Abstract
Pulmonary tumor embolism is commonly discovered at autopsy, but is rarely suspected ante-mortem. Microangiopathy is an uncommon and distinct form of simple tumor pulmonary embolism. Here, we present a 52-year-old male with tumor thrombotic microangiopathy and pulmonary infarction, which might have originated from intraductal papillary mucinous tumor of the pancreas. Multiple wedge-shaped consolidations were found initially and aggravated with cavitation. These CT features of pulmonary infarction were pathologically confirmed to result from pulmonary tumor thrombotic microangiopathy.
Keywords: Pulmonary embolism, Tumor embolism, Pulmonary tumor thrombotic microangiopathy, Pulmonary infarction, Intraductal papillary mucinous neoplasm
INTRODUCTION
Pulmonary tumor thrombotic microangiopathy (PTTM) of a metastatic tumor is a rare and completely different form of simple tumor pulmonary embolism. The most important pathological feature of PTTM is extensive pulmonary vascular fibrocellular intimal hyperplasia concomitant with tumor emboli (1). According to previous reports, the tree-in-bud pattern and multiple small nodular opacities on thin-section CT are characteristic radiological findings of PTTM (1,2). Here, we report a case of pulmonary tumor thrombotic microangiopathy in which no small nodules or tree-in-bud pattern were apparent on CT. However, progressive wedge-shaped consolidations with cavitation developed, which were pathologically identified as pulmonary infarction by means of a video-assisted thoracoscopic lung biopsy. To the best of our knowledge, this is the first report of pulmonary cavitary infarction caused by PTTM. The origin of metastasis in our case may have been intraductal papillary mucinous neoplasm (IPMN) of the pancreas, which is also a very rare extrathoracic malignancy resulting in PTTM. This case report was approved by the Institutional Review Board of our institution, and the need for informed consent was waived.
CASE REPORT
