Abstract
In patients with non-ischemic cardiomyopathy (NICM), risk stratification for sudden cardiac death (SCD) and selection of patients who would benefit from prophylactic implantable cardioverter-defibrillators remains challenging. We aim to discuss the evidence of cardiac magnetic resonance (CMR)-derived myocardial scar for the prediction of adverse cardiovascular outcomes in NICM. From the 15 studies analyzed, with a total of 2747 patients, the average prevalence of myocardial scar was 41%. In patients with myocardial scar, the risk for adverse cardiac events was more than 3-fold higher, and risk for arrhythmic events 5-fold higher, as compared to patients without scar. Based on the available observational, single center studies, CMR scar assessment may be a promising new tool for SCD risk stratification, which merits further investigation.
Keywords: Non-ischemic cardiomyopathy, Sudden cardiac death, Cardiac magnetic resonance
INTRODUCTION
Non-ischemic cardiomyopathy (NICM) refers to a broad spectrum of myocardial conditions characterized by the reduction in left ventricular (LV) systolic function, in the absence of significant coronary artery disease (CAD). The prevalence of NICM in adults is more than 1:2500, and it is one of the leading causes of mortality (1,2). In Korea, NICM is identified as the reason in 27% acute heart failure (HF) presentations, and is associated with significant morbidity and mortality (3,4). Although progressive HF is an important cause of mortality, prior studies in Western patients found that one-third of deaths maybe due to sudden cardiac death (SCD) (5), which may be lower in Korean NICM patients (6).
A meta-analysis of five primary prevention trials in 1854 patients with NICM reported that implantable cardioverter-defibrillators (ICD) result in a 31% reduction in mortality (7). However, no single prospective randomized clinical trial of ICD therapy in NICM, which used reduced LV ejection fraction (LVEF) mainly below 30-35% as the primary risk marker, has demonstrated convincing evidence of mortality reduction (CAT (8), AMIOVIRT (9), DEFINITE (10), SCD-HeFT (11), and COMPANION (12)). This suggests that patients with NICM and depressed LVEF may be quite heterogeneous in both their risks for mortality and SCD, if stratified by their LVEF.
Nonetheless, based on the results of these trials, LVEF has become a major criterion for selection of ICD recipient in clinical practice guidelines in both the United States (13) and Korea (14). However, analysis of primary prevention trials suggest that only 20-25% of primary prevention ICD patients receive appropriate shocks within 5 years of implantation, and that many nominally eligible patients do not benefit from ICD therapy (15,16). A recent meta-analysis of commonly used risk stratification tests for arrhythmic events in patients with NICM, including functional parameters, depolarization and repolarization abnormalities, and arrhythmias, found that the available tests provide only a modest predictive value (17). For example, LVEF was found to have a sensitivity of only 72% and specificity of 51% for the prediction of arrhythmic events.
Recently, delayed-enhancement cardiac magnetic resonance imaging (DE-CMR) has become a widely available clinical test to visualize in-vivo myocardial scar and fibrosis in ischemic (18) and non-ischemic cardiomyopathies (19). Considering that the anatomic substrate for ventricular tachycardia (VT) is directly visualized, this test may be useful to identify patients with NICM who are at a risk for ventricular tachyarrhythmia, and for predicting SCD. In this review, we aim to discuss the evidence of cardiac magnetic resonance (CMR) scar imaging for the prediction of adverse cardiovascular outcomes in NICM, and in particular for SCD risk stratification.
Myocardial Scar in NICM and Ventricular Arrhythmias
Myocardial scar tissue provides the electrophysiological basis for VT in the context of chronic myocardial infarction (20). While SCD in patients with prior myocardial infarction is thought mainly to be the result of reentrant ventricular arrhythmias originating from the subendocardial surface of infarcted myocardium, the mechanism in NICM is less well understood. Electro-anatomical mapping in patients with NICM however, has demonstrated abnormal electrocardiogram recordings favoring the occurrence of reentry circuits located in the LV base in the myocardium, frequently involving the perivalvular region (21,22).
In this context, it is interesting that myocardial scar can be seen in the basal septum located in the term should be mid-myocardium ("midwall fibrosis" or "midwall striae") in about 30% of patients with NICM (19,23); this non-ischemic type of fibrosis can be visualized with post-contrast DE-CMR imaging. Non-ischemic scar in NICM can also be found in epicardial location, or in a patchy pattern of fibrosis, which typically spares the endocardium (Fig. 1). Features of ischemic injury are the involvement of the endocardium, and location in a region consistent with a coronary perfusion territory. This type of endocardial scar from ischemic injury can also be found in NICM without the presence of significant epicardial coronary disease, and can result from embolic events, coronary vasospasm, or spontaneously recanalized plaque rupture with insignificant stenosis (24). The pattern and localization of myocardial fibrosis seen on DE-CMR can help identify the etiology of cardiomyopathy and is frequently used in the work-up of patients with new diagnosis of reduced LVEF or congestive heart failure symptoms (25).
