Abstract
Lymphocyte development and function are regulated by tyrosine kinase and G-protein coupled receptors. Each of these classes of receptors activates PI3K. Here we summarize current understanding of how PI3K contribute to key aspects of the adaptive immune system.
2 Introduction
The adaptive immune response protects the host from recurring infections by viruses, bacteria and parasites. The enormous diversity and exquisite specificity of the antigen receptors expressed by B cell and T lymphocytes ensures that any pathogen can be recognized while clonal expansion and the generation of long-lived memory cells ensures effective clearance and, in many cases, life-long immunity from re-infection by the same pathogen. In addition, natural killer (NK) lymphocytes provide more immediate protection and can, in some cases, serve as a back-up mechanism to kill pathogens that evade immune recognition by T cells by suppressing major histocompatibility complex (MHC) expression. The diversity of the antigen receptor repertoire is generated by homologous recombination of gene components in B cell and T cell precursors. After gene recombination, each clone is selected so that clones bearing non-functional or autoreactive antigen receptors are eliminated. In the case of T cells, clones bearing antigen receptors capable of recognizing MHC structures are positively selected. This system originally evolved in teleost fishes and is required for protection against a wide variety of common pathogens, many of which have co-evolved mechanisms to evade the adaptive immune response. The extreme susceptibility of severe combined or acquired immune deficiency patients to common infections illustrates how dependent we are on the adaptive immune responses for survival. However, the evolution of the adaptive immune system has also come at a cost, particularly in long-lived animals such as humans. Self-nonself discrimination is an imperfect process and several common autoimmune diseases are caused by autoreactive T cells and/or B cells. In addition, the gut, vaginal tract, respiratory tract and skin are host to a rich microbial flora of mostly harmless or beneficial microbes. Inappropriate activation of the immune system against commensal bacteria causes unwarranted inflammation and disease. Moreover, the immune system can respond inappropriately to innocuous substances such as pollen and cause allergic reactions. Finally, the rejection of transplanted organs is mediated by the adaptive immune response. Therefore, therapeutic strategies are aimed at dampening unwanted immune responses without rendering the patient unprotected from infections.
Currently, the most widely used immunosuppressive drugs are corticosteroids, cyclosporine, and cytotoxic or cytostatic drugs such as methotrexate or rapamycin. In addition, various monoclonal antibody therapies have been developed; the most widely used of these have been the anti-tumor necrosis factor-α drugs used to treat rheumatoid arthritis and other autoimmune diseases [1]. Abatacept (CTLA4-Ig) and Rituximab (anti-CD20) are more recent examples of agents that target T cells and B cells specifically and help treat autoimmunity [2, 3]. Yet, there remains significant clinical need for additional drugs that either suppress or modulate adaptive immune responses as not all patients respond favorably to currently available drugs. The phosphoinositide 3-kinase (PI3K) enzymes, which are the focus of this series, have received considerable interest in this context [4, 5]. PI3Ks play central roles in the signaling not only by antigen receptors, but also by various costimulatory, cytokine and chemokine receptors that control lymphocyte biology [6, 7]. In general, the PI3K isoforms p110α and p110β transmit signals from tyrosine kinases and G protein coupled receptors (GPCRs), respectively. However, in lymphocytes, tyrosine-kinase associated receptors signal primarily through p110δ whereas GPCRs signal primarily through p110γ, although there are notable exceptions that we will highlight in this review. Since p110δ and p110γ are expressed at low or undetectable levels in most organs, inhibitors against p110δ and p110γ should, in theory, be selective for the immune system and non-toxic. In this chapter, we will consider the roles played by PI3K during development and activation of lymphocytes and also consider both the advantages and liabilities associated with pharmacological targeting of these PI3K isoforms.
Our understanding of the roles of PI3Ks in lymphocyte biology has been advanced both by the development of small molecule inhibitors that target the PI3Ks with high selectivity, and by gene mutation through homologous recombination in mice (for a list of gene targeting studies, see table 1). Initial studies demonstrated that B cell and T cell proliferation could be blocked by the broad-spectrum PI3K inhibitors wortmannin and LY294002 [8]. However, these compounds have many off-target effects and a specific role for PI3K could therefore not always be established unequivocally. The demonstration that p85α knockout mice showed impaired B cell development and humoral immune responses provided the clear evidence that PI3Ks are intimately involved in signal transduction by the B cell antigen receptor (BcR) [9, 10]. These phenotypes were also evident in mice where p110δ had been inactivated by deletion or by point mutations in the genes [11-13]. Thus a picture emerged, in which p85α would recruit p110δ to antigen and coreceptor complexes at the plasma membrane and that the PI3K activity thus generated was essential for B cell proliferation. The situation for T cells was more complicated. While p85α was dispensable for normal T cell proliferation and cytokine production, p110δ did contribute to these responses - especially when T cells were stimulated with peptide-MHC rather than antibodies [12, 14]. This apparent discrepancy was in part resolved subsequently when it was demonstrated that p85α and p85β play mutually redundant roles in transmitting signals from the T cell antigen receptor (TcR) [15]. Nonetheless, in terms of development and proliferative responses by purified cells, the T cells appeared to be less severely affected by PI3K deficiency than B cells. However, while the development of various mature subsets and their proliferative responses are the most immediately obvious measurements to make, both T cells and B cells live complicated lives and recently a more detailed appreciation of how PI3Ks can not only enhance, but also suppress certain B cell and T cell responses. The challenge of understanding the apparently contradictory roles of PI3K in adaptive immune function is a significant one. Understanding of these roles is going to have a significant impact on the development of small molecules against the PI3Ks expressed in lymphocytes.
Table 1.
| PI3K isoform(s) targeted | Genetic or pharmacological | Cell Type or animal model | References |
|---|---|---|---|
| p110δ | Null allele | T and B cells B cells NK cells |
[11, 13] [20, 151] [145, 147, 152] |
| p110δ | Kinase-dead D910A knock-in |
T and B cells T cells B cells NK or NKT |
[12, 54] [14, 77, 81, 82, 87, 90, 104, 106, 142] [36, 39] [148, 152, 153] |
| p110δ | Selective inhibitor (IC87114) |
B cells In vivo (asthma) |
[39, 50] [154] |
| p110γ | Null or kinase-dead | T and B cells T cells NK |
[54, 55, 58, 155, 156] [77, 130-132] [146, 148, 152] |
| p110γ | Selective inhibitors | In vivo (Arthritis, lupus) | [157, 158] |
| p110δ/p110γ | Double knockout or knockout/knock-in |
T cells NK cells In vivo (arthritis) |
[60, 61, 63, 103] [146] [159] |
| p85α | Null | B cells T cells |
[10, 17, 18, 37, 57] [62] |
| p85α/p55α/p50α | Null | B cells | [9] |
| p85α/p55α/p50α | T cell-specific conditional | T cells | [15] |
| p85α/p55α/p50α | B cell-specific conditional | B cells | [160] |
| p85β | Null | T and B cells T cells |
[161] [162] |
| p85α/p55α/p50α/p85β | p85 dko = double knockout (conditional p85α/p55α/p50α, null p85β) |
T cells B cells |
[15, 105] [160] |
3 PI3K in B cells
3.1 B cell development
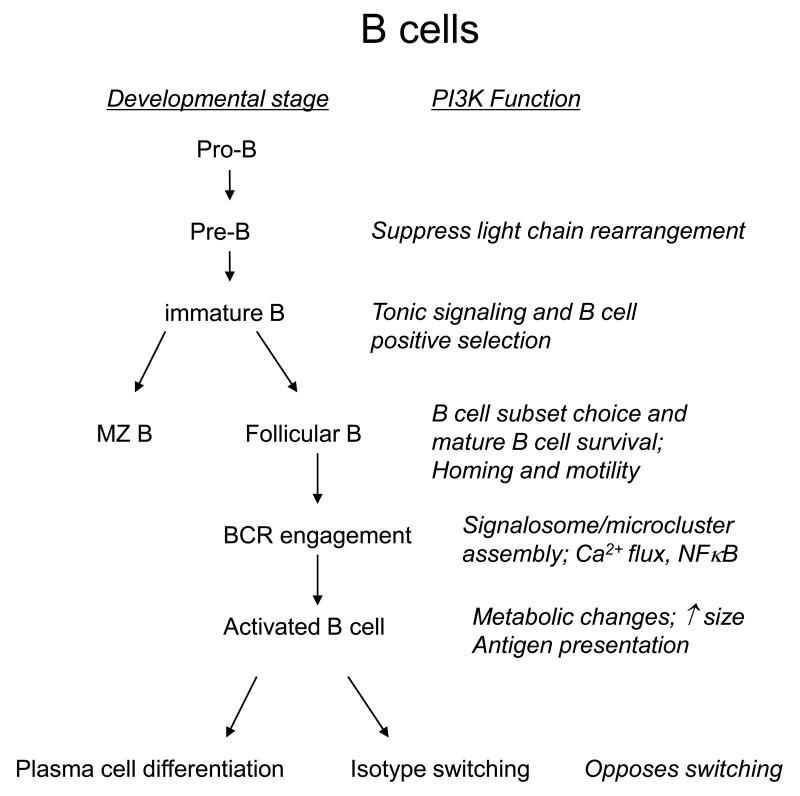
B cells develop from lymphoid progenitors in the fetal liver and bone marrow [16]. At an early stage, a lineage split occurs resulting in two general subsets: B-1 B cells that reside in body cavities, and B-2 B cells that reside in secondary lymphoid organs. The B-2 subset is further sub-divided into marginal zone (MZ) B cells, which occupy a unique anatomical and functional niche in the marginal zone of the spleen, and follicular (FO) B cells, which recirculate through blood, lymph and secondary lymphoid tissues. Mice lacking p85α or p110δ have markedly fewer B-1 and MZ B cells as well as reduced numbers of FO cells [9-13, 17, 18].
Developing B cells undergo an ordered series of gene rearrangement steps [19]. First, heavy chain rearrangement occurs at the pro-B cell stage. If successful, light chain rearrangement proceeds at the pre-B cell stage. Cells that make productive heavy and light chain rearrangements express surface IgM and are classified as immature B cells. These cells are then screened for self-reactivity in the bone marrow and spleen, and autoreactive cells are either anergized, deleted, or induced to re-express recombination-activating gene (RAG) proteins and undergo receptor editing. In the absence of self-antigen, immature B cells suppress RAG expression via tonic BcR signaling and advance to the mature B cell state. Mice lacking p85α or p110δ have reduced numbers of cells making it through the pro-B to pre-B transition, as well as defects in tonic signaling at the immature stage [9-12, 20, 21]. In addition, PI3K inhibitors can lead to de-differentiation of immature B cells in vitro [22].
Activation of naive B cells by foreign antigen, in the presence of T cell help or Toll-like receptor (TLR) costimulation, results in clonal expansion and differentiation. Some activated B cells develop rapidly into antibody-secreting plasma cells to provide an early source of antibody. Other B cell clones continue differentiation within the germinal centers within lymphoid follicles, where the antibody constant region is changed by isotype switching, and the variable region is modified by somatic hypermutation. B cells surviving the germinal center reaction then develop either into plasma cells secreting high affinity antibody, or memory B cells capable of mounting rapid secondary responses. B cells lacking p85α or p110δ mediate reduced T-independent antibody responses in response to polymeric antigens in vivo [9-13].