A 52-year-old man presented with diffuse abdominal and back pain and respiratory symptoms (mild cough and whitish sputum) for several days. He had a history of surgical treatment for IPMN associated with recurrent pancreatitis 10 years previously, for which further pathological details were not available. Physical examination revealed abdominal rigidity and fine crackles in both lungs. The patient had anemia, diabetes, and hypoxemia. His oxygen saturation level was below 90% (room air). Laboratory findings were as follows: white blood cells, 6940/µL; Hb, 7.0 g/dL; platelet count, 22.9 × 104/µL; amylase, 9 IU/L; lipase, 18 IU/L; glucose, 149 mg/dL; and CRP, 14.5 mg/dL. Carbohydrate antigen 19-9 was 4332 U/mL. Echocardiography revealed mild resting pulmonary arterial hypertension.
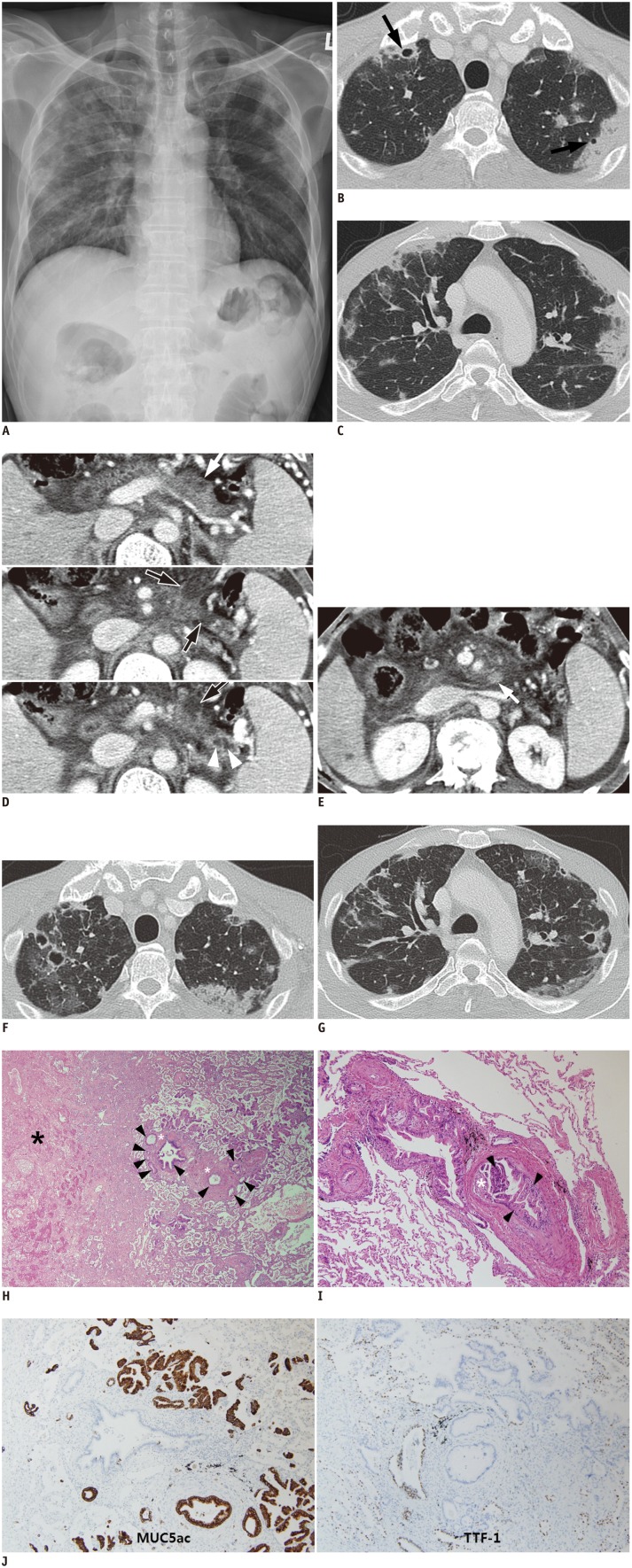
Chest radiographs showed bilateral multifocal consolidations, which were predominantly in both upper lung fields and particularly prevalent close to the pleura (Fig. 1A). Chest computed tomography (CT) images (lung window setting: window width, 1500 Hounsfield unit [HU]; window level, -700 HU) revealed ill-defined wedge-shaped consolidations and ground glass opacities predominantly in the peripheral portions of the lungs. There was cavitation in some of the consolidations (Fig. 1B, C). Enhanced abdominal CT scans showed multiple cystic lesions suggesting IPMN in the remnant pancreas and peripancreatic lymphadenopathy (Fig. 1D, E). However, pathological evaluation of the pancreatic lesion and complete oncological evaluation were not performed at that time. We started empirical antibiotics and antifungal agents under the working diagnosis of septic emboli or fungal infection. However, chest CT images 3 weeks later demonstrated that the wedge-shaped consolidations had rapidly become aggravated, and cavitation had progressively developed in the consolidations, despite antibiotic and antifungal therapy (Fig. 1F, G). Furthermore, subjective symptoms of dyspnea and chest radiograph findings had deteriorated. Therefore, we obtained specimens from the cavitary consolidations by percutaneous transthoracic needle biopsy. However, the biopsy specimens only showed numerous neutrophils and necrosis without any organisms such as bacteria or fungus. The patient then underwent a video-assisted thoracoscopic lung biopsy for an accurate pathological diagnosis. Two weeks after the operative biopsy, the patient died despite intensive supportive care.
Fig. 1. 52-year-old man with cavitary infarction caused by pulmonary tumor thrombotic microangiopathy originated from pancreatic intraductal papillary mucinous neoplasm.