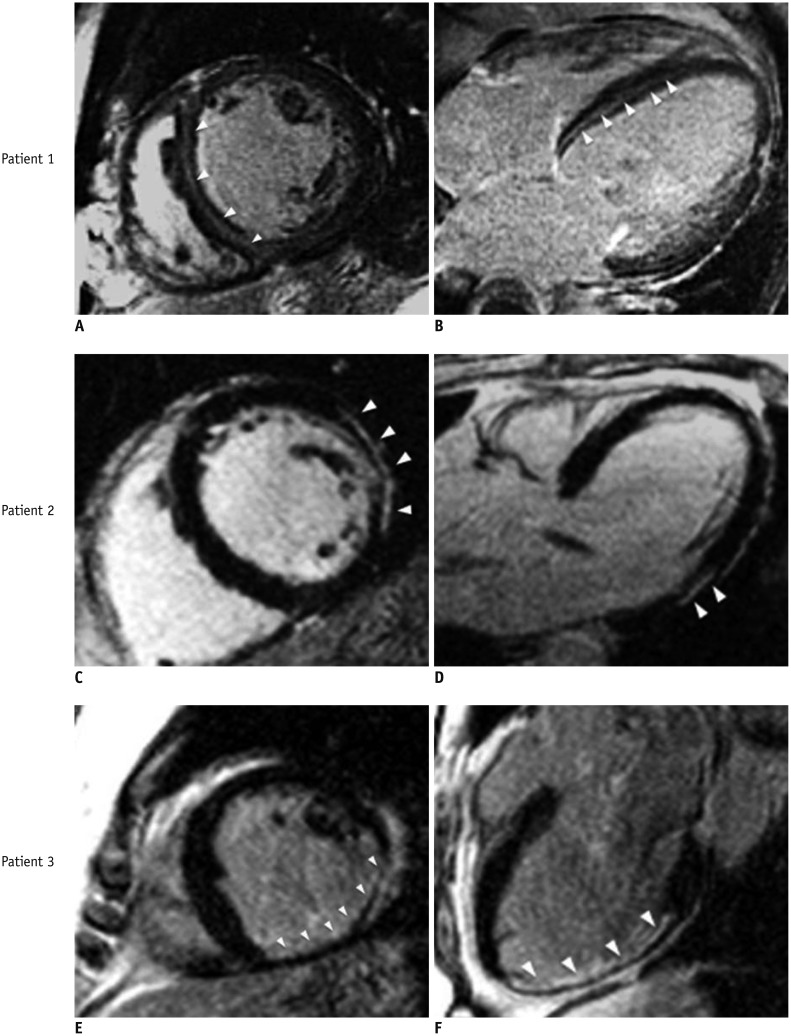
Fig. 1. Different delayed enhancement patterns in non-ischemic cardiomyopathy.
Patient 1. 48-year-old female with history of dilated cardiomyopathy. Delayed enhancement images demonstrate intramural contrast enhancement in septum (midwall striae, arrowheads). A. Mid-ventricular short-axis view. B. 4-chamber-view. Patient 2. 50-year-old male with remote history of biopsy-proven viral myocarditis presented with left ventricular dysfunction. Delayed enhancement images demonstrate epicardial hyperenhancement localized at basal inferolateral wall (arrowheads). C. Basal-short axis view. D. 3-chamber-view. Patient 3. 68-year-old male presented with progressive left ventricular dysfunction found to have insignificant coronary stenosis (25% middle left anterior descending artery lesion) on invasive coronary angiography. Delayed enhancement images demonstrate subendocardial hyperenhancement with 75% transmurality (arrowheads) involving inferior and inferolateral walls from middle ventricular level extending through apex consistent with ischemic injury. E. Mid-ventricular short axis view. F. 3-chamber-view.
It is important to note that the presence and extent of the myocardial scar might not be concordant with LVEF. For instance, some patients with extensive scar might have relatively preserved LVEF either because the scar is not of full-thickness and/or because there is hyperkinesia of remote walls (26,27). Conversely, some NICM patients, even with severely reduced LVEF, may have no myocardial scar.
Study Population Characteristics
Studies evaluating the relationship of myocardial scar and outcomes in NICM are summarized in Table 1. The inclusion and exclusion criteria used in these studies reflect the heterogeneous nature of NICM, which clinically has as a common denominator the absence of obstructive epicardial CAD. Several studies reference the World Health Organization classification of cardiomyopathies from 1996 (19,23,28,29,30,31,32) as inclusion criterion, whereby "dilated cardiomyopathy" is defined as "dilation and impaired contraction ... may be idiopathic, familial/genetic, viral, immune, alcohol/toxic, or associated with recognized cardiovascular disease in which the degree of myocardial dysfunction is not explained by the abnormal loading conditions or the extent of ischemic damage" (33). Clinical, non-MRI-based exclusion criteria were variable; thus, some studies included a wider range of etiologies of NICM (23,30,34), whereas others had a narrow spectrum (35). The former is more representative of the traditional clinical approach of patients with new onset of HF, which is to assess for the presence of CAD with invasive or non-invasive tests. Once CAD is excluded, the diagnosis of NICM is made and patients are treated alike, without further investigation of myocardial disease (36).
Table 1. Studies Evaluating Relation of Myocardial Scar and Outcomes in Non-Ischemic Heart Disease and Cardiomyopathy.
| First Author (Reference) | Inclusion Criteria | Exclusion Criteria | Primary Prophylaxis ICD | Primary and Secondary Prophylaxis ICD* |
|---|---|---|---|---|
| Assomull (19) | DCM > 12 months duration | LVEF > 56%, significant valvular disease, HCM, infiltrative heart disease | O | |
| Wu (27) | NICM and LVEF ≤ 35% referred to primary prophylaxis ICD | NYHA class IV, acute myocarditis, CHD, HCM, infiltrative heart disease | O | |
| Hombach (28) | Idiopathic dilated CMP > 12 months duration | Inflammatory heart disease based on cardiac biopsy | O | |
| Cheong (42) | All patients with CMR and no CAD | HCM, myocarditis, sarcoidosis or other infiltrative cardiomyopathy | O | |
| Cho (32) | NICM with LVEF < 35% | Life expectancy < 6 months, significant primary valvular disease, prior myocarditis | O | |
| Lehrke (29) | DCM > 12 months duration with LVEF < 50% | Valvular disease, hypertensive heart disease, myocarditis, CHD, endocardial scar, LVEF > 55% | O | |
| Iles (46) | NICM referred to primary prophylaxis ICD | Myocarditis | O | |
| Klem (43) | All patients with CMR and no CAD | None | O | |
| Wu (44) | NICM referred to primary prophylaxis ICD | Acute myocarditis, acute sarcoidosis or infiltrative disorders such as amyloidosis, hemochromatosis, CHD, HCM | O | |
| Gao (34) | NICM referred to EP service for consideration of ICD | None | O | |
| Müller (41) | NICM < 4 weeks duration | Endocardial scar or history of MI | O | |
| Neilan (30) | NICM referred to primary prophylaxis ICD | Infiltrative CMP | O | |
| Gulati (23) | DCM > 6 months duration with LV end diastolic volume above and LVEF below normal values | Endocardial scar of prior MI | O | |
| Perazzolo Marra (31) | NICM and LVEF < 50% | Valvular or hypertensive heart disease, CHD, < 1 month duration of CHF, HCM, ARVC, suspected infiltrative heart disease | O | |
| Masci (35) | NICM with reduced LVEF and no history of CHF | Recent myocarditis, peripartum CMP, ARVC, severe primary valvular disease, untreated hypertension, end-stage HCM, cardiac amyloidosis | O |
"O" indicates included patient. In four of studies, only patients with NICM referred to ICD implantation for primary prophylaxis of SCD were included. *Patients with prior sudden cardiac arrest, ventricular tachycardia or other indications for secondary ICD prophylaxis were included, or not specified. ARVC = arrhythmogenic right ventricular cardiomyopathy, CAD = coronary artery disease, CHD = congenital heart disease, CHF = congestive heart failure, CMP = cardiomyopathy, CMR = cardiac magnetic resonance, DCM = non-ischemic dilated cardiomyopathy, HCM = hypertrophic cardiomyopathy, ICD = implantable cardioverter-defibrillator, ICM = ischemic cardiomyopathy, LVEF = left ventricular ejection fraction, MI = myocardial infarction, NICM = non-ischemic cardiomyopathy, NYHA = New York Heart Association
