.3 software (Cary, NC).
RESULTS
Patient Demographics
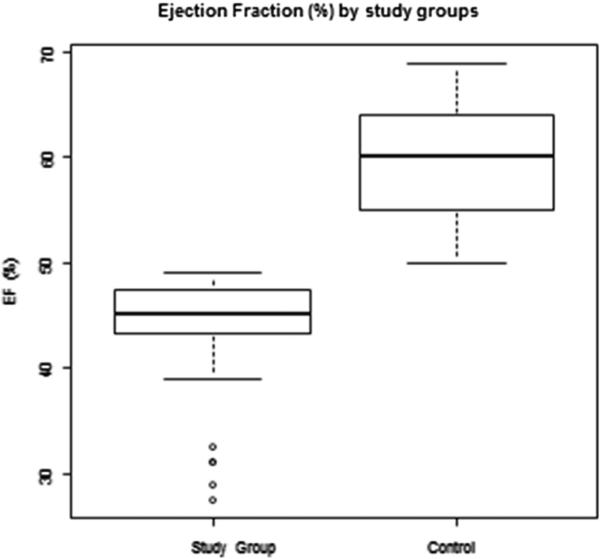
Patient characteristics were similar between study and control group (Table 2). The median age at time of transplantation was 52.3 (range, 19.1 to 69.2) years for the study group and 54.3 (range, 20.7 to 72.5) for the control group (P = .89). The majority of subjects in both groups were male and received RIC. Acute myeloid leukemia was the most common indication for transplantation. Nearly all subjects received conditioning with cyclophosphamide (n = 47 [96%] cases; n = 48 [98%] controls) and total body irradiation (n = 47 [96%] cases; n = 46 [94%] controls). The hematopoietic stem cell source was from a single donor (umbilical cord blood, peripheral blood stem cells, or bone marrow) for 25 subjects (51%) in each group, whereas the remaining 24 subjects (49%) in each group received double umbilical cord blood (P = 1.00). The median LVEF was 45% (range, 27.5% to 49%) for the study group, compared with 60% (range, 50% to 69%) for the control group (P < .01) (Figure 1).
Table 2.
Patient Characteristics
| Characteristic | Case (n = 49) | Control (n = 49) |
|---|---|---|
| Male | 31 (63.3%) | 31 (63.3%) |
| Female | 18 (36.7%) | 18 (36.7%) |
| Age, median (range), yr | 52.3 (19.1-69.2) | 54.3 (20.7-72.5) |
| Transplantation indication | ||
| ALL | 3 (6.1%) | 3 (6.1%) |
| AML | 22 (44.9%) | 22 (44.9%) |
| Other leukemia | 3 (6.1%) | 3 (6.1%) |
| Myelodysplasia | 3 (6.1%) | 3 (6.1%) |
| Non-Hodgkin lymphoma | 14 (28.6%) | 14 (28.6%) |
| Hodgkin lymphoma | 3 (6.1%) | 3 (6.1%) |
| Myeloproliferative disease | 1 (2.0%) | 1 (2.0%) |
| Conditioning | ||
| Myeloablative conditioning | 9 (18.4%) | 9 (18.4%) |
| RIC | 40 (81.6%) | 40 (81.6%) |
| Stem cell source | ||
| Bone marrow | 2 (4%) | 1 (2%) |
| PBSC | 19 (39%) | 16 (33%) |
| UCB | 28 (57%) | 32 (65%) |
ALL indicates acute lymphoblastic leukemia; AML, acute myeloid leukemia; PBSC, peripheral blood stem cells; UCB, umbilical cord blood.
Data presented are n (%) unless otherwise indicated.
Figure 1.
Box plot comparing the range of ejection fractions of the 2 groups.
Comorbidities were similar between the study and control groups (Table 1) with no significant difference in a history of the following: tobacco use, arrhythmia, CHF, diabetes mellitus, chronic obstructive pulmonary disease, hypertension, dyslipidemia, or MI. However, subjects in the study group were more likely to have a diagnosis of CAD compared with patients in the control group (n = 6 [12.2%] versus n = 1 [2%], P = .05). The number of patients exposed to ≥ 400 mg/m2 of anthracycline before transplantation was similar (n = 10[20.4%] versus n = 4 [8.2%], P =.35) between the study and control groups, respectively. Five subjects in the study group and 1 subject in the control group (diastolic dysfunction) had a history of symptomatic CHF.
