Abstract
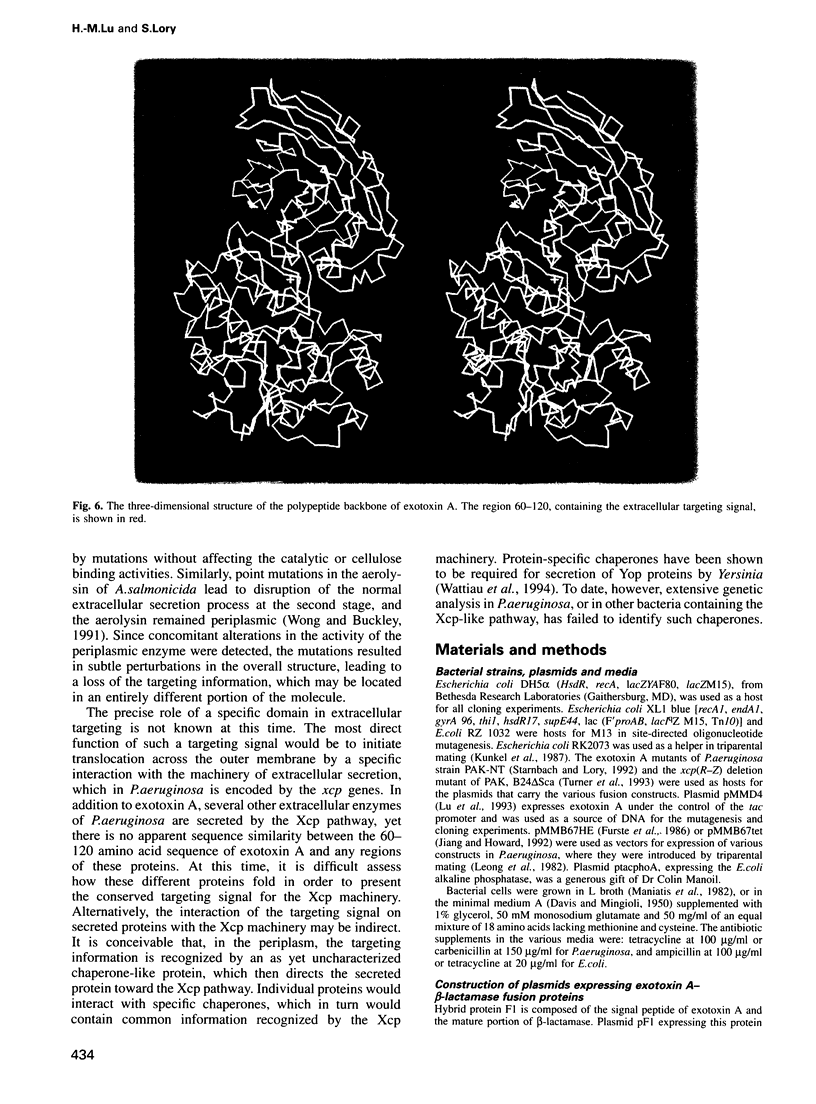
A number of Gram-negative bacteria, including Pseudomonas aeruginosa, actively secrete a subset of periplasmic proteins into their surrounding medium. The presence of a putative extracellular targeting signal within one such protein, exotoxin A, was investigated. A series of exotoxin A truncates, fused to beta-lactamase, was constructed. Hybrid proteins, which carry at their N- termini 120, 255, 355 or the entire 613 residues of the mature exotoxin A, were stable and were secreted into the extracellular medium. Hybrid proteins which carry residues 1-30 and 1-60 of the mature exotoxin A were unstable; however, they could be detected entirely within the cells after a short labeling period. A hybrid with beta-lactamase was constructed which carried only the N-terminal residues 1-3 and region 60-120 of exotoxin A. It was also secreted into the culture medium, suggesting that a specific 60 amino acid domain contains the necessary targeting information for translocation of exotoxin A across the outer membrane. The secretion of the hybrid proteins is independent of the passenger protein, since a similar exotoxin A-murine interleukin 4 hybrid protein was also secreted. The extracellular targeting signal between amino acids 60 and 120 is rich in anti-parallel beta-sheets. It has been shown previously to be involved in the interaction of the exotoxin A with the receptors of the eukaryotic cells. In the three- dimensional view, the targeting region is on the toxin surface where it is easily accessible to the components of the extracellular secretion machinery.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allured V. S., Collier R. J., Carroll S. F., McKay D. B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bally M., Filloux A., Akrim M., Ball G., Lazdunski A., Tommassen J. Protein secretion in Pseudomonas aeruginosa: characterization of seven xcp genes and processing of secretory apparatus components by prepilin peptidase. Mol Microbiol. 1992 May;6(9):1121–1131. doi: 10.1111/j.1365-2958.1992.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Bortoli-German I., Brun E., Py B., Chippaux M., Barras F. Periplasmic disulphide bond formation is essential for cellulase secretion by the plant pathogen Erwinia chrysanthemi. Mol Microbiol. 1994 Feb;11(3):545–553. doi: 10.1111/j.1365-2958.1994.tb00335.x. [DOI] [PubMed] [Google Scholar]
- Chaudhary V. K., Xu Y. H., FitzGerald D., Adhya S., Pastan I. Role of domain II of Pseudomonas exotoxin in the secretion of proteins into the periplasm and medium by Escherichia coli. Proc Natl Acad Sci U S A. 1988 May;85(9):2939–2943. doi: 10.1073/pnas.85.9.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G. R. Yersinia pathogenicity factors. Curr Top Microbiol Immunol. 1994;192:243–263. doi: 10.1007/978-3-642-78624-2_11. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Guidi-Rontani C., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: evidence that domain I functions in receptor binding. Mol Microbiol. 1987 Jul;1(1):67–72. doi: 10.1111/j.1365-2958.1987.