Since DE-CMR allows the non-invasive and in-vivo assessment of myocardial tissue pathology, investigation for myocardial fibrosis, infiltration, and scarring to determine the etiology of cardiomyopathy has become a routine approach in clinical practice (25). Similar to excluding certain etiologies of NICM based on clinical grounds alone (prior to CMR), the analyzed studies have taken a diverse approach in excluding etiologies after the knowledge of DE-CMR findings. Table 2 lists the specific study characteristics, among them the scar patterns that were included. Of interest is that several studies have excluded subendocardial scar, which is suggestive of prior ischemic damage, and a priori appears not compatible with the diagnosis of NICM. However, it has long been known from autopsy studies that chronic infarcts can be found in 12-14% of patients having NICM and no coronary disease (37,38). The interpretation of ischemic damage in NICM is not always clear, but the occurrence of "ischemic injury" after spontaneous recanalization of an occlusive coronary event, embolization, or vasospasm has been documented without epicardial coronary obstructive lesions (39). Importantly, the knowledge of prior myocardial ischemic injury in a patient without CAD on cardiac catheterization, without a history of myocardial infarction, or Q-waves on electrocardiography (ECG) is obscure without CMR (40); based on established clinical criteria, these patients are considered as having "non-ischemic" CMP. Therefore, what deserves to be evaluated is how the unique information provided by the CMR study in patients with clinically established diagnosis of NICM, which primarily relies on coronary evaluation, relates to prognosis. Specifically, whether the finding of endocardial scar in a patient with NICM portends unique prognostic information, which is otherwise not available from other clinical tests, e.g., negative troponins, no stenosis on angiogram and no Q-wave on ECG. To fully ascertain the prognostic utility of CMR, the entire spectrum of clinically diagnosed patients who underwent CMR needs to be investigated. This prognosis-specific consideration of study design however, should be separated from how we use CMR to generate differential diagnosis of cardiomyopathies by better understanding the "tissue physiology". Some studies took a highly-focused approach by including solely the "midwall fibrosis" pattern of scar into their analyses (19,23); however, they do not specify how NICM patients who were found to have other patterns of scar were considered. It is not reported how many patients had other scar patterns, and whether these patients were excluded or considered as not having any scar in the analysis.
Table 2. Study Characteristics and Results.
| First Author (Reference) | Number of Patients | Follow-up Duration (Months)* | Mean LVEF (%) | Scar Patterns Included | Scar Prevalence (%) | Scar Quantification Method† | Scar Size (% LV Mass)* | Primary Endpoint |
|---|---|---|---|---|---|---|---|---|
| Assomull (19) | 101 | 22 | 35 | Midwall fibrosis | 35 | > 2SD | 4.6 (0.8-21) | All cause death, hospitalization for cardiovascular disease |
| Wu (27) | 65 | 17 | 24 | Midwall, endocardial, patchy foci often including RV insertion sites | 42 | > Peak remote | 10 (3-16) | Cardiovascular death, ICD shock, CHF hospitalization |
| Hombach (28) | 141 | 45 | 32 | Midwall, epicardial, diffuse | 26 | Manual planimetry | 5.6 (1.2-25.6) | Cardiac death |
| Cheong (42) | 215 | 53 | 52 | Midwall fibrosis and other patterns not specified | 18 | Visual scoring | [Scar index: 1.00] | All cause death, heart transplant |
| Cho (32) | 79 | 33 | 33 | Focal patchy, diffuse | 53 | Not quantified | - | All cause death, CHF hospitalization, transplant |
| Lehrke (29) | 184 | 22 | 38 | Midwall, epicardial, patchy/foci, diffuse; endocardial excluded | 39 | > 2SD | 2.9 | Cardiac death, ICD shock, CHF hospitalization |
| Iles (46) | 61 | 19 | 25 | Midwall, patchy | 51 | > 2SD | Not reported | Appropriate ICD shock |
| Klem (43) | 554 | 29 | 56 | Any | 26 | Visual scoring | [4 segments] | All cause death |
| Wu (44) | 98 | 43 | 25 | Any | 41 | FWHM | [0 (IQR 0-5.4) g] | Cardiac death, appropriate ICD shock |
| Gao (34) | 65 | 21 | 27 | Midwall, epicardial, endocardial | 71 | > 2, 3, and 5SD‡ | [34.5 g] | Appropriate ICD shock, SCD, survived cardiac arrest |
| Müller (41) | 185 | 21 | 43 | Midwall and non-midwall, excluding endocardial | 51 | Not quantified | - | All cause mortality, transplant, aborted SCD, sustained VT, CHF hospitalization |
| Neilan (30) | 162 | 29 | 28 | Midwall, epicardial, RV insertion, diffuse | 50 | > 2SD/FWHM§ | 9 (2SD)/6 (FWHM) | Cardiovascular death, ICD shock |
| Gulati (23) | 472 | 64 | 37 | Midwall fibrosis | 30 | FWHM | 2.5 (1.2-4.8) | All-cause death |
| Perazzolo Marra (31) | 137 | 36 | 32 | Epicardial, midwall, patchy/junctional; endocardial excluded | 56 | > 2SD | 9 (4-15) | SCD, ICD shock, VF, sustained VT |
| Masci (35) | 228 | 23 | 43 | Midwall, epicardial | 27 | Visual scoring∥ | [3 segments (1-10)] | Cardiac death, CHF onset, aborted SCD |
| Averaged | 183 | 32 | 35 | 41 | 6.2 |
*Mean or median, range as reported in study, if other measure of scar placed in parenthesis and not included in summary analysis, †Presence or absence of scar was determined by visual interpretation of experienced readers first in all studies, ‡Various thresholds were tested, optimal threshold were > 2SD in ischemic and > 5SD in non-ischemic subgroups, §Both methods used, showing similar and robust results with respect to prognostic association, ∥Visual scoring only of segments when hyperintense myocardium involved > 50% of its circumferential extent. CHF = congestive heart failure, FWHM = full width at half maximum, ICD = implantable cardioverter-defibrillator, IQR = interquartile range, LV = left ventricle, LVEF = left ventricular ejection fraction, RV = right ventricle, SCD = sudden cardiac death, SD = standard deviation, VF = ventricular fibrillation, VT = ventricular tachycardia
With respect to arrhythmia specific risk of the study populations, the majority of studies has included a general NICM population and did not exclude patients with prior arrhythmic events. In four of the studies, only patients with NICM referred for ICD implantation for primary prophylaxis of SCD were included, which are limited to patients with an LVEF ≤ 35%.