At the time of transplantation, subjects in the study group were more likely to be on beta blockers (n = 16 [32%] versus n = 2 [4.1%], P < .01), ACE inhibitors (n = 15 [30.6%] versus n = 3 [6.1%]; P < .01), aspirin (n = 6 [12.2%] versus n = 0[0%], P =.01), and statins (n = 9 [18.4%] versus n = 2 [4.1%], P = .03) compared with subjects in the control group (Table 1). There was no difference in the use of angiotensin receptor blockers (n = 3 [6.1%] versus n = 1 [2%], P = .31), clopidogrel (n = 2 [4.1%] versus n = 0 [0%], P = .15), or loop diuretics (n = 4 [8.2%] versus n = 1 [2%] P = .17) between the study and control groups, respectively.
TRM and OS
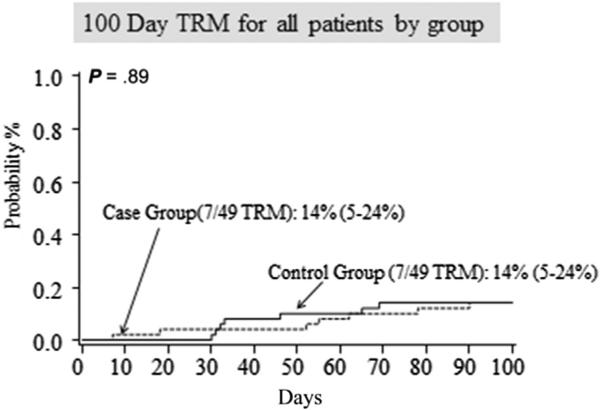
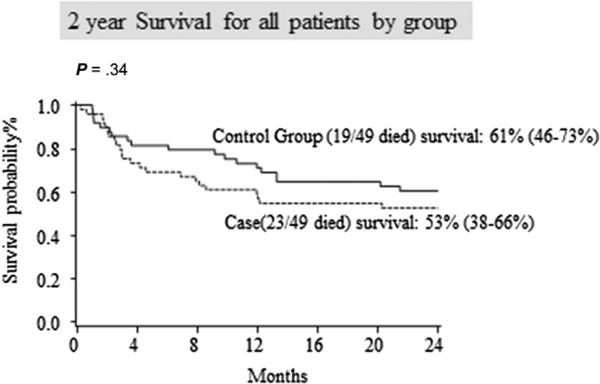
TRM and OS are reported in Table 3 and in Figures 2 and 3. There was no difference in the 100-day TRM. There were 7 transplantation-related deaths for a cumulative incidence of 14%, (95% confidence interval [CI], 5% to 24%) in the study group and 7 transplantation-related deaths for a cumulative incidence of 14% (95% CI, 5% to 24%) in the controls (P = .89). TRM was identical between the study and control at 1 year (n = 8 [16%]; 95% CI, 6% to 27% for both groups; P = .87) and 2 years (n = 9 [19%]; 95% CI, 8% to 30% for both groups; P = .88). Additionally, there was no difference in OS at 100 days (n = 37 [76%]; 95% CI, 61% to 85% versus n = 42 [86%]; 95% CI, 72% to 93%; P = .24), at 1 year (n = 29 [59%]; 95% CI, 44% to 71% versus n = 35 [71%]; 95% CI, 57% to 82%; P = .20), or at 2 years (n = 26 [53%]; 95% CI, 38% to 66% versus n = 30 [61%]; 95% CI, 46% to 73%; P = .30) between the study and control groups, respectively.
Table 3.