3.2 BcR signaling, antigen presentation and metabolism
Each of the B cell fate decisions outlined above is controlled by signaling through the BcR (Fig 1). Considering the various defects in PI3K-deficient B cells; it is therefore not surprising that PI3K activation is a fundamental aspect of BcR signaling. The mechanism through which the BcR is coupled to PI3K signaling has recently been resolved. Two proteins with YxxM motifs (providing the optimal binding site for the Src homology-2 (SH2) domains of p85) are known to be important for B cell signaling: CD19 and B cell adaptor protein (BCAP). Whereas deleting either of these alone is insufficient to ablate PI3K signaling in B cells, combined deficiency of CD19 and BCAP reduces PI3K signaling below a detectable threshold and strongly impacts B cell development at the stage where the B cell progenitors first express a pre-BcR (composed of an immunoglobulin heavy chain and a surrogate light chain) [23]. As CD19 and BCAP are both substrates for BcR dependent Syk activity, we can conclude that we now have a detailed model of how the BcR connects with PI3K activity. However, it should be noted that the guanine exchange factor Vav and its substrate Rac have also be implicated in the activation of PI3K downstream of the BcR [24-26]. In recognition of this complexity, we have proposed a “signalosome” model of BcR signaling in which PI3K, its lipid products, and other components function as an integrated molecular machine in which all parts are functionally interconnected [27].
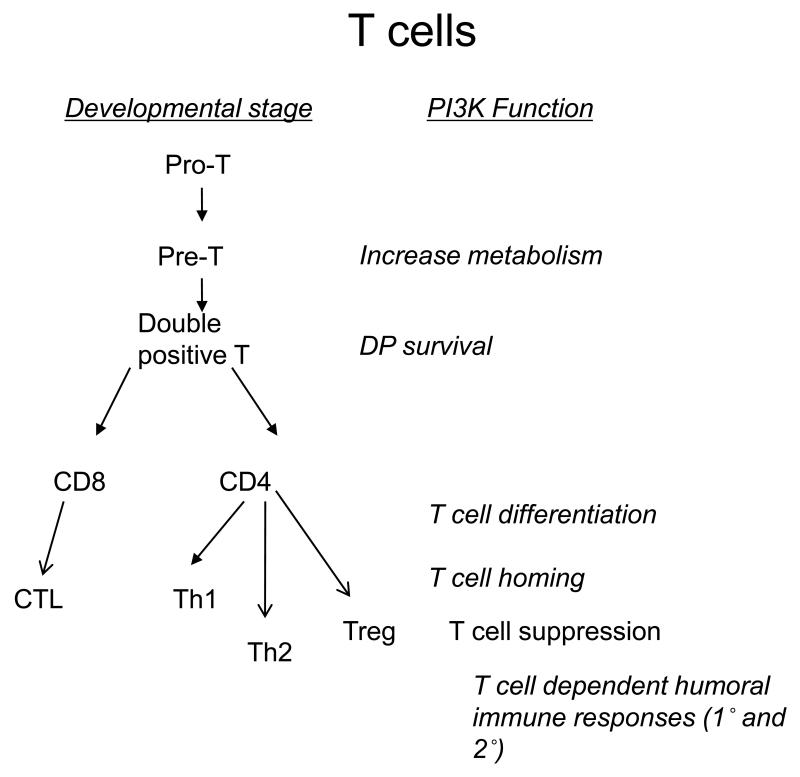
Figure 1.
Simplified flow charts of lymphocyte development and function, showing key points of action of PI3K in B cells (A) and T cells (B).
A primary role of PI3K in BcR signaling is to promote signalosome assembly and membrane localization, leading to phospholipase C-gamma (PLCγ) activation and phosphatidylinositol-4,5-bisphosphate (PIP2) hydrolysis [7, 17]. Thus, signaling events controlled by PLCγ such as elevation of cytoplasmic calcium and activation of protein kinase C-beta (PKCβ) are also impaired in PI3K-deficient B cells. B cells lacking PI3K or CD19 exhibit defective accumulation of protein aggregates, termed microsignalosomes, at or near the plasma membrane [28, 29]. Consequently, PI3K-deficient B cells proliferate poorly in responses to antigen receptor triggering. This response can be rescued by the provision of signals via CD40 or TLR4, which may explain why humoral immune responses can be realized in PI3K-deficient mice, albeit at reduced levels. Engagement of CD40 or TLRs provides a mechanism for activating the NFκB pathway, whose induction by BcR signaling is attenuated in PI3K-deficient cells. Interestingly, a B cell-intrinsic mechanism to limit activation involves engagement of the inhibitory receptor FcγRIIb1, which recruits the inositol lipid phosphatase SHIP1 and reduces phosphatidylinositol-3,4,5-trisphosphate (PIP3) levels. Mice lacking FcγRIIb1develop a lupus-like syndrome, and some human lupus patients show decreased expression or function of FcγRIIb1 which indirectly suggests a role for SHIP in preventing autoimmunity [30-32]. Of interest, BcR-mediated PIP3 production is suppressed in anergic B cells, providing another example of how intrinsic attenuation of PI3K activation limits B cell responsiveness [33].
BcR clustering promotes antigen internalization, processing and presentation of the antigen to T cells. Several studies using anti-Ig antibodies as model antigens suggested a role for PI3K signaling in BcR aggregation, internalization and early stages of antigen presentation since internalization was inhibited by coligation of FcγRIIB [34, 35]. A more recent study suggested that p110δ is required for efficient antigen presentation, but through regulation of a later step in antigen processing [36]. Although defective antigen presentation may contribute to the reduced T-dependent antibody responses seen in p110δ-deficient mice [11-13], other functions for p110δ in B and T cells are also likely to determine this phenotype.
In addition to providing “core” signals required for proliferation and differentiation, PI3Ks contribute to the fitness of B cells by increasing cellular growth and metabolism. These functions are mediated in part by the Akt serine/threonine kinases, which are activated by BcR and/or CD19 engagement in a PI3K-dependent manner. As in most cells, Akt contributes to B cell size increase through promoting activation of mammalian target of rapamycin (mTOR) complex-1 (mTORC1) [37]. However, this mechanism is stimulus-dependent, with lipopolysaccharide (LPS) inducing mTORC1 activity in a PI3K-independent manner. MZ cells exhibit high basal mTORC1 activity that might contribute to the larger size and more rapid activation of these cells compared to FO cells. Akt is also thought to mediate changes in glucose metabolism in activated B cells. BcR engagement induces a glycolytic switch and this can be mimicked by expression of an inducible, constitutively active Akt construct [38]. Activation of the PI3K/Akt signaling axis also contributes to survival in response to interleukin-4 (IL-4), B cell activation factor of the tumor necrosis factor family (BAFF), and other cytokines [39, 40]. Thus PI3Ks contribute to diverse signaling events that control B cell development and antibody production.
3.3 Immunoglobulin gene rearrangement and isotype switching
A surprising observation was that SH2 domain containing protein of 65kDa (SLP-65) (otherwise known as BLNK), an adaptor protein important for signalosome assembly, appears to suppress PI3K signaling in pre-B cells [41]. The mechanism by which SLP-65 suppresses PI3K signaling is not known. However, a picture of why it may be important to suppress PI3K signaling at various stages of development has recently emerged.
The only group of transcription factors whose activity is entirely dependent on PI3K signaling in diverse cell types is the Forkhead Box Subgroup O (Foxo) proteins [42]. There are four Foxo isoforms; among these, Foxo1 and Foxo3a appear to be the most important in B cells and T cells [43-47]. In its unphosphorylated form, Foxo translocates to the nucleus where it drives the transcription of a number of genes whose products regulate apoptosis and cell cycle progression such as Bim, p27 and FasL [42]. More recently, it was found that Foxo promotes expression of the Rag-1 and Rag-2 genes [43, 44]. When phosphorylated by Akt, Foxo is found in the cytoplasm associated with 14-3-3 proteins and unable to regulate gene transcription. Thus, active PI3K/Akt signaling inhibits Foxo-dependent transcription. With respect to the Rag genes, it has been proposed that suppression of PI3K signaling is important for recombination of the immunoglobulin light chain locus to occur. Furthermore, it seems that subsequent reactivation of PI3K is also important to suppress Rag expression and to prevent inappropriate expression of a second light chain [20, 41, 44]. Concurrently, tonic signaling by surface IgM on immature B cells acts through PI3K to positively select cells for further differentiation into mature B cell subsets and to promote survival of mature B cells [21, 22, 48].
Immunoglobulin isotype switching is also regulated by the suppression of Foxo function, and hence indirectly by PI3K. Class-switch recombination was enhanced in the presence of p110δ inhibitors and increased further by constitutively active Foxo proteins, but suppressed in Pten-deficient B cells where PI3K signaling was elevated [49]. These results seemed to contradict other studies with p85α or p110δ deficient mice that showed reduced switching to other isotypes after immunization. However, further analysis revealed increased propensity for both p85α and p110δ deficient B cells to produce immunoglobulins of the IgE subclass, both in vitro and in vivo [50, 51]. A similar effect was observed in mice that were treated with the p110δ-selective inhibitor IC87114 [50]. An increased percentage of cells showed sequential switching from IgG1 to IgE, consistent with unrestrained class-switch recombination in the absence of p110δ activity. The excessive IgE production in p85α−/−, p110δD910A and IC87114-treated mice suggest that therapeutic use of p110δ inhibitors could lead to increased allergic responses. In part, this should be compensated by diminished mast cell function, but only as long as the inhibitor is administered [52, 53]. Thus, while in general PI3Ks are thought to integrate signals from the BcR, costimulatory and cytokine receptors to promote B cell activation and differentiation, PI3Ks also negatively regulate the expression of Rag proteins and genes involved in immunoglobulin class-switch recombination and may thus paradoxically also limit aspects of B cell development and differentiation.
3.4 B cell chemotaxis and trafficking
B cell trafficking is strongly influenced by PI3K signaling. Treatment of B cells with wortmannin reduces chemokine-driven migration in vitro, and alters homing after adoptive transfer to mice [54-56]. B cell chemotaxis is partially dependent on the class IA isoform p110δ, an exception to the rule that class IB (p110γ) functions downstream of GPCRs in lymphocytes. Wortmannin-treated B cells and p85α-deficient B cells also display reduced basal motility in lymph nodes [56]. Proper recirculation of B cells requires the lymph node homing receptor L-selectin (CD62L), whose expression is controlled by Foxo1 [43]. BcR stimulation down-regulates L-selectin expression, and this is partially prevented in p85α-deficient B cells [57].
Figure 1A outlines key actions of PI3K in B cell development and function, as highlighted in this section.