Although several studies specifically included patients with chronic stable cardiomyopathy, Müller et al. (41) included in their study only patients with recent (less than 4 weeks) diagnosis of NICM. On the other hand, Masci et al. (35) limited the study population to asymptomatic patients not having any HF symptoms.
Two investigations which we included in the present analysis, studied a general referral population undergoing CMR assessment of scar and function, and reported results separately for sub-populations with and without CAD (42,43). Although the subgroups of patients without CAD, did not specifically have reduced LVEF and/or cardiomyopathy, the studies did investigate the impact of scar on hard outcomes (both had all-cause mortality as primary endpoint) in large non-ischemic study cohorts.
To summarize, since NICM is a heterogeneous disorder with often clinically vague and ill-defined boundaries as opposed to more specific disease entities, and possibly having several coexisting pathologies in the same patient, it is challenging to make homogeneous groups based on clinical data. This is true in clinical practice, where systolic dysfunction in the absence of epicardial CADs on coronary angiograms or stress tests, or other loading conditions, typically defines NICM. The same problem exists in the published studies evaluating the prognostic role of DE-CMR in NICM. Moreover, attempts to create more homogeneous groups by excluding a priori certain subpopulations of NICM based on clinical grounds, and retrospectively considering the information from CMR hampers the comparison between studies and limits the translation of the study findings into clinical practice. A simpler and more generalized approach would be to include the entire spectrum of NICM as it is diagnosed clinically-by systolic dysfunction and absence of CAD-and then explore how well the finding of myocardial scar on DE-CMR can risk stratify this broad population. This would also better determine the incremental value of performing an MRI study when evaluating clinical patients with cardiomyopathy.
Relation of Myocardial Scar and Outcomes
In the 15 studies included in this analysis a total of 2747 patients were enrolled, with an average population size of 183 patients (range 61-554). The average follow-up time was 32 months (range 19-64 months). The mean LVEF was 35%, which was indicative of a wide range of individual study populations and LVEF; the range was from 25% (in studies which included patients undergoing only ICD placement for primary prophylaxis) (30,34,44) to 52-56% (in more generalized patients without CAD) (42,43).
The prevalence of "scar", e.g., myocardial delayed enhancement suggestive of myocardial damage, was on average 41%, the range being 18% to 71%. This was not surprising, considering the heterogeneity of exclusion criteria for patient groups and scar patterns. In all studies, the presence of scar was diagnosed by visual interpretation by an experienced reader. To improve the specificity, most studies required the documentation of scar in an identical location, on orthogonal imaging planes. The methods used for scar quantification included visual scoring of the number of segments with scar, manual planimetry, and signal thresholding techniques to determine scar borders. For the latter approach, most often the "2-standard deviation (SD)"-technique was used, as described originally by Kim et al. (18) for myocardial infarction. In 2 studies, the "full-width at half maximum" (FWHM) technique was used. Despite possible differences in measured scar size due to different methods of analysis, the average scar size was small, typically around 6% of LV mass (range 0.8-25.6%). Few studies provided comparisons of measurements of scar size using different techniques. Gao et al. (34) measured a 50% larger scar size going from 5-SD to 2-SD thresholds above remote myocardial signal intensity. Neilan et al. (30) found that scar size was, on average, 50% greater using the 2-SD technique versus the FWHM technique (9 ± 5% by 2-SD method vs. 6 ± 4% by FWHM method); however, there was close correlation between both the measurements (r = 0.92, p < 0.001), and more importantly, both methods of quantification showed robust prognostic association.
The primary study endpoints were heterogeneous. Few studies have considered all-cause or cardiac mortality only, but most had a combined endpoint of mortality with HF events (HF hospitalization, heart transplant) and/or arrhythmic events (survived SCD, ICD shock, and sustained ventricular arrhythmias). Some studies have focused on an arrhythmic endpoint by combining SCD, appropriate ICD shocks, aborted SCD, and sustained ventricular arrhythmias.
Presence of Scar and Major Cardiac Events
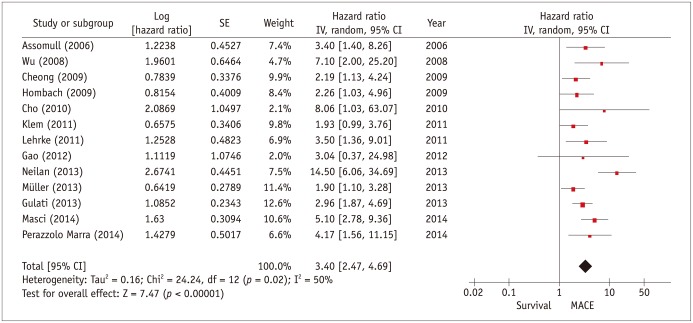
Figure 2 summarizes the univariate associations of the presence of myocardial scar to the primary study endpoints, which are heterogeneous in the 13 studies analyzed (see Table 2 for list of primary endpoints), and therefore summarized as major adverse cardiac events (MACE). Overall, patients with myocardial scar had a more than 3-fold higher risk for MACE compared with patients without scar (hazard ratio [HR], 3.40; 95% confidence interval, 2.47-4.69). Two studies were not included in this analysis. Wu et al. (45) focused on the prognostic role of "grey-zone" of intermediate signal intensity scar, and provide limited data on total scar. The authors presented total scar data only stratified in tertiles, wherein the tertile with largest scar extent has a higher event risk compared to the lowest tertile (HR, 3.4; 95% confidence interval, 1.6-7.0). Iles et al. (46) demonstrated that NICM patients with scar have a higher event risk than NICM patients without a scar and a group of patients with ICM; however, they did not provide a HR.