A. Chest radiograph demonstrates bilateral multifocal consolidations in peripheral portions of both upper lungs. Axial computed tomography images (lung window setting; window width, 1500 Hounsfield unit [HU]; window level, -700 HU) show ill-defined wedge-shaped consolidations and ground glass opacities in peripheral portion of both upper lobes (B, C). Cavitation was present in some consolidations (B, arrows). D. Contiguous contrast-enhanced axial CT images show multiple cystic lesions (black arrows with white border) with minimal disproportional pancreatic duct dilatation (branch ducts, white arrowheads) in body and tail of pancreas. Ill-defined soft tissue lesion is present in one cyst (white arrow), which is presumed to be mural nodule of malignant intraductal papillary mucinous neoplasm. E. Lymphadenopathy in peripancreatic space (arrow) and ascites are also seen. F, G. Follow-up axial CT images (lung window setting: window width, 1500 Hounsfield unit [HU]; window level, -700 HU) 3 weeks after initial CT scan demonstrate that wedge-shape consolidations are extended or newly developed. Additionally, cavities in consolidations are newly developed or changed, predominantly in peripheral portion of lungs. H. Photomicrograph of histopathological specimen shows tumor emboli (black arrowheads) and intimal hyperplasia (white asterisks) in vasculature. These tumor emboli and intimal hyperplasia may obstruct vascular lumen and cause irregular vascular shape. Distal lung parenchyma of obstructed vascular lumen is necrotic (black asterisk) (hematoxylin-eosin, original magnification, × 40). I. Photomicrograph of histopathological specimen shows tumor cells, columnar mucin-producing glands (black arrowheads) in pulmonary vasculature, and marked fibrocellular intimal hyperplasia (white asterisk) (hematoxylin-eosin, original magnification, × 100). There is little fibrin thrombosis in vascular lumen. J. Immunohistochemical stain for MUC5ac (× 100), to which metastatic adenocarcinomas demonstrate positive reaction (brown color) but bronchial epithelium and alveolar pneumocytes are negative. In contrast to MUC5ac, basal cells of bronchial epithelium and alveolar pneumocytes demonstrate positive reaction (brown color) to immunohistochemical stain for TTF-1 (× 100), but metastatic adenocarcinomas are negative.
The pathological features of the specimen explained the cause of the unexplained lung lesions and the patient's condition. Tumor emboli were found involving multiple levels of the microscopic pulmonary vasculature, from medium-sized arteries down to small arteries or arterioles. The areas surrounding these tumor emboli-containing vessels consisted of necrotic tissue that appeared to have been caused by pulmonary infarction (Fig. 1H). Pulmonary arteries and arterioles were stenosed and occluded by fibrocellular intimal proliferation induced by intraluminal tumor emboli composed of compact nests of columnar mucin-producing glands and papillae with a fibrovascular core. However, contrary to previously reported cases of thrombotic microangiopathy, there was little fibrinous thrombosis within the involved pulmonary microvasculature (Fig. 1I). Histologically, pulmonary mucinous adenocarcinoma shows extremely well differentiated and tumor cells float within the pool of mucin, but tumor cells of this case looked like intestinal columnar cells, gastric foveolar cells and pancreatobiliary cells with papillae. Additional immunohistochemical findings demonstrated that tumor cells are positive reaction for mucin 5AC (MUC5AC), but negative for thyroid transcription factor-1 (TTF-1) (Fig. 1J).
DISCUSSION
Intravascular tumor embolization leading to the occlusion of pulmonary vessels by neoplastic cells is reported in 2.4-26% of autopsies on patients with extrathoracic malignancy (1). These tumor emboli originate from various malignancies and are usually located in small- or medium-sized pulmonary arteries, which makes radiological diagnosis difficult (3). Patients may present with respiratory symptoms such as unexplained progressive dyspnea, chest pain, cough, hypoxia, and respiratory alkalosis (4). Oxygen saturation is decreased, and right-sided heart failure and pulmonary hypertension are occasionally reported (4). Adenocarcinoma is the tumor type most frequently associated with pulmonary tumor embolism, and common primary organs are the breast, stomach, lung, colon, and liver (5). However, pancreatic origin is extremely rare (5). Mostly pulmonary tumor emboli occur at an advanced stage in a known cancer, although embolism can develop at any time from an extrathoracic malignancy. Rarely, pulmonary tumor embolism can be the initial manifestation of a latent cancer, which can make diagnosis difficult (5). To our knowledge, this is the first case of pulmonary tumor embolism possibly caused by pancreatic IPMN to be reported in the literature, although complete pathological proof was lacking. Two cases of pulmonary tumor embolism from other types of pancreatic cancer have been described (5).