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Hamood A. N., Olson J. C., Vincent T. S., Iglewski B. H. Regions of toxin A involved in toxin A excretion in Pseudomonas aeruginosa. J Bacteriol. 1989 Apr;171(4):1817–1824. doi: 10.1128/jb.171.4.1817-1824.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst T. R., Holmgren J. Conformation of protein secreted across bacterial outer membranes: a study of enterotoxin translocation from Vibrio cholerae. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7418–7422. doi: 10.1073/pnas.84.21.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs M., Mattick J. S. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol Microbiol. 1993 Oct;10(2):233–243. doi: 10.1111/j.1365-2958.1993.tb01949.x. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Kageyama M. Purification and properties of a binding protein for branched-chain amino acids in Pseudomonas aeruginosa. J Bacteriol. 1980 Mar;141(3):1055–1063. doi: 10.1128/jb.141.3.1055-1063.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B., Howard S. P. The Aeromonas hydrophila exeE gene, required both for protein secretion and normal outer membrane biogenesis, is a member of a general secretion pathway. Mol Microbiol. 1992 May;6(10):1351–1361. doi: 10.1111/j.1365-2958.1992.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leong S. A., Ditta G. S., Helinski D. R. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. J Biol Chem. 1982 Aug 10;257(15):8724–8730. [PubMed] [Google Scholar]
- Lory S. Determinants of extracellular protein secretion in gram-negative bacteria. J Bacteriol. 1992 Jun;174(11):3423–3428. doi: 10.1128/jb.174.11.3423-3428.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lory S., Strom M. S., Johnson K. Expression and secretion of the cloned Pseudomonas aeruginosa exotoxin A by Escherichia coli. J Bacteriol. 1988 Feb;170(2):714–719. doi: 10.1128/jb.170.2.714-719.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. M., Mizushima S., Lory S. A periplasmic intermediate in the extracellular secretion pathway of Pseudomonas aeruginosa exotoxin A. J Bacteriol. 1993 Nov;175(22):7463–7467. doi: 10.1128/jb.175.22.7463-7467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn D. N., Lory S. Components of the protein-excretion apparatus of Pseudomonas aeruginosa are processed by the type IV prepilin peptidase. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):47–51. doi: 10.1073/pnas.89.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbye L. J., Sandkvist M., Bagdasarian M. Genes required for extracellular secretion of enterotoxin are clustered in Vibrio cholerae. Gene. 1993 Sep 30;132(1):101–106. doi: 10.1016/0378-1119(93)90520-d. [DOI] [PubMed] [Google Scholar]
- Pohlner J., Halter R., Beyreuther K., Meyer T. F. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. 1987 Jan 29-Feb 4Nature. 325(6103):458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- Poquet I., Faucher D., Pugsley A. P. Stable periplasmic secretion intermediate in the general secretory pathway of Escherichia coli. EMBO J. 1993 Jan;12(1):271–278. doi: 10.1002/j.1460-2075.1993.tb05653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993 Mar;57(1):50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P. Translocation of a folded protein across the outer membrane in Escherichia coli. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12058–12062. doi: 10.1073/pnas.89.24.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., d'Enfert C., Reyss I., Kornacker M. G. Genetics of extracellular protein secretion by gram-negative bacteria. Annu Rev Genet. 1990;24:67–90. doi: 10.1146/annurev.ge.24.120190.000435. [DOI] [PubMed] [Google Scholar]
- Py B., Chippaux M., Barras F. Mutagenesis of cellulase EGZ for studying the general protein secretory pathway in Erwinia chrysanthemi. Mol Microbiol. 1993 Mar;7(5):785–793. doi: 10.1111/j.1365-2958.1993.tb01169.x. [DOI] [PubMed] [Google Scholar]
- Salmond G. P., Reeves P. J. Membrane traffic wardens and protein secretion in gram-negative bacteria. Trends Biochem Sci. 1993 Jan;18(1):7–12. doi: 10.1016/0968-0004(93)90080-7. [DOI] [PubMed] [Google Scholar]
- Starnbach M. N., Lory S. The fliA (rpoF) gene of Pseudomonas aeruginosa encodes an alternative sigma factor required for flagellin synthesis. Mol Microbiol. 1992 Feb;6(4):459–469. doi: 10.1111/j.1365-2958.1992.tb01490.x. [DOI] [PubMed] [Google Scholar]
- Strom M. S., Lory S. Structure-function and biogenesis of the type IV pili. Annu Rev Microbiol. 1993;47:565–596. doi: 10.1146/annurev.mi.47.100193.003025. [DOI] [PubMed] [Google Scholar]
- Strom M. S., Nunn D., Lory S. Multiple roles of the pilus biogenesis protein pilD: involvement of pilD in excretion of enzymes from Pseudomonas aeruginosa. J Bacteriol. 1991 Feb;173(3):1175–1180. doi: 10.1128/jb.173.3.1175-1180.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., Filloux A., Bally M., Murgier M., Lazdunski A. Protein secretion in Pseudomonas aeruginosa. FEMS Microbiol Rev. 1992 Sep;9(1):73–90. doi: 10.1016/0378-1097(92)90336-m. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner L. R., Lara J. C., Nunn D. N., Lory S. Mutations in the consensus ATP-binding sites of XcpR and PilB eliminate extracellular protein secretion and pilus biogenesis in Pseudomonas aeruginosa. J Bacteriol. 1993 Aug;175(16):4962–4969. doi: 10.1128/jb.175.16.4962-4969.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C. Secretion across the bacterial outer membrane. Trends Genet. 1992 Sep;8(9):317–322. doi: 10.1016/0168-9525(92)90264-5. [DOI] [PubMed] [Google Scholar]
- Wattiau P., Bernier B., Deslée P., Michiels T., Cornelis G. R. Individual chaperones required for Yop secretion by Yersinia. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10493–10497. doi: 10.1073/pnas.91.22.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick M. J., Frank D. W., Storey D. G., Iglewski B. H. Structure, function, and regulation of Pseudomonas aeruginosa exotoxin A. Annu Rev Microbiol. 1990;44:335–363. doi: 10.1146/annurev.mi.44.100190.002003. [DOI] [PubMed] [Google Scholar]
- Wong K. R., Buckley J. T. Site-directed mutagenesis of a single tryptophan near the middle of the channel-forming toxin aerolysin inhibits its transfer across the outer membrane of Aeromonas salmonicida. J Biol Chem. 1991 Aug 5;266(22):14451–14456. [PubMed] [Google Scholar]