4 PI3K in T cells
4.1 T cell development and differentiation
T cells develop in the thymus from CD4−CD8−TcR− precursors that arrive from the bone marrow. These precursors are referred to as double negative (DN) cells (due to the lack of expression of CD4 or CD8) and are subdivided into DN1-4 based on the differential expression of CD44 and CD25. At the DN3 stage, T cells express RAG enzymes that rearrange the TcR β-chain genes so that a TcRβ chain can be expressed on the cell surface. T cells that express a productively rearranged TcRβ chain are selected to become CD4+CD8+ double-positive (DP) cells that rearrange and express TcRα chains. Concurrent with this developmental stage, TcRαβ+ DP T cells are positively selected to become CD4 or CD8 single positive mature thymocytes if they bind self-peptide presented by MHC class I or II molecules with intermediate affinity. DP cells are negatively selected though apoptosis if they bind peptide-MHC (pMHC) with high affinity. Most DP thymocytes die by neglect, however, as they show no apparent affinity for pMHC molecules. Selected CD4+ or CD8+ thymocytes then migrate through the blood and lymph to populate the lymph nodes and spleen. PI3K controls T cell biology at multiple junctions of their development (see Fig. 1b).
4.1.1 A surprising redundancy between p110δ and p110γ during T cell development
Both p110δ and p85 double knockout (p85α deleted in T cells, p85β germline deleted)(dko) mice produce near normal number of thymocytes whereas p110γ-deficient mice have reduced numbers of thymocytes, which was attributed to increased apoptosis of DP thymocytes [12, 15, 58, 59]. Strikingly, p110δ-p110γ double-deficient mice show a dramatic reduction in thymocyte numbers caused by a block at the DN3-DN4 stage of development in addition to reduced DP cell survival [60, 61]. Pre-TcR signaling as this stage was mostly controlled by p85α and p110δ [62]. The unexpected redundancy between p110δ and p110γ therefore suggested that GPCRs may play a greater role at this developmental stage than previously appreciated. Indeed, further investigations demonstrated that coordinated activation of p110δ by the pre-TcR and p110γ by the chemokine receptor CXCR4 is essential for optimal development of double positive T cells [63]. A key role for PI3K signaling during thymocyte development has also been revealed from mice with T cell-specific deletions of Akt or Pdk1 [64-67]. In each case, DP cell numbers were dramatically reduced. By contrast, deletion of Pten in pre-T cells led to the development of double positive T cells in the absence of pre-TcR expression, suggesting that unrestrained PI3K signaling can substitute for the signals that normally drive β-selection [68]. One key observation was that the transition from DN3 to DN4 stage was accompanied by increased metabolic activity, increased cell size and by the expression of nutrient receptors such as CD71 (the transferrin receptor) and CD98 (an amino acid transporter), presumably required to support the increased growth and proliferative burst [69, 70]. Pdk1−/− thymocytes failed to upregulate these receptors and Pdk1−/− DN4 thymocytes were smaller than normal [70]. There is also a requirement for Notch signaling at the DN3 to DP thymocyte transition and PI3K and Akt were required for Notch-dependent increase in metabolism and cell growth at this stage [65, 69, 70]. Notch is unlikely to directly regulate the recruitment of PI3K to the plasma membrane. Instead, it has been proposed that notch can increase PI3K signaling by suppressing the expression of Pten [71]. This would be consistent with the observation that deletion of Pten was sufficient to promote DP thymocyte development in the absence of TcR signaling [68]. Interestingly, Pten deficiency was also permissive for DP T cell development in absence of Pdk1, thus implicating additional PI3K-dependent signaling pathways in early T cell development [72].
4.2 TcR signaling and costimulation
The mature T cell receptor activates PI3K signaling by recruiting p85/p110 to the immune synapse [73]. Precisely how PI3K becomes recruited to the TcR is less clear than it is in the case of BcR signaling. The major TcR-associated tyrosine kinase substrates CD3 and Lat lack obvious p85 docking sites. T cells do express the transmembrane adapter protein TRIM, which contains YxxM motifs that become phosphorylated by ZAP-70 and to which PI3K could dock [74]. However, Akt phosphorylation was unimpaired in TRIM-deficient T cells [75]. CD28 has often been considered to play an analogous role in T cells as CD19 does in B cells; that is by acting as a docking site for PI3K after phosphorylation by Lck or other TcR associated kinases [76]. Consistent with this model is the presence of a p85 SH2 binding motif (Y170MNM) in the CD28 cytoplasmic domain. However, while CD28−/− T cells showed reduced PI3K activity in response to stimulation by pMHC on antigen presenting cells (APCs), retrogenic expression of a CD28Y170F mutant rescued CD28-dependent PI3K activity despite the lack of association between p85-SH2 and CD28Y170F [77]. In addition, recent imaging experiments that followed the segregation of the TcR and CD28 into different microclusters revealed that in the mature synapse, PI3K co-localised with the TcR whereas, surprisingly, protein kinase C-theta (PKCθ) co-localised with CD28 [78]. Indeed, PKCθ recruitment to the immune synapse is more diffuse in T cells lacking CD28 [77, 79, 80]. Thus, while initial models proposed that CD28-dependent PI3K activity would complement TcR dependent PKC activity, recent genetic and imaging experiments implicate the TcR as the main activator of PI3K (helped by CD28 through undefined mechanisms) and that CD28 instead contributes to sustained or enhanced PKC activity (again as initiated by the TcR). It remains possible that subsequent to initial activation and in certain T cell lines, CD28 may act more independently of the TcR which may have contributed to the original notion that CD28 is a major activator of PI3K. Indeed, CD28-dependent migration of memory T cells to peripheral tissues was dependent both on the PI3K binding motif in CD28 and on p110δ [81, 82]. Where CD28 does bind p85, there appears to be a preference for the association with the p85β isoform [83]. The CD28-related protein ICOS, expressed mainly by primed T cells ,is also an important PI3K activator. ICOS contains a p85-SH2 binding motif that shows a higher affinity than CD28 for p85 [84]. Recent evidence also indicates that ICOS may recruit the smaller p50α subunit, which appears to correlate with greater PI3K enzyme activation [85]. A tyrosine to phenylalanine mutation in ICOS mimics the ICOS deficient phenotype showing that PI3K engagement is essential for ICOS function. Thus ICOSY181F mice lacked follicular helper T cells which support the germinal cell reaction that leads to class switching and high affinity antibody production [86]. Mice lacking p110δ activity have a similar phenotype to ICOS deficient mice with regards to reduced IL-10 production by T cells, reduced Th2 responses and impaired germinal centre formation in the spleen [12, 14, 87-90](see section 4.3). Thus, PI3K may be an important component of T cell costimulatory signaling, but the rules of engagement are different than initially assumed.
In addition to the tyrosine-based recruitment mechanisms described here, p110δ has recently also been shown to bind the atypical Ras family member Rras2 (also known as Tc21) which localizes to the T cell and B cell antigen receptor complexes [91]. Thus p85α and p110δ recruitment to the BcR and TcR was impaired in Rras2−/− B cells and T cells and aspects of the Rras2−/− phenotype, such as the lack of MZ B cells, was reminiscent of that observed in the context of p85α or p110δ-deficiency [91]. To what extent Rras2 complements or supplements tyrosine-based recruitment mechanisms remains to be further explored.
4.2.1 PI3K regulates some, but not all, TcR-dependent signaling pathways
The activation of Akt is entirely dependent on the p85s and p110δ in peripheral T cells stimulated through the antigen receptor with or without CD28 [12, 14, 15, 77]. PI3K deficiency also has a particularly strong impact on Erk phosphorylation in T cells, but a more modest effect on Ca2+ release into the cytosol [12, 15]. The modest impact on Ca2+ flux suggest that PI3K may act on pathways downstream of, or in parallel to, PLCγ. In this context, it is of interest to note that the PI3K effector Bam32 was required for full Erk activation in T cells [92]. PI3K may also contribute to NF-κB nuclear translocation, though this is a subject of considerable debate. Several mechanisms exist for stimulating the nuclear translocation and activation of NF-κB [93]. In response to TcR signaling, the main pathway leading to NF-κB activation involves the activation of PKCθ and sequential engagement of Carma, Bcl10 and Malt1 leading to the activation of IκB kinase [94]. Where PI3K has been proposed to contribute, it has been suggested to be either via the capacity of Pdk1 to interact with and phosphorylate PKCθ or by Akt-dependent phosphorylation and activation of Cot (also known as Tpl2 or Map3k8) [95-98] . The capacity of Pdk1 to phosphorylate AGC kinases, including PKCs, is generally thought to be PI3K-independent so a requirement for Pdk1 in activating PKCθ does not necessarily imply a requirement for PI3K. Indeed, a knockin mutation in the PH domain of Pdk1 which renders Pdk1 insensitive to PI3K signaling and which attenuates Akt phosphorylation, did not appear to have any effect on PKCθ activation [99]. In addition, the extent to which the putative Pdk1 phosphorylation site in PKCθ regulates downstream signaling events has been questioned [100]. Although Akt-dependent phosphorylation of Cot may be critical in some cells, NF-κB activation and IL-2 production are unimpaired in Map3k8-deficient T cells suggesting that Akt-dependent Cot phosphorylation is also non-essential in this context [101, 102]. Pertinently, PKCθ recruitment to the synapse and NF-κB translocation to the nucleus were intact in antigen-stimulated p110δ-deficient T cells [14, 77]. These data suggest that PI3K contribution to NF-κB signaling in T cells is non-essential. However, PI3K signaling does play a key role in regulating the Foxo family of transcription factors as these are direct targets for phosphorylation by Akt. Although this may in part relieve T cells from anti-proliferative and apoptosis inducing signals [42], more recent data suggest a key role for Foxo family proteins in the regulation of T cell trafficking which will be discussed further in section 4.6.
4.3 T helper cell differentiation
Both p110δ-deficient and p85 dko T cells show reduced proliferation and cytokine production [12, 14, 15, 87, 90, 103-105]. In general, the defect in cytokine production is more pronounced for the effector cytokines interferon-gamma (IFN-γ) and IL-4 that are mainly produced by antigen experienced differentiated T-helper type-1 (Th1) or T-helper type-2 (Th2) cells than it is for interleukin-2 (IL-2) which can be produced by naïve T cells. Thus, PI3K may contribute to Th lineage-specific differentiation programs. In addition, p110δ-inhibitors blocked cytokine production by both mouse and human memory T cells, suggesting that even after a T helper cell has differentiated, it remains dependent on PI3K activity for optimal cytokine secretion [106]. However, defective cytokine secretion has not been observed in all experimental models. For instance, under Th2 skewing conditions in vitro or in vivo, IFN-γ production by p110δ-deficient T cells was enhanced, perhaps reflecting reduced interleukin-10 (IL-10) production that strongly antagonizes IFN-γ production [50, 87]. Similarly, mice with a T cell-specific deletion of SHIP showed enhanced Th1 but reduced Th2 responses under Th2 skewing immunization conditions [107]. In addition, both p110δ-deficiency and Ship deficiency are associated with reduced IL-17 production by T cells [106, 108]. These are curious observations, as one would anticipate that p110δ-deficiency and SHIP-deficiency would have opposing effects on T cell function as the former is associated with reduced PIP3 levels whereas the latter is associated with elevated PIP3 levels. However, it should be noted that PtdIns(3,4)P2 produced by Ship-mediated hydrolysis of PIP3 may contribute to T cell activation by mechanisms that have yet to be fully appreciated [109].