The CT findings in pulmonary tumor embolism include multifocal dilatations and a beaded appearance of the peripheral subsegmental arteries (3). Episodes of infarction induced by pulmonary embolism were estimated to occur in only 10% of all pulmonary embolisms (6). When pulmonary infarction is caused by emboli, it classically appears as pleural-based consolidations or ground glass opacities with their apexes directed towards the thoracic hilum (6). These imaging findings are correlated with histological changes including intra-alveolar hemorrhage and interstitial edema without destruction of lung parenchyma (7).
Pulmonary tumor thrombotic microangiopathy is a rare entity, and a separate disease distinct from tumor embolism. While pulmonary tumor embolism indicates the occlusion of pulmonary vascular spaces by tumor cells themselves, with or without accompanying local thrombosis, the most important finding in PTTM is intimal proliferation resulting in increased vascular resistance (8). This entity is characterized histopathologically by extensive pulmonary arterial or arteriolar fibrocellular intimal hyperplasia that is concomitant with tumor microemboli (1). This microangiopathy has been reported at autopsy in only 0.9-3.3% of cadavers with extrathoracic malignancies (1). Widespread pulmonary tumor vascular occlusion in patients with thrombotic microangiopathy leads to increased vascular resistance. Consequently, progressive dyspnea, cough, hypoxia, and pulmonary hypertension develop in patients with pulmonary tumor thrombotic microangiopathy (1). Franquet et al. (1) described CT findings of thrombotic microangiopathy in pulmonary tumors. The tree-in-bud pattern on thin-section CT is a characteristic finding similar to infectious bronchiolitis. Pathologically, it indicates that fibrocellular intimal hyperplasia in small arteries and arterioles has caused obliteration of the vascular lumen rather than the tumor emboli themselves. Small groups of tumor cells in PTTM metastasize at a microscopic level to the pulmonary vasculature and adhere to the vascular endothelium (9). These tumor cells do not cause simple mechanical obstruction of involved vessels as a true tumor embolus, but rather are thought to activate the coagulation cascade and release of inflammatory mediators, which might result in deposition of platelets and fibrin microthrombi, fibrocellular subintimal proliferation, and smooth muscle colonization of the lesional complexes. These proliferations lead to diffuse affected pulmonary arterial luminal narrowing and increased vascular resistance. However, the precise mechanisms of PTTM are unclear (10). The pathological features of our case were extensive tumor emboli and marked fibrocellular intimal hyperplasia in the small arteries and arterioles. However, there were few thrombi in the affected vascular lumen. It remains unclear why minor thrombosis occurred, disproportionate to the extensive metastatic tumor emboli in the pulmonary vessels.
Pulmonary embolism causes infarction in fewer than 15% of cases, and only about 5% of infarctions develop cavitation (11). The previously reported prevalence of cavity formation was 3.4% (12). In our case, extensive tumor emboli were present in medium-sized and small arteries, and arterioles were accompanied by widespread vascular intimal hyperplasia (microangiopathy). These massive tumor emboli not only mechanically obstruct the pulmonary vessels but also result in fibrocellular intimal hyperplasia that then leads to intimal proliferation, luminal stenosis, and ultimately total occlusion. They are presumed to cause massive pulmonary infarction with necrosis, which presented as cavitary consolidation on CT. We believe that this finding is an extremely rare complication of pulmonary tumor emboli and may be correlated with poor prognosis. In a previous report, over 75% of the developed cavities were located in the middle and upper zones (apical and posterior segments of the upper lobes and superior segments of the lower lobes), and they were more predominant in the right lung (12). Conversely, non-cavitary infarcts occurred more frequently in the lower zone. On CT of our case, the lesions involving pulmonary infarction with cavitation were also predominantly located in the upper lobes.