Survival Outcomes and Proportion of Complications
| Outcome | Case (n = 49) | Control (n = 49) | P Value |
|---|---|---|---|
| TRM at day 100 | 7 (14.3%) | 7 (14.3%) | 1.00 |
| TRM at 1 year | 8 (17%) | 8 (17%) | .87 |
| TRM at 2 years | 9 (19%) | 9 (19%) | .88 |
| OS at day 100 | 37 (75.5%) | 42 (85.7%) | .20 |
| OS at 1 year | 28 (57.1%) | 34 (69.4%) | .21 |
| OS at 2 years | 25 (51.0%) | 29 (59.2%) | .42 |
| Minor cardiac complication | 6 (12.2%) | 4 (8.2%) | .50 |
| Serious cardiac complication | 2 (4.1%) | 1 (2.0%) | .56 |
| Hemodialysis initiation by day 100 | 3 (6.1%) | 3 (6.1%) | 1.00 |
| Mechanical ventilation by day 100 | 11 (22.4%) | 8 (16.3%) | .44 |
Figure 2.
TRM was identical between the study and control groups at 100 days.
Figure 3.
Kaplan-Meier curves show similar survival between the study and control groups.
CAD was the only comorbidity that was associated with a significantly increased risk of TRM at 100 days (hazard ratio [HR], 4.35; 95% CI, 1.24 to 15.32; P = .02) and 1 year (HR, 3.77; 95% CI, 1.06 to 13.43; P = .04). Other comorbidities or use of cardiac medications were not associated with differences in TRM (Table 4).
Table 4.
Comorbidity and Medication Hazard Ratios
| Parameter | HR (95% CI) | P Value |
|---|---|---|
| CAD | 4.35 (1.24-15.32) | .02 |
| Hypertension | .84 (.19-3.67) | .81 |
| CHF | 1.27 (.15-10.59) | .83 |
| Dyslipidemia | 3.04 (.95-9.74) | .06 |
| Prior MI | 2.68 (.38-18.75) | .32 |
| High prior anthracycline (>300 mg/m2) | .14 (.02-1.05) | .06 |
| ACE inhibitor | 1.87 (.59-5.86) | .29 |
| Beta blocker | 1.24 (.35-4.39) | .74 |
| Aspirin | 3.08 (.66-14.37) | .15 |
| Statin | 2.55 (.68-9.53) | .17 |
| Clopidogrel | 4.46 (.69-28.95) | .12 |
Day 100 TRM univariate regression.
Subjects with an LVEF < 43%, in the lowest 10th percentile, had significantly lower OS at 1 year compared to the remainder of the cohort (HR, 2.79; 95% CI, 1.15 to 6.78; P = .02). The 1-year survival was only 33% (95% CI, 8% to 62%) versus 69% (95% CI, 58% to 77%) for the others (P = .02).
Cardiac Complications
Overall, there were few cardiac complications in both the study group and the controls. There was no significant difference in the total number of minor (n = 6 [12.2%] versus n = 4 [8.2%], P = .50] or serious cardiac complications (n = 2 [4.1%] versus n = 1 [2%], P = .56) between the study and control groups, respectively (Table 3). The minor complications in the study group were CHF exacerbation (n = 4) and atrial fibrillation (n = 3); 1 subject had both. Two in the control group had CHF exacerbations, 1 had atrial fibrillation, and 1 had an MI. The serious (life-threatening or fatal) complications in the study group were CHF exacerbation requiring mechanical ventilation (n = 1) and idiopathic cardiac tamponade requiring an emergent pericardial window procedure (n = 1). There were no deaths in the study group attributed to a cardiac complication. There was 1 fatal MI in the control group.
DISCUSSION
Our findings illustrate that asymptomatic patients with borderline systolic dysfunction can undergo HCT without increased risk of mortality or complication. However, patients with an LVEF < 43% had significantly worse outcomes. This suggest that LVEF < 43% could be considered an important risk factor in candidates for HCT and requires further study for validation. Subjects with a history of CAD were at increased risk for TRM, but we found no association between the use of specific cardiac medications and outcome.