As a consequence of impaired T cell development in the thymus, p110δ-p110γ double deficient mice were lymphopenic, particularly with respect to T cell numbers [60, 61]. The T cells found in the periphery secreted elevated amounts of Th2 cytokines. The serum from these mice was also enriched in IgE, had normal levels of IgG1 and reduced levels of IgG2a [103]. The enhanced Th2-like phenotype in the p110γ-p110δ double deficient mice seems paradoxical considering the reduced Th2 responses observed in p110δ-deficient mice [87], but is most likely explained by stress caused by lymphopenia rather than any role for p110δ or p110γ in suppressing Th2 responses. The requirement for PI3K in promoting Th1 differentiation may be mitigated by a role for PI3K in the negative regulation of TLR signaling in dendritic cells (DCs). Thus, PI3K-deficient DCs secreted larger amounts of IL-12, which promoted Th1 differentiation in p85α knockout mice [110]. Thus, while PI3K seems to play a positive and T cell intrinsic role in promoting cytokine production, in the context of additional stimuli this defect may not always be apparent.
4.4 Survival and glucose homeostasis in mature T cells
PI3K can also regulate glucose uptake in T cells by stimulating Akt, which controls Glut1 translocation to the membrane [111]. This was put forward as an alternative mechanism for CD28 to promote costimulation as it was known that CD28 has minimal impact on the transcription profile of T cells [112, 113]. However, there is no evidence that the CD28 PI3K binding motif was required for this response and more recent evidence might suggest that CD28 contributes in concert with the TcR to trigger optimal PI3K and Akt activity required for glucose uptake. CD28 and PI3K were also required for the upregulation of Bcl-xL which promotes survival of T cells [114, 115]. The serine/threonine Pim kinases, whose expression is regulated by IL-2 in T cells, may also play a partial and PI3K-independent role in stimulating T cell metabolism and/or survival through mechanisms that remain to be fully defined [116]. In this context it is important to note that both Pim kinases and mTOR can be inhibited by the commonly used tool compound LY294002 – thus some studies using this inhibitor alone to probe PI3K function need to be reinterpreted [117]. Therefore, while PI3K may promote cellular fitness by augmenting metabolic and pro-survival responses, other pathways such as Pim and PI3K-independent activation of mTOR may also contribute which may explain why it is possible to stimulate PI3K-deficient T cells to grow and proliferate in the absence of detectable PI3K activity [15].
4.5 PI3K controls the development and suppressive function of regulatory T cells (Tregs)
Tregs play an essential life preserving function by reining in the adaptive immune system [118, 119]. Tregs can develop either in the thymus from immature progenitors or in the periphery from mature CD4 T cells. Both peripheral and thymic development of Treg require TGF-β signaling [120]. p110δ-deficient mice showed increased proportions of Treg in the thymus, whereas over-expression of myristoylated Akt in thymocytes inhibited Treg development, suggesting that PI3K negatively regulates thymic development of Tregs via Akt [90, 121]. By contrast, Treg were found in reduced numbers in the spleen and lymph nodes of p110δ-deficient mice, suggesting a possible role for PI3K in the homeostatic maintenance or peripheral development of Tregs. Consistent with this notion, Ship−/− mice harbored increased proportions of Treg in the spleen [108]. Curiously, treatment of naïve T cells with PI3K and mTOR inhibitors 18 hours after activation through the antigen receptor led to the induction of Foxp3 expression in the absence of TGF-β [122]. In addition, genetic deletion of mTor in T cells led to enhanced Treg development at the expense the Th lineages [123]. The physiological relevance of how interrupted PI3K and mTOR signaling could lead to induction of Foxp3 expression remains to be defined; nonetheless, these results, together with the p110δD910A and Akt over-expressing thymocytes, show that PI3K signaling is intimately linked to the production and maintenance of Foxp3+ Tregs.
Treg from p110δ mice are defective in their capacity to suppress T helper cell proliferation in vitro and to secrete IL-10 [90]. One apparent consequence of reduced Treg function in p110δ mice is that they were shown to clear Leishmania infections more efficiently than WT mice despite impaired Th1 responses [104]. However, acute or chronic depletion of Treg cells can also lead to massive lymphoproliferation, lymphocyte tissue infiltration and multiple organ failure [124]. More subtle defects in Treg function or Treg depletion in the absence of intact adaptive immunity, frequently leads to colon inflammation stimulated by commensal flora in the gut [125]. Indeed, p110δ-deficient Treg failed to prevent disease in an experimental model of colitis [90]. Accordingly, p110δD910A mice showed signs of inflammatory bowel disease but no other autoimmune symptoms were evident [12]. By contrast, p85 dko mice showed symptoms reminiscent of Sjogren’s syndrome, including dry eyes, lacrimal gland destruction and autoantibodies [105]. Whether the different symptoms described in the different mice represent real differences in how p85 vs p110δ deficiency affects Treg function or whether other environmental or tissue-specific factors explain the different symptoms is not yet clear. PI3K signaling in response to IL-2 stimulation was reduced in Treg compared to conventional CD4 T cells [126]. Deletion of Pten resulted in elevated PI3K signaling and enhanced proliferation of Treg in response to IL-2 – even in absence of TcR signaling which is required for the proliferation of conventional Treg [127]. However, deletion of Ship or Pten had no effect on Treg-mediated suppression [108, 127]. However, one study showed that the expression of transgenic Akt in mouse Treg enhanced suppression [128] whereas another study showed that in human Tregs basal PI3K signaling was also lower than in conventional T cells and that ectopic expression of myristoylated Akt interfered with Treg-mediated suppression [129]. Thus, PI3Ks regulate many facets of Treg development, homeostasis and suppression through mechanisms that are still incompletely understood. What seems clear is that the timing and magnitude of the PI3K and mTOR signals have strong impacts on the homeostasis and function of Treg.
4.6 T cell chemotaxis and migration in lymph nodes: not all about PI3K
The role of p110γ in peripheral T cells is debated. While some investigators observe reduced proliferation and cytokine production by p110γ−/− T cells [58, 130], others observe that p110γ−/− T cells proliferate normally [131, 132]. Recent evidence suggests that the TcR can form complexes with chemokine receptors, especially in memory T cells, and may hence influence TcR-dependent events under some circumstances [133, 134]. Other factors such as the health status of the mice, cell purification protocols and media components may also influence to what extent p110γ contributes to T cell activation by antigen receptor stimulation in vitro by altering the levels of available GPCR ligands. Perhaps surprisingly, chemotactic responses to CCL19, CCL21 and CXCL12 were only moderately affected by p110γ deficiency despite the capacity of these agonists to stimulate PI3K activity in a p110γ-dependent manner [54, 55, 81]. Instead, the Rac guanine nucleotide exchange factor (GEF) Dock2 was required for T cell chemotaxis in a mostly PI3K-independent manner [55, 135]. More recently, the role of PI3Ks in regulating T cell migration within the lymph nodes has been investigated using intravital two-photon microscopy or by fluorescent microscopy of lymph node slices [56, 135, 136]. Constitutive migration of T cells within lymph nodes is promoted by chemokines secreted by and adhered to fibroblastic reticular cells [137]. However, the effects of inhibiting PI3K were more subtle. Interestingly, by some criteria, the loss of the p85 adapter subunits had a stronger effect than inhibition by wortmannin. These observations suggest that p85 may regulate some aspects of cell motility independently of PI3K in T cells as had previously been shown in fibroblasts [56, 138].
In addition to promoting chemotaxis, CCL19 and CCL21 are important for T cell homeostasis and survival [139]. Thus an intriguing possibility is that chemokines use p110γ in T cells to promote survival or metabolic fitness rather than to detect chemotactic gradients. Consistent with this interpretation is the observation that p110γ-deficiency could alleviate autoimmunity caused by the overexpression of an activating form of p85 (referred to as p65) and that this alleviation was correlated with reduced accumulation of memory T cells rather than by reduced infiltration of T cells into affected organs [140]. Chemokines can also provide costimulatory signals to T cells to enhance proliferation, but this is not p110γ or p110δ dependent [141]. More recent data has provided evidence for a key role for p110γ in mediating chemotactic signaling in previously activated T cells in response to inflammatory chemokine CCL5 or the lipid chemotractant leukotriene B4 [131]. This was correlated with a defective recruitment of p110γ−/− T cells to the site of vaccinia virus infection and diminished ability to resist infection. Similarly, p110γ−/−CD4 T cells showed reduced response to the chemokine CCL22 [132].
4.7 T cell trafficking
Several lines of evidence have indicated key roles for class IA PI3Ks in regulating lymphocyte motility and trafficking. p85-deficient T cells show reduced motility in intact lymph nodes and p110δ-deficient T cells show reduced recruitment to sites of inflammation or skin allografts [56, 81]. These defects do not appear to reflect a role for p85 or p110δ in chemokine signaling as PI3K plays a minor role in T cell chemotaxis. Where there is a role for PI3K, p110γ appears to be the relevant isoform. Instead, PI3K, via its activation of Akt controls the exclusion of Foxo from the nucleus and hence prevents Foxo from driving the transcription of Krüppel-like factor-2 (KLF2) and a number of genes that are critical for lymphocyte trafficking and homing, such as L-selectin (CD62L), CCR7 and receptors for sphingosine-1-phosphate (S1P) and interleukin-7 (IL-7) [45, 47, 142, 143]. L-selectin, CCR7 and S1P receptor expression can also be inhibited by rapamycin, suggesting a novel capacity of mTORC1 to inhibit transcription [142]. CD62L is required for the movement of lymphocytes across the high endothelial venules during entry into lymph nodes. CCR7 contributes to the recruitment of T cells into the T cell areas of lymph nodes, whereas S1P promotes the exit of T cells from lymph nodes. IL-7 is a key regulator of homeostatic survival of T cells and it found in limiting amounts in lymph nodes. Because the effects of the defined Foxo targets are pleiotropic and can promote recruitment or exit from lymph nodes, the effect of PI3K inhibition (and hence sustained expression of these trafficking molecules) may not always be easily predicted.
In memory T cells, the TcR and CD28 can independently stimulate T cell trafficking to peripheral tissues by mechanisms that remain to be defined but which are absolutely dependent on p110δ activity [81, 82]. The cells used in these studies had been generated by repeated immunizations followed by in vitro cultures, hence both CCR7 and CD62L expression was low also on the p110δD910A T cells resulting in few T cells of either genotype being recruited to the lymph nodes in these studies [81]. Circulating T cells interact with the capillary and post-capillary endothelium and translocate through the endothelial barrier if they detect the expression of foreign antigen or presence of costimulatory ligands. How PI3K is required for this process is presently unclear but may involve the clustering or otherwise alteration of selected integrin affinity or avidity [82] or additional Foxo or KLF2 targets that have yet to be fully characterized. In this context it is of interest to note that KLF2 deficient lymphocytes have been found to accumulate preferentially in non-lymphoid tissues [144]. Given that PI3K suppresses KLF2 [142], this suggests that p110δ-deficient cells may lack the expression of certain genes that would facilitate entry into non-lymphoid organs. These observations have potentially important clinical implications as they indicate that p110δ inhibitors could reduce the recruitment of T cells to sites of inflammation where the T cells otherwise might increase the inflammatory response.