In conclusion, this case illustrates that extensive tumor emboli and pulmonary tumor thrombotic microangiopathy can lead to consequent progressive necrotic pulmonary infarction presenting as cavitary consolidation or ground glass opacity on CT.
References
- 1.Franquet T, Giménez A, Prats R, Rodríguez-Arias JM, Rodríguez C. Thrombotic microangiopathy of pulmonary tumors: a vascular cause of tree-in-bud pattern on CT. AJR Am J Roentgenol. 2002;179:897–899. doi: 10.2214/ajr.179.4.1790897. [DOI] [PubMed] [Google Scholar]
- 2.Shepard JA, Moore EH, Templeton PA, McLoud TC. Pulmonary intravascular tumor emboli: dilated and beaded peripheral pulmonary arteries at CT. Radiology. 1993;187:797–801. doi: 10.1148/radiology.187.3.8497633. [DOI] [PubMed] [Google Scholar]
- 3.Seo JB, Im JG, Goo JM, Chung MJ, Kim MY. Atypical pulmonary metastases: spectrum of radiologic findings. Radiographics. 2001;21:403–417. doi: 10.1148/radiographics.21.2.g01mr17403. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, Choi Y, Beom SH, Kim JW, Joen YK, Kim NJ, et al. A case report of breast cancer with extensive pulmonary lymphovascular tumor emboli. J Breast Cancer. 2012;15:128–132. doi: 10.4048/jbc.2012.15.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sancho-Chust JN, Ferreres J, Pineda J, Molla MA, Giner F, Juan M, et al. Pulmonary tumor embolism as an initial manifestation of pancreatic adenocarcinoma. Respir Care. 2009;54:1732–1735. [PubMed] [Google Scholar]
- 6.Greaves SM, Hart EM, Brown K, Young DA, Batra P, Aberle DR. Pulmonary thromboembolism: spectrum of findings on CT. AJR Am J Roentgenol. 1995;165:1359–1363. doi: 10.2214/ajr.165.6.7484563. [DOI] [PubMed] [Google Scholar]
- 7.Redline S, Tomashefski JF, Jr, Altose MD. Cavitating lung infarction after bland pulmonary thromboembolism in patients with the adult respiratory distress syndrome. Thorax. 1985;40:915–919. doi: 10.1136/thx.40.12.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babu RV, Romero A, Sharma G. A 39-year-old man with epigastric pain, intermittent chest pain, and progressive dyspnea. Chest. 2007;132:2012–2015. doi: 10.1378/chest.07-0878. [DOI] [PubMed] [Google Scholar]
- 9.Pinckard JK, Wick MR. Tumor-related thrombotic pulmonary microangiopathy: review of pathologic findings and pathophysiologic mechanisms. Ann Diagn Pathol. 2000;4:154–157. doi: 10.1016/s1092-9134(00)90038-8. [DOI] [PubMed] [Google Scholar]
- 10.Chinen K, Kazumoto T, Ohkura Y, Matsubara O, Tsuchiya E. Pulmonary tumor thrombotic microangiopathy caused by a gastric carcinoma expressing vascular endothelial growth factor and tissue factor. Pathol Int. 2005;55:27–31. doi: 10.1111/j.1440-1827.2005.01783.x. [DOI] [PubMed] [Google Scholar]
- 11.Morgenthaler TI, Ryu JH, Utz JP. Cavitary pulmonary infarct in immunocompromised hosts. Mayo Clin Proc. 1995;70:66–68. doi: 10.1016/S0025-6196(11)64668-5. [DOI] [PubMed] [Google Scholar]
- 12.Harris H, Barraclough R, Davies C, Armstrong I, Kiely DG, van Beek E., Jr Cavitating lung lesions in chronic thromboembolic pulmonary hypertension. J Radiol Case Rep. 2008;2:11–21. doi: 10.3941/jrcr.v2i3.50. [DOI] [PMC free article] [PubMed] [Google Scholar]