As the HCT population ages, better understanding of management of patient comorbidities during transplantation is needed. Prior studies on the significance of a reduced LVEF in patients undergoing HCT are conflicting, with 4 reports that suggesting a reduced LVEF is a risk for serious cardiac complication [6,7,14-19]. We focused on the association of impaired LVEF and TRM. Our data are consistent with the findings of Qazilbash et al. that patients with impaired LVEF can safely undergo transplantation without increased risk of TRM or cardiac complication. Their study noted an increase in cardiac complications in patients with 1 of the following risk factors: history of tobacco use, hyper-tension, hyperlipidemia, CAD, arrhythmia, prior MI, and CHF. We investigated whether the presence of these risk factors placed patients at increased risk of TRM and found that only a history of CAD was a risk factor for increased TRM at day 100 and 1 year. We did not find any risk factors that increased the risk of cardiac complications.
There is little specific data on how to manage systolic dysfunction in patients undergoing HCT; thus, its management during transplantation should be the same as the general population with beta blockers and ACE inhibitors per the American Heart Association and American College of Cardiology guidelines [20]. Evidence that beta blockers (particularly carvedilol) and ACE inhibitors are effective for preventing anthracycline-induced cardiomyopathy provides further rational for this practice in oncology patients [21-24]. Furthermore, these medications have been shown to improve LVEF in subjects with anthracycline-induced cardiomyopathy [25]. Our data did not show any association with cardiac medication use and TRM, but since cardiac complications were rare, we could not identify any best practices in our cohorts.
Limitations
The small number of subjects in our study group and infrequent cardiac events limit the power for conclusions from our study. Because most of the subjects in our study group had minimally reduced LVEF, we are unable to make conclusions about HCT outcomes in patients with more severely depressed LVEF. Also, the majority of subjects in our study group did not have symptomatic CHF, so our results apply to those without symptomatic systolic dysfunction. Because of several variables, including age and possibly decreased LVEF, the majority of subjects in our study group underwent RIC. Additionally, a potential for selection bias of the study group exist as it is possible that patients with severe or symptomatic heart failure may not have been referred for transplantation. At our institution, though, few patients have been denied transplantation based only on depressed LVEF without symptomatic CHF. Our practice for patients with significant cardiac comorbidity is to obtain a consultation from a cardiologist with expertise in cardiooncology to optimize the patient's cardiac status and closely manage patients during the transplantation process.
Clinical Significance of Findings
Evaluation of cardiac systolic function by multiple gated acquisition scan, echocardiogram, or cardiac magnetic resonance imaging is usually part of a pretransplantation evaluation. Our results help clinicians interpret the significance of depressed LVEF in HCT candidates. We suggest that patients with minimally depressed LVEF (>43%) remain candidates for transplantation with RIC and are not at particularly higher risk for mortality or complications. Lower LVEF or a history of CAD were risk factors in our study, and such patients need extra attention during the pretransplantation evaluation and throughout their course.
Footnotes
Financial disclosure: The authors have nothing to disclose.
Conflict of interest statement: There are no conflicts of interest to report.
REFERENCES
- 1.Popplewell LL, Forman SJ. Is there an upper age limit for bone marrow transplantation? Bone Marrow Transplant. 2002;29:277–284. doi: 10.1038/sj.bmt.1703382. [DOI] [PubMed] [Google Scholar]
- 2.Brunner AM, Kim HT, Coughlin E, et al. Outcomes in patients age 70 or older undergoing allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2002;19:1374–1380. doi: 10.1016/j.bbmt.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 3. [March 1, 2014];Current use and outcome of hematopoietic stem cell transplantation: CIBMTR summary slides. 2012 Available at: http://www.cibmtr.org.