4.8 Nonessential role for PI3K in cytotoxic responses
p85 or p110δ do not appear to be essential for CD8 T cell-mediated killing in vivo of either virally infected cells or of hematopoietic allografts [15, 77]. Also, the capacity of NK cells to kill targets was either unaffected or modestly affected in p110δ or p110γ deficient mice [145-148]. These results were of particular interest as the NKG2D receptor, which recognizes proteins on cancer and virally infected cells, is linked to PI3K via the adapter DAP10 [149]. PI3K-deficient NK cells showed defective IFN-γ secretion and both p110γ and p110δ played an important role in the recruitment of NK cells to sites of infection and to tumors [145, 146, 150]. The precise role of PI3K in CD8 T cells and NK cells remain to be fully understood, but the retention of significant cytotoxicity in the absence of PI3K activity does suggest that while PI3K inhibitors may limit inflammation and autoimmune responses, they will not necessarily compromise resistance to common viral infections.
Figure 1B outlines key actions of PI3K in T cell development and function, as highlighted in this section.
5 Prospects for PI3K inhibitors in inflammation and autoimmunity
As will be discussed in other chapters in this book, PI3K inhibitors have been used with notable success in preclinical models of lupus, arthritis, asthma and allergy. While much of this effort has focused on the important capacity of PI3K inhibitors to reduce neutrophil recruitment and reactive oxygen species generation, p110δ inhibitors may block T cell and B cell dependent inflammation, autoimmunity or graft rejection with minimal impact on PI3K signaling in other tissues. By a similar vein, the therapeutic potential of p110γ inhibitors may in part be mediated by suppressing the survival of memory T cells or they may prevent T cells from being recruited to sites of inflammation. To what extent p110δ or p110γ inhibition will compromise resistance to infection is currently not known, but this will be an important area of investigation as inhibitors of these isoforms enter clinical trials. In addition, attention needs to be paid to the possibility that PI3K inhibition can enhance certain immune responses by blocking regulatory T cells or by enhancing DC function or B cell class switch recombination.
6 Acknowledgements
Work cited from our laboratories has been funded by grants from the BBSRC (to K.O.) and from the National Institutes of health and the American Cancer Society (to D.A.F.). We are grateful to Dalya Soond and Dan Patton for constructive comments on the manuscript.
7 References
- 1.Feldmann M, Maini SR. Role of cytokines in rheumatoid arthritis: an education in pathophysiology and therapeutics. Immunol Rev. 2008;223:7–19. doi: 10.1111/j.1600-065X.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone JA, St Clair EW, Turka LA. CTLA4Ig: bridging the basic immunology with clinical application. Immunity. 2006;24:233–238. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Edwards JC, Leandro MJ, Cambridge G. B lymphocyte depletion therapy with rituximab in rheumatoid arthritis. Rheum Dis Clin North Am. 2004;30:393–403. viii. doi: 10.1016/j.rdc.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Rommel C, Camps M, Ji H. PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol. 2007;7:191–201. doi: 10.1038/nri2036. [DOI] [PubMed] [Google Scholar]
- 5.Ruckle T, Schwarz MK, Rommel C. PI3Kgamma inhibition: towards an ‘aspirin of the 21st century’? Nat Rev Drug Discov. 2006;5:903–918. doi: 10.1038/nrd2145. [DOI] [PubMed] [Google Scholar]
- 6.Okkenhaug K, Ali K, Vanhaesebroeck B. Antigen receptor signalling: a distinctive role for the p110delta isoform of PI3K. Trends Immunol. 2007;28:80–87. doi: 10.1016/j.it.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fruman DA, Bismuth G. Fine tuning the immune response with PI3K. Immunol Rev. 2009;228:253–272. doi: 10.1111/j.1600-065X.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- 8.Crabbe T, Welham MJ, Ward SG. The PI3K inhibitor arsenal: choose your weapon! Trends Biochem Sci. 2007;32:450–456. doi: 10.1016/j.tibs.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Fruman DA, Snapper SB, Yballe CM, Davidson L, Yu JY, Alt FW, Cantley LC. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science. 1999;283:393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki H, Terauchi Y, Fujiwara M, Aizawa S, Yazaki Y, Kadowaki T, Koyasu S. Xid-like immunodeficiency in mice with disruption of the p85alpha subunit of phosphoinositide 3-kinase. Science. 1999;283:390–392. doi: 10.1126/science.283.5400.390. [DOI] [PubMed] [Google Scholar]
- 11.Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, Humphries LA, Rawlings D, Reynolds H, Vigorito E, Turner M. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med. 2002;196:753–763. doi: 10.1084/jem.20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, Smith AJ, Vanhaesebroeck B. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 13.Jou ST, Carpino N, Takahashi Y, Piekorz R, Chao JR, Carpino N, Wang D, Ihle JN. Essential, nonredundant role for the phosphoinositide 3-kinase p110delta in signaling by the B-cell receptor complex. Mol Cell Biol. 2002;22:8580–8591. doi: 10.1128/MCB.22.24.8580-8591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okkenhaug K, Patton DT, Bilancio A, Garcon F, Rowan WC, Vanhaesebroeck B. The p110delta isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J Immunol. 2006;177:5122–5128. doi: 10.4049/jimmunol.177.8.5122. [DOI] [PubMed] [Google Scholar]
- 15.Deane JA, Kharas MG, Oak JS, Stiles LN, Luo J, Moore TI, Ji H, Rommel C, Cantley LC, Lane TE, Fruman DA. T cell function is partially maintained in the absence of class IA phosphoinositide 3-kinase signaling. Blood. 2007;109:2894–2902. doi: 10.1182/blood-2006-07-038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardy RR, Kincade PW, Dorshkind K. The protean nature of cells in the B lymphocyte lineage. Immunity. 2007;26:703–714. doi: 10.1016/j.immuni.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Donahue AC, Hess KL, Ng KL, Fruman DA. Altered splenic B cell subset development in mice lacking phosphoinositide 3-kinase p85alpha. Int Immunol. 2004;16:1789–1798. doi: 10.1093/intimm/dxh180. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Matsuda S, Terauchi Y, Fujiwara M, Ohteki T, Asano T, Behrens TW, Kouro T, Takatsu K, Kadowaki T, Koyasu S. PI3K and Btk differentially regulate B cell antigen receptor-mediated signal transduction. Nat Immunol. 2003;4:280–286. doi: 10.1038/ni890. [DOI] [PubMed] [Google Scholar]
- 19.Herzog S, Reth M, Jumaa H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat Rev Immunol. 2009;9:195–205. doi: 10.1038/nri2491. [DOI] [PubMed] [Google Scholar]
- 20.Llorian M, Stamataki Z, Hill S, Turner M, Martensson IL. The PI3K p110delta is required for down-regulation of RAG expression in immature B cells. J Immunol. 2007;178:1981–1985. doi: 10.4049/jimmunol.178.4.1981. [DOI] [PubMed] [Google Scholar]
- 21.Verkoczy L, Duong B, Skog P, Ait-Azzouzene D, Puri K, Vela JL, Nemazee D. Basal B cell receptor-directed phosphatidylinositol 3-kinase signaling turns off RAGs and promotes B cell-positive selection. J Immunol. 2007;178:6332–6341. doi: 10.4049/jimmunol.178.10.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tze LE, Schram BR, Lam KP, Hogquist KA, Hippen KL, Liu J, Shinton SA, Otipoby KL, Rodine PR, Vegoe AL, Kraus M, Hardy RR, Schlissel MS, Rajewsky K, Behrens TW. Basal immunoglobulin signaling actively maintains developmental stage in immature B cells. PLoS Biol. 2005;3:e82. doi: 10.1371/journal.pbio.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aiba Y, Kameyama M, Yamazaki T, Tedder TF, Kurosaki T. Regulation of B-cell development by BCAP and CD19 through their binding to phosphoinositide 3-kinase. Blood. 2008;111:1497–1503. doi: 10.1182/blood-2007-08-109769. [DOI] [PubMed] [Google Scholar]
- 24.Vigorito E, Bardi G, Glassford J, Lam EW, Clayton E, Turner M. Vav-dependent and vav-independent phosphatidylinositol 3-kinase activation in murine B cells determined by the nature of the stimulus. J Immunol. 2004;173:3209–3214. doi: 10.4049/jimmunol.173.5.3209. [DOI] [PubMed] [Google Scholar]
- 25.Inabe K, Ishiai M, Scharenberg AM, Freshney N, Downward J, Kurosaki T. Vav3 modulates B cell receptor responses by regulating phosphoinositide 3-kinase activation. J Exp Med. 2002;195:189–200. doi: 10.1084/jem.20011571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walmsley MJ, Ooi SK, Reynolds LF, Smith SH, Ruf S, Mathiot A, Vanes L, Williams DA, Cancro MP, Tybulewicz VL. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302:459–462. doi: 10.1126/science.1089709. [DOI] [PubMed] [Google Scholar]
- 27.Fruman DA, Satterthwaite AB, Witte ON. Xid-like phenotypes: a B cell signalosome takes shape. Immunity. 2000;13:1–3. doi: 10.1016/s1074-7613(00)00002-9. [DOI] [PubMed] [Google Scholar]
- 28.Depoil D, Fleire S, Treanor BL, Weber M, Harwood NE, Marchbank KL, Tybulewicz VL, Batista FD. CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat Immunol. 2008;9:63–72. doi: 10.1038/ni1547. [DOI] [PubMed] [Google Scholar]
- 29.Weber M, Treanor B, Depoil D, Shinohara H, Harwood NE, Hikida M, Kurosaki T, Batista FD. Phospholipase C-gamma2 and Vav cooperate within signaling microclusters to propagate B cell spreading in response to membrane-bound antigen. J Exp Med. 2008;205:853–868. doi: 10.1084/jem.20072619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Floto RA, Clatworthy MR, Heilbronn KR, Rosner DR, MacAry PA, Rankin A, Lehner PJ, Ouwehand WH, Allen JM, Watkins NA, Smith KG. Loss of function of a lupus-associated FcgammaRIIb polymorphism through exclusion from lipid rafts. Nat Med. 2005;11:1056–1058. doi: 10.1038/nm1288. [DOI] [PubMed] [Google Scholar]
- 31.Brauweiler A, Tamir I, Dal Porto J, Benschop RJ, Helgason CD, Humphries RK, Freed JH, Cambier JC. Differential regulation of B cell development, activation, and death by the src homology 2 domain-containing 5′ inositol phosphatase (SHIP) J Exp Med. 2000;191:1545–1554. doi: 10.1084/jem.191.9.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarasenko T, Dean JA, Bolland S. FcgammaRIIB as a modulator of autoimmune disease susceptibility. Autoimmunity. 2007;40:409–417. doi: 10.1080/08916930701464665. [DOI] [PubMed] [Google Scholar]