Fig. 2. Individual and pooled hazard ratios from univariate Cox proportional hazards analysis for risk of major cardiovascular events.
Forest plot comparing prognosis of NICM patients with and without scar, detected by delayed-enhancement CMR. CI = confidence interval, MACE = major adverse cardiovascular event, NICM = non-ischemic cardiomyopathy
Multivariable analysis with scar presence included as a covariate was performed in 11 studies, and was found to be independently associated with MACE in 9 studies. In the 2 negative studies, Müller et al. (41) included only those patients with a new diagnosis (< 4 weeks) of NICM, and found LVEF ≤ 40% and positive troponin I to be the only significant independent predictors, whereas brain natriuretic peptide, New York Heart Association (NYHA) Classes, QRS duration, and scar presence were not found to be independent predictors. The other study with a negative multivariable analysis was performed by Hombach et al. (28), who found that neither LVEF nor scar presence were independent predictors of cardiac death, when adjusted for diabetes, QRS-duration on ECG, cardiac index, as well as right ventricular volumes. The 9 studies with positive findings on multivariable analysis included different clinical, ECG, and CMR covariates in the multivariable models, and notably, scar was shown to be a robust predictor of MACE independent of LVEF in 8 out of 9 studies.
Three studies with 1241 patients and a mean follow-up of 4 years, reported the relation of scar presence to all-cause mortality and heart transplant in NICM patients. As shown in Figure 3, patients with scar had a higher event rate compared to patients without scar (HR, 2.5; 95% confidence interval, 1.8-3.4). Eight studies with a total of 1367 patients reported the relation of myocardial scar to arrhythmic events, which were mostly SCD, aborted SCD, appropriate ICD shocks, and sustained VT (Fig. 4). In this analysis, we included results from all studies that considered arrhythmic events either as primary study endpoint (31,34,46), secondary endpoint or composite of a combined endpoint (19,23,28,30). Patients with myocardial scar had a 5-fold risk of arrhythmic events compared to those NICM patients without scar (HR, 5.0; 95% confidence interval, 3.2-7.7).
Fig. 3. Individual and pooled hazard ratios from univariate Cox proportional hazards analysis for risk of all-cause mortality.
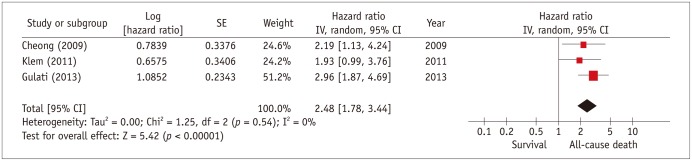
Forest plot comparing prognosis of NICM patients with and without scar, detected by delayed-enhancement MRI. CI = confidence interval, NICM = non-ischemic cardiomyopathy, SE = standard error
Fig. 4. Individual and pooled hazard ratios from univariate Cox proportional hazards analysis for risk of arrhythmic events.
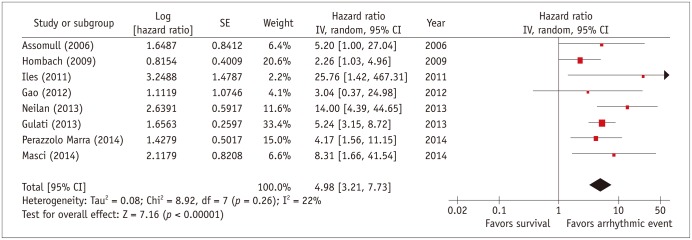
Forest plot comparing prognosis of NICM patients with and without scar, detected by delayed-enhancement MRI. CI = confidence interval, NICM = non-ischemic cardiomyopathy, SE = standard error
Extent of Scar and Major Cardiac Events
An important aspect of scar as a risk marker is whether there is a quantitative relationship between scar extent and increasing event risk. Although different methods for scar quantification have been used, several studies have demonstrated that larger myocardial scar in NICM portends a higher risk for adverse outcomes (23,30,34,35,42,43). A simple index of scar extent based on visual scoring of 17 segments, was associated with increasing risks of all-cause mortality or heart transplant (HR, 5.7; 95% confidence interval, 1.7-18.3) (42). Neilan et al. (30) demonstrated that for every 1% of LV mass increase in scar size, the risk of cardiovascular death or ventricular arrhythmia increases by 15%. This relationship was similar whether scar size was measured using the 2-SD method (HR, 1.15; 95% confidence interval, 1.12-1.18) or the FWHM-method (HR, 1.16; 95% confidence interval, 1.12-1.20). When only arrhythmic events were considered, the extent of scar was again associated with higher event risk (HR, 1.17 for each 1% absolute increase in scar size; 95% confidence interval, 1.12-1.22). Similar results were reported by Gulati et al. (23), for each percent scar extent the risk of all-cause mortality was increased by 11% (HR, 1.11; 95% confidence interval, 1.06-1.16), and the risk for arrhythmic events was increased by 10% (HR, 1.10; 95% confidence interval, 1.05-1.16).
Scar Cutoff and Major Cardiac Events
Few studies have investigated if there is an optimized cutoff for scar size, which best stratifies NICM patients into high and low risk subgroups. Some studies have used median scar size to stratify patients (31,34,44), while others identified an optimal cut-point with receiver-operating characteristic (ROC) analysis (19,29,30). Using the median scar size did not improve risk stratification, as reported in the studies by Perazzolo Marra et al. (31) and Gao et al. (34). Perazzolo Marra et al. (31) found that the event-free survival did not differ between patients having scar size measured below the median, and patients with values above the median (log-rank p = 0.295). Gao et al. (34) did find a higher cumulative event risk in patients with scar above the median scar of 18.7 g; however, this was not statistically significant (HR, 1.8; 95% confidence interval, 0.4-7.6).