- 4.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murdych T, Weisdorf DJ. Serious cardiac complications during bone marrow transplantation at the University of Minnesota, 1977-1997. Bone Marrow Transplant. 2001;28:283–287. doi: 10.1038/sj.bmt.1703133. [DOI] [PubMed] [Google Scholar]
- 6.Qazilbash M, Amjad A, Qureshi S, et al. Outcome of allogeneic hematopoietic stem cell transplantation in patients with low left ventricular ejection fraction. Biol Blood Marrow Transplant. 2009;10:1265–1270. doi: 10.1016/j.bbmt.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertenstein B, Stefanic M, Schmeiser T, et al. Cardiac toxicity of bone marrow transplantation: Predictive value of cardiologic evaluation before treatment. J Clin Oncol. 1994;12:998–1004. doi: 10.1200/JCO.1994.12.5.998. [DOI] [PubMed] [Google Scholar]
- 8.Anderlini P, Benjamin RS, Wong FC, et al. Idarubicin cardiotoxicity: A retrospective study in acute myeloid leukemia and myelodysplasia. J Clin Oncol. 1995;13:2827–2834. doi: 10.1200/JCO.1995.13.11.2827. [DOI] [PubMed] [Google Scholar]
- 9.Crossley RJ. Clinical safety and tolerance of mitoxantrone. Semin Oncol. 1984;11:54–58. [PubMed] [Google Scholar]
- 10.Von Hoff DD, Rozencweig M, Layard M, et al. Daunomycin-induced cardiotoxicity in children and adults. A review of 110 cases. Am J Med. 1977;62:200–208. doi: 10.1016/0002-9343(77)90315-1. [DOI] [PubMed] [Google Scholar]
- 11.US Department of Health and Human Services Common Terminology Criteria for Adverse Events, Version 4.0 (version 4.03 ed.) 2009 Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.
- 12.Cox DR. Regression models and life-tables. J Roy Statist Soc Ser B Methodol. 1972;34:187–220. [Google Scholar]
- 13.Fine JP, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 14.Fujimaki K, Maruta A, Yoshida M, et al. Severe cardiac toxicity in hematological stem cell transplantation: Predictive value of a reduced left ventricular ejection fraction. Bone Marrow Transplant. 2001;27:307–310. doi: 10.1038/sj.bmt.1702783. [DOI] [PubMed] [Google Scholar]
- 15.Braverman AC, Antin JH, Pappert MT, et al. Cyclophosphamide cardiotoxicity in bone marrow transplantation: A prospective evaluation of new dosing regimens. J Clinic Oncol. 1991;9:1215–1223. doi: 10.1200/JCO.1991.9.7.1215. [DOI] [PubMed] [Google Scholar]
- 16.Sakata-Yanagimoto M, Kanda Y, Nakagawa M, et al. Predictors for severe cardiac complications after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;33:1043–1047. doi: 10.1038/sj.bmt.1704487. [DOI] [PubMed] [Google Scholar]
- 17.Peres E, Levine JE, Khaled YE, et al. Cardiac complications in patients undergoing a reduced-intensity conditioning hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:149–152. doi: 10.1038/bmt.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg SL, Klumpp TR, Magdalinski AJ, Mangan KF. Value of the pretransplant evaluation in predicting toxic day-100 mortality among blood stem-cell and bone marrow transplant recipients. J Clin Oncol. 1998;16:3796–3802. doi: 10.1200/JCO.1998.16.12.3796. [DOI] [PubMed] [Google Scholar]
- 19.Zangari M, Henzlova MJ, Ahmad S, et al. Predictive value of left ventricular ejection fraction in stem cell transplantation. Bone Marrow Transplant. 1999;23:917–920. doi: 10.1038/sj.bmt.1701734. [DOI] [PubMed] [Google Scholar]
- 20.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 21.Kalay N, Basar E, Ozdogru, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48:2258–2262. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 22.Blaes AH, Gaillard P, Peterson BA, et al. Angiotensin converting enzyme inhibitors may be protective against cardiac complications following anthracycline chemotherapy. Breast Cancer Res Treat. 2010;122:585–590. doi: 10.1007/s10549-009-0730-5. [DOI] [PubMed] [Google Scholar]
- 23.Cardinale D, Bacchiani G, Beggiato M, et al. Strategies to prevent and treat cardiovascular risk in cancer patients. Semin Oncol. 2013;40:186–198. doi: 10.1053/j.seminoncol.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Bosch X, Rovira M, Sitges M, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: The OVERCOME trial (preventiOn of left ventricular dysfunction with enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of malignant hEmopathies). J Am Coll Cardiol. 2013;61:2355–2362. doi: 10.1016/j.jacc.2013.02.072. [DOI] [PubMed] [Google Scholar]
- 25.Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–220. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]