- 33.Browne CD, Del Nagro CJ, Cato MH, Dengler HS, Rickert RC. Suppression of phosphatidylinositol 3,4,5-trisphosphate production is a key determinant of B cell anergy. Immunity. 2009;31:749–760. doi: 10.1016/j.immuni.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phee H, Rodgers W, Coggeshall KM. Visualization of negative signaling in B cells by quantitative confocal microscopy. Mol Cell Biol. 2001;21:8615–8625. doi: 10.1128/MCB.21.24.8615-8625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagle NM, Faassen AE, Kim JH, Pierce SK. Regulation of B cell receptor-mediated MHC class II antigen processing by FcgammaRIIB1. J Immunol. 1999;162:2732–2740. [PubMed] [Google Scholar]
- 36.Al-Alwan MM, Okkenhaug K, Vanhaesebroeck B, Hayflick JS, Marshall AJ. Requirement for phosphoinositide 3-kinase p110delta signaling in B cell antigen receptor-mediated antigen presentation. J Immunol. 2007;178:2328–2335. doi: 10.4049/jimmunol.178.4.2328. [DOI] [PubMed] [Google Scholar]
- 37.Donahue AC, Fruman DA. Distinct signaling mechanisms activate the target of rapamycin in response to different B-cell stimuli. Eur J Immunol. 2007;37:2923–2936. doi: 10.1002/eji.200737281. [DOI] [PubMed] [Google Scholar]
- 38.Doughty CA, Bleiman BF, Wagner DJ, Dufort FJ, Mataraza JM, Roberts MF, Chiles TC. Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood. 2006;107:4458–4465. doi: 10.1182/blood-2005-12-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bilancio A, Okkenhaug K, Camps M, Emery JL, Ruckle T, Rommel C, Vanhaesebroeck B. Key role of the p110delta isoform of PI3K in B-cell antigen and IL-4 receptor signaling: comparative analysis of genetic and pharmacologic interference with p110delta function in B cells. Blood. 2006;107:642–650. doi: 10.1182/blood-2005-07-3041. [DOI] [PubMed] [Google Scholar]
- 40.Henley T, Kovesdi D, Turner M. B-cell responses to B-cell activation factor of the TNF family (BAFF) are impaired in the absence of PI3K delta. Eur J Immunol. 2008;38:3543–3548. doi: 10.1002/eji.200838618. [DOI] [PubMed] [Google Scholar]
- 41.Herzog S, Hug E, Meixlsperger S, Paik JH, DePinho RA, Reth M, Jumaa H. SLP-65 regulates immunoglobulin light chain gene recombination through the PI(3)K-PKB-Foxo pathway. Nat Immunol. 2008;9:623–631. doi: 10.1038/ni.1616. [DOI] [PubMed] [Google Scholar]
- 42.Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol. 2004;4:889–899. doi: 10.1038/nri1488. [DOI] [PubMed] [Google Scholar]
- 43.Dengler HS, Baracho GV, Omori SA, Bruckner S, Arden KC, Castrillon DH, DePinho RA, Rickert RC. Distinct functions for the transcription factor Foxo1 at various stages of B cell differentiation. Nat Immunol. 2008;9:1388–1398. doi: 10.1038/ni.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amin RH, Schlissel MS. Foxo1 directly regulates the transcription of recombination-activating genes during B cell development. Nat Immunol. 2008;9:613–622. doi: 10.1038/ni.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, Hedrick SM. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin L, Hron JD, Peng SL. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21:203–213. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 47.Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkheadbox transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity. 2009;30:358–371. doi: 10.1016/j.immuni.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, Kutok JL, Kearney JF, Otipoby KL, Rajewsky K. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Omori SA, Cato MH, Anzelon-Mills A, Puri KD, Shapiro-Shelef M, Calame K, Rickert RC. Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity. 2006;25:545–557. doi: 10.1016/j.immuni.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 50.Zhang TT, Okkenhaug K, Nashed BF, Puri KD, Knight ZA, Shokat KM, Vanhaesebroeck B, Marshall AJ. Genetic or pharmaceutical blockade of p110delta phosphoinositide 3-kinase enhances IgE production. J Allergy Clin Immunol. 2008;122:811–819. e812. doi: 10.1016/j.jaci.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Doi T, Obayashi K, Kadowaki T, Fujii H, Koyasu S. PI3K is a negative regulator of IgE production. Int Immunol. 2008;20:499–508. doi: 10.1093/intimm/dxn009. [DOI] [PubMed] [Google Scholar]
- 52.Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- 53.Ali K, Camps M, Pearce WP, Ji H, Ruckle T, Kuehn N, Pasquali C, Chabert C, Rommel C, Vanhaesebroeck B. Isoform-specific functions of phosphoinositide 3-kinases: p110 delta but not p110 gamma promotes optimal allergic responses in vivo. J Immunol. 2008;180:2538–2544. doi: 10.4049/jimmunol.180.4.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reif K, Okkenhaug K, Sasaki T, Penninger JM, Vanhaesebroeck B, Cyster JG. Cutting edge: differential roles for phosphoinositide 3-kinases, p110gamma and p110delta, in lymphocyte chemotaxis and homing. J Immunol. 2004;173:2236–2240. doi: 10.4049/jimmunol.173.4.2236. [DOI] [PubMed] [Google Scholar]
- 55.Nombela-Arrieta C, Lacalle RA, Montoya MC, Kunisaki Y, Megias D, Marques M, Carrera AC, Manes S, Fukui Y, Martinez AC, Stein JV. Differential requirements for DOCK2 and phosphoinositide-3-kinase gamma during T and B lymphocyte homing. Immunity. 2004;21:429–441. doi: 10.1016/j.immuni.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 56.Matheu MP, Deane JA, Parker I, Fruman DA, Cahalan MD. Class IA phosphoinositide 3-kinase modulates basal lymphocyte motility in the lymph node. J Immunol. 2007;179:2261–2269. doi: 10.4049/jimmunol.179.4.2261. [DOI] [PubMed] [Google Scholar]
- 57.Hess KL, Donahue AC, Ng KL, Moore TI, Oak J, Fruman DA. Frontline: The p85alpha isoform of phosphoinositide 3-kinase is essential for a subset of B cell receptor-initiated signaling responses. Eur J Immunol. 2004;34:2968–2976. doi: 10.1002/eji.200425326. [DOI] [PubMed] [Google Scholar]
- 58.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak TW, Ohashi PS, Suzuki A, Penninger JM. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez-Borlado L, Barber DF, Hernandez C, Rodriguez-Marcos MA, Sanchez A, Hirsch E, Wymann M, Martinez AC, Carrera AC. Phosphatidylinositol 3-kinase regulates the CD4/CD8 T cell differentiation ratio. J Immunol. 2003;170:4475–4482. doi: 10.4049/jimmunol.170.9.4475. [DOI] [PubMed] [Google Scholar]
- 60.Webb LM, Vigorito E, Wymann MP, Hirsch E, Turner M. Cutting edge: T cell development requires the combined activities of the p110gamma and p110delta catalytic isoforms of phosphatidylinositol 3-kinase. J Immunol. 2005;175:2783–2787. doi: 10.4049/jimmunol.175.5.2783. [DOI] [PubMed] [Google Scholar]
- 61.Swat W, Montgrain V, Doggett TA, Douangpanya J, Puri K, Vermi W, Diacovo TG. Essential role of PI3Kdelta and PI3Kgamma in thymocyte survival. Blood. 2006;107:2415–2422. doi: 10.1182/blood-2005-08-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shiroki F, Matsuda S, Doi T, Fujiwara M, Mochizuki Y, Kadowaki T, Suzuki H, Koyasu S. The p85alpha regulatory subunit of class IA phosphoinositide 3-kinase regulates beta-selection in thymocyte development. J Immunol. 2007;178:1349–1356. doi: 10.4049/jimmunol.178.3.1349. [DOI] [PubMed] [Google Scholar]
- 63.Janas ML, Varano G, Gudmundsson K, Noda M, Nagasawa T, Turner M. Thymic development beyond beta-selection requires phosphatidylinositol 3-kinase activation by CXCR4. J Exp Med. 2010;207:247–261. S241–242. doi: 10.1084/jem.20091430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hinton HJ, Alessi DR, Cantrell DA. The serine kinase phosphoinositide-dependent kinase 1 (PDK1) regulates T cell development. Nat Immunol. 2004;5:539–545. doi: 10.1038/ni1062. [DOI] [PubMed] [Google Scholar]
- 65.Juntilla MM, Wofford JA, Birnbaum MJ, Rathmell JC, Koretzky GA. Akt1 and Akt2 are required for alphabeta thymocyte survival and differentiation. Proc Natl Acad Sci USA. 2007;104:12105–12110. doi: 10.1073/pnas.0705285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fayard E, Gill J, Paolino M, Hynx D, Hollander GA, Hemmings BA. Deletion of PKBalpha/Akt1 affects thymic development. PLoS ONE. 2007;2:e992. doi: 10.1371/journal.pone.0000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mao C, Tili EG, Dose M, Haks MC, Bear SE, Maroulakou I, Horie K, Gaitanaris GA, Fidanza V, Ludwig T, Wiest DL, Gounari F, Tsichlis PN. Unequal contribution of Akt isoforms in the double-negative to double-positive thymocyte transition. J Immunol. 2007;178:5443–5453. doi: 10.4049/jimmunol.178.9.5443. [DOI] [PubMed] [Google Scholar]
- 68.Hagenbeek TJ, Naspetti M, Malergue F, Garcon F, Nunes JA, Cleutjens KB, Trapman J, Krimpenfort P, Spits H. The loss of PTEN allows TCR alphabeta lineage thymocytes to bypass IL-7 and Pre-TCR-mediated signaling. J Exp Med. 2004;200:883–894. doi: 10.1084/jem.20040495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ciofani M, Zuniga-Pflucker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- 70.Kelly AP, Finlay DK, Hinton HJ, Clarke RG, Fiorini E, Radtke F, Cantrell DA. Notch-induced T cell development requires phosphoinositide-dependent kinase 1. Embo J. 2007;26:3441–3450. doi: 10.1038/sj.emboj.7601761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palomero T, Sulis ML, Cortina M, Real PJ, Barnes K, Ciofani M, Caparros E, Buteau J, Brown K, Perkins SL, Bhagat G, Agarwal AM, Basso G, Castillo M, Nagase S, Cordon-Cardo C, Parsons R, Zuniga-Pflucker JC, Dominguez M, Ferrando AA. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat Med. 2007;13:1203–1210. doi: 10.1038/nm1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Finlay DK, Sinclair LV, Feijoo C, Waugh CM, Hagenbeek TJ, Spits H, Cantrell DA. Phosphoinositide-dependent kinase 1 controls migration and malignant transformation but not cell growth and proliferation in PTEN-null lymphocytes. The J Exp Med. 2009;206:2441–2454. doi: 10.1084/jem.20090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fabre S, Lang V, Harriague J, Jobart A, Unterman TG, Trautmann A, Bismuth G. Stable activation of phosphatidylinositol 3-kinase in the T cell immunological synapse stimulates Akt signaling to FoxO1 nuclear exclusion and cell growth control. J Immunol. 2005;174:4161–4171. doi: 10.4049/jimmunol.174.7.4161. [DOI] [PubMed] [Google Scholar]