Three studies report an optimal cutoff for risk stratification to be around 5% of LV mass, which was determined using ROC analysis. Notably, in all three studies, scar was measured using the same the 2-SD technique (19,29,30). Assomull et al. (19) studied 101 patients with NICM. The best threshold was calculated using ROC analysis in 35 patients having midwall fibrosis. When they subdivided the patients with midwall fibrosis into groups with scar < 4.8% and scar > 4.8%, Kaplan-Meier analysis showed a strong trend towards significant difference in outcome between the two groups for their primary endpoint of all-cause death and cardiovascular hospitalization (p = 0.07). In a subsequent study, the same center expanded their study population to 472 NICM patients with a longer follow-up, and found a strong correlation in the presence and the extent of midwall fibrosis, with all-cause death, arrhythmic event, and a combined HF endpoint. However, no data on scar cutoff was reported (23). Neilan et al. (30) studied 162 NICM patients and performed ROC in 81 patients with scar, which was more inclusive when compared to the study of Assomull et al. (19) and Gulati et al. (23), with midwall fibrosis, epicardial scar, focal/involving the right ventricular insertion points, or diffuse scar. They found a scar size of > 6.1% by 2-SD (> 4.4% by FWHM technique) to have a sensitivity of 90% and specificity of 95% for the prediction of the cardiovascular death or appropriate ICD therapy. Lehrke et al. (29) found a percentage scar of 4.4 among NICM patients who had midwall, epicardial, patchy/foci, or diffuse scar, as optimal discriminator (HR, 5.3; 95% confidence interval, 1.8-15.5).
Distribution of Scar and Major Cardiac Events
The analyzed studies are heterogeneous with respect to how different morphological scar patterns were considered. Some studies included only patients with NICM who either had midwall fibrosis or no scar; it was not reported whether other scar patterns were encountered, and if so, whether those patients were excluded (19,23). The majority of the analyzed studies included other non-ischemic patterns of scar (excluding only subendocardial scar), and few study included all types of scars (34,43,44,45).
A number of studies explored the relationship of adverse outcomes to specific patterns of scar. Wu et al. (44) found that the rates of their combined endpoint (cardiovascular death, HF hospitalization, or ICD shock) occurrence was similar among the three patterns of scar they reported (38% midwall fibrosis, 43% transmural pattern, 50% patchy scar). Similarly Lehrke et al. (29) found that the rates of cardiac death, ICD shock for VT/ventricular fibrillation, or hospitalization for HF were similar among patients with midwall, patchy focal, epicardial, and diffuse enhancement patterns. Neilan et al. (30) found the highest risk in patients with diffuse scar, but likely due to small number of patients in each category of scar (epicardial, midwall, and focal insertion point), and they also found that the location of scar was not associated with MACE (cardiovascular death and appropriate ICD shock) or an arrhythmic endpoint alone. In summary, based on the present evidence, we cannot conclude that any specific scar pattern, either ischemic versus non-ischemic, or one of the different non-ischemic patterns, has a stronger correlation to adverse outcomes when compared to other patterns.
DISCUSSION
There is rapidly growing evidence that the presence of myocardial scar detected by CMR provides prognostic value in patients with NICM. In this review, we have summarized data from 15 studies mostly published in the last 5 years, with over 2700 patients, which demonstrate that myocardial scar is associated with 2.5-fold risk of all-cause mortality, and a more than 3-fold risk of major cardiovascular events. However, the strongest association is seen with arrhythmic risks, with a near 5-fold increased rate of ventricular tachyarrhythmia or SCD in patients with NICM who have scar.
The average prevalence of myocardial scar in NICM patients was 41% which, from a practical standpoint, is an important pre-requisite for a valuable risk stratification test, in that a large group of low-risk patients can be separated from a small group of patients with highest risk.
This potential of myocardial scar for risk stratification appears to be particularly appealing, given the modest specificity of LVEF for identifying appropriate ICD recipients. Analysis of the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial), which evaluated the benefit of ICD treatment in the primary prevention of SCD in patients with ICM or NICM, an LVEF ≤ 35%, and NYHA II or III functional class, showed that although there is a mortality risk reduction, only about 20-25% of patients received an appropriate ICD shock in 5 years (16). Thus, the number of patients who need to be treated to prevent one event is very high if patients are selected primarily based on LVEF criteria, and many eligible patients did not benefit from this valuable, but costly, ICD therapy. Although there are several possible explanations, some insights in particular for the low specificity may be gleaned from the pathophysiologic mechanisms of ventricular tachyarrhythmia. Although LVEF as a global parameter of systolic function does strongly relate to all-cause mortality (47,48), LVEF itself does not directly represent a substrate for ventricular fibrillation or fibrillation. Moreover, it only indirectly indicates that a substrate for arrhythmia (for example ischemia or myocardial scar) may be present (49). However, many patients have systolic dysfunction without having a substrate for ventricular tachyarrhythmia, and therefore despite having a reduced LVEF they are at lower risk for arrhythmic death.
Conversely, LVEF has also limited sensitivity for predicting SCD. Community-based studies from the United States and Europe demonstrated that of the patients who had LV function assessed before cardiac arrest, up to 70% of SCD victims had a preserved LVEF prior to the cardiac arrest (50,51), and did not meet current LVEF criteria according the guidelines for ICD prophylaxis. Thus, a large group of patients who are at risk for SCD are not appropriately identified with current risk stratification tools.
Before scar can be used widely for SCD risk stratification, there are few open questions. Is the presence of scar alone the best criterion, or should a scar cutoff be used? Although few studies have explored a scar cutoff for optimized risk stratification, to date the largest study by Gulati et al. has shown that its presence itself is a strong risk parameter, and therefore may obviate the requirement for scar quantification (26). Nonetheless, several studies have shown a quantitative relation between extent of scar and adverse outcome. This important question-presence or cutoff-requires further investigation.
A related issue is the method to be used for scar quantification. First, before any quantification method is used, the presence or absence of scar has to be determined by an experienced reader, particularly because scar sizes in NICM are usually small, and often very subtle changes with intermediate signal intensities. Several studies used a segmental scoring approach, which is simple and fast. For quantification, the "2-SD" threshold method has been most widely used, and would therefore be a good approach for future studies to allow comparison of results. Few investigations have used the "FWHM" threshold method, which measures smaller scar sizes as compared to the 2-SD technique. However, there are no comparisons to a gold standard of pathology examinations in NICM patients.