- 74.Bruyns E, Marie-Cardine A, Kirchgessner H, Sagolla K, Shevchenko A, Mann M, Autschbach F, Bensussan A, Meuer S, Schraven B. T cell receptor (TCR) interacting molecule (TRIM), a novel disulfide-linked dimer associated with the TCR-CD3-zeta complex, recruits intracellular signaling proteins to the plasma membrane. J Exp Med. 1998;188:561–575. doi: 10.1084/jem.188.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kolsch U, Arndt B, Reinhold D, Lindquist JA, Juling N, Kliche S, Pfeffer K, Bruyns E, Schraven B, Simeoni L. Normal T-cell development and immune functions in TRIM-deficient mice. Mol Cell Biol. 2006;26:3639–3648. doi: 10.1128/MCB.26.9.3639-3648.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat Rev Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 77.Garcon F, Patton DT, Emery JL, Hirsch E, Rottapel R, Sasaki T, Okkenhaug K. CD28 provides T-cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood. 2008;111:1464–1471. doi: 10.1182/blood-2007-08-108050. [DOI] [PubMed] [Google Scholar]
- 78.Yokosuka T, Kobayashi W, Sakata-Sogawa K, Takamatsu M, Hashimoto-Tane A, Dustin ML, Tokunaga M, Saito T. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanchez-Lockhart M, Marin E, Graf B, Abe R, Harada Y, Sedwick CE, Miller J. Cutting edge: CD28-mediated transcriptional and posttranscriptional regulation of IL-2 expression are controlled through different signaling pathways. J Immunol. 2004;173:7120–7124. doi: 10.4049/jimmunol.173.12.7120. [DOI] [PubMed] [Google Scholar]
- 80.Sanchez-Lockhart M, Graf B, Miller J. Signals and sequences that control CD28 localization to the central region of the immunological synapse. J Immunol. 2008;181:7639–7648. doi: 10.4049/jimmunol.181.11.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jarmin SJ, David R, Ma L, Chai JG, Dewchand H, Takesono A, Ridley AJ, Okkenhaug K, Marelli-Berg FM. T cell receptor-induced phosphoinositide-3-kinase p110delta activity is required for T cell localization to antigenic tissue in mice. J Clin Invest. 2008;118:1154–1164. doi: 10.1172/JCI33267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mirenda V, Jarmin SJ, David R, Dyson J, Scott D, Gu Y, Lechler RI, Okkenhaug K, Marelli-Berg FM. Physiologic and aberrant regulation of memory T-cell trafficking by the costimulatory molecule CD28. Blood. 2007;109:2968–2977. doi: 10.1182/blood-2006-10-050724. [DOI] [PubMed] [Google Scholar]
- 83.Alcazar I, Cortes I, Zaballos A, Hernandez C, Fruman DA, Barber DF, Carrera AC. p85beta phosphoinositide 3-kinase regulates CD28 coreceptor function. Blood. 2009;113:3198–3208. doi: 10.1182/blood-2008-04-152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parry RV, Rumbley CA, Vandenberghe LH, June CH, Riley JL. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J Immunol. 2003;171:166–174. doi: 10.4049/jimmunol.171.1.166. [DOI] [PubMed] [Google Scholar]
- 85.Fos C, Salles A, Lang V, Carrette F, Audebert S, Pastor S, Ghiotto M, Olive D, Bismuth G, Nunes JA. ICOS ligation recruits the p50alpha PI3K regulatory subunit to the immunological synapse. J Immunol. 2008;181:1969–1977. doi: 10.4049/jimmunol.181.3.1969. [DOI] [PubMed] [Google Scholar]
- 86.Gigoux M, Shang J, Pak Y, Xu M, Choe J, Mak TW, Suh WK. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc Natl Acad Sci USA. 2009;106:20371–20376. doi: 10.1073/pnas.0911573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nashed BF, Zhang T, Al-Alwan M, Srinivasan G, Halayko AJ, Okkenhaug K, Vanhaesebroeck B, Hayglass KT, Marshall AJ. Role of the phosphoinositide 3-kinase p110delta in generation of type 2 cytokine responses and allergic airway inflammation. Eur J Immunol. 2007;37:416–424. doi: 10.1002/eji.200636401. [DOI] [PubMed] [Google Scholar]
- 88.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 89.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 90.Patton DT, Garden OA, Pearce WP, Clough LE, Monk CR, Leung E, Rowan WC, Sancho S, Walker LS, Vanhaesebroeck B, Okkenhaug K. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6598–6602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 91.Delgado P, Cubelos B, Calleja E, Martinez-Martin N, Cipres A, Merida I, Bellas C, Bustelo XR, Alarcon B. Essential function for the GTPase TC21 in homeostatic antigen receptor signaling. Nat Immunol. 2009;10:880–888. doi: 10.1038/ni.1749. [DOI] [PubMed] [Google Scholar]
- 92.Sommers CL, Gurson JM, Surana R, Barda-Saad M, Lee J, Kishor A, Li W, Gasser AJ, Barr VA, Miyaji M, Love PE, Samelson LE. Bam32: a novel mediator of Erk activation in T cells. Int Immunol. 2008;20:811–818. doi: 10.1093/intimm/dxn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schulze-Luehrmann J, Ghosh S. Antigen-receptor signaling to nuclear factor kappa B. Immunity. 2006;25:701–715. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 94.Thome M. CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nat Rev Immunol. 2004;4:348–359. doi: 10.1038/nri1352. [DOI] [PubMed] [Google Scholar]
- 95.Lee KY, D’Acquisto F, Hayden MS, Shim JH, Ghosh S. PDK1 nucleates T cell receptor-induced signaling complex for NF-kappaB activation. Science. 2005;308:114–118. doi: 10.1126/science.1107107. [DOI] [PubMed] [Google Scholar]
- 96.Park SG, Schulze-Luehrman J, Hayden MS, Hashimoto N, Ogawa W, Kasuga M, Ghosh S. The kinase PDK1 integrates T cell antigen receptor and CD28 coreceptor signaling to induce NF-kappaB and activate T cells. Nat Immunol. 2009;10:158–166. doi: 10.1038/ni.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kane LP, Mollenauer MN, Xu Z, Turck CW, Weiss A. Akt-dependent phosphorylation specifically regulates Cot induction of NF-kappa B-dependent transcription. Mol Cell Biol. 2002;22:5962–5974. doi: 10.1128/MCB.22.16.5962-5974.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Narayan P, Holt B, Tosti R, Kane LP. CARMA1 is required for Akt-mediated NF-kappaB activation in T cells. Mol Cell Biol. 2006;26:2327–2336. doi: 10.1128/MCB.26.6.2327-2336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Waugh C, Sinclair L, Finlay D, Bayascas JR, Cantrell D. Phosphoinositide (3,4,5)-triphosphate binding to phosphoinositide-dependent kinase 1 regulates a protein kinase B/Akt signaling threshold that dictates T-cell migration, not proliferation. Mol Cell Biol. 2009;29:5952–5962. doi: 10.1128/MCB.00585-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gruber T, Freeley M, Thuille N, Heit I, Shaw S, Long A, Baier G. Comment on “PDK1 nucleates T cell receptor-induced signaling complex for NF-kappaB activation”. Science. 2006;312:55. doi: 10.1126/science.1115362. author reply 55. [DOI] [PubMed] [Google Scholar]
- 101.Sriskantharajah S, Belich MP, Papoutsopoulou S, Janzen J, Tybulewicz V, Seddon B, Ley SC. Proteolysis of NF-kappaB1 p105 is essential for T cell antigen receptor-induced proliferation. Nat Immunol. 2009;10:38–47. doi: 10.1038/ni.1685. [DOI] [PubMed] [Google Scholar]
- 102.Tsatsanis C, Vaporidi K, Zacharioudaki V, Androulidaki A, Sykulev Y, Margioris AN, Tsichlis PN. Tpl2 and ERK transduce antiproliferative T cell receptor signals and inhibit transformation of chronically stimulated T cells. Proc Natl Acad Sci USA. 2008;105:2987–2992. doi: 10.1073/pnas.0708381104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ji H, Rintelen F, Waltzinger C, Bertschy Meier D, Bilancio A, Pearce W, Hirsch E, Wymann MP, Ruckle T, Camps M, Vanhaesebroeck B, Okkenhaug K, Rommel C. Inactivation of PI3Kgamma and PI3Kdelta distorts T-cell development and causes multiple organ inflammation. Blood. 2007;110:2940–2947. doi: 10.1182/blood-2007-04-086751. [DOI] [PubMed] [Google Scholar]
- 104.Liu D, Zhang T, Marshall AJ, Okkenhaug K, Vanhaesebroeck B, Uzonna JE. The p110delta isoform of phosphatidylinositol 3-kinase controls susceptibility to Leishmania major by regulating expansion and tissue homing of regulatory T cells. J Immunol. 2009;183:1921–1933. doi: 10.4049/jimmunol.0901099. [DOI] [PubMed] [Google Scholar]
- 105.Oak JS, Deane JA, Kharas MG, Luo J, Lane TE, Cantley LC, Fruman DA. Sjogren’s syndrome-like disease in mice with T cells lacking class 1A phosphoinositide-3-kinase. Proc Natl Acad Sci USA. 2006;103:16882–16887. doi: 10.1073/pnas.0607984103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soond DR, Bjorgo E, Moltu K, Dale VQ, Patton DT, Torgersen KM, Galleway F, Twomey B, Clark J, Gaston JH, Tasken K, Bunyard P, Okkenhaug K. PI3K p110{delta} regulates T cell cytokine production during primary and secondary immune responses in mice and humans. Blood. 2010 doi: 10.1182/blood-2009-07-232330. Epub Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tarasenko T, Kole HK, Chi AW, Mentink-Kane MM, Wynn TA, Bolland S. T cell-specific deletion of the inositol phosphatase SHIP reveals its role in regulating Th1/Th2 and cytotoxic responses. Proc Natl Acad Sci USA. 2007;104:11382–11387. doi: 10.1073/pnas.0704853104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Locke NR, Patterson SJ, Hamilton MJ, Sly LM, Krystal G, Levings MK. SHIP regulates the reciprocal development of T regulatory and Th17 cells. J Immunol. 2009;183:975–983. doi: 10.4049/jimmunol.0803749. [DOI] [PubMed] [Google Scholar]
- 109.Parry RV, Harris SJ, Ward SG. Fine tuning T lymphocytes: A role for the lipid phosphatase SHIP-1. Biochim Biophys Acta. 2010;1804:592–597. doi: 10.1016/j.bbapap.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 110.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 111.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 112.Riley JL, Mao M, Kobayashi S, Biery M, Burchard J, Cavet G, Gregson BP, June CH, Linsley PS. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci USA. 2002;99:11790–11795. doi: 10.1073/pnas.162359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Diehn M, Alizadeh AA, Rando OJ, Liu CL, Stankunas K, Botstein D, Crabtree GR, Brown PO. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc Natl Acad Sci USA. 2002;99:11796–11801. doi: 10.1073/pnas.092284399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Okkenhaug K, Wu L, Garza KM, La Rose J, Khoo W, Odermatt B, Mak TW, Ohashi PS, Rottapel R. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat Immunol. 2001;2:325–332. doi: 10.1038/86327. [DOI] [PubMed] [Google Scholar]
- 115.Burr JS, Savage ND, Messah GE, Kimzey SL, Shaw AS, Arch RH, Green JM. Cutting edge: distinct motifs within CD28 regulate T cell proliferation and induction of Bcl-XL. J Immunol. 2001;166:5331–5335. doi: 10.4049/jimmunol.166.9.5331. [DOI] [PubMed] [Google Scholar]
- 116.Fox CJ, Hammerman PS, Thompson CB. The Pim kinases control rapamycin-resistant T cell survival and activation. J Exp Med. 2005;201:259–266. doi: 10.1084/jem.20042020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jacobs MD, Black J, Futer O, Swenson L, Hare B, Fleming M, Saxena K. Pim-1 Ligand-bound Structures Reveal the Mechanism of Serine/Threonine Kinase Inhibition by LY294002. J Biol Chem. 2005;280:13728–13734. doi: 10.1074/jbc.M413155200. [DOI] [PubMed] [Google Scholar]
- 118.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 119.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 120.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 125.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 126.Bensinger SJ, Walsh PT, Zhang J, Carroll M, Parsons R, Rathmell JC, Thompson CB, Burchill MA, Farrar MA, Turka LA. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Walsh PT, Buckler JL, Zhang J, Gelman AE, Dalton NM, Taylor DK, Bensinger SJ, Hancock WW, Turka LA. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J Clin Invest. 2006;116:2521–2531. doi: 10.1172/JCI28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pierau M, Engelmann S, Reinhold D, Lapp T, Schraven B, Bommhardt UH. Protein kinase B/Akt signals impair Th17 differentiation and support natural regulatory T cell function and induced regulatory T cell formation. J Immunol. 2009;183:6124–6134. doi: 10.4049/jimmunol.0900246. [DOI] [PubMed] [Google Scholar]
- 129.Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood. 2007;109:2014–2022. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- 130.Alcazar I, Marques M, Kumar A, Hirsch E, Wymann M, Carrera AC, Barber DF. Phosphoinositide 3-kinase gamma participates in T cell receptor-induced T cell activation. J Exp Med. 2007;204:2977–2987. doi: 10.1084/jem.20070366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martin AL, Schwartz MD, Jameson SC, Shimizu Y. Selective regulation of CD8 effector T cell migration by the p110 gamma isoform of phosphatidylinositol 3-kinase. J Immunol. 2008;180:2081–2088. doi: 10.4049/jimmunol.180.4.2081. [DOI] [PubMed] [Google Scholar]
- 132.Thomas MS, Mitchell JS, DeNucci CC, Martin AL, Shimizu Y. The p110gamma isoform of phosphatidylinositol 3-kinase regulates migration of effector CD4 T lymphocytes into peripheral inflammatory sites. J Leukoc Biol. 2008;84:814–823. doi: 10.1189/jlb.0807561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Molon B, Gri G, Bettella M, Gomez-Mouton C, Lanzavecchia A, Martinez AC, Manes S, Viola A. T cell costimulation by chemokine receptors. Nat Immunol. 2005;6:465–471. doi: 10.1038/ni1191. [DOI] [PubMed] [Google Scholar]
- 134.Kumar A, Humphreys TD, Kremer KN, Bramati PS, Bradfield L, Edgar CE, Hedin KE. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity. 2006;25:213–224. doi: 10.1016/j.immuni.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 135.Nombela-Arrieta C, Mempel TR, Soriano SF, Mazo I, Wymann MP, Hirsch E, Martinez AC, Fukui Y, von Andrian UH, Stein JV. A central role for DOCK2 during interstitial lymphocyte motility and sphingosine-1-phosphate-mediated egress. J Exp Med. 2007;204:497–510. doi: 10.1084/jem.20061780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Asperti-Boursin F, Real E, Bismuth G, Trautmann A, Donnadieu E. CCR7 ligands control basal T cell motility within lymph node slices in a phosphoinositide 3-kinase-independent manner. J Exp Med. 2007;204:1167–1179. doi: 10.1084/jem.20062079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jimenez C, Portela RA, Mellado M, Rodriguez-Frade JM, Collard J, Serrano A, Martinez AC, Avila J, Carrera AC. Role of the PI3K regulatory subunit in the control of actin organization and cell migration. J Cell Biol. 2000;151:249–262. doi: 10.1083/jcb.151.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 140.Barber DF, Bartolome A, Hernandez C, Flores JM, Fernandez-Arias C, Rodriguez-Borlado L, Hirsch E, Wymann M, Balomenos D, Carrera AC. Class IB-phosphatidylinositol 3-kinase (PI3K) deficiency ameliorates IA-PI3K-induced systemic lupus but not T cell invasion. J Immunol. 2006;176:589–593. doi: 10.4049/jimmunol.176.1.589. [DOI] [PubMed] [Google Scholar]
- 141.Gollmer K, Asperti-Boursin F, Tanaka Y, Okkenhaug K, Vanhaesebroeck B, Peterson JR, Fukui Y, Donnadieu E, Stein JV. CCL21 mediates CD4+ T cell costimulation via a DOCK2/Rac-dependent pathway. Blood. 2009;114:580–588. doi: 10.1182/blood-2009-01-200923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fabre S, Carrette F, Chen J, Lang V, Semichon M, Denoyelle C, Lazar V, Cagnard N, Dubart-Kupperschmitt A, Mangeney M, Fruman DA, Bismuth G. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J Immunol. 2008;181:2980–2989. doi: 10.4049/jimmunol.181.5.2980. [DOI] [PubMed] [Google Scholar]
- 144.Sebzda E, Zou Z, Lee JS, Wang T, Kahn ML. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nat Immunol. 2008;9:292–300. doi: 10.1038/ni1565. [DOI] [PubMed] [Google Scholar]
- 145.Kim N, Saudemont A, Webb L, Camps M, Ruckle T, Hirsch E, Turner M, Colucci F. The p110delta catalytic isoform of PI3K is a key player in NK-cell development and cytokine secretion. Blood. 2007;110:3202–3208. doi: 10.1182/blood-2007-02-075366. [DOI] [PubMed] [Google Scholar]
- 146.Tassi I, Cella M, Gilfillan S, Turnbull I, Diacovo TG, Penninger JM, Colonna M. p110gamma and p110delta phosphoinositide 3-kinase signaling pathways synergize to control development and functions of murine NK cells. Immunity. 2007;27:214–227. doi: 10.1016/j.immuni.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 147.Zebedin E, Simma O, Schuster C, Putz EM, Fajmann S, Warsch W, Eckelhart E, Stoiber D, Weisz E, Schmid JA, Pickl WF, Baumgartner C, Valent P, Piekorz RP, Freissmuth M, Sexl V. Leukemic challenge unmasks a requirement for PI3Kdelta in NK cell-mediated tumor surveillance. Blood. 2008;112:4655–4664. doi: 10.1182/blood-2008-02-139105. [DOI] [PubMed] [Google Scholar]
- 148.Guo H, Samarakoon A, Vanhaesebroeck B, Malarkannan S. The p110 delta of PI3K plays a critical role in NK cell terminal maturation and cytokine/chemokine generation. J Exp Med. 2008;205:2419–2435. doi: 10.1084/jem.20072327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zompi S, Hamerman JA, Ogasawara K, Schweighoffer E, Tybulewicz VL, Di Santo JP, Lanier LL, Colucci F. NKG2D triggers cytotoxicity in mouse NK cells lacking DAP12 or Syk family kinases. Nat Immunol. 2003;4:565–572. doi: 10.1038/ni930. [DOI] [PubMed] [Google Scholar]
- 150.Saudemont A, Garcon F, Yadi H, Roche-Molina M, Kim N, Segonds-Pichon A, Martin-Fontecha A, Okkenhaug K, Colucci F. p110gamma and p110delta isoforms of phosphoinositide 3-kinase differentially regulate natural killer cell migration in health and disease. Proc Natl Acad Sci USA. 2009;106:5795–5800. doi: 10.1073/pnas.0808594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Janas ML, Hodson D, Stamataki Z, Hill S, Welch K, Gambardella L, Trotman LC, Pandolfi PP, Vigorito E, Turner M. The effect of deleting p110delta on the phenotype and function of PTEN-deficient B cells. J Immunol. 2008;180:739–746. doi: 10.4049/jimmunol.180.2.739. [DOI] [PubMed] [Google Scholar]
- 152.Saudemont A, Garcon F, Yadi H, Roche-Molina M, Kim N, Segonds-Pichon A, Martin-Fontecha A, Okkenhaug K, Colucci F. p110{gamma} and p110{delta} isoforms of phosphoinositide 3-kinase differentially regulate natural killer cell migration in health and disease. Proc Natl Acad Sci USA. 2009;106:5795–5800. doi: 10.1073/pnas.0808594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kishimoto H, Ohteki T, Yajima N, Kawahara K, Natsui M, Kawarasaki S, Hamada K, Horie Y, Kubo Y, Arase S, Taniguchi M, Vanhaesebroeck B, Mak TW, Nakano T, Koyasu S, Sasaki T, Suzuki A. The Pten/PI3K pathway governs the homeostasis of Valpha14iNKT cells. Blood. 2007;109:3316–3324. doi: 10.1182/blood-2006-07-038059. [DOI] [PubMed] [Google Scholar]
- 154.Lee KS, Lee HK, Hayflick JS, Lee YC, Puri KD. Inhibition of phosphoinositide 3-kinase delta attenuates allergic airway inflammation and hyperresponsiveness in murine asthma model. Faseb J. 2006;20:455–465. doi: 10.1096/fj.05-5045com. [DOI] [PubMed] [Google Scholar]
- 155.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 156.Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- 157.Barber DF, Bartolome A, Hernandez C, Flores JM, Redondo C, Fernandez-Arias C, Camps M, Ruckle T, Schwarz MK, Rodriguez S, Martinez AC, Balomenos D, Rommel C, Carrera AC. PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med. 2005;11:933–935. doi: 10.1038/nm1291. [DOI] [PubMed] [Google Scholar]
- 158.Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Francon B, Martin T, Gretener D, Perrin D, Leroy D, Vitte PA, Hirsch E, Wymann MP, Cirillo R, Schwarz MK, Rommel C. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 159.Randis TM, Puri KD, Zhou H, Diacovo TG. Role of PI3Kdelta and PI3Kgamma in inflammatory arthritis and tissue localization of neutrophils. Eur J Immunol. 2008;38:1215–1224. doi: 10.1002/eji.200838266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Oak JS, Chen J, Peralta RQ, Deane JA, Fruman DA. The p85beta regulatory subunit of phosphoinositide 3-kinase has unique and redundant functions in B cells. Autoimmunity. 2009;442:447–458. doi: 10.1080/08916930902911746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Deane JA, Trifilo MJ, Yballe CM, Choi S, Lane TE, Fruman DA. Enhanced T cell proliferation in mice lacking the p85beta subunit of phosphoinositide 3-kinase. J Immunol. 2004;172:6615–6625. doi: 10.4049/jimmunol.172.11.6615. [DOI] [PubMed] [Google Scholar]
- 162.Alcazar I, Cortes I, Zaballos A, Hernandez C, Fruman DA, Barber DF, Carrera AC. p85{beta} phosphoinositide 3-kinase regulates CD28 co-receptor function. Blood. 2009;113:3198–3208. doi: 10.1182/blood-2008-04-152942. [DOI] [PMC free article] [PubMed] [Google Scholar]