Another open question relates to the relative importance of different scar patterns seen in NICM patients. Based on the presently available data, we can conclude that the absence of any scar portends a low risk for adverse cardiovascular events, and therefore CMR scar imaging in addition to LVEF determination should be considered in patients undergoing risk stratification for ventricular arrhythmias. If scar is present though, there is still limited data on the relative prognostic importance of different scar patterns. In the reported literature, subendocardial scar has particularly been excluded from most analyses. Nonetheless, this pattern can be encountered in 12-15% of patients with NICM (40,41,52), and therefore its prognostic value is currently not well established in these patients. Furthermore, the relative prognostic importance among different non-ischemic patterns has been investigated only by few studies, and with a small numbers of patients for each pattern; therefore, more studies are needed before definite conclusions can be established.
Finally, in the majority of the analyzed studies, patient selection bias is present, which is introduced by an attempt to refine the NICM population by excluding patients based either on clinical "pre-CMR" grounds, or after the knowledge of the CMR results based on certain CMR patterns. However, this limits the generalizability of findings, because patients are clinically being diagnosed with NICM typically if they have systolic dysfunction after exclusion of epicardial CAD and abnormal loading conditions. The true value of CMR can only be assessed, if one chooses the same approach clinicians use to evaluate patients, e.g., if CAD is excluded by coronary angiography with or without stress testing, abnormal loading (primary valvular disease, hypertrophic obstructive cardiomyopathy) by echocardiography, and specific diseases, based on non-CMR data such as clinical history, laboratory results, biopsy etc. Excluding patient groups after the CMR results (either new diagnosed ischemic damage, infiltrative disease etc.) not only limits the generalizability but also the evaluation of the full strength of this test.
Cardiac magnetic resonance imaging is now widely used in several fields of cardiovascular disease assessment. Even if there is a practical utility of this parameter for risk stratification in patients with cardiomyopathy, CMR is not yet widely accepted among clinicians as a routine screening test for risk assessment in patients with NICM. Recently, Korean guidelines for appropriate utilization of CMR have been published, which recommends myocardial scar evaluation with CMR to determine candidates of ICD or cardiac resynchronization therapy (CRT) therapy in HF patients (Level of evidence, A; Appropriateness criteria, A) (53). On the basis of these recommendations, expanding the clinical applications of CMR would be beneficial for the management of patients with NICM.
Footnotes
I. Klem receives research support from Medtronic Inc.
References
- 1.Jefferies JL, Towbin JA. Dilated cardiomyopathy. Lancet. 2010;375:752–762. doi: 10.1016/S0140-6736(09)62023-7. [DOI] [PubMed] [Google Scholar]
- 2.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 3.Lee SE, Cho HJ, Lee HY, Yang HM, Choi JO, Jeon ES, et al. A multicentre cohort study of acute heart failure syndromes in Korea: rationale, design, and interim observations of the Korean Acute Heart Failure (KorAHF) registry. Eur J Heart Fail. 2014;16:700–708. doi: 10.1002/ejhf.91. [DOI] [PubMed] [Google Scholar]
- 4.Choi DJ, Han S, Jeon ES, Cho MC, Kim JJ, Yoo BS, et al. Characteristics, outcomes and predictors of long-term mortality for patients hospitalized for acute heart failure: a report from the Korean heart failure registry. Korean Circ J. 2011;41:363–371. doi: 10.4070/kcj.2011.41.7.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamburro P, Wilber D. Sudden death in idiopathic dilated cardiomyopathy. Am Heart J. 1992;124:1035–1045. doi: 10.1016/0002-8703(92)90989-9. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Choi EK, Lee MH, Kang DY, Sung YJ, Lee DW, et al. The relevance of the primary prevention criteria for implantable cardioverter defibrillator implantation in Korean symptomatic severe heart failure patients. Korean Circ J. 2012;42:173–183. doi: 10.4070/kcj.2012.42.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai AS, Fang JC, Maisel WH, Baughman KL. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: a meta-analysis of randomized controlled trials. JAMA. 2004;292:2874–2879. doi: 10.1001/jama.292.23.2874. [DOI] [PubMed] [Google Scholar]
- 8.Bänsch D, Antz M, Boczor S, Volkmer M, Tebbenjohanns J, Seidl K, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT) Circulation. 2002;105:1453–1458. doi: 10.1161/01.cir.0000012350.99718.ad. [DOI] [PubMed] [Google Scholar]
- 9.Strickberger SA, Hummel JD, Bartlett TG, Frumin HI, Schuger CD, Beau SL, et al. Amiodarone versus implantable cardioverter-defibrillator: randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia--AMIOVIRT. J Am Coll Cardiol. 2003;41:1707–1712. doi: 10.1016/s0735-1097(03)00297-3. [DOI] [PubMed] [Google Scholar]
- 10.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 11.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 12.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 13.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1–e62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Kim JS. Guidelines for using the implantable cardioverter-defibrillator (ICD) Arrhythmia. 2012;13:4–6. [Google Scholar]
- 15.Ezekowitz JA, Rowe BH, Dryden DM, Hooton N, Vandermeer B, Spooner C, et al. Systematic review: implantable cardioverter defibrillators for adults with left ventricular systolic dysfunction. Ann Intern Med. 2007;147:251–262. doi: 10.7326/0003-4819-147-4-200708210-00007. [DOI] [PubMed] [Google Scholar]
- 16.Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozaffarian D, Linker DT, et al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation. 2009;120:835–842. doi: 10.1161/CIRCULATIONAHA.108.816884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberger JJ, Subačius H, Patel T, Cunnane R, Kadish AH. Sudden cardiac death risk stratification in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2014;63:1879–1889. doi: 10.1016/j.jacc.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 19.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 20.Richards DA, Blake GJ, Spear JF, Moore EN. Electrophysiologic substrate for ventricular tachycardia: correlation of properties in vivo and in vitro. Circulation. 1984;69:369–381. doi: 10.1161/01.cir.69.2.369. [DOI] [PubMed] [Google Scholar]
- 21.Hsia HH, Callans DJ, Marchlinski FE. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation. 2003;108:704–710. doi: 10.1161/01.CIR.0000083725.72693.EA. [DOI] [PubMed] [Google Scholar]
- 22.Soejima K, Stevenson WG, Sapp JL, Selwyn AP, Couper G, Epstein LM. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: the importance of low-voltage scars. J Am Coll Cardiol. 2004;43:1834–1842. doi: 10.1016/j.jacc.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 23.Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 24.Senthilkumar A, Majmudar MD, Shenoy C, Kim HW, Kim RJ. Identifying the etiology: a systematic approach using delayed-enhancement cardiovascular magnetic resonance. Heart Fail Clin. 2009;5:349–367. vi. doi: 10.1016/j.hfc.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Writing Committee Members. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 26.Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–1977. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet. 2001;357:21–28. doi: 10.1016/S0140-6736(00)03567-4. [DOI] [PubMed] [Google Scholar]
- 28.Hombach V, Merkle N, Torzewski J, Kraus JM, Kunze M, Zimmermann O, et al. Electrocardiographic and cardiac magnetic resonance imaging parameters as predictors of a worse outcome in patients with idiopathic dilated cardiomyopathy. Eur Heart J. 2009;30:2011–2018. doi: 10.1093/eurheartj/ehp293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehrke S, Lossnitzer D, Schöb M, Steen H, Merten C, Kemmling H, et al. Use of cardiovascular magnetic resonance for risk stratification in chronic heart failure: prognostic value of late gadolinium enhancement in patients with non-ischaemic dilated cardiomyopathy. Heart. 2011;97:727–732. doi: 10.1136/hrt.2010.205542. [DOI] [PubMed] [Google Scholar]
- 30.Neilan TG, Coelho-Filho OR, Danik SB, Shah RV, Dodson JA, Verdini DJ, et al. CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. JACC Cardiovasc Imaging. 2013;6:944–954. doi: 10.1016/j.jcmg.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perazzolo Marra M, De Lazzari M, Zorzi A, Migliore F, Zilio F, Calore C, et al. Impact of the presence and amount of myocardial fibrosis by cardiac magnetic resonance on arrhythmic outcome and sudden cardiac death in nonischemic dilated cardiomyopathy. Heart Rhythm. 2014;11:856–863. doi: 10.1016/j.hrthm.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Cho JR, Park S, Choi BW, Kang SM, Ha JW, Chung N, et al. Delayed enhancement magnetic resonance imaging is a significant prognostic factor in patients with non-ischemic cardiomyopathy. Circ J. 2010;74:476–483. doi: 10.1253/circj.cj-09-0446. [DOI] [PubMed] [Google Scholar]
- 33.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 34.Gao P, Yee R, Gula L, Krahn AD, Skanes A, Leong-Sit P, et al. Prediction of arrhythmic events in ischemic and dilated cardiomyopathy patients referred for implantable cardiac defibrillator: evaluation of multiple scar quantification measures for late gadolinium enhancement magnetic resonance imaging. Circ Cardiovasc Imaging. 2012;5:448–456. doi: 10.1161/CIRCIMAGING.111.971549. [DOI] [PubMed] [Google Scholar]
- 35.Masci PG, Doulaptsis C, Bertella E, Del Torto A, Symons R, Pontone G, et al. Incremental prognostic value of myocardial fibrosis in patients with non-ischemic cardiomyopathy without congestive heart failure. Circ Heart Fail. 2014;7:448–456. doi: 10.1161/CIRCHEARTFAILURE.113.000996. [DOI] [PubMed] [Google Scholar]
- 36.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 37.Uretsky BF, Thygesen K, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, et al. Acute coronary findings at autopsy in heart failure patients with sudden death: results from the assessment of treatment with lisinopril and survival (ATLAS) trial. Circulation. 2000;102:611–616. doi: 10.1161/01.cir.102.6.611. [DOI] [PubMed] [Google Scholar]
- 38.Roberts WC, Siegel RJ, McManus BM. Idiopathic dilated cardiomyopathy: analysis of 152 necropsy patients. Am J Cardiol. 1987;60:1340–1355. doi: 10.1016/0002-9149(87)90618-7. [DOI] [PubMed] [Google Scholar]
- 39.Topol EJ, Yadav JS. Recognition of the importance of embolization in atherosclerotic vascular disease. Circulation. 2000;101:570–580. doi: 10.1161/01.cir.101.5.570. [DOI] [PubMed] [Google Scholar]
- 40.Kim HW, Klem I, Shah DJ, Wu E, Meyers SN, Parker MA, et al. Unrecognized non-Q-wave myocardial infarction: prevalence and prognostic significance in patients with suspected coronary disease. PLoS Med. 2009;6:e1000057. doi: 10.1371/journal.pmed.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Müller KA, Müller I, Kramer U, Kandolf R, Gawaz M, Bauer A, et al. Prognostic value of contrast-enhanced cardiac magnetic resonance imaging in patients with newly diagnosed non-ischemic cardiomyopathy: cohort study. PLoS One. 2013;8:e57077. doi: 10.1371/journal.pone.0057077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheong BY, Muthupillai R, Wilson JM, Sung A, Huber S, Amin S, et al. Prognostic significance of delayed-enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation. 2009;120:2069–2076. doi: 10.1161/CIRCULATIONAHA.109.852517. [DOI] [PubMed] [Google Scholar]
- 43.Klem I, Weinsaft JW, Bahnson TD, Hegland D, Kim HW, Hayes B, et al. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol. 2012;60:408–420. doi: 10.1016/j.jacc.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu KC, Gerstenblith G, Guallar E, Marine JE, Dalal D, Cheng A, et al. Combined cardiac magnetic resonance imaging and C-reactive protein levels identify a cohort at low risk for defibrillator firings and death. Circ Cardiovasc Imaging. 2012;5:178–186. doi: 10.1161/CIRCIMAGING.111.968024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iles L, Pfluger H, Lefkovits L, Butler MJ, Kistler PM, Kaye DM, et al. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2011;57:821–828. doi: 10.1016/j.jacc.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed A, Rich MW, Love TE, Lloyd-Jones DM, Aban IB, Colucci WS, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27:178–186. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 49.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 50.Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47:1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 51.Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest--the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24:1204–1209. doi: 10.1016/s0195-668x(03)00191-x. [DOI] [PubMed] [Google Scholar]
- 52.McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 53.Yoon YE, Hong YJ, Kim HK, Kim JA, Na JO, Yang DH, et al. 2014 Korean guidelines for appropriate utilization of cardiovascular magnetic resonance imaging: a joint report of the Korean Society of Cardiology and the Korean Society of Radiology. Korean Circ J. 2014;44:359–385. doi: 10.4070/kcj.2014.